Abstract
Objective:
Autologous matrix-induced chondrogenesis (AMIC) is a 1-step cartilage restoration technique that combines microfracture with the use of an exogenous scaffold. This matrix covers and mechanically stabilizes the clot. There have been an increasing number of studies performed related to the AMIC technique and an update of its use and results is warranted.
Design and methods:
Using the PubMed database, a literature search was performed using the terms “AMIC” or “Autologous Matrix Induced Chondrogenesis.” A total of 19 basic science and clinical articles were identified.
Results:
Ten studies that were published on the use of AMIC for knee chondral defects were identified and the results of 219 patients were analyzed. The improvements in Knee Injury and Osteoarthritis Outcome Score, International Knee Documentation Committee Subjective, Lysholm and Tegner scores at 2 years were comparable to the published results from autologous chondrocyte implantation (ACI) and matrix ACI techniques for cartilage repair.
Conclusions:
Our systematic review of the current state of the AMIC technique suggests that it is a promising 1-stage cartilage repair technique. The short-term clinical outcomes and magnetic resonance imaging results are comparable to other cell-based methods. Further studies with AMIC in randomized studies versus other repair techniques such as ACI are needed in the future.
Keywords: cartilage, microfracture, autologous matrix-induced chondrogenesis (AMIC)
Introduction
Marrow stimulating techniques have been used to treat cartilage defects since 1959 when Pridie1 introduced subchondral drilling. Steadman et al.2 refined this with microfracture techniques to avoid heat necrosis from drilling. The bleeding from the subchondral bone forms a clot that contains growth factors and attracts ingrowth of mesenchymal stem cells (MSCs). The clot induces a repair that covers the cartilage defect with a combination of fibrous and hyaline-like cartilage.3,4
The limitation for marrow stimulation techniques is that the bone marrow stem cells and growth factors are released into the joint rather than being contained at the site of the defect. It is also suggested that the newly formed clot is not mechanically stable to withstand tangential forces.5
Some authors have modified the traditional marrow stimulating techniques to enhance its efficacy; by combining it with an intraarticular injection of platelet-rich plasma (PRP) or in vitro manipulated MSCs.6-9
Autologous matrix-induced chondrogenesis (AMIC) is a novel technique of cartilage restoration. It is a one-step procedure that combines microfracture with the fixation of a biological scaffold, such as a porcine collagen matrix. This matrix covers the blood clot, permitting the ingrowing of MSCs to differentiate into the chondrogenic lineage. The matrix acts as a temporary structure to allow the cells to be seeded and establish a 3-dimensional structure.
Over the past few years, there has been an increasing number of reports of AMIC-like techniques used for cartilage resurfacing in the knee and ankle joint.10,11 There has also been a variation in the surgical techniques, the type of matrix used, and the addition of supplementary growth factors and exogenous cell transplant. Thus, it is necessary to discuss the differences in these variants in AMIC techniques.
Our aim is to review the basic science rationale, the various techniques and results of AMIC for knee cartilage repair. We also hope that this can be a basis for standardization during reporting of surgical techniques and comparing outcomes of AMIC in future.
Methods
Using the PubMed database a literature search was performed using the terms “AMIC” or “Autologous Matrix Induced Chondrogenesis.” A total of 11 publications published in English were identified with this initial search.10,12-20 Following this, the summary and references of each publication were reviewed to identify other relevant studies. Another 8 studies were identified and included in this review.21-28
In total, we identified 4 technical notes explaining AMIC surgical techniques,10,12,15,19 10 outcome reports of the AMIC technique for chondral defects in the knee5,13,14,17,18,23,24,26-28 and 5 basic science studies on the AMIC technique16,29-31,34 The full text of these publications were reviewed and summarized by the primary author for this review.
Basic Science Rationale
Gille et al.29 initially proposed that the use of a matrix provides a stimulus for chondrogenic differentiation. Benthien and Behrens10 also proposed that covering the chondral defect with a matrix after microfracture concentrates the MSCs and growth factors released from the bone marrow. Kramer et al.30 reported from their studies that when a matrix was used, the bone marrow cells were contained by the collagen matrix. This was based on their in vivo study where they could regularly isolate MSCs from the matrix.30
Tallheden et al.25 reported that MSCs from the microfracture had the same phenotypic plasticity as chondrogenic cells in the cartilage basal zone. They found that 1 cm3 of blood from a microfracture hole had 8,000 CD34+ MSCs. With AMIC, MSCs were distributed on the rough part of the membrane and the membrane acted as the roof of a “biological chamber.”25
Knee surgeons have made use of different scaffolds for the AMIC technique in cartilage repair. The ideal scaffold should mimic biology, architecture, and structural properties of the native tissue, facilitating cell infiltration, attachment, proliferation, and differentiation. It should first support tissue formation and then gradually be replaced by the regenerating tissue with no harmful breakdown products released.
There are different types of scaffolds available: natural protein–based or carbohydrate-based scaffolds, and synthetic scaffolds. The 3 scaffolds that have been reported in the literature for AMIC are ChondroGide (Geistlich Biomaterials, Wolhausen, Switzerland), Hyalofast (Fidia Advanced Biopolymers, Padua, Italy), and Chondrotissue (BioTissue, Zurich, Switzerland). There are other commercially available collagen and alginate scaffolds that have been used for cartilage repairs that are reported in the literature.32,33
ChondroGide (Geistlich Biomaterials, Switzerland)
The porcine-derived type I/III collagen membrane ChondroGide is the commonest type of matrix used. This is a protein-based natural bilayer collagen matrix that exists as a porous cell adhesive and a smooth cell occlusive layer.
The cell adhesive layer ensures that the MSCs are attached to the collagen fibers for the proliferation of stem cells and the differentiation into chondrocytes. The second cell occlusive nonporous layer of ChondroGide makes sure that the super clot remains in the defect.
Gille et al.29 has shown that cells grown on ChondroGide form a multilayered apical cell sheet with chondrocyte-like cells. The ChondroGide matrix (collagen I/III) will be resorbed within 6 to 24 weeks after implantation. They reported from laboratory studies that the collagen I/III has better properties for chondrogenesis compared with collagen II matrices.29
Breinan et al.34 reported the results of using a collagen type II scaffold for management of articular cartilage defects in their canine study. They found that total defect fill was best with the AMIC group, compared with 2 other groups that had only microfracture done and the other that was seeded with chondrocytes.34
Gigante et al.16 reported that chondral lesions treated with AMIC augmented with bone marrow concentrate had a nearly normal morphologic appearance at second-look arthroscopy. The second-look biopsies from 5 patients with isolated medial femoral condyle lesions (mean size 3.7 cm2) at 12 months postsurgery had a mean histological score of 60.16 (International cartilage Repair Society [ICRS] II histology score range 0-100)
Chondrotissue (BioTissue, Switzerland)
Chondrotissue is an absorbable polyglycolic acid (PGA) textile treated with hyaluronan.31 In AMIC, the Chondrotissue scaffold acts as a sponge which holds the clot and MSCs within the defect.31
This hypothesis was reinforced by the work of Erggelet et al.21 who evaluated the combined use of microfracture and Chondrotissue matrix against microfracture alone for cartilage resurfacing in an ovine model. Six months after implantation, the histological scores documented improvement with the AMIC technique, compared with the microfracture group. They showed that the AMIC group had repair tissue with type II collagen and was rich in proteoglycans compared with the microfracture group that had fibrocartilage repair tissue.21
Hyalofast (Fidia Advanced Biopolymers, Italy)
Hyalofast is made from semisynthetic derivative of hyaluronic acid. Hyaluronic acid has been shown to induce MSCs from the bone marrow to differentiate along the chondrogenic lineage.9,35
Hyalofast is nonwoven, porous, 3-dimensional structure consisting of 10- to 15-µm thick fibers. The interstices of variable sizes allow cell contact, cluster formation, and extracellular matrix deposition.36 It also helps entrap the MSCs. It is resorbed following the breakdown pathway of endogenous hyaluronic acid. Buda et al.27 and Vannini et al.28 used the Hyalofast matrix for in AMIC combined with PRP and MSCs.
Surgical Technique
The original AMIC technique was described by Benthien and Behrens10,12 and performed via an arthrotomy. The surgery begins with an arthroscopy to verify the size and location of the defect. Their indications for AMIC are symptomatic full thickness chondral or osteochondral lesions that do not exceed 1.5 cm2. For osteochondral lesions, they recommend harvesting the cancellous bone from the proximal tibia and impacted into the defect before the scaffold is attached on the defect.
After an assessment of the defect, the chondral lesion is debrided till a stable shoulder surrounds the cartilage defect (Figure 1). Microfracture was performed in the original technique using microfracture awls.12 Other authors describe subchondral drilling using 1.1 mm K-wires where multiple holes are drilled at 5-mm intervals37 (Figure 2).
Figure 1.
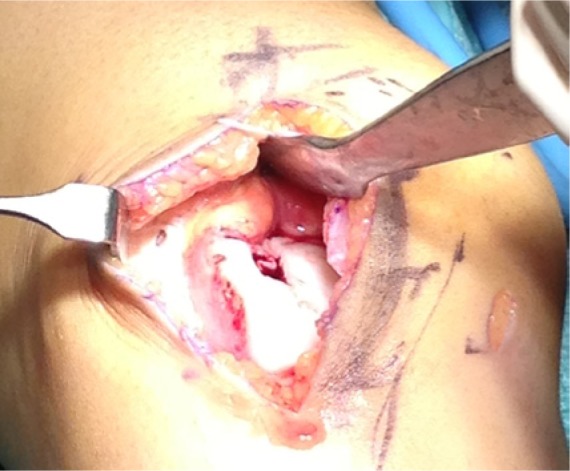
Preparation of the defect with stable shoulders around the defect.
Figure 2.
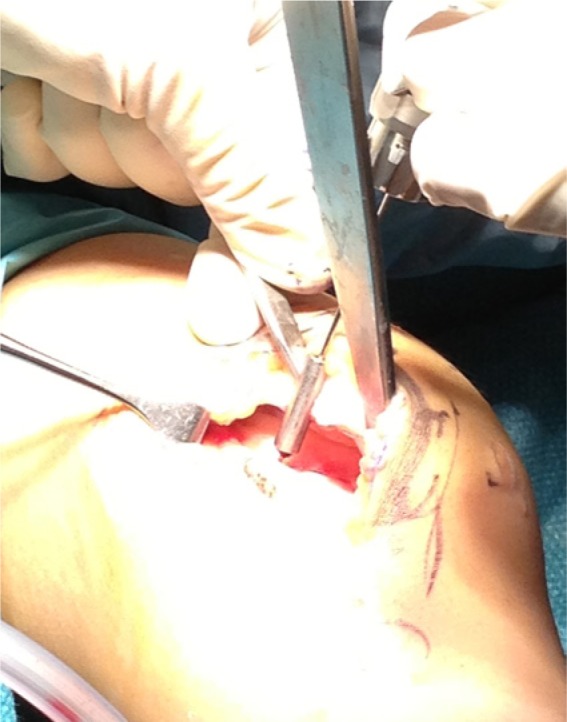
Subchondral drilling with K-wires.
The matrix is trimmed to the size of the defect. Most authors use a size that is slightly smaller than the actual lesion and others make multiple patches to cover the defect. If multiple patches are used, the patches are overlapped over each other. The ChondroGide matrix is moistened with physiological saline, and the Chondrotissue matrix is immersed in autologous serum10,19 (Figure 3).
Figure 3.
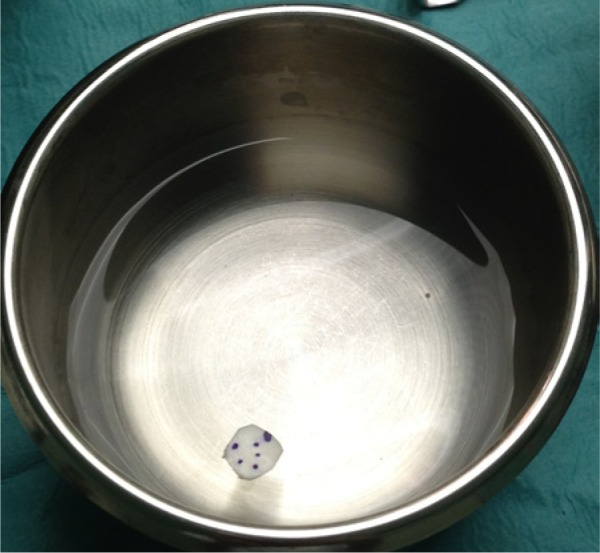
Preparing the ChondroGide matrix prior to implantation. Note the dots placed on the surface to indicate the nonporous side (ie, the side not on bone).
The bilayer ChondroGide matrix is placed in the defect with the porous surface of the membrane (rough) is facing the bone surface. Fibrin glue (Tissucol, Baxter, Warsaw, Poland) is applied over the membrane-covered areas and left for 5 minutes to set10,12 (Figures 4 and 5). The knee is then brought through a series of flexion and extension movements to check the stability of matrix in the defect before wound closure.
Figure 4.
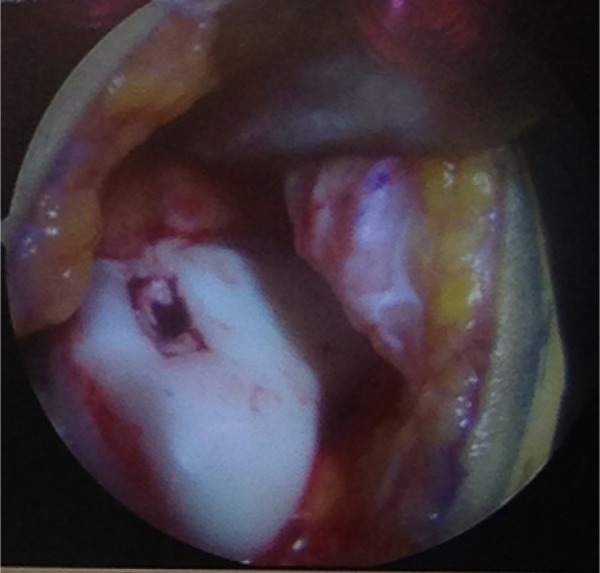
Defect covered with the ChondroGide matrix.
Figure 5.
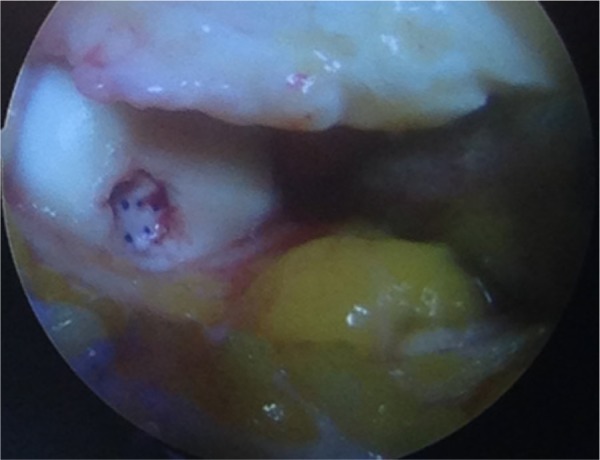
Femoral condyle defect with a ChondroGide matrix.
Some authors have reported the use of sutures to prevent matrix from being dislodged.14 However, Hunziker and Stähli38 in their studies have suggested that suturing of the articular cartilage can induce local damage, with histology changes seen that are similar to the early osteoarthritis.
The mechanical stability of some scaffolds, such as Chondrotissue allows ease of handling and secure fixation with resorbable pins.30 Zantop et al.39 recommended the use of 1 or 2 biodegradable pins to fix the matrix when AMIC is used in larger chondral defects.
For the authors who use Hyalofast for AMIC, their technique is often supplemented with bone marrow–derived MSCs and PRP.27,28 They harvest 60 mL of bone marrow aspirate from the posterior iliac crest and this is processed with a cell separator to obtain 6 mL of bone marrow–derived MSCs. Together with PRP centrifuged from the patients’ blood, these MSCs and growth factors are delivered onto the Hyalofast matrix and inserted into the defect.
Piontek et al.19 described an all-arthroscopic variant to the AMIC technique. The authors make use of a punch to establish vertical shoulders at the site of the cartilage defect. The subchondral drilling of the lesion is performed via a dry arthroscopy.19 After an estimation of the lesion size, the matrix is inserted under dry arthroscopy. Fibrin glue is next applied to the membrane covered surface and then allowed to set.
In the postoperative phase, some authors recommend knee immobilization for a period of time.17 In addition, other authors limit the knee to non-weightbearing for between 2 and 6 weeks13,14,17,24,26-28 and Kusano et al.18 allowed their patients partial weightbearing of 15 kg for 6 weeks. Like other cartilage repair techniques, most authors only allow high-impact activities after 6 months and return to sports only after 1 year.13,14,17,24,26-28
Clinical Results
Ten studies that were published on the use of AMIC for knee chondral defects and the results of 219 patients are summarized in Table 1.5,13,14,17,18,23,24,26-28 The first 6 reviewed studies made use of ChondroGide as the matrix.5,13,17,18,23,26
Table 1.
Summary of Clinical Studies on AMIC for knee chondral defects.
| Authors | No. | Male (M)/Female (F) | Age (Years) | Follow-up (Months) | Technique Drilling/Scaffold/Fixation | Location | No. of Osteochondral Lesions | Defect Size (cm2) | KOOS/Lysholm/Tegner/VAS/IKDC | MRI Evaluation/MOCART | Additional Surgery | Rehabilitation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gille et al.26 | 57 | 38 M/ 19 F | 37.3 | 24 | Awl ChondroGide fibrin | 32 MFC, 6 LFC, 4 T, 15 P | No mention | 3.4 (1-12) | Lysholm pre:50 post: 85.2 VAS pre: 7 post: 2 |
— | 5 osteotomy | — |
| Kusanoet al.18 | 38 | 23 M/17 F | 25.9 | 28.2 | Awl ChondroGide suture/fibrin | 16 MFC, 4 LFC, 20 P | 11 | 3.87 | Tegner pre: 3.0 post: 4.3 IKDC pre: 47.7 post:76.5 |
— | — | PWB 6 wk 0-60 4 wks |
| Panni et al.5 | 17 | 10 M/7 F | 39 | 36 | Awl ChondroGide | — | No mention | No mention | Lysholm pre: 38 post:74 IKDC pre: 32 post:82 |
— | — | — |
| Pascarella et al.23 | 19 | 12 M/7 F | 26 | 24 | Wire drilling ChondroGide fibrin | 63% MFC, 28% LFC, 9 % P | 9% | No mention | Lysholm pre: 54 post:98 IKDC pre: 30 post: 83 |
53% showed significant reduction in defect area | 2 osteotomy | |
| Dhollander et al.14 | 5 | 3 M/2 F | 27 | 24 | Awl ChondroGide PRP sutures | 5 P | Nil | 2 (1-3) | KOOS pre: 208 post: 357 Tegner pre: 2 post: 3 VAS pre: 52 post: 14 |
12 mo: 53%, 24 mo: 53% | 3 ostetotomy | NWB 2 wk 0-90 4 wk FROM 8 wk RTS 12 mo |
| Siclariet al.24 | 52 | 20 M/32 F | 44.3 | 12 | Wire drilling Chondrotissue PRO Pin (MFC) | 12 MFC, 31 MTP, 9 LTP | Nil | 2.75 (1.5-5.0) | KOOS pre: 198 post: 320 | — | — | NWB 2 wk, PWB 2 wk, FWB 4 wk |
| Dhollander et al.13 | 5 | 4 M/1 F | 36 | 24 | Awl Chondrotissue Pin | 2 MFC, 2 LFC, 1 T | 1 | 2.3 (1.5-5.0) | KOOS pre: 188 post: 365 Tegner pre: 3 post: 4 VAS pre: 72 post: 50 |
12 mo: 67%, 24 mo: 67% | — | NWB 2 wk, 0-90 4 wk FROM 8 wk, RTS 12 mo |
| Budaet al. 27 | 20 | 12 M/8 F | <50 | 24 | No drilling Hyalofast PRP BMA | 14 MFC, 4 LFC, 2 both | Nil | No mention | KOOS pre: 47.1 post 93.3 IKDC pre: 32.9 post: 90.4 |
14 complete repair, 4 hypertrophy, 2 incomplete | 3 osteotomy | NWB 4 wk, run 6 mo, RTS, 12 mo |
| Vanniniet al.28 | 6 | 4 F/2 M | 16 | 36 | No drilling Hyalofast PRP BMA | — | All | 4.6 (±1.5) | IKDC pre: 61 post 96 | 4 complete, 2 hypertrophic | — | NWB 4 wk, run 6 mo, RTS, 12 mo |
Abbreviations: MRI, magnetic resonance imaging; MFC, medial femoral condyle; LFC, lateral femoral condyle; MTP, medial tibial condyle; LTP, lateral tibial plateau; T, troclear; P, patella; NWB, non-weightbearing; PWB, partial weightbearing; FWB, full weightbearing; KOOS, Knee Injury and Osteoarthritis Outcome Score; VAS, visual analog scale; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue; IKDC, International Knee Documentation Committee; PRP, platelet-rich plasma; BMA, bone marrow aspirate; RTS, return to sports.
In 2010, Gille et al.17 reported the results of 32 chondral lesions in 27 patients treated with AMIC at a mean of 37 months. In their technique, they kept the patients on protected weightbearing for 6 weeks. They reported significant improvement in Lysholm, Tegner, and ICRS scores that was observed as early as 12 months after AMIC. The postoperative magnetic resonance images (MRIs) showed moderate to complete filling in most cases. They also reported that male patients had significantly higher ICRS scores than female patients.
Gille et al.26 evaluated 57 patients at 2 years after surgery as part of the ChondroGide AMIC Registry. This registry group studied included their original series of patients.17 Using Lysholm and visual analog scale (VAS) scores, they reported significant improvement in both parameters. They concluded that AMIC was a safe and effective method of treating symptomatic chondral defects in the knee.26
Kusano et al. evaluated the clinical and MRI outcomes of 38 patients treated with AMIC, at a mean of 29 months (13-51 months) after surgery. Even though they reported that the tissue fill was not complete in most cases, they found significant improvements in IKDC, Lysholm, Tegner, and VAS pain scores. The largest improvements were found in the osteochondral subgroup.18
Panni et al.5 prospectively evaluated 17 patients treated with AMIC for focal cartilage defects. At a mean follow-up of 36 months, the subjective IKDC and Lysholm scores improved from 32 to 82 and from 38 to 74, respectively. The follow-up MRIs showed a reduction of the defect area in 59% of the cases.
Pascarella et al.23 reported that the preoperative IKDC score of 30 improved to 83 and the preoperative Lysholm score of 54 improved to 98 at 24 months after AMIC surgery in 19 patients. Ten postoperative MRIs (53% of cohort) showed a significant reduction of the chondral defect area.23 Dhollander et al.13 also reported improvements in Knee Injury and Osteoarthritis Outcome Score (KOOS), VAS, and Tegner scores in their 2-year results of AMIC combined with PRP for the treatment of patellar cartilage defect, using the ChondroGide collagen matrix.
Siclari et al.24 evaluated 52 patients that had arthroscopic AMIC with Chondrotissue and PRP for articular cartilage defects of the knee. The KOOS subscores improved for pain (from 55 to 91), symptoms (from 57 to 88), activities of daily living (from 69 to 86), sports and recreation (from 36 to 70), and quality of life (from 38 to 73). The histologic evaluation showed a homogenous hyaline-like cartilage repair tissue.24
Dhollander et al.13 reviewed their results of 5 patients treated with the AMIC using the Chondrotissue (BioTissue, Switzerland) matrix fixed with a bioabsorbable screw. They found that the patients had a gradual clinical improvement in KOOS, VAS, and Tegner activity scale during the 2-year follow-up.
Using the Hyalofast matrix, Buda et al.27 and Vannini et al.28 published the outcome studies of their version of AMIC. Buda et al.27 reported improved IKDC and KOOS scores as well as 70% complete repairs of the defects on follow-up MRIs at 24 months. Vannini et al.28 reported the use of this technique for the treatment of 6 osteochondritis dissecans with large improvements in IKDC scores and 4 out of 6 complete repairs on follow-up MRIs at 3 years.
Discussion
Knutsen et al.40 had reported that microfracture techniques produce results comparable to autologous chondrocyte implantation (ACI). However, Mithoefer et al.41 found that the initial improved knee function after microfracture was not sustained. They suggested that the shortcomings of microfracture were limited hyaline repair tissue, variable repair cartilage volume, and gradual functional deterioration. With AMIC surgery, Gille et al.17 reported that the results remained stable up to 60 months.
The initial results of ACI by Peterson et al.42 reported good to excellent outcomes in 76.5% of patients. Various authors have also reported good outcomes in 72% to 87% of patients treated with ACI and matrix ACI (MACI) for chondral defects.42-45 From the results of 10 AMIC studies, the improvements in KOOS, Subjective IKDC, Lysholm, and Tegner scores at 2 years were comparable to published results from ACI and MACI techniques.5,13,14,17,18,23,24,26-28
These encouraging outcome results and the concept of a single-stage cartilage resurfacing technique are attractive for many sports surgeons. However, there are many factors from a basic science standpoint that are not completely understood in AMIC.
The cartilage repair from AMIC is postulated to be from MSC chondrogenesis. De Girolamo et al.15 studied bone marrow samples, from iliac crest and microfracture sites; 0.04% of concentrated bone marrow cells harvested from the iliac crest had MSC phenotype, compared to 0.02% at microfractures sites.15
In ACI, the chondrocyte density required for treatment is 106 cells per cm2.46 The cell density required for chondrogenesis from MSC is thought to be higher than ACI.30,40, 46 These studies highlight the benefit of using a matrix to concentrate the MSCs within the defect in the AMIC technique.
Using a rabbit model comparing microfracture awls versus subchondral drilling, Chen et al.47,48 reported that subchondral drilling does not have a deleterious effect on the subchondral bone. Instead it helps gain better access to the bone marrow stroma.47,48 The authors also suggested that drilling to a depth of 6 mm had superior results in an animal model compared with drilling to 2 mm.47
The ideal scaffold for cartilage resurfacing is still a matter of debate. When we look at the outcome studies of these 3 matrices: ChondroGuide, Chondrotissue, and Hyalofast, they all have comparable clinical results as well as follow-up MRI. However, the ChondroGide matrix is more well studied and has the most published literature when compared with the other 2 matrices.
From the basic science perspective, various authors suggest that hyaluronic acid–enriched microenvironment seems to induce chondrogenesis.49,50 Wu et al.49 found that the addition of hyaluronic acid to a poly-l-lactic-co-glycolic acid (PLGA) scaffold produces higher levels of gylcoaminoglycans and collagen type II compared with those cultures in a PLGA scaffold. Erickson et al.50 reported that the use of hyaluronic acid hydrogel led to a high density of MSC seeding and the engineered cartilage had native tissue properties.
However, Welsch et al.20 has shown that the repair tissue was better with the collagen based scaffold than the hyaluronic acid–based scaffold at 2 years of follow-up based on MRI scoring and T2 mapping. In addition, Gille et al.29 tested the biomechanical behavior stability of PGA and PLGA, collagen membranes and gel matrices by loading in tension until failure. They found that the PGA scaffolds withstood the highest failure loads with all fixation techniques, compared with the PLGA scaffold and collagen membranes.29
There has been a trend toward the use of PRP and MSC to supplement the AMIC technique. This has led to some confusion as these were called AMIC plus techniques. PRP has shown to stimulate cartilage repair after microfracture in a sheep model.51
In their rabbit animal model, Qi et al.52 reported that the group with PRP and collagen scaffold had the largest amount of cartilage repair tissue compared with the group with the collagen scaffold alone. They suggested that the addition of PRP could possibly resurface a large chondral defect.52 The other question that needs to be answered is the type of PRP that has best chondrogenesis. Filardo et al.53 found similar improvements in the subjective scores at 12 months after intra-articular injection of single and double spinning of PRP for osteoarthritis.
From our review, we find that there is a need to standardize reporting of the AMIC technique. This will enable future comparison to determine the efficacy of these techniques and determine if various technical variations can potentially affect outcomes. The technical factors that should be reported are
arthroscopic or open surgery
method of subchondral drilling or microfracture
type of matrix or scaffold used
matrix is seeded with PRP or bone marrow stem cells
fixation of the matrix or scaffold
postoperative rehabilitation program
In addition, we suggest that the outcome measures should be standardized. The commonest outcome instruments are the KOOS, Lysholm, Tegner, and IKDC scoring. We suggest that these 4 instruments should be used in all studies at a minimum at 1 year and 2 years to allow comparison.
Most studies use the MRI protocol for cartilage assessment such as the modified MOCART score by by Marlovits et al.54,55 This should be continued to allow for comparison of follow-up radiographic studies. The modified MOCART score takes into account that subchondral lamina perforation is part of the AMIC technique and its integrity cannot be evaluated as suggested in the original MOCART scoring.
The key limitation for AMIC is its use in larger defects. Minas et al.41 also reported that the results of ACI in defects that had prior microfracture failed at 3 times the rate of nontreated defects. The authors suggest that marrow stimulation techniques may have a negative effect on subsequent ACI surgery. Therefore, AMIC should be used judiciously in larger cartilage defects that may require future treatment with ACI. As such, the authors do not recommend the AMIC technique in large cartilage defects.
Conclusion
Our review of the current state of the AMIC technique suggests that this is a promising cartilage resurfacing technique. The outcome scores and MRI results are comparable to other cell-based cartilage methods. It should be pointed out that we still lack understanding on the ideal conditions for chondrogenesis.
Footnotes
Acknowledgment and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Pridie K. A method of resurfacing osteoarthritic knee joints. J Bone Joint Surg Br. 1959;41(3):618-9. [Google Scholar]
- 2. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 3. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 4. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982; 64(3):460-6. [PubMed] [Google Scholar]
- 5. Panni SA, Cerciello S, Vasso M. The management of knee cartilage defects with modified AMIC technique: preliminary results. Int J Immunopathol Pharmacol. 2011;24(1 Suppl. 2):149-52. [DOI] [PubMed] [Google Scholar]
- 6. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-37. [DOI] [PubMed] [Google Scholar]
- 7. Jakob RP. AMIC technique for cartilage repair: a single-step surgical intervention as compared to other methods. Eur Cell Mater. 2006;12(Suppl. 1):26.16941384 [Google Scholar]
- 8. Lee KB.1,Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects—a porcine model. Stem Cells. 2007;25(11):2964-71. [DOI] [PubMed] [Google Scholar]
- 9. Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, Jee CS, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27(4):493-506. [DOI] [PubMed] [Google Scholar]
- 10. Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC). A one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg. 2010;76(2):260-3. [PubMed] [Google Scholar]
- 11. Wiewiorski M, Leumann A, Buettner O, Pagenstert G, Horisberger M, Valderrabano V. Autologous matrix-induced chondrogenesis aided reconstruction of a large focal osteochondral lesion of the talus. Arch Orthop Trauma Surg. 2011;131(3):293-6. [DOI] [PubMed] [Google Scholar]
- 12. Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316-9. [DOI] [PubMed] [Google Scholar]
- 13. Dhollander AA, Verdonk PC, Lambrecht S, Almqvist KF, Elewaut D, Verbruggen G, et al. The combination of microfracture and cell-free polymer based implant immersed with autologous serum for cartilage defect coverage. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1773-80. [DOI] [PubMed] [Google Scholar]
- 14. Dhollander AA, De Neve F, Almqvist KF, Verdonk R, Lambrecht S, Elewaut D, et al. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg Sports Traumatol Arthrosc. 2010;19(4):536-42. [DOI] [PubMed] [Google Scholar]
- 15. De Girolamo L, Bertolini G, Cervellin M, Sozzi G, Volpi P. Treatment of chondral defects of the knee with one-step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41(11):1172-7. [DOI] [PubMed] [Google Scholar]
- 16. Gigante A, Calcagno S, Cecconi S, Ramazzotti D, Manzotti S, Enea D. Use of collagen scaffold and autologous bone marrow concentrate as a one-step cartilage repair in the knee: histological results of second-look biopsies at 1 year follow-up. Int J Immunopathol Pharmacol. 2011;24(1 Suppl. 2):69-72. [DOI] [PubMed] [Google Scholar]
- 17. Gille J, Schuseil E, Wimmer J, Gellissen J, Schulz AP, Behrens P. Mid-term results of autologous matrix induced chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18:1456-64. [DOI] [PubMed] [Google Scholar]
- 18. Kusano T, Jakob RP, Gautier E, Magnussen RA, Hoogewoud H, Jacobi M. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2109-15. [DOI] [PubMed] [Google Scholar]
- 19. Piontek T, Ciemniewska-Gorzela K, Szulc A, Naczk J, Slomczykowski M. All-arthroscopic AMIC procedure for repair of cartilage defects of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Welsch GH, Mamisch TC, Zak L, Blanke M, Oik A, Marovits S, et al. Evaluation of cartilage repair tissue after matrix associated autologous chondrocyte transplantation using a hyaluronic-based or collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38(5):934-42. [DOI] [PubMed] [Google Scholar]
- 21. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe L, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res. 2009;27(10):1353-60. [DOI] [PubMed] [Google Scholar]
- 22. Knecht S, Erggelet C, Endres M, Sittinger M, Kaps C, Stüssi E. Mechanical testing of fixation techniques for scaffold-based tissue-engineered grafts. J Biomed Mater Res B Appl Biomater. 2007;83(1):50-7. [DOI] [PubMed] [Google Scholar]
- 23. Pascarella A, Ciatti R, Pascarella F, Latte C, Di Salvatore MG, Liguori L, et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):509-13. [DOI] [PubMed] [Google Scholar]
- 24. Siclari A, Mascaro G, Gentili C, Cancedda R, Boux E. A cell-free scaffold-based cartilage repair provides improved function hyaline-like repair at one year. Clin Orthop Related Res. 2012;470(3):910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tallheden T, Dennis JE, Lennon DP, Sjögren-Jansson E, Caplan AI, Lindahl A. Phenotypic plasticity of human articular chondrocytes. J Bone Joint Surg Am. 2003;85A(Suppl 2):93-100. [DOI] [PubMed] [Google Scholar]
- 26. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcomes of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC registry. Arch Orthop Trauma Surg. 2013;133(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one step repair technique with bone marrow derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2-11. [DOI] [PubMed] [Google Scholar]
- 28. Vannini F, Battaglia M, Buda R, Cavallo M, Giannini S. “One step” treatment of juvenile osteochondritis dessecans in the knee: clinical results and T2 mapping characterisation. Orthop Clin North Am. 2012;43(2):237-44. [DOI] [PubMed] [Google Scholar]
- 29. Gille J, Meisner U, Ehlers EM, Müller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005;37(5):339-48. [DOI] [PubMed] [Google Scholar]
- 30. Kramer J, Böhrnsen F, Lindner U, Behrens P, Schlenke P, Rohwedel J. In vivo matrix-guided human mesenchymal stem cells. Cell Mol Life Sci. 2006;63(5):616-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patrascu JM, Freymann U, Kaps C, Poenaru DV. Repair of a post-traumatic cartilage defect with a cell-free polymer-based cartilage. J Bone Joint Surg Br. 2010;92(8):1160-3. [DOI] [PubMed] [Google Scholar]
- 32. Crawford DC, Deberadino TM, Williams RJ., 3rd NeoCart, an autologous cartilage tissue implant, compared to microfracture for treatment of distal femoral lesions: an FDA phase II prospective trial after two years. J Bone Joint Surg Am. 2012;94(11):979-89. [DOI] [PubMed] [Google Scholar]
- 33. Dhollander AA, Verdonk PC, Lambrecht S, Verdonk R, Elewaut D, Almqvist KF. Midterm results of the treatment of cartilage defects in the knee using alginate beads containing human allogenic chondrocytes. Am J Sports Med. 2012;40(1):75-82. [DOI] [PubMed] [Google Scholar]
- 34. Breinan HA, Martin SD, Hsu HP, Spevctor M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18:781-9. [DOI] [PubMed] [Google Scholar]
- 35. Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36(6):431-8. [DOI] [PubMed] [Google Scholar]
- 36. Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, et al. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42(2):172-81. [DOI] [PubMed] [Google Scholar]
- 37. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in surgical management. Knee Surg Sports Arthrosc. 2010;18(4):434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hunziker EB, Stähli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16(9):1067-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zantop T, Petersen W. Arthroscopic implantation of a matrix to cover large chondral defect during microfracture. Arthroscopy. 2009;25(11):1354-60. [DOI] [PubMed] [Google Scholar]
- 40. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 41. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 42. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 43. Minas T. Autologous chondrocyte implantation for focal chondral defects in the knee joint. Clin Orthop Relat Res. 2001;(391 Suppl):S349-61. [DOI] [PubMed] [Google Scholar]
- 44. Mithöfer K, Petersen L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-46. [DOI] [PubMed] [Google Scholar]
- 45. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36(12):2336-44. [DOI] [PubMed] [Google Scholar]
- 46. Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J. Transplantation of cartilage-like tissue made by tissue engineering in treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84(4):571-584 [DOI] [PubMed] [Google Scholar]
- 47. Chen H, Hoemann CD, Sun J, Chevrier A, McKee MD, Shive MS, et al. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. 2001;29(8):1178-84. [DOI] [PubMed] [Google Scholar]
- 48. Chen H, Sun J, Hoemann C, Lascau-Coman V, Ouyang W, Trankhanh N, et al. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009;11:1432-8. [DOI] [PubMed] [Google Scholar]
- 49. Wu SC, Chang JK, Wang CK, Wang GJ, Ho ML. Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials. 2010;31(4):631-4. [DOI] [PubMed] [Google Scholar]
- 50. Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012;8(8):3027-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Milano G, Sanna Passino E, Deriu L, Careddu G, Manunta L, Manunta A, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects. An experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971-80. [DOI] [PubMed] [Google Scholar]
- 52. Qi YY, Chen X, Jiang YZ, Cai HX, Wang LL, Song XH, et al. Local delivery of autologous platelet in collagen matrix stimulated in situ articular cartilage repair. Cell Transplant. 2009;18(10):1161-9. [DOI] [PubMed] [Google Scholar]
- 53. Filardo G, Kon E, Pereira Ruiz MT, Vaccaro F, Guitaldi R, Di Martino A, et al. Platelet-rich plamsa intra-articular injections for articular cartilage degeneration and osteoarthritis: single versus double spinning approach. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):2082-91. [DOI] [PubMed] [Google Scholar]
- 54. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 55. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. [DOI] [PubMed] [Google Scholar]


