Abstract
Background:
The 3-dimensional autologous chondrocyte transplantation (ACT3D) comprises isolation of chondrocytes from cartilage biopsies, cultivation to spheroids, and transplantation into the cartilage defect.
Objectives:
To evaluate the patients’ general health and functionality and to assess the defect repair after ACT3D with spheroids by MRI and MOCART scoring.
Methods:
Thirty-seven patients with isolated chondral lesions of the knee underwent ACT3D with spheroids through medial arthrotomy. Patient-administered scores were assessed at baseline (day before transplantation), at 6 weeks, and at 3, 6, and 12 months. MRI and MOCART scoring were performed at 3 and 12 months after ACT3D.
Results:
Patients were diagnosed with full-thickness patellofemoral (n = 16), femoral condylar (n = 18), or both defect types (n = 3), International Cartilage Repair Society (ICRS) grade 3 or 4, with defect sizes between 1.0 and 12.0 cm2. On average, 59.5 spheroids/cm2 in defect size were transplanted. An overall statistically significant improvement from baseline to 12 months was observed for all assessment scores (Lysholm, International Knee Documentation Committee [IKDC], SF-36, Tegner) combined with a significant reduction in the visual analog scale (VAS) for pain and an advanced defect filling. Subgroup analyses revealed a positive clinical outcome independent on defect size, defect locations, spheroid dosage, age, duration of symptoms, and severity of complaints at baseline. Seven patients experienced in total 8 adverse events, of which knee joint effusion and blocking were assessed as possibly or probably related to ACT3D.
Conclusions:
The patient-administered assessment scores along with the fast defect filling with ACT3D using spheroids demonstrated an increase in activity level and quality of life after a 1-year follow-up.
Keywords: autologous chondrocytes, spheroids, ACT, ACT3D, Lysholm, IKDC, SF-36, Tegner, VAS, cartilage defect
Introduction
Articular cartilage is an avascular, aneural tissue that has poor regenerative capacity compared to other mesenchymal tissues. As a consequence, adult articular chondral defects often progress into osteoarthritis.1 Articular cartilage injuries are a common orthopedic problem, affecting more than 1 million people per year in the United States.2 As severe and chronic forms of knee cartilage damage, osteoarthritis is one of the most common disabling disorders, affecting more than 10% of the Western population.3 Approximately 175,000 total knee replacement operations are performed annually in Germany as a consequence of osteoarthritis.4 Reduced joint function and pain often result in the disability to work or to follow daily life activities. Generally, clinical efficacy of any repair method should therefore demonstrate the improvement of clinical symptoms together with a hopefully structural repair as surrogate parameters for the prevention of osteoarthritis.5 The filling of the defect with hyaline-like repair cartilage tissue is proposed to be an essential factor in long-term clinical success of cartilage repair methods.6,7
One of the approaches seen to be most successful in longitudinal analyses is the autologous chondrocyte transplantation (ACT).8,9 The principle of ACT is based on the biopsy from healthy cartilage containing the patient’s own chondrocytes, which are in vitro cultured and subsequently transplanted into the cartilage defect. Arthroscopic findings after ACT are known to display favorable filling with repair tissue, good adherence to underlying bone, seamless integration with adjacent cartilage, and stiffness close to that of the adjacent tissue.10,11 ACT is regarded to achieve a high tissue quality in cartilage repair as the implantation of autologous cultured chondrocytes into a chondral defect may replace damaged cartilage with hyaline or hyaline-like cartilage.6,12,13 Marrow stimulation techniques such as microfracture14 aim to provide fibrocartilaginous repair tissue that however seems to develop, in a long-term sense, mechanical properties inferior to those of hyaline cartilage as it has been shown to degenerate over time due to shear forces.15,16 Thus, microfracture is recommended only in smaller, isolated cartilage defects (1-3 cm2) in young, active patients.17
ACT products have been used since 1987 (first generation) to treat cartilage defects.12 A further development is the 3-dimensional autologous chondrocyte transplantation (ACT3D). Here, 3-dimensional chondrocyte constructs or structures, either purely autologous or associated with scaffolds, are transplanted, offering several advantages.18-20 The 3-dimensional structure is known to be important for growth and is assumed to contribute to the phenotypic stability of chondrocytes. However, due to the xenogenous fixation materials in many products, there might be a risk of immune responses, infections, or healing problems. It was reported in a goat model that the suturing of articular cartilage in the femoral groove induced severe local damage, which was progressive and reminiscent of that associated with the early stages of osteoarthritis. Compared to control regions, chondrocytes were lost from the perisutural area, and the fissures did not heal.21
Another approach was the development of the autologous 3-dimensional chondrocyte product co.don chondrosphere (co.don AG, Teltow, Germany), which has been marketed in Germany and other European countries since 2004. co.don chondrosphere consists of human autologous spheroids in 0.9% NaCl suspension and is used for transplantation into isolated cartilage defects of joints. The spheroids derive from human autologous chondrocytes, which can produce cartilage-specific matrix and are able to build a 3-dimensional structure under defined cell culture conditions. The major advantage of these spheroids is that they are purely autologous in a sense that neither during the manufacturing process nor for the transplantation is xenogenous material is used, which reduces the risk of rejections or incompatibilities or viral contaminations. While chondrocyte cell suspensions do not start differentiating and secreting matrix proteins until their transplantation into the cartilage defect, the chondrocytes in the spheroids are in a more advanced differentiation state already before being transplanted. Due to the higher differentiated state and a pronounced formation of extracellular matrix, the spheroids may provide a faster refilling of the defect with hyaline cartilage than the cell suspension products.22,24 It could be demonstrated in in vitro investigations as well as in the minipig animal model that the spheroids start fusing already 1 day after application to the cell culture dish or bone plate, respectively. This fusion construct is the start for repair tissue. In vivo, this tissue develops to hyaline cartilage. A special feature of co.don chondrosphere is also the adhesion and direct contact of spheroids to the native cartilage tissue without any additional matrices or scaffolds as described above.23 The chondrogenic potential of the spheroids was demonstrated in in vivo animal studies as well as functional in vitro tests by detection of respective hyaline cartilage–specific markers.17,24 In the SCID mouse model, the differentiation of spheroid chondrocytes as well as the adhesion and integration of spheroids into native cartilage after subcutaneous transplantation was observed.25 An integration of the spheroids into generated cartilage lesions, no gaps between cartilage and spheroids, and direct cell-matrix contacts could be observed after 12 weeks.
Since 2004, co.don chondrosphere has been used in clinical practice for the treatment of chondral defects in the knee up to 10 cm2 and in some cases even up to 15 cm2, classified as Outerbridge or International Cartilage Repair Society (ICRS) grade 3 or 4. However, a systematic evaluation of the improvement in functionality, mental and physical health, quality of life, and tolerability in association with the cartilage repair after transplantation of co.don chondrosphere has not yet been performed.
Regarding the locations of the cartilage defect in the knee, most clinical studies with classic ACT were performed to treat defects on the femoral condyle, although recently, published long-term results demonstrate that ACT with the use of periosteum is a valid treatment for patellofemoral lesions.26,27 Because of the 3-dimensional nature of co.don chondrosphere, it was suggested that this product may also be suitable for defect locations in the knee other than at the femoral condyle. Particularly for patellofemoral defects, ACT3D offers a promising treatment option without the use of periosteum with favorable results at short- and long-term follow-up.28
Therefore, this investigator-initiated trial (IIT) was conducted to include patients from the daily practice with cartilage defects with a broader range regarding age, defect size, defect, body mass index (BMI), and spheroid dosage when compared to the stringent conditions of a randomized controlled phase III clinical trial (RCT). The objective of this IIT was to evaluate the patients’ subjective assessments for general health and functionality before and at 6 weeks and 3, 6, and 12 months after transplantation. Furthermore, the results of MRI are presented, having been taken before and 3 and 12 months after transplantation in order to assess cartilage defects after treatment with co.don chondrosphere.
Methods
This prospective IIT was conducted at the University Medical Center Mannheim, University of Heidelberg (Germany), according to the principles of the Declaration of Helsinki (World Medical Association). Approval was obtained by the local independent ethics committee. The University Medical Center Mannheim is authorized for withdrawal of starting material for donation and procurement according to directive 2004/23/EC.
Participants
Thirty-seven patients with focal chondral lesions of the knee were screened and selected for the IIT between 2008 and 2010. From all participating patients, a signed informed consent form was obtained. Twenty-two men and 15 women were included in the IIT and underwent ACT3D with spheroids (co.don chondrosphere) (Table 1). A final assessment was performed at 12 months after treatment.
Table 1.
Demographic Data and Baseline Characteristics
| Patient data | Variables |
|---|---|
| Patients, n | 37 |
| Gender, male/female, n | 22/15 |
| Age, mean (range), y | 35.5 (15-53) |
| ≤30 | 11 |
| 30-40 | 16 |
| >40 | 10 |
| Body mass index, mean (range), n = 30a | 26.9 (14.0-44.8) |
| Defect size, mean (range), cm2, n = 36a | 4.4 (1.0-12.0) |
| Duration of symptoms before inclusion, months, n = 29a | 27.7 (2-132) |
| ≤12 | 16 |
| >12 | 13 |
| Defect locations, n (%) of patients | 37 (100) |
| Patellofemoral | 16 (43.2) |
| Femoral condyle | 18 (48.6) |
| Both defect types in the same patient | 3 (8.1) |
| Left knee | 20 (54.1) |
| Right knee | 17 (45.9) |
| Defect size, n (%) of patients, cm2 | 37a (100) |
| ≤3.0 | 16 (43.2) |
| 3.1-5.0 | 7 (18.9) |
| >5.0 | 13 (35.1) |
Data were not assessed for 1 patient.
Treatment with the Investigational Product
The treatment with spheroids, as with any other ACT product, is a 2-step surgical procedure requiring, at the first step, a diagnostic arthroscopy. For cultivation of the autologous chondrocytes, cartilage biopsies were taken during arthroscopy from the medial femoral condyle rim outside of the loaded area with a 6-mm gauge.
In addition, approximately 240 mL of blood was taken from the patient to obtain serum for the cultivation of the chondrocytes. The advantage of using the patient’s own blood for obtaining serum for chondrocyte cultivation is that no addition of antibiotics, antimycotics, growth factors, and other allogenic or xenogenic substances (e.g., fetal calf serum) is required.
The tissue biopsy and blood samples were transported to co.don AG (Teltow, Germany), a Good Manufacturing Practice (GMP)–approved facility, for manufacturing of the spheroids. For this purpose, chondrocytes were isolated from the biopsies and cultured under defined cell culture conditions, until they had developed to 3-dimensional spheroids. From clinical experience with doses of co.don chondrosphere, a range of 10 to 70 spheroids/cm2 of defects was applied in clinical practice in the majority of cases treated since 2004. Thus, this dose was aimed for being transplanted in this clinical trial, which requires a total cultivation period between 5 and 10 weeks, depending on the individual proliferation behavior of the cells. The spheroids were then harvested, washed, and transferred in physiological saline solution directly into a sterile syringe, which was placed aseptically in sterile packaging and transported back to the clinics in a box with wet ice. The transplantation (surgical step 2) of the spheroids was performed using medial arthrotomy at the knee. After debridement of the defect and complete removal of any cartilage tissue and calcified cartilage within the defect area, the spheroids were applied. In most cases, no bleeding from the subchondral bone plate was seen after debridement. A few patients showed minimal bleeding of the subchondral plate, and spheroids were applied after the bleeding was stopped. After approximately 20 minutes, the attachment of the spheroids to the bone plate had occurred, and additional covering was not required.23,29
Endpoints
Primary endpoint was the subjective improvement of symptoms and functionality and the repair of the cartilage defect at 12 months after ACT3D compared to baseline (day before transplantation).
Secondary endpoints were
clinical assessments of knee functionality and subjective patient-reported outcome at 6 weeks and 3 and 6 months after ACT3D compared to baseline, and
the change of knee functionality and subjective patient-reported outcome from baseline to 6 weeks, from baseline to 3 months, from 6 weeks to 3 months, from 3 to 6 months, and from 6 to 12 months.
Trial Conduct
At screening, inclusion and exclusion criteria were checked. Inclusion criteria were male and female patients between 15 and 53 years of age, isolated cartilage defects according to ICRS grades 3 and 4, presence of subchondral bone lamella at the medial and lateral femoral condyle and at the trochlea and retropatellar, cartilage surrounding the defect had to be intact, and informed consent form signed by the patient.
Patients were excluded from the IIT if they had defects on both knees at the same time, radiological signs of osteoarthritis, any signs of knee instability, rheumatoid arthritis, parainfectious or infectious arthritis, any condition after these diseases, cartilage lesion on the corresponding joint area, valgus or varus malalignment, total resection of the meniscus and partial resection of the meniscus with more than 50% resection of the meniscus diameter, alcohol or drug (medication) abuse, current diagnosis of human immunodeficiency virus (HIV-1, -2) and/or hepatitis C virus (HCV) infection, acute infectious diseases, autoimmune diseases, cancer, chronic heart diseases, endocrine or metabolic diseases, coagulation dysfunction, chondropathy, patellar dysplasia, any concomitant painful or disabling disease of the spine or hips or lower limbs that would interfere with the rehabilitation program, were pregnant or nursing, and were participating in concurrent clinical trials.
Demographic data, baseline characteristics, routine laboratory parameters, body temperature, HIV and hepatitis testing according to directive 2006/17/EC30, concomitant diseases, and medications/measures were recorded in the case report form (CRF). Lower extremities were investigated regarding ligament stability, relation of the axes, and appearance of the skin. MRI pictures and conventional radiographs not older than 8 weeks were used to assess the cartilage defect and to determine the eligibility of the patient for participating in this IIT.
On the day before arthroscopy, inclusion and exclusion criteria were checked again, and patients filled in the questionnaires to assess their current state of health, pain, and functionality. During arthroscopy, the cartilage defect was classified macroscopically according to ICRS grades, and biopsies and blood samples were taken for the cultivation of the chondrocytes as described above.
In a second surgical step after 5 to 10 weeks, patients were transplanted with their individual spheroids. After postsurgery immobilization for 48 hours, the patient had to follow a strict standardized rehabilitation program. Regular continuous passive motion (CPM) therapy was organized for up to 6 weeks for a minimum of 6 hours daily. The rehabilitation program included partial weightbearing for 6 weeks.
Patients visited the investigator at 6 weeks and 3, 6, and 12 months after ACT3D, where the clinical status was checked and blood samples were taken for laboratory analyses. The consumption of pain medication, any changes in other concomitant medications, tolerability of the treatment, and the occurrence of any adverse events (AEs) were recorded in the CRF.
MRI
High-resolution MRI of the respective knee was performed at 1.5 T (Magnetom Avanto, Siemens Healthcare Sector, Erlangen, Germany) using a circular, polarized 8-channel knee coil (Siemens Healthcare Sector) at 3 and 12 months after ACT3D. All examinations were performed in a feet-first supine position using established MRI sequences31 of a standard knee MRI protocol including proton density, fast spin echo, and gradient-echo acquisitions in sagittal, coronal, and axial orientations for cartilage and bone marrow evaluation.
MRI scans were obtained from 11 patients both at 3 and 12 months. Nine patients received MRI only at 3 months and 3 patients only at 12 months. The 9 patients at the 3-month follow-up were not willing to take additional MRI scans at the 12-month follow-up; 3 patients were lost at the 3-month follow-up. Seventeen patients were not scanned at all due to unwillingness to get additional MRI.
Cartilage Assessment
For the evaluation of the cartilage repair tissue, the previously published and validated classification system MOCART was used.32 The MOCART system includes 9 variables that are used to describe the morphology and signal intensity of the repair tissue compared to the adjacent native cartilage. Every parameter on the anonymous MRI pictures was assessed by 2 independent, experienced readers (one radiologist with 7 years of cartilage MRI experience, and one orthopedic surgeon with 10 years of cartilage MRI experience), and a certain score value was given to each patient. For every patient, a maximum score value was calculated, summarizing the 9 parameters for the first and second follow-up MRI examination after ACT3D.
Instruments for Subjective Assessments
The following subjective questionnaires were used to evaluate patient outcome at baseline, which was defined as baseline, and at 6 weeks and 3, 6, and 12 months after ACT3D: 1) Lysholm score assessing pain and functional parameters of daily living33; 2) International Knee Documentation Committee (IKDC) subjective knee evaluation form rating symptoms, sports activities, and function; 3) 100-mm visual analog scale (VAS) for pain; 4) Short Form Health Survey (SF-36) evaluating the general health with mental and physical components; and 5) Tegner score covering activities in daily life and recreational and competitive sports.33
Safety
The AEs were assessed with regard to seriousness, intensity, and causality and classified by system organ class (SOC) and preferred term (PT) according to MedDRA version 13.1.
Statistical Analysis
For statistical analysis, PASW Statistics version 18.0 (IBM SPSS Statistics, Ehningen, Germany) was used. Changes from baseline (day before transplantation) to final assessment at 12 months were statistically evaluated using the Mann-Whitney U test. Significances of differences between the subgroups were analyzed with the Kruskal-Wallis test. P values <0.05 were considered as statistically significant. Data are presented as median (range) or as mean ± standard deviation (SD) as indicated.
Results
Baseline Characteristics
Thirty-seven patients (22 men, 15 women) aged between 15 and 53 years were included in the study for treatment with the spheroids (Table 1). For the 15-year-old male patient, closed epiphyses were confirmed by conventional x-rays; therefore, this patient was assessed as eligible for the treatment with ACT3D.
Patients were diagnosed with full-thickness patellofemoral defects, femoral condylar defects, or both types of defects in the knee. The majority of patients had defect sizes either ≤3 cm2 (n = 16) or >5 to 12 cm2 (n = 13). Seven patients had defect sizes between 3 and 5 cm2. For one patient, the defect size was not assessed. At screening, all defects were classified as Outerbridge grade 3 (fissuring to the level of subchondral bone in an area with a diameter more than 1.5 cm) or grade 4 (exposed subchondral bone). The duration of symptoms due to the cartilage defect (pain, impairment in general health, and daily life functions) varied between 2 months and 11 years. Sixteen patients had symptoms for ≤12 months, whereas 13 patients had symptoms for more than 12 months before inclusion in this IIT. For 8 patients, no information was available.
Before consultation at the University Hospital, 17 patients had no operations relating to their cartilage defect or regarding any other complaints in the affected knee. The other patients had already undergone surgical treatments (anterior cruciate ligament [ACL] reconstruction, meniscal surgery, or high tibial osteotomy) (Table 2). In total, there were 15 patients with 1, 3 patients with 2, 1 patient with 3, and 1 patient with 6 previous surgical treatments.
Table 2.
Operations Performed in the Affected Knee before Consultation of the Patients at the University Hospital
| Type of previous operation | Patients, n (%) |
|---|---|
| None | 17 (45.9) |
| Arthroscopy | 3 (8.1) |
| Surgical treatment of the cartilage (e.g., smoothing, abrasion, microfracture, mosaicplasty) | 5 (13.5) |
| ACL reconstruction | 3 (8.1) |
| ACL reconstruction + meniscal surgery (e.g., suturing, partial resection) | 2 (5.4) |
| Meniscal surgery (e.g., suturing, partial resection) | 6 (16.2) |
| High tibial osteotomy | 1 (2.7) |
| Total | 37 (100.0) |
Note: ACL = anterior cruciate ligament.
During the first surgical step, that is, the diagnostic arthroscopy, 5 patients received additional treatments in the affected knee: ACL reconstruction in 1 patient, meniscal surgery (e.g., suturing, partial resection) in 1 patient, and high tibial osteotomy in 3 patients.
Operation Time and Additional Treatments of the Affected Knee
The operation time for the ACT3D was on average 60.12 minutes (range, 39-148 minutes) and varied depending on the additional surgeries in the operated knee. During the transplantation of the spheroids, lateral release was performed in 2 patients, ACL reconstruction in 1 patient, and meniscal surgery in 1 patient. The operation time for 1 of the 2 patients with the ACL reconstruction took 148 minutes. Duration of the other operations varied between 39 and 95 minutes.
Spheroid Dosage
On average, 59.5 spheroids/cm2 in defect size were transplanted. Doses varied between 13.8 and 170.0 spheroids/cm2. Retrospectively, patients were subdivided into 3 groups to analyze the subjective improvement in symptoms and function depending on the transplanted spheroid dose. Nine patients (25.0%) received ≤35.0 spheroids/cm2, while 15 patients (41.7%) were transplanted with 35.1 to 60.0 spheroids/cm2 and 12 patients (33.3%) with >60 to 170.0 spheroids/cm2.
MRI Assessment of Cartilage Repair with the MOCART Score
Assessments of the defect filling using MRI and 2-dimensional MOCART score were performed by 2 independent readers in 20 patients at 3 months after ACT3D and in 14 patients at 12 months after ACT3D (Table 3). Both readers gave higher scorings for all subdomains at 12 months as compared to 3 months after transplantation. In particular, reader 1 assessed a significant improvement in synovitis (subdomain 9) from 3 to 12 months, while reader 2 assessed a significant improvement in the subdomains 3 and 4 describing the surface and the structure of the repair tissue, respectively. A nearly complete integration was observed, and the defect filling was assessed with 15 of 20 points. However, the overall increase of the MOCART total score from 3 to 12 months after transplantation was not significant for the assessments of both readers, suggesting that the defect repair at 3 months was already at an advanced stage. Of a maximum score of 100 points, the scores reached 68.9 ± 27.56 and 71.1 ± 25.2 points, respectively, after 12 months (Table 3). Examples of MRI pictures of 2 patients at 3 and 12 months after ACT3D are presented in Figure 1. The evaluations of the subchondral lamina and bone marrow edema are part of the MOCART score and reflected by 5 points for each criterion. After 1 year, bone marrow edema was still present in 8 patients. The subchondral lamina was not intact in 6 patients and intact in 8 patients.
Table 3.
Assessment of Defect Repair with the MOCART Score at 3 and 12 Months after Transplantation of Spheroids
| MOCART score | ||||
|---|---|---|---|---|
| Subdomains (points) | 3 months after transplantation: R1 | 3 months after transplantation: R2 | 12 months after transplantation: R1 | 12 months after transplantation: R2 |
| 1. Degree of defect repair and filling (0-20) | 12.25 ± 6.38 | 13.25 ± 7.3 | 15.35 ± 6.03 | 14.66 ± 7.18 |
| 2. Integration to border zones (0-15) | 12.25 ± 3.79 | 11.75 ± 4.06 | 12.14 ± 4.25 | 12.5 ± 3.25 |
| 3. Surface (0-10) | 6 ± 3.47 | 6.31 ± 3.66 | 7.85 ± 3.23 | 8.57 ± 2.34* |
| 4. Structure of the repair tissue (0-5) | 1.75 ± 2.44 | 1.25 ± 2.22 | 2.85 ± 2.56 | 3.21 ± 2.48* |
| 5. Signal intensity of the repair tissue: | ||||
| Dual T2-FSE (0-15) | 7.22 ± 4.60 | 7.22 ± 4.91 | 9.16 ± 6.33 | 9.58 ± 5.82 |
| 3D-GRE-FSE (0-15) | 7.05 ± 4.69 | 8.23 ± 4.65 | 9.16 ± 6.68 | 10.41 ± 5.82 |
| 6. Subchondral lamina (0-5) | 3.94 ± 2.09 | 3.25 ± 2.44 | 3.21 ± 2.48 | 3.21 ± 2.48 |
| 7. Subchondral bone (0-5) | 1.75 ± 2.44 | 1.25 ± 2.22 | 1.78 ± 2.48 | 1.42 ± 2.34 |
| 8. Adhesions (0-5) | 4.5 ± 1.53 | 4.25 ± 1.83 | 5 ± 0 | 4.64 ± 1.33 |
| 9. Synovitis (0-5) | 4 ± 2.05 | 4 ± 2.05 | 5 ± 0* | 4.64 ± 1.33 |
| Total | 58.75 ± 20.25 | 60 ± 22.99 | 68.92 ± 27.46 | 71.07 ± 25.20 |
Note: Values in parentheses represent minimal and maximal possible points. R1 = reader 1; R2 = reader 2.
P < 0.05 (t test): significant difference to the 3-month assessment of reader 1.
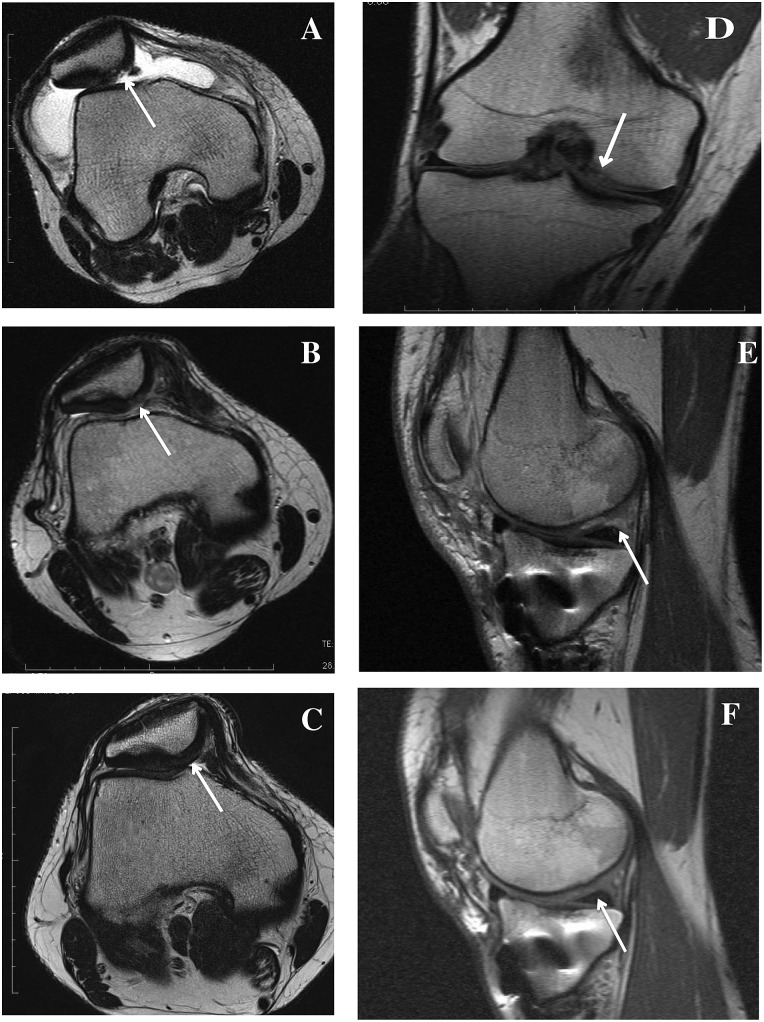
Figure 1.
MRI scans of the knee before and after ACT3D. (A-C) Transversal T2-weighted images of a 25-year-old man with a posttraumatic retropatellar defect before arthroscopy. A white arrow marks the defect (A). (B) Defect filling 3 months after ACT3D; there is a slightly hyperintense signal of the graft tissue. (C) The graft tissue is now only partially moderately hypointense compared to the adjacent cartilage and well integrated into the adjacent cartilage 12 months after ACT3D. (D-F) Coronal (D) and sagittal (E, F) T1-weighted MRI scans of a 37-year-old man with a full-thickness articular cartilage defect of the medial femoral condyle before treatment (white arrow) (D). (E) Image 3 months after ACT3D showing complete defect filling with smooth surface. (F) Image 1 year after ACT3D with complete integration of the graft.
Patient-Administered Assessment Scores
Clinical efficacy of ACT3D was investigated in terms of functional, symptomatic testing according to several clinical outcome scores addressing motility, pain, and postoperative physical burden as recommended by medical societies like the German Joint Working group on Tissue Regeneration and Tissue Replacement and the European Committee for Advanced Therapies (CAT).17,34 Also, socioeconomic and quality of life aspects were addressed. An overall statistically significant improvement from baseline to 12 months was observed for all scores assessed by the patients as it is shown in detail for each score in the following.
Lysholm score
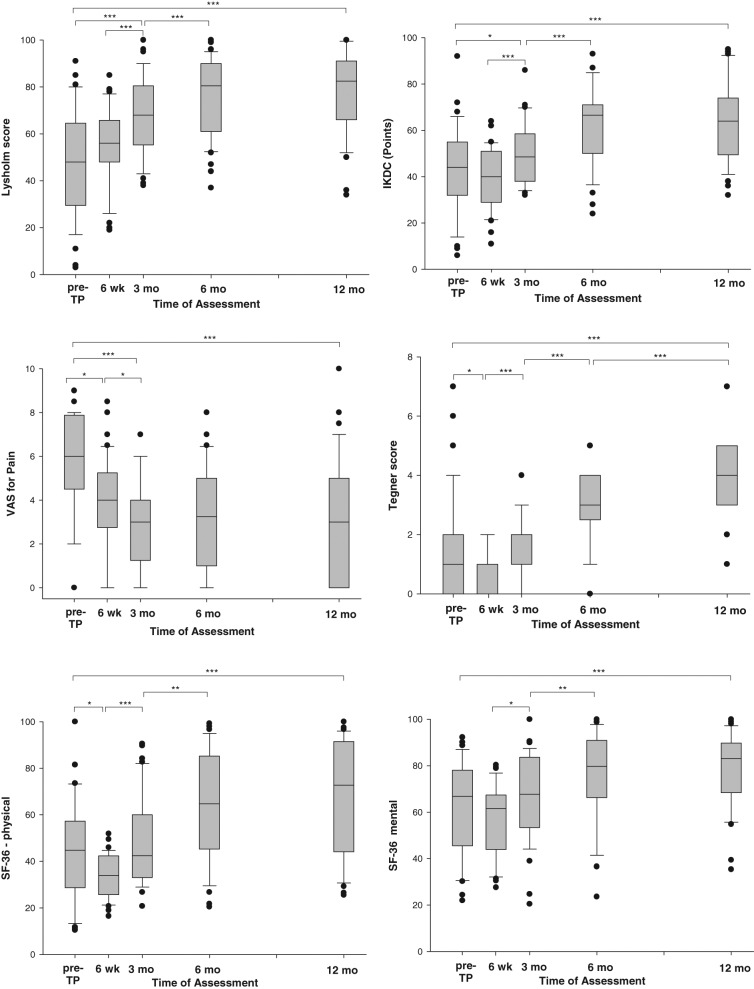
The median Lysholm score significantly increased from 48.0 (range, 3-91 points) at baseline to 82.5 (range, 34-100) points after 12 months (Fig. 2). The increase from baseline to 6 weeks after ACT3D was not significant. However, from baseline to 3 months, from 6 weeks to 3 months, and from 3 to 6 months after ACT3D, a further significant improvement in the Lysholm total score was observed. From 6 to 12 months, the level of responses remained constant; the slight increase was not significant.
Figure 2.
Changes in the self-administered assessment scores from the day before ACT3D (baseline) to the final assessment at 12 months. The boxes indicate the 25th and 75th percentiles; the error bars (whiskers) indicate the 10th and 90th percentiles. The bullet points mark the outlying values. wk = weeks; mo = months; pre-TP = day before transplantation. *P < 0.05. **P < 0.001. ***P < 0.0001.
IKDC
A significant increase of the median IKDC total score was observed from 44.0 points at baseline to 64.0 points at 12 months after ACT3D (Fig. 2). The strongest increase was observed between 3 and 6 months. Consistent with the Lysholm score, the maximal improvement was already reached after 6 months and remained at a constant level up to 12 months.
VAS for pain
When evaluating the assessments of the VAS for pain, a significant reduction in pain symptoms rated 6.0 points (median) before transplantation to 4.0 points (median) was reported by the patients already at 6 weeks after ACT3D (Fig. 2). The lowest level of pain with 3 points (median) was reached after 3 months and remained constant up to 12 months.
Tegner score
The total median Tegner score at baseline was 1.0 point with a range between 0 and 7 points. The total Tegner score from baseline to 12 months was significantly increased (Fig. 2). An improvement to 2.0 points was reported between 6 weeks and 3 months, with a further increase of this score to 3.0 points at 6 months and to 4.0 points at 12 months after ACT3D.
SF-36
Before transplantation, the majority of patients scored low for the total SF-36 (data not shown) and of physical and mental health (Fig. 2). After 12 months, significant improvement in total SF-36 as well as the physical and mental subscores was observed (P < 0.0001 for each score). A significant improvement in the physical and mental health was reported by all patients from 3 to 6 months after ACT3D. A slight increase in the scoring was observed for both subscores from 6 to 12 months, which however was not significant.
Subgroup Analyses
Duration of symptoms
The duration of symptoms before inclusion into the IIT was classified into 2 groups with ≤12 months (16 patients) or >12 months (13 patients) (Table 1). No statistically significant differences between the groups in responses at baseline and at posttransplantation assessment time points were observed for the changes of the Lysholm score, IKDC, VAS for pain, and SF-36 physical and SF-36 mental score (data not shown). For only the Tegner score, a significant difference was observed between both groups at 12 months after ACT3D. Those 16 patients with a duration of symptoms ≤12 months gave significantly higher ratings for their activity level (4.42 ± 1.16 points) than the 13 patients with a duration of symptoms >12 months (3.27 ± 1.1 points) (P = 0.032, Mann-Whitney test). This implies that 12 months after treatment with the spheroids, the level of sports and daily life activities of patients were less pronounced when symptoms in the affected knee persisted for more than 1 year.
Spheroid dosage
No statistically significant differences between the dose groups (≤35.0, 35.1-60.0, or >60-170.0 spheroids/cm2) regarding any of the subjective assessment scores were observed at any time (Fig. 3A). This demonstrates that spheroid dosage within the applied range did not seem to be a critical parameter for the improvement in general health, pain, functionality, and physical activity.
Figure 3.
Changes in the self-administered assessment scores from baseline to 12 months after ACT3D depending on the spheroid dosage (A), defect size (B), and defect location (C). Numbers on top of the bars indicate the numbers of patients.
Cartilage defect size
Patients were categorized in 3 subgroups with respect to defect size (≤3.0 cm2, 3.1-5.0 cm2, and >5.0 cm2) (Table 1). At baseline, patients with defect sizes >5.0 cm2 had a tendency to higher ratings for the mean Lysholm score, IKDC score, Tegner score, SF-36 physical score, and a lower VAS for pain rating compared with the mean values of these scores for the other defect size groups. This seems to illustrate a higher activity level and a lower level of symptoms in patients with larger defect sizes at baseline compared with the patients with smaller defect sizes. However, it is questionable whether this observation is of clinical relevance as the differences at baseline were not statistically significant for most scores; only the IKDC was significantly different between the groups.
A clear improvement in performance and reduction in pain and symptoms could be observed for all groups at 12 months after ACT3D (Fig. 3B). No statistically significant differences between these groups at 12 months after ACT3D were observed for all scores.
Location and number of cartilage defects
The subjective assessments were evaluated with respect to the defect locations at the femoral condyle or patellofemoral (Table 1). For the IKDC, patients with defects at the femoral condyle gave lower ratings compared with patients with patellofemoral defects at baseline, implying that they suffered from stronger complaints before transplantation. At 12 months after ACT3D, the relative change of all scores was comparable between these 2 groups (Fig. 3C). In the group with both defect types, the preoperative-postoperative differences for IKDC and SF-36 were less pronounced.
The analysis of IKDC, Tegner score, Lysholm score, VAS for pain, and SF-36 for physical and mental health revealed no statistically significant differences in the assessments between the defect location at any time point.
Age subgroups
For the purpose of this analysis, patients were divided into 3 age groups: ≤30, 30 to 40, and >40 years of age. No statistically significant differences between the age groups regarding any of the subjective assessment scores were found at 12 months after ACT3D (Table 4). However, a tendency towards a better outcome in younger patients ≤30 years old as compared to older patients could be observed, although it has to be considered that the group with the younger patients seemed to have a better health status indicated by higher functionality scorings and lower pain scoring at baseline compared to the other groups with older patients. Thus, the relative change from baseline to 12 months was comparable between the groups.
Table 4.
Changes in Self-Administered Assessment Scores from Baseline to 12 Months after Transplantation Depending on Age
| Age at time point of transplantation | |||||||
|---|---|---|---|---|---|---|---|
| ≤30 y | 31-40 y | >40 y | |||||
| Outcome variables | Time point of assessment | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) |
| Lysholm score | Baseline | 11 | 42.6 (29.4) | 14 | 55.3 (18.1) | 10 | 45.9 (22.8) |
| 12 mo | 10 | 81.2 (21.3) | 14 | 77.5 (18.3) | 6 | 73.7 (14.6) | |
| IKDC | Baseline | 9 | 53.8 (21.7) | 12 | 38.3 (17.0) | 9 | 37.5 (19.6) |
| 12 mo | 10 | 72.2 (18.8) | 14 | 63.0 (18.0) | 7 | 54.7 (11.8) | |
| VAS for pain | Baseline | 11 | 5.5 (3.1) | 14 | 5.4 (2.1) | 10 | 6.1 (2.2) |
| 12 mo | 10 | 2.5 (2.7) | 14 | 3.1 (3.2) | 6 | 4.6 (1.8) | |
| Tegner score | Baseline | 11 | 0.9 (1.6) | 14 | 1.2 (1.5) | 10 | 2.3 (2.5) |
| 12 mo | 10 | 4.2 (1.4) | 14 | 3.7 (1.1) | 6 | 3.5 (0.5) | |
| SF-36 physical | Baseline | 9 | 56.1 (23.6) | 12 | 38.2 (15.8) | 6 | 40.0 (27.0) |
| 12 mo | 10 | 75.0 (21.4) | 14 | 65.7 (26.9) | 7 | 59.8 (25.4) | |
| SF-36 mental | Baseline | 9 | 67.7 (15.7) | 12 | 64.1 (18.8) | 7 | 49.0 (26.9) |
| 12 mo | 10 | 80.1 (14.7) | 13 | 77.2 (18.5) | 7 | 78.1 (18.5) | |
Note: SD = standard deviation; IKDC = International Knee Documentation Committee; VAS = visual analog scale; SF-36 = Short Form (36) Health Survey.
IKDC ≤30 or >30
Patients were divided into 2 groups with regard to their IKDC ratings of ≤30 or >30 points at baseline. The majority of patients (n = 23) had an IKDC of >30, indicating a lower level of symptoms and a higher level of sports activities and function, while 7 patients had an IKDC ≤30. For 7 patients, no ratings are available. Consistent with the higher IKDC of >30, the baseline assessments for most of the other scores reflect less pronounced symptoms and a better functionality when compared with the patients with the IKDC ≤30.
Significant differences in all scores were observed between the groups at different assessment time points between baseline and 6 months. However, at 12 months after ACT3D, there was no longer any statistically significant difference between the groups detectable (Fig. 4).
Figure 4.
Changes in the self-administered assessment scores from baseline to 12 months after ACT3D depending on IKDC ≤30 versus >30 at baseline. IKDC = International Knee Documentation Committee; SD = standard deviation; SF-36 = Short Form (36) Health Survey; VAS = visual analog scale. *P < 0.05, Mann-Whitney U test. Numbers on top of the bars indicate the numbers of patients.
Adverse Events
All reported AEs and serious AEs (SAEs) during the first 12 months of the trial are presented as reported by system organ class (SOC) and preferred term (PT) according to MedDRA (version 13.1) (Table 5). Of 37 patients being treated with the spheroids, 7 patients experienced in total 8 AEs during the 12-month follow-up period.
Table 5.
Adverse Events (AEs) Reported during the 12-Month Time Course of the Investigator-Initiated Trial with the Spheroids in co.don chondrosphere
| AE (reported term) | MedDRA preferred term (PT) version 13.1 | MedDRA system organ class (SOC) version 13.1 | No. of patients with an AEa | Intensity of the AE | Seriousness of the AE | Relation to transplantation surgery | Causally related to co.don chondrosphere |
|---|---|---|---|---|---|---|---|
| Swelling | Swelling | General disorders and administration site conditions | 1 | Mild | Nonserious | Yes | No |
| Knee joint effusion | Joint effusion | Musculoskeletal and connective tissue disorders | 1 | Mild | Nonserious | Yes | Probable |
| Blocking | Joint lock | Musculoskeletal and connective tissue disorders | 2 | Moderate | Nonserious | Yes | Possible |
| Thrombosis (superficial) | Thrombophlebitis (superficial) | Vascular disorders | 1 | Moderate | Nonserious | Yes | No |
| Infection | Infection | Infections and infestations | 1 | Severe | Nonserious | Yes | No |
| Deep vein thrombosis | Deep vein thrombosis | Vascular disorders | 1 | Severe | Serious | Yes | No |
| Pulmonary embolism | Pulmonary embolism | Respiratory, thoracic, and mediastinal disorders | 1 | Severe | Serious | Yes | No |
A patient may have experienced more than one AE.
Two patients reported swelling, and 2 patients had knee joint effusion, both of mild intensity and up to 3 months after transplantation. Joint effusion was assessed as probably being related to the spheroid application.
Two patients had blocking of the knee joint in relation to loading, 1 patient at 6 months after transplantation, and 1 patient throughout the whole follow-up period. Blocking was assessed as moderate and possibly related to the treatment with spheroids. In the clinical examination, no movement restriction was raised. On MRI, there was no evidence for loose bodies or other causes, which could explain the blocking. One of these patients in addition had superficial vein thrombosis.
One patient experienced deep vein thrombosis directly after surgery, leading to pulmonary embolism. This 46-year-old female patient in addition had to undergo ACL surgery during the transplantation procedure. The duration of the transplantation was 148 minutes and thus took more time than the average duration of the transplantation procedures in this trial (average, 60.12 minutes). The thromboembolic event occurred during hospitalization after transplantation; however, the patient recovered within 3 months from this SAE. These SAEs had no causal relationship to the spheroid application but to the surgical procedure.
Discussion
The present IIT was conducted to prospectively evaluate the efficacy of the treatment with the 3-dimensional autologous chondrocyte product co.don chondrosphere with respect to mental and physical health, pain, functionality, and tolerability in a relatively heterogenous group of patients with isolated cartilage defects in the knee presenting at the hospital.
Special features of the spheroids in co.don chondrosphere are that they already contain chondrocytes in an advanced differentiation state, producing cartilage-specific matrix before being transplanted, and that adhesion and direct association of spheroids with the native cartilage tissue and the bone plate are possible without any additional materials for fixation.23 It was proposed that this treatment may accelerate the process of defect filling and consequently the improvement in functioning in daily life and sports activities.
MRI has become a widely used noninvasive method for the assessment of articular cartilage defects and repairs. The main advantages of MRI for cartilage imaging are not only its noninvasiveness but also its reproducibility and accuracy by using standardized MRI protocols.
The MOCART classification system includes 9 variables for the accurate description of cartilage repair tissue with high correlation to the clinical and arthroscopic outcome of the procedures.32 Its purpose is to categorize visual information on the cartilage transplant. This scoring system was used here as it is the best validated and internationally most widely accepted method to visually assess cartilage repair sites. Assessments of the defect filling using MRI and 2-dimensional MOCART score were performed by 2 independent readers. Both readers gave higher scorings at 12 months as compared to 3 months after transplantation. However, the overall increase of the MOCART total score from 3 to 12 months after transplantation was not significant for the assessments of both readers, which seems to indicate that the defect repair tissue has already developed to a large extent at 3 months. This corresponds to the observation that the maximal reduction of pain was reported after 3 months. It also corresponds to the outcome of the various patient scores, indicating pronounced improvement after 3 to 6 months regarding different parameters.
The results in the IIT demonstrate that all self-administered subjective assessment scores reached their maximal levels of improvement and minimal levels of complaints already at 6 months after treatment with the spheroids and, in case of pain reduction, even after 3 months. Other 3-dimensional products with scaffold-associated chondrocytes for cartilage repair were confirmed in a prospective cohort study to show a more rapid improvement in ACT3D-treated patients compared to conventional ACT.35 Particularly when timely IKDC score curves were compared, the curve of the ACT3D group in the study by Ferruzzi et al.35 reached the plateau earlier at about 18 months, while the curve for the ACT group reached a similar maximum level at 24 months.
Factors in the present IIT that may potentially have influenced the results of the ACT3D using spheroids were defect size, defect locations, spheroid dosage, age, duration of symptoms previous to the clinical trial, and severity of complaints.
Several clinical studies, some of them randomized and prospective, showed that conventional ACT and ACT3D provide one of the safest and most satisfying and reliable methods of cartilage reconstruction in adults with relatively large defects exceeding 4 cm2.7 The spheroids in co.don chondrosphere have been used for the treatment of chondral defects up to 10 cm2, classified in our clinical practice either as grade 3 or 4 Outerbridge or ICRS grades 3 and 4. In isolated cases, the spheroids have also successfully been used for the treatment of even larger defects up to 15 to 20 cm2. In the present IIT, 13 patients with defect sizes >5 cm2, including 2 patients with defect sizes of even 11.2 and 12 cm2, respectively, were successfully treated with the spheroids, and no remarkable difference in the subjective assessments was observed when compared with the outcome reported by patients in the subgroups with smaller cartilage lesions.
Interestingly, the spheroid dosage does not seem to play a major critical role in cartilage repair of different defect sizes as the range of 13.8 and 170.0 spheroids/cm2 applied in patients of this study did not reveal significant differences in the patient-reported outcome.
The principle for spheroid dosage differs substantially from the dosing of chondrocyte cell suspensions. The relevant dosage to be applied is concerning the number of spheroids and not the number of cells within the spheroids. For ACT3D, a defined number of spheroids per 1-cm2 defect has to be applied. After transfer of the chondrocytes to the 3-dimensional system, the cells aggregate, and the spheroids become tighter (matrix formation) and smaller (matrix condensation). Matrix formation and maturation are accompanied by cell death. The area of dead cells within a spheroid is refilled by matrix. Thus, the number of cells within a spheroid does not reflect the capacity of a specific spheroid number to fill a defined defect area. From clinical experience with doses of co.don chondrosphere, a range of 10 to 70 spheroids/cm2 of defects was applied in the majority of cases treated since 2004.
Results on age dependency of the clinical success of the ACT method are controversial. Younger patients (less than 40 years of age) were seen to have better outcomes in some studies.36-38 However, other studies did not find a relationship between age and clinical success.39,40 Rosenberger et al. and Niemeyer et al. found a comparable rate of failures and reoperations in older patients (45-60 years) compared to results reported for younger patients.41,42 The Joint Advisory Board of the German Societies for Traumatology (DGU) and Orthopaedic Surgery (DGOOC) proposes an approximate age limit of 50 years of age. However, it was reported that chondrocytes can also be cultivated successfully beyond 50 years of age depending on the “fitness” of chondrocytes.15 This view is shared by the working group of the Belgian Orthopaedic Societies on cartilage repair in the knee.3 When using primary mesenchymal stem cells as cell-based strategy to treat musculoskeletal diseases including critical-sized bone defects or cartilage defects, it was observed that donor age did not affect the osteogenic43 and chondrogenic differentiation potential, respectively, and thus, it should not be used as an exclusion criterion for autologous transplantation of human adult mesenchymal stem cells.
Thus, older patients might still show good recovery as observed in this study. The age range of the patients in the present IIT was between 15 and 53 years, with 2 patients of 51 and 53 years of age, respectively, and 1 patient being 15 years old at the time point of transplantation. A comparison of the defined age groups of ≤30-, 30- to 40-, and >40-year-old patients did not reveal a significant difference in subjective and clinical outcome. A tendency towards slightly better ratings was observed for the younger patients ≤30 years old; however, this was already observed at baseline, and the relative improvement in symptoms was comparable between the groups. Thus, an age-dependent success of ACT3D with the spheroids could not be observed in this IIT.
Interestingly, a recent study suggested that results from published RCTs may not be representative of the gross cartilage population in the clinical practice.44 This applies also to the IIT presented here, where patients were included, being heterogenous in etiology and the anatomic locations and size of cartilage lesions. The authors stated that these limitations, which are necessary to achieve a high internal validity due to the study design of RCTs, may naturally interfere with the external validity and clinical applicability of them. Often, concomitant injuries in the knee are defined as exclusion criteria. However, in daily practice, concomitant injuries are common, with meniscus injury as the most common one. Here, it was observed that the operation time may vary substantially between a single transplantation procedure and a surgery including repair of coinjuries. Therefore, a conclusion towards a shorter operation time using ACT3D compared to other surgical techniques for cartilage repair, for example, microfracture, cannot be drawn from this IIT.
It was reported earlier that anatomic locations may affect the clinical outcome of ACT, with femoral condyle as the most favorable one and patellae as the most challenging one.45 Lesions on the femoral condyles also showed more improvement when treated with chondrocyte cell suspensions than lesions on the patellae and trochlea. However, the situation for the 3-dimensional spheroids is different as they are in a more advanced differentiation state, expressing a more pronounced extracellular matrix structure and potentially leading to stronger adhesion at the time point of transplantation. Spheroids adhere within 20 minutes without any fixation.46 In the present IIT, where patients with either type or both types of these particular defects were treated with spheroids, no differences could be observed in the MRI regarding the defect filling. There was even a higher tendency for improvement with patellofemoral lesions than lesions on the femoral condyle. Also regarding the patient-reported outcome, no significant differences between both groups were found.
Recently, it was reported that more than 1 lesion in the knee was significantly associated with a lower Lysholm score.44 A t test comparing the Lysholm score between those patients with 1 lesion and those with 2 or more had yielded P < 0.002. Although in the present IIT, a difference in the clinical outcome with regard to the number of defects could not be observed, the results have to be interpreted with caution due to the low number of patients with more than 1 lesion.
The duration of symptoms previous to treatment was suggested to be relevant for the outcome of the ACT3D treatment particularly with respect to the activity level. In a study reported by Saris et al.,8 in the group treated with characterized chondrocyte implantation (CCI) using an autologous cell suspension product, improvement in overall Knee injury and Osteoarthritis Outcome Score (KOOS) was 28% and 71% higher when symptom onset was <2 years and <3 years, respectively, compared with ≥2 and ≥3 years.
Recently, an evaluation of the duration of symptoms as efficacy parameter for clinical outcome was performed in a 5-year follow-up study with CCI.47 The time to treatment was shown to affect the outcome at 5 years postsurgery, indicating that an early treatment of cartilage defects with a chondrocyte-derived product had improved long-term clinical outcome assessed with the KOOS. Such a correlation was observed in the present IIT only for the activity level of the patients. The results of the Tegner score assessing activities in recreational and competitive sports and daily life showed a significant difference between patients with shorter duration of symptoms for ≤12 months compared to those with symptoms persisting for more than 12 months before participating in the IIT. The other assessment scores did not display significant differences depending on the duration of symptoms before treatment. Importantly, the previous surgical treatments regarding the cartilage defect (Table 2) had no remarkable impact on the clinical and subjective outcome at 1 year after ACT3D with spheroids (data not shown).
The BMI range of the patients in the present IIT varied between 14.0 and 44.8. Of 30 patients, for whom BMI values were available, 6 patients had a BMI >30, which is above the limit that is usually accepted under the condition of controlled clinical trials. In most published RCTs, a BMI of >30 is defined as exclusion criterion.48 In the present trial, no correlation between BMI and clinical outcome was detected (data not shown), indicating that the treatment with the spheroids is an option for cartilage repair also for obese patients with a BMI >30.
The AEs and SAEs observed in this clinical trial are common for ACT procedures and have been reported for different ACT products.49 AEs with third-generation autologous chondrocyte–derived products (particularly matrix associated) include hypertrophy, infections, treatment failure, and subsequent surgical procedures. Incidence rates are low (0%-6.3%). Thus, the AEs reported in the present IIT for ACT3D with co.don chondrosphere (thrombosis [superficial], infection, thrombosis [deep vein], pulmonary embolism) are consistent with the known AE spectrum for this type of product and are likely related to the surgical procedure of the transplantation but could also be caused by underlying conditions of the patient. Due to its strictly autologous nature, co.don chondrosphere may be regarded as a safe product without immunological or toxic reactions.
Conclusions
Overall, the final scores along with the fast defect filling after the treatment with the spheroids in co.don chondrosphere revealed a statistically significant increased level of activity and quality of life already after a 6-month follow-up with a further slight increase at the 1-year final assessment. Admittedly, this 1-year assessment is a weakness of this analysis as long-term observations under defined conditions of a clinical trial are required to provide further evidence for the clinical efficacy and safety of the treatment with the spheroids. A 2-year follow-up assessment is therefore planned and will be published in due course. Two randomized controlled clinical trials (1 phase II dose-finding study and 1 phase III study with microfracture as comparator) are currently running to substantiate the findings of this IIT.
In this study, an open surgery was performed in all cases. The spheroids allow also an arthroscopic application, which offers the possibility of reducing surgical complications and optimizing the safety profile of the product. A further point to consider is that the spheroids are the only purely autologous chondrocyte product; that is, no xenogenous material is needed for fixation. This represents a further safety advantage by minimizing toxicity or viral contamination, thereby reducing the risk for treatment failure.
Acknowledgments
The authors thank Dr. Joachim Brade (Department of Medical Statistics, Faculty of Medicine Mannheim, University of Heidelberg) for the data management and the statistical analysis of the clinical data. They thank Dr. Zaklina Buljovcic and Dr. Monika Thulke-Gross for their scientific support in preparing this article and Dr. Holger-Maria Rohde (all at PharmaLex GmbH, Mannheim Germany) for performing the classification of the adverse events according to MedDRA.
Footnotes
The authors received no financial support for the research and/or authorship of this article.
The authors declared the following potential conflicts of interest with respect to the authorship and/or publication of this article: M. Kaszkin-Bettag is a consultant for co.don AG (Teltow, Germany).
References
- 1. Umlauf D, Frank S, Pap T, Bertrand J. Cartilage biology, pathology, and repair. Cell Mol Life Sci. 2010;67:4197-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Melero-Martin JM, Al-Rubeai M. In vitro expansion of chondrocytes. In: Ashammakhi N, Reis R, Chiellini E. editors. Topics in tissue engeneering. 3 2007. p. 1-37. http://www.oulu.fi/spareparts/ebook_topics_in_t_e_vol3/abstracts/al-rubeai_chapter_01.pdf
- 3. Vanlauwe J, Almqvist F, Bellemans J, Huskin JP, Verdonk R, Victor J. Repair of symptomatic cartilage lesions of the knee: the place of autologous chondrocyte implantation. Acta Orthop Belg. 2007;73:145-58. [PubMed] [Google Scholar]
- 4. Statistisches Bundesamt. Krankenhausreport 2010. http://www.krankenhaus-report-online.de/
- 5. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92:2220-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vijayan S, Bentley G, Briggs T, Skinner J, Carrington R, Pollock R, Flanagan A. Cartilage repair: a review of Stanmore experience in the treatment of osteochondral defects in the knee with various surgical techniques. Indian J Orthop. 2010;44:238-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bedi A, Feeley BT, Williams RJ., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:994-1009. [DOI] [PubMed] [Google Scholar]
- 8. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP; TIG/ACT/01/2000&EXT Study Group. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37 Suppl 1:10S-9S. [DOI] [PubMed] [Google Scholar]
- 9. Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943-9. [DOI] [PubMed] [Google Scholar]
- 10. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation: biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 11. Vasara AI, Nieminen MT, Jurvelin JS, Peterson L, Lindahl A, Kiviranta I. Indentation stiffness of repair tissue after autologous chondrocyte transplantation. Clin Orthop Relat Res. 2005;433:233-42. [DOI] [PubMed] [Google Scholar]
- 12. Brittberg M, Lidahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 13. Bentley G, Biant LC, Carrington RWJ, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85-B:223-30. [DOI] [PubMed] [Google Scholar]
- 14. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 15. Behrens P, Bosch U, Bruns J, Erggelet C, Esenwein SA, Gaissmaier C, et al. Recommendations of the joint working group on tissue regeneration and tissue replacement for the indication and application of autologous chondrocyte transplantation (ACT). Z Orthop Unfall. 2004;142:529-39. [DOI] [PubMed] [Google Scholar]
- 16. Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. Injury. 2008;39 Suppl 1:S26-31. [DOI] [PubMed] [Google Scholar]
- 17. Behrens P, Bosch U, Bruns J, Erggelet C, Esenwein SA, Gaissmaier C, et al. Recommendations of the joint working group on tissue regeneration and tissue replacement for the indication and application of autologous chondrocyte transplantation (ACT). Z Orthop Unfall. 2004;142:529-39. [DOI] [PubMed] [Google Scholar]
- 18. Getgood A, Brooks R, Fortier L, Rushton N. Articular cartilage tissue engineering: today’s research, tomorrow’s practice? J Bone Joint Surg Br. 2009;91-B:565-76. [DOI] [PubMed] [Google Scholar]
- 19. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41. [DOI] [PubMed] [Google Scholar]
- 20. Iwasa J, Engebretsen L, Shima Y, Ochi M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol Arthrosc. 2009;17:561-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hunziker EB, Stähli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16:1067-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Libera J, Ruhnau K, Baum P, Lüthi U, Schreyer T, Meyer U, et al. Cartilage engineering. Berlin: Springer Verlag; 2009. [Google Scholar]
- 23. Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Joint Surg Br. 2002;17:1420-9. [DOI] [PubMed] [Google Scholar]
- 24. Libera J, Luethi U, Alasevic OJ. co.don chondrosphere® (co.don® AG): autologous matrix-induced engineered cartilage transplantation. In: Zanasi S, Brittberg M, Maracacci M. editors. Basic science, clinical repair and reconstruction of articular cartilage defects: current status and prospects. Bologna, Italy: Timeo Editore; Vol. 1 2006. p. 591-600. [Google Scholar]
- 25. Schubert T, Schedel J, Anders S, Neumann E, Grifka J, Müller-Ladner U, Libera J. In vivo integration and maturation of in vitro engineered cartilage tissue investigated in the SCID mouse model. 6th ICRS Symposium; 2006 January 8-11; San Diego, CA. [Google Scholar]
- 26. Krishnan SP, Skinner JA, Bartlett W, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88-B:61-4. [DOI] [PubMed] [Google Scholar]
- 27. Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L. Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2011;19:452-7. [DOI] [PubMed] [Google Scholar]
- 28. Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37:1083-92. [DOI] [PubMed] [Google Scholar]
- 29. Libera J, Gralla A, Walentin K, Höll T, Josimović-Alasecić O. Adhesion and integration of autologous in vitro engineered chondrocytic tissue for articular and disc cartilage repair: an in vitro study. 6th ICRS Symposium; 2006 January 8-11; San Diego, CA. [Google Scholar]
- 30. Commission directive 2006/17/EC implementing directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. 2006. February 8 http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:038:0040:0052:EN:PDF
- 31. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, Trattnig S. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging, Eur J Radiol. 2004;52:310-9. [DOI] [PubMed] [Google Scholar]
- 32. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interreader variability and correlation to clinical outcome after 2 years: Eur J Radiol. 2006;57:16-23. [DOI] [PubMed] [Google Scholar]
- 33. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-9. [PubMed] [Google Scholar]
- 34. EMA/CAT/CPWP/568181/2009. Reflection paper on in vitro cultured chondrocytes containing products for cartilage repair of the knee. 2010. April 16 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004223.pdf
- 35. Ferruzzi A, Buda R, Faldini C, Vannini F, Di Caprio F, Luciani D, Giannini S. Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J Bone Joint Surg Am. 2008;90 Suppl 4:90-101. [DOI] [PubMed] [Google Scholar]
- 36. Krishnan SP, Skinner JA, Bartlett W, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006;88-B:61-4. [DOI] [PubMed] [Google Scholar]
- 37. Micheli LJ, Moseley JB, Anderson AF, Browne JE, Erggelet C, Arciero R, et al. Articular cartilage defects of the distal femur in children and adolescents: treatment with autologous chondrocyte implantation. J Pediatr Orthop. 2006;26:455-60. [DOI] [PubMed] [Google Scholar]
- 38. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873-80. [DOI] [PubMed] [Google Scholar]
- 39. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-24. [DOI] [PubMed] [Google Scholar]
- 40. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77-95. [DOI] [PubMed] [Google Scholar]
- 41. Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med. 2008;36:2336-44. [DOI] [PubMed] [Google Scholar]
- 42. Niemeyer P, Köstler W, Salzmann GM, Lenz P, Kreuz PC, Südkamp NP. Autologous chondrocyte implantation for treatment of focal cartilage defects in patients age 40 years and older: a matched-pair analysis with 2-year follow-up. Am J Sports Med. 2010;38:2410-6. [DOI] [PubMed] [Google Scholar]
- 43. Fickert S, chröter-Bobsin U, Groß AF, Hempel U, Wojciechowski C, Rentsch C, et al. Human mesenchymal stem cell proliferation and differentiation during long-term ex vivo cultivation is not age dependent. J Bone Miner Metab. In press. [DOI] [PubMed] [Google Scholar]
- 44. Engen CN, Engebretsen L, Aroen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brittberg M, Peterson L, Sjögren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation: a review of recent developments. J Bone Joint Surg Am. 2003;85-A Suppl 3:109-15. [DOI] [PubMed] [Google Scholar]
- 46. Rössing S. Neue technik zur arthroskopischen, autologen chondrozytentransplantation mittels chondrospheren. Orthopädie und Unfallchirurgie Aktuell. 2007;145:276-7. [DOI] [PubMed] [Google Scholar]
- 47. Vanlauwe J, Saris DB, Victor J, Almqvist F, Bellemans J, Verdonk R, Luyten FP; TIG/ACT/01/2000&EXT Study Group. Characterized chondrocyte implantation versus microfracture for the treatment of symptomatic cartilage defects of the knee in patients with short onset of symptoms: 60 months follow-up. ICRS Sitges, Spain; 2010. September 26-29 e-Poster 3880. [Google Scholar]
- 48. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 49. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259-71. [DOI] [PubMed] [Google Scholar]