Abstract
The Lifestyle Improvement through Food and Exercise (LIFE) study is a community-based randomized-controlled trial to measure the effectiveness of a lifestyle intervention to improve glycemic control among African Americans with type 2 diabetes attending safety net clinics. The study enrolled African American adults with a diagnosis of type 2 diabetes and HbA1c ≥ 7.0 who had attended specific safety net community clinics in the prior year. 210 patients will be enrolled and randomized to either the LIFE intervention or a standard of care control group, which consists of two dietitian-led diabetes self-management classes. The LIFE intervention was delivered in 30 group sessions over 12-months and focused on improving diet through dietitian-led culturally-tailored nutrition education, increasing physical activity through self-monitoring using an accelerometer, increasing ability to manage blood sugar through modifications to lifestyle, and providing social support for behavior change. In addition to the group sessions, peer supporters made regular telephone calls to participants to monitor progress towards behavioral goals and provide social support. The 12-month intervention phase was followed by a six-month maintenance phase consisting of two group sessions. The primary outcome of the study is change in A1C from baseline to 12-months, and an additional follow-up will occur at 18-months. The hypothesis of the study is that the participants in the LIFE intervention will show a greater improvement in glycemic control over 12-months than participants in the control group.
Keywords: African American, type 2 diabetes, self-management intervention, health disparities, glycemic control, community-based intervention
African Americans are more likely than whites to suffer from diabetes-related complications [1-3] and experience higher rates of diabetes hospitalization.[4-6] The disproportionately high rate of diabetes morbidity in low-income African Americans is due to higher hemoglobin A1C (A1C) and blood pressure.[7, 8] This disparity in diabetes outcomes is preventable; improved glycemic control results in reductions in micro- and macrovascular complications as well as reduced healthcare utilization and costs.[9-11]
Racial differences in diabetes outcomes are driven by social-environmental and medical system factors. Social-environmental factors include lower socioeconomic status and the high-risk environments experienced by African Americans,[12, 13], including lack of access to affordable low energy density foods[14-16] and fewer opportunities for physical activity[17, 18]. Medical factors include poorer quality of care[19, 20] and lack of insurance.[21] Evidence also suggests that poor health literacy, especially diabetes numeracy, plays a critical role in racial and socioeconomic disparities in glycemic control.[22, 23]
Glycemic control can be improved by medications, changes to diet, and improved physical activity. Among disadvantaged African Americans, improved diet and exercise and improved self-management skills may result in better glycemic control. This is especially important due to low medication adherence in this group [24-27] and the potential of preventing medication side effects such as weight gain, which may increase already high cardiovascular risk.[28, 29] Moreover, improving diet and physical activity could also help decrease blood pressure, an important contributing cause of diabetes morbidity in African Americans.[30-32] Approaches that improve diabetes self-management among low-income African Americans could, in the long-term, substantially reduce the high rate of avoidable hospitalizations and diabetes complications in this population.
The current study describes the design of a randomized-controlled trial to test the effectiveness of the Lifestyle Improvement through Food and Exercise (LIFE) intervention, a literacy-sensitive, culturally-tailored community-based group intervention designed to help disadvantaged African Americans with type 2 diabetes achieve glycemic control through dietary modification and physical activity. The intervention was community-based in order to contextualize diabetes self-management as a lifestyle issue rather than a medical one, and to increase participant exposure to community resources for diabetes self-management (e.g. exercise venues and grocery stores in their community), and to decrease medical mistrust as a potential barrier to participation.[33]
The LIFE intervention consists of four main components: 1) literacy-sensitive and culturally-tailored diabetes nutrition education delivered by a registered dietitian (RD), 2) physical activity, 3) self monitoring of blood glucose (SMBG) and interpretation and use of the results for decision making about self management, and 4) social support. These components are delivered in group sessions and peer supporter telephone calls over a 12-month intervention period, followed by a 6-month maintenance program. The LIFE intervention will be compared with a control intervention which includes two 2-hour diabetes self-management education group classes delivered by a RD, consistent with the Medicare reimbursement schedule for diabetes self-management training.[34]
Methods
Study Design
The effect of the LIFE Program on glycemic control will be evaluated in an 18-month randomized controlled trial. Participants are randomized to either: (1) a control group with 2 standard diabetes education classes in the first 6-months, and (2) a 12-month culturally-tailored behavioral intervention followed by a 6-month maintenance phase. An overview of the study design is provided in Figure 1. The trial is registered with ClinicaTtrials.gov, NCT#01901952. Approval for the use of human subjects was provided by Rush University Medical Center’s Institutional Review Board, and the study is monitored by a Data and Safety Monitoring Board.
Figure 1.
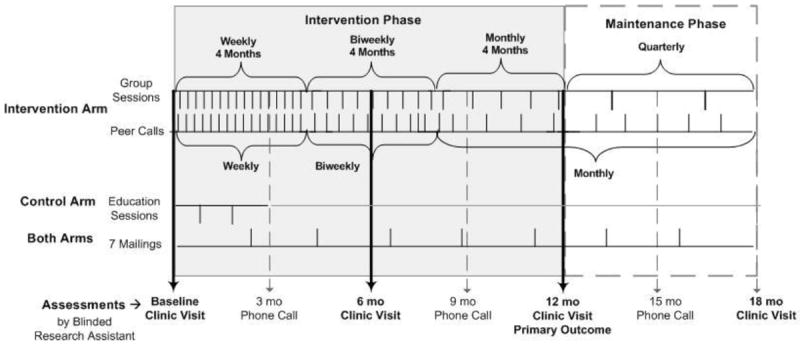
Diagram of study design for the Lifestyle Improvement through Food and Exercise (LIFE) Study.
Eligibility Criteria
Eligibility criteria were designed to maximize the generalizability of results by targeting a broad range of individuals with diabetes at varying risk, and to assure that individuals are healthy enough to benefit from lifestyle changes. Inclusion criteria include a diagnosis of type 2 diabetes mellitus, uncontrolled diabetes (A1C ≥ 7.0%), African American race, age 18 or older, and seen at least once in the past 12 months by a primary care physician in a designated Cook County Health and Hospitals System (CCHHS) primary care clinic. Participants were required to be available at the time the group sessions were scheduled. Intervention and control group sessions were held at the same day and time.
Exclusion criteria included BMI < 18.5 kg/m2, end-stage renal disease (GFR<15 mL/min/1.73 m2), stroke with paresis, congestive heart failure (New York Heart Association class 2-4) or major end-organ complications of diabetes, conditions limiting probable lifespan to <4 years, current pregnancy, diagnosis of a major psychiatric condition, use of prednisone (“steroids”) in the prior 3 months, weight loss surgery, lack of access to telephone, inability to walk two blocks without stopping, impaired cognitive function, not cleared for participation by a primary care physician, or living in the same household as an active study participant. Medical questions related to eligibility and safety of participants are adjudicated by the study endocrinologist.
Recruitment
Patient lists were provided by Cook County Health system staff and patient medical records were screened by research assistants for potential eligibility. Research assistants contacted potentially eligible patients from each clinic and completed final screening. Eligible patients were invited to an information session where they were given details about the study and told the importance of completing the study if they chose to enroll. Patients who were still interested completed the consent process and were given a point-of-care A1C test. If A1C was ≥ 7.0, patients were scheduled for a baseline assessment. Upon completion of the baseline assessment, patients were enrolled in the study and randomized. Recruitment started in March of 2012 and was completed in April 2014.
Measures
Trained study staff members who were blinded to group assignments conducted all assessments at the patient clinic. Full assessments were conducted at baseline, 12-months and 18-months. An abbreviated assessment was conducted at 6 months. Full assessments were completed in two clinic visits separated by one week. During the first visit, participants provided medical history and completed a range of measures, including a 24-hour diet recall interview. They also received an accelerometer, which they were asked to wear around their waist at least 10 hours daily for 7 days. Participants visited the clinic one week later to return the accelerometer and complete another 24-hour food recall. If participants had worn the accelerometer for less than 4 days, they were asked to re-wear it. Participants were also asked about changes in contact information, health care utilization, and adverse events such as hospitalizations and ER visits every three months. See Figure 1 for a diagram of the assessment schedule.
The primary outcome of this study was glycemic control, reflected in hemoglobin A1c levels (A1C). Secondary outcomes included blood pressure, body mass index, dietary intake, and physical activity.
Glycemic control
Glycemic control was measured as the difference in A1C between baseline and follow-up. A1C was measured using an Axis-Shield Afinion A1C POC device, which requires a fingerstick blood sample.[35]
Blood Pressure
Blood pressure was measured in the resting state as the average of 3 readings taken 2 minutes apart by using an Omron digital blood pressure monitor (Omron Healthcare, Inc, Lake Forest, Illinois).
Body mass index (BMI)
Weight was measured by using a balance-beam scale; participants wore light-weight clothes and no shoes. Height was measured using a wall-mounted stadiometer. BMI is calculated by dividing weight (in kilograms [kg]) by height (in meters squared [m2]).
Dietary intake
Dietary intake data were collected using the Nutrition Data System for Research software (Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN). The software utilizes the multiple-pass interview method and data collection is standardized by built-in software interview prompts. Research assistants received 6 hours of training by an experienced PhD-level dietitian. Participants were provided with a booklet that contained pictures of food portion sizes to assist in reporting. Each interview took approximately 30 minutes.
Physical Activity
The accelerometer used to capture physical activity was the Actigraph GT3X (Actigraph, LLC, Fort Walton Beach, FL). This is a tri-axial accelerometer measuring acceleration in three axes (vertical, anteroposterior, mediolateral), as well as incline and step count. The epoch interval was set at one minute and output was expressed as mean counts per minute. The counts obtained in one epoch were linearly related to the intensity of the participants’ activity during that period.[36] Study participants wore the accelerometer around the hip or waist using an adjustable fabric belt. They were asked to wear the device for at least 10 hours on at least 7 days. A composite measure of counts from all three axes was used to calculate intensity of activity during each epoch (minute) the device is worn. Classification of minutes of activity as sedentary, light, moderate, vigorous, and very vigorous was based on previously published thresholds.[37, 38] Daily step count was also calculated.
Intermediate outcomes included nutrition knowledge, medication adherence measured by self-report and using the outpatient pharmacy database[39], diabetes self-management behavior, diabetes-related quality of life and social support. Potential moderators included depressive symptoms, perceived stress, and health numeracy. Available longitudinal data of kidney function (plasma creatinine, eGFR, urine albumin creatinine ratio), lipids levels (total cholesterol, LDL, HDL, triglycerides) was collected from the electronic medical record.
After fulfilling study entry criteria and successful completion of all baseline components, participants were randomized in a 1:1 ratio, stratified by recruitment clinic, to one of two treatment arms: intervention and control.
The LIFE intervention
The LIFE intervention was guided by cognitive behavioral models of behavior change, specifically that behavior change is mediated by cognitions but also requires motivation, skills and social support.[40] The LIFE intervention included culturally-tailored, literacy-sensitive nutrition education as well as behavior modification techniques and social support to encourage change in diet, increase in physical activity, and self- monitoring of blood glucose (SMBG). The intervention was structured to encourage participants to discover the relationship between their diet and physical activity and their blood glucose levels, and to learn effective lifestyle strategies for reducing their blood glucose. Goal-setting improves self-efficacy and is an effective strategy for helping people change their self-management behavior.[41] Participants set goals for diet, physical activity and SMBG, and social support was provided to help participants achieve goals.
The intervention consisted of three phases of decreasing contact over 12 months. Each phase was 4 months long. Phase 1 consisted of 16 weekly 2-hour group sessions, phase 2 consisted of 8 bi-weekly group sessions, and phase 3 consisted of 4 monthly group sessions. The 6-month maintenance phase consisted of 2 group sessions. Fifteen to twenty participants were assigned to each group. Peer supporters offered participants weekly telephone contact throughout the intervention. The intervention team for each group consisted of a registered dietitian, a group facilitator, and 1-2 peer supporters. A clinical psychologist supervised peer supporters and an endocrinologist was available for consultation regarding emerging issues related to medical management of diabetes. The format of the 2 hour group sessions is shown in Table 1.
Table 1. Group session format for Lifestyle Improvement through Food and Exercise (LIFE) intervention.*.
| Activity | Description |
|---|---|
| 1. Data collection and individual goal setting (15 min) | Goal setting and self-monitoring. Weigh participants; review food logs and create individual diet goals; record pedometer steps and create individualized activity goals. |
| 2. Prayer (2 min) | Motivation. Participant or peer supporter leads prayer. |
| 3. Culturally tailored educational content (45 min) | Nutrition education and behavioral modification. Nutrition and diabetes education, glucose self-monitoring skills, behavioral modification techniques, interactive activities to reinforce educational content. |
| 4. Physical activity (10 min) | Social support and role modeling. Peer supporter leads participants in moderate aerobic activity along with music. |
| 5. Healthy snack (15 min) | Nutrition education. Healthful snack is provided and new eating behaviors are demonstrated (eg, healthy portion sizes, new healthy foods). |
| 6. Listening (25 min) | Emotional and social support, role modeling. Participants share their struggles and victories in making behavior changes. |
| 7. Goal setting (10 min) | Goal setting and self-monitoring. Participants set/review goals for activity, diet, and blood glucose monitoring for each week. |
from Lynch, E. B., et al. (2014). “A self-management intervention for African Americans with comorbid diabetes and hypertension: a pilot randomized controlled trial.” Prev Chronic Dis 11: E90.
Nutrition Education
The dietary intake goals of the LIFE intervention were designed to improve glycemic control and reduce cardiovascular risk. To improve glycemic control, participants were encouraged to consume a consistent level of carbohydrate intake across each day.[42] The LIFE program advocated for a diet consistent with the American Diabetes Association nutrition guidelines, characterized by low saturated fat intake, increased consumption of fruits, vegetables and whole grains, and decreased sodium.[43]
The nutrition education component was grounded in the information processing theory of food choice which assumes that changes in dietary behavior require changes to the content and processing of the mental representations underlying dietary behavior.[44-46] Design of the curriculum was guided by a series of cognitive anthropology studies which elucidated the conceptual landscape of food and health among low-income African American mothers.[47-49] Findings from those studies suggested that the target population had very limited knowledge of the food sources of macronutrients and the health impact associated with specific foods. Those studies also revealed that lay food concepts are organized around functional, rather than nutritional, dimensions of food (e.g. meals, snacks, food preparation versus proteins, carbohydrates, etc.), and that fruits and vegetables are frequently referred to as “God’s food.” Evidence indicates that learning of new information is facilitated if it is taught in a way that is consistent with preexisting mental representations [50]. The nutrition education curriculum focuses on teaching necessary nutrition information in terms of functional food categories. That is, the curriculum teaches how to create healthy meals and snacks using appropriate portions of healthy carbohydrate, protein and non-starchy vegetable foods. The LIFE curriculum described here was informed by a pilot study conducted using an earlier version of the curriculum.[51]
The nutrition education curriculum was delivered by a registered dietitian using an interactive teaching style. The curriculum is shown in Table 2. The curriculum was centered around the modified plate method,[52] referred to as “the Plate of LIFE,” which illustrates the components of a healthy meal. The “Plate of LIFE” (shown in Appendix A) is a plate divided into three sections: half the plate is filled with non-starchy vegetables, 1/4 with carbohydrate and 1/4 with protein. Participants were encouraged to use this plate to construct lunches and dinners and to use the top half of the plate (carbohydrate and protein) to create breakfasts. Knowledge of macronutrient content of foods is necessary for participants to utilize the Plate of LIFE, however prior research suggested that a majority of participants would lack this knowledge.[48, 49] Therefore, participants were explicitly taught which foods are carbohydrates (including starchy vegetables), proteins and fats. Categorized lists of culturally familiar and commonly eaten foods were provided in the manual and participants practiced categorizing foods in the group sessions and with their food logs.
Table 2.
Nutrition education curriculum of Lifestyle Improvement through Food and Exercise (LIFE) intervention.
| Phase 1: Portioning and Food Label Reading 4 months, weekly sessions | ||
|---|---|---|
| Session | Nutrition Education Content and Selected Activities | |
| 1 |
Introduction to Program and Self Monitoring
|
|
| 2 |
Plate of LIFE
|
|
| 3 |
Blood Glucose and Carbohdyrates 1
|
|
| 4 |
Blood Glucose and Carbohdyrates 2
|
|
| 5 |
Beverages
|
|
| 6 |
Eat Less Fat
|
|
| 7 |
Review
|
|
| 8 |
Breakfast
|
|
| 9 |
Lunch
|
|
| 10 |
Meal timing and snacks
|
|
| 11 |
Dinner
|
|
| 12 |
Tricks for Better Eating
|
|
| 13 |
Carb Counting 1
|
|
| 14 |
Carb Counting 2
|
|
| 15 |
Food Labels 1
|
|
| 16 |
Food Labels 2
|
|
|
Phase 2: Selection and Preparation of Higher Quality Foods 4 months, biweekly sessions | ||
| 17 |
Review/Relapse Prevention
|
|
| 18 |
Eat More Vegetables
|
|
| 19 |
God’s Food: Whole Foods
|
|
| 20 |
God’s Food: Sodium
|
|
| 21 |
Relapse Prevention
|
|
| 22 |
Shopping Preparation
|
|
| 23 |
Grocery Store Tour
|
|
| 24 |
Eating Out
|
|
|
Phase 3: Review and Preparation for Maintenance 4 months, monthly sessions | ||
| 25 |
Preventive care for diabetes
|
|
| 26 |
SMBG Review
|
|
| 27 |
Maintenance of Behavior Changes
|
|
| 28 |
Planning for the Future
|
|
|
Maintenance Phase 6 months, quarterly sessions | ||
| 29 |
Jeopardy Review
|
|
| 30 |
Celebrate LIFE
|
|
In Phase 1 of the curriculum (weekly phase), participants were introduced to the plate and other key nutritional concepts (fat and sugar-sweetened beverages) and were taught two additional portioning methods: the hand guide for portion control (see Appendix A) and carbohydrate counting. The hand guide for portion control recommends eating a portion of vegetables the size of two fists (approximately 2 cups), a portion of carbohydrate the size of one fist (approximately 1 cup), a portion of protein the size of 1 palm (approximately 4-6 ounces) and a portion of fat the size of a thumb (approximately one tablespoon) per meal. Participants were also introduced to carbohydrate counting as an additional, optional method of portioning carbohydrates. During the carbohydrate counting section of the curriculum, participants were encouraged to consume 3-4 carbs (45-60 grams of carbohydrate) per meal, and 1-2 carbs (15-30 grams) per snack. Participants were also taught to calculate carbohydrate content using food labels. Phase 2 (bi-weekly) focused on how to select and prepare higher quality foods from each Plate of LIFE food category; that is, foods with more complex carbohydrates, less sodium and less fat. Participants also learned how to identify fiber and sodium content using food labels. During Phase 2, the lay concept “God’s Food,” identified in prior studies with the target population, was used to refer to natural, non-processed foods with high fiber and low sodium. During the first two phases of the curriculum, there were interactive, hands-on activities during each lesson. For example, participants worked with actual food labels of common foods, practiced portioning real foods such as pasta, rice, cookies, etc., and measured out portions of sugar and fat contained in common foods. Phase 3 (monthly) focused on discussion among participants regarding topics related to prevention of complications, relapse prevention and problem-solving, and maintenance of behavioral changes. The curriculum was designed to be repetitive. Information was reviewed in different ways across multiple sessions. This was to address literacy issues and also to accommodate individuals who could not attend every class. Participants who missed a class were taught the information by the peer supporters during the group classes they attended and on telephone calls.
Literacy limitations were addressed by tailoring the intervention to specific information deficits that had been previously identified in the target population, as described above. In addition, during instruction, literacy barriers were addressed through frequent use of graphics, simplified food lists, and physical demonstrations and hands-on activities to reinforce more abstract concepts. Numeracy barriers were addressed by repeated visual and tangible exercises counting out carb portions (using real food), connecting carb portions to blood sugar readings, and frequent reinforcement of blood sugar targets and A1C goals.
At each session, participants were given a healthy snack and at one session participants were offered a meal, consistent with the plate of LIFE with healthy versions of culturally-favored foods. The snacks and meal gave participants an opportunity to try new foods and to experience appropriate portion sizes. Participants also received recipes for all new foods.
Participants received a color manual divided into chapters for each of the lessons. The manual contained simple graphic illustrations of each of the concepts taught and worksheets for participants to use during group activities. Worksheets were included to assist participants in planning meals, generating food substitutions (e.g. substitute whole grain foods for refined carbohydrate foods), and practicing counting carbohydrates and interpreting food labels. The manual was accompanied by a detailed leader’s guide for the intervention staff which described the lesson, interactive activities, and materials needed for each session. Participants also received daily self-monitoring logs in which they were encouraged to keep a daily record of all food eaten as well as pre- and post-prandial blood sugar values. The LIFE log (Appendix B) depicted pictures of the Plate of LIFE in which participants could record their food intake for lunch and dinner. The LIFE log served as reinforcement of the core nutrition teaching tool of the intervention and encouraged participants to learn macronutrient categories of foods.
The nutrition portion of the intervention includes individual tailoring for each participant. At the beginning of each session, the dietitian reviewed the daily logs collaboratively with each participant. The dietitian encouraged the participant to problem-solve to discover the influence of his or her food choices on pre and post-prandial blood sugar values. This helped increase participant self-efficacy and helped participants to self-identify food changes that were more likely to improve glycemic control
The nutrition education curriculum was delivered by a registered dietitian in 30-45 minutes of the two hour group session (see Table 1). Sessions were held in community centers near each clinic. Each clinic had a kitchen, used to prepare food for the class, and sufficient space for the group exercise activity.
Self-monitoring of blood glucose
Self-monitoring of blood glucose is associated with improvement in glycemic control when it is used by participants to make changes in diabetes management.[42] A central goal of the curriculum was to encourage participants to discover the relationship between their food intake and their blood glucose levels, which allowed them to optimize self-management of blood glucose through adjustments in food decisions.
All study participants were supplied with a glucometer by the clinic. Intervention participants were provided with extra blood glucose test strips as needed, in order to meet the recommended blood sugar testing schedule. Participants were asked to test and record their blood sugar throughout the intervention in their daily log books. During the first four weeks, participants were asked to record their fasting blood sugar at least two times per week. During this phase, if fasting blood sugar was consistently high, participants were encouraged to see their provider to adjust medications. During weeks 5-7, participants were asked to test and record their blood sugar at fasting and 2 hours after breakfast at least twice per week. During the remaining weeks, participants were encouraged to monitor their blood sugar before and two hours after lunch or dinner and adjust their carbohydrate consumption accordingly. Participants recorded blood glucose readings in the LIFE log (Appendix B). If their post-prandial glucose was > 180 mg/dL, the dietitian helped the participant to problem solve to identify excess carbohydrate consumption and/or medication non-adherence. Throughout the intervention, if the dietitian became aware of consistently high blood glucose or frequent hypoglycemic events, the dietitian advised the participant to discuss medication changes with his or her primary care provider.
As needed, the dietitian tailored blood glucose self-monitoring goals to the specific needs and circumstances of individual participants during the individual food log reviews at each group session. During weekly phone calls, peer supporters recorded blood sugar readings and encouraged participants to check their blood sugars at the recommended times.
Physical activity
Higher frequency of aerobic activity and resistance training are associated with better glycemic control.[53-55] Participants were given resistance bands and a 10-minute resistance band workout was included in every group session (see Table 1). Participants were also given a New Lifestyles NL-800 Accelerometer with 7-day memory (New Lifestyles, Lees Summit, MO) to self-monitor physical activity. At every group session, peer supporters recorded participants’ average daily steps for the prior week from the device memory, and participants were encouraged to write their steps and other activity in their LIFE logs. Participants set a daily step goal at every session and were encouraged to increase their number of daily steps in increments up to 10,000 per day. Peer supporters checked progress toward step goals during telephone calls.
Social support
Abundant evidence suggests that social support improves diabetes self-management behavior, attendance at diabetes self management classes and motivation.[56-59] Two components of the intervention were designed to provide social support to participants. First, approximately 25 minutes of each small group session was dedicated to a group discussion among participants about the barriers and successes they experienced in their journey to improve their diabetes self-management (Table 1). These group support sessions were called “listening sessions” because the primary goal was to enable participants to share their struggles as well as their communal wisdom and expertise. This process was meant to provide further opportunity for self-discovery and self-empowerment. This aspect of the intervention also encouraged trust and bonding among participants, who come to see one another as role models. The listening section of the class was led by the group facilitator on the intervention team, an African American individual with extensive experience in group facilitation.
Social support was also provided through the use of peer supporters. The intervention team included African American peer supporters with successfully controlled type 2 diabetes, from the same communities as the participants. The peer supporters completed 8 hours of training from a clinical psychologist in the techniques and processes described below. Each participant was assigned a single peer supporter with whom they interacted throughout the intervention.
In each group session, peer supporters worked with their participants to set individualized behavioral goals that were specific, measurable, attainable, relevant to the program goals, and time-limited (SMART). Typically, one goal was set for each of three LIFE program goals (diet, physical activity, and self-monitoring of blood glucose). Between group sessions, peer supporters contacted participants by telephone to provide social support and assess progress with each goal. Peer supporters were trained to reinforce progress on goals with verbal praise. When goals were not achieved, peer supporters probed to identify barriers to progress and determine whether those barriers stemmed from either motivational ambivalence about change (e.g., participant is unmotivated to perform finger stick to self-monitor blood glucose) or a logistical problem (e.g., ran out of glucose test strips). Peer supporters were trained in the application of simplified motivational interviewing techniques to address motivational ambivalence, and problem-solving strategies to help participants overcome logistical barriers to meeting their goals. Peer supporters recorded participant goals at the group sessions, and documented progress with goals and the application of motivational interviewing and problem-solving techniques during peer support phone calls. Peer supporters were supervised by a clinical psychologist and presented their caseload at weekly case conferences attended by the supervising clinical psychologist, the dietitians, and group facilitators.
Control Group
The control group received two group classes covering diabetes self-management training and diabetes nutrition education, taught in the clinics by a registered dietitian. This amount of diabetes education corresponds to the type and amount of diabetes education covered by the Centers for Medicare and Medicaid Services after the first year of diagnosis.[34] Like the intervention group classes, the content of the control group classes covered topics recommended in the National Standards for Diabetes Self-Management Education.[41] The first class covered the pathophysiology of diabetes, prevention of acute and long-term complications, signs and treatment of hypoglycemia and hyperglycemia and self-monitoring of blood glucose. The second class began with a discussion of participant behavior changes since the first class and a review of the first class, followed by instruction and discussion on the importance of meal and snack timing, carbohydrate management and the role of physical activity and stress reduction in diabetes management. At the end of each class, participants were encouraged to identify one behavior (medication adherence, blood glucose monitoring, diet or exercise behavior) they were ready to change. One class was offered per month and each of the two classes was offered three times over the first six months of the intervention period to maximize the ability of study participants to attend both classes. Patient education materials were uniform throughout all the control cohorts and consisted of blood glucose logs, ABC’s of diabetes target goal sheets, carbohydrate counting instruction sheets, food models and food labels. All control group participants were called and reminded to attend the classes.
Educational Mailings
Through the duration of the study, participants in both groups received educational material from the American Association of Diabetes Educators every 10 weeks. Topics covered the AADE 7 self-care behaviors and include: Being Active, Healthy Coping, Healthy Eating, Self-monitoring, Problem-solving, Reducing Risks, and Taking Medications.
Fidelity
Fidelity to intervention and control group sessions was monitored using checklists developed for each session to assess content delivery. All group sessions were recorded using a digital voice recorder. The project director reviewed fidelity data and provided group leaders with feedback to ensure all topics were discussed over the duration of the study. Peer supporters completed protocol forms for each peer support call which indicated whether specific topics were addressed and provided a quick evaluation of participant comprehension of content. Investigators randomly recorded peer supporter phone calls in each cohort to assess delivery of intervention content. Supervisory meetings were held weekly by the study psychologist to provide feedback to peer supporters and problem-solve individual participant cases.
Enactment of treatment skills was assessed via food logs, pedometer readings, and blood glucose monitoring records collected and reviewed at each group session. Pedometer steps, food intake, and blood glucose monitoring were also monitored through evaluation by peer supporter calls.
Statistical analysis
The primary outcome is change in glycemic control at 1 year. The hypothesis is that participants in the intervention group will experience a greater reduction in A1C than participants in the control group at 12-months. The following analyses will be performed to assess the effect of the intervention on change in A1C: 1) For the primary analysis, baseline A1C will be subtracted from the 12-month A1C measurement. A two-sided, 2-sample, t-test (or the Mann-Whitney test if the distribution of the change of A1C is not well approximated by a Normal distribution) will be used to compare mean change in A1C measures between the two groups. 2) Linear regression will be used to assess the effect of the intervention on the 12-month change in A1C (from baseline) adjusting for covariates such as age, gender, baseline A1C, length of time with diabetes, and insulin-use. 3) To assess the influence of group dynamics in both treatment arms an indicator for treatment delivery group will be added as a covariate to the linear regression described above. 4) To assess the change in A1C over the first 12 months (from baseline, 6 months and 12 months), linear mixed models will be employed, utilizing a time by treatment arm interaction variable to test for an interaction between time (3 A1C measurements) and treatment group, along with a random intercept and any other appropriate random effects. 5) The previous model will be repeated adjusting for age, gender, length of time with diabetes, recruitment site and insulin-use. As with the earlier analysis, a group indicator variable will be added as a covariate to assess the influence of the group construct to be employed within the intervention arm.
To measure maintenance of any intervention effects, we will repeat analyses 1-5 above using 18-month A1C values and covariates. An additional analysis will be run to determine whether intervention effects were maintained between 12 and 18 months. An indicator variable will be added to model 5 above to allow for piecewise modeling of slopes during the intervention and maintenance time periods.
Sample size computation
The study sample size was determined based on the primary analysis that compares mean change in A1C between the two treatment groups. It was hypothesized that the mean change in A1C, from baseline to month 12, among those in the intervention group will be at least 0.7% lower than the mean change among those in the control group. This hypothesis is supported by data obtained from the LIFE pilot study.[51] We further assume that the standard deviation of this change in A1C is approximately 1.6, as was observed in both the LIFE pilot study and the MATCH study, a two-year community-based study of Mexican-Americans with diabetes.[60, 61] A two-sample, two-sided, t-test will be used for the primary analysis. In order to detect a 0.7% difference in the means, at a significance level of 0.05 and 80% power, the t-test requires a total sample size of 166. Further, adjusting for an assumed 20% dropout rate and the need to recruit participants in cohorts of size 15, 210 participants in total will be required, with 105 participants in each group.
Discussion
The existence of black-white health disparities in complications of type 2 diabetes is an ongoing problem that can be addressed by increasing the evidence-base for diabetes self-management interventions for low-income African Americans. Because disparities appear to be associated with lower literacy and lower socioeconomic status, interventions must be sensitive to those vulnerabilities. In addition, diabetes self-management interventions should target lifestyle behaviors, improve low medication adherence and decrease cardiovascular risk factors. The current intervention combines literacy-sensitive, culturally-tailored nutrition education, evidence-based behavioral modification strategies of self-monitoring and goal-setting, and extensive social support to facilitate improved self-management among African Americans with type 2 diabetes attending safety net primary care clinics.
To our knowledge, there are no community-based interventions that have achieved long-term improvements in glycemic control in low-income African Americans by targeting diet and physical activity. Three diet and physical activity interventions showed short-term improvements in glycemic control but none showed improvements at six months following intervention.[62-64] The recent failure of the Look AHEAD intensive lifestyle intervention to show long-term reduction in cardiovascular outcomes,[65] highlights the need for novel approaches to diabetes self-management that will result in long-term reductions in diabetes complications. The LIFE intervention can also be used to inform development of an adaptive intervention by identifying tailoring variables that may distinguish between early, late and non-responders.[66]
The LIFE intervention was specifically designed to decrease disparities in diabetes outcomes by targeting low-income African Americans. The intervention used a multi-component strategy based on components that have been shown to be effective at improving diabetes self-management in higher-risk groups, including cultural tailoring, one-on-one contact with individualized assessment and reassessment, a focus on behavior-related tasks, use of feedback about participant’s control of disease, and a high-intensity intervention delivered over a long duration.[67] Unique aspects of this intervention include the extent of developmental research that informed the tailoring of the nutrition education curriculum, the extensive social support provided by the intervention, the focus on using blood glucose readings to manage food choice decisions, and the extent of individual tailoring that was incorporated.
Recent recommendations for nutrition therapy for participants with type 2 diabetes recommend that it be provided by a registered dietitian and individualized for each patient.[43, 68] One-on-one sessions with a dietitian can be resource intensive and may not be practical in a public health or safety net clinic setting. The current intervention was designed to provide the benefits of dietitian-delivered individual counseling in a cost-effective way, with the addition of the critical ongoing social support necessary for behavior change in populations with numerous barriers to effective self-management. We hypothesize that this model will prove to be a sustainable and cost-effective way to provide a high risk population with comprehensive support for diabetes self-management that will also maximize other health benefits, such as improved blood pressure and weight.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK092271. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Joellen Wilbur, PhD and Don Waddell, MA provided assistance with early design of this project, Tangula Jefferson, Candace Nicks, and Syed Quadri provided assistance with data collection. Wilamina Mims and Denise Mason served as peer supporters, and Don Waddell, Francine Stark, Sheila Reed and LaDawne Jenkins served as group facilitators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. The New England Journal of Medicine. 1989;321:1074–9. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Valsania P, Phillips CL. Diabetes mellitus and nontraumatic lower extremity amputation in black and white Americans: The national health and nutrition examination survey epidemiologic follow-up study, 1971-1992. Archives of Internal Medicine. 1999;159:2470–5. doi: 10.1001/archinte.159.20.2470. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Saaddine JB, Chou C-F, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of Diabetic Retinopathy in the United States, 2005-2008. JAMA: The Journal of the American Medical Association. 2010;304:649–56. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis SK, Liu Y, Gibbons GH. Disparities in trends of hospitalization for potentially preventable chronic conditions among African Americans during the 1990s: Implications and benchmarks. American Journal of Public Health. 2003;93:447–55. doi: 10.2105/ajph.93.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook CB, Naylor DB, Hentz JG, Miller WJ, Tsui C, Ziemer DC, et al. Disparities in diabetes-related hospitalizations: Relationship of age, sex, and race/ethnicity with hospital discharges, lengths of stay, and direct inpatient charges. Ethnicity and Disease. 2006;16:126–31. [PubMed] [Google Scholar]
- 6.Cdc. National Diabetes Surveillance System.
- 7.Cook CB, Tsui C, Ziemer DC, Naylor DB, Miller WJ, Hentz JG. Common reasons for hospitalization in urban diabetes patients. Ethnicity and Disease. 2006;16:391–7. [PubMed] [Google Scholar]
- 8.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association Clinical Practice Recommendations Among U.S. Adults With Diabetes, 1999-2002: The National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–7. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 9.Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. Journal of the American Medical Association. 2001;285:182–9. doi: 10.1001/jama.285.2.182. [DOI] [PubMed] [Google Scholar]
- 10.Turner R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 11.Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. British medical journal. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing Diabetes Prevalence Between African Americans and Whites of Similar Socioeconomic Status. American Journal of Public Health. 2007;97:2260–7. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaVeist TA, Thorpe RJ, Jr, Galarraga JE, Bower KM, Gary-Webb TL. Environmental and socioeconomic factors as contributors to racial disparities in diabetes prevalence. Journal of general internal medicine. 2009;24:1144–8. doi: 10.1007/s11606-009-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darmon N, Drewnowski A. Does social class predict diet quality? The American Journal of Clinical Nutrition. 2008;87:1107–17. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 15.Drewnowski A, Moudon AV, Jiao J, Aggarwal A, Charreire H, Chaix B. Food environment and socioeconomic status influence obesity rates in Seattle and in Paris. Int J Obes (Lond) 2014;38:306–14. doi: 10.1038/ijo.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horowitz CR, Colson KA, Hebert PL, Lancaster K. Barriers to buying healthy foods for people with diabetes: Evidence of environmental disparities. American Journal of Public Health. 2004;94:1549–54. doi: 10.2105/ajph.94.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parks SE, Housemann RA, Brownson RC. Differential correlates of physical activity in urban and rural adults of various socioeconomic backgrounds in the United States. J Epidemiol Community Health. 2003;57:29–35. doi: 10.1136/jech.57.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siceloff ER, Coulon SM, Wilson DK. Physical activity as a mediator linking neighborhood environmental supports and obesity in African Americans in the path trial. Health Psychol. 2014;33:481–9. doi: 10.1037/a0032758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivedi AN, Zaslavsky AM, Schneider EC, Ayanian JZ. Relationship between quality of care and racial disparities in Medicare health plans. JAMA. 2006;296:1998–2004. doi: 10.1001/jama.296.16.1998. [DOI] [PubMed] [Google Scholar]
- 20.Sequist TD, Fitzmaurice GM, Marshall R, Shaykevich S, Safran DG, Ayanian JZ. Physician performance and racial disparities in diabetes mellitus care. Archives of Internal Medicine. 2008;168:1145–51. doi: 10.1001/archinte.168.11.1145. [DOI] [PubMed] [Google Scholar]
- 21.Miller ST, Schlundt DG, Larson C, Reid R, Pichert JW, Hargreaves M, et al. Exploring ethnic disparities in diabetes, diabetes care, and lifestyle behaviors: The Nashville REACH 2010 community baseline survey. Ethnicity and Disease. 2004;14 [PubMed] [Google Scholar]
- 22.Osborn CY, Cavanaugh K, Wallston KA, White RO, Rothman RL. Diabetes numeracy: An overlooked factor in understanding racial disparities in glycemic control. Diabetes care. 2009;32:1614–9. doi: 10.2337/dc09-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schillinger D, Barton LR, Karter AJ, Wang F, Adler N. Does literacy mediate the relationship between education and health outcomes? A study of a low-income population with diabetes. Public Health Reports. 2006;121:245–54. doi: 10.1177/003335490612100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes care. 2002;25:1015–21. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 25.Shenolikar RA, Balkrishnan R, Camacho FT, Whitmire JT, Anderson RT. Race and medication adherence in medicaid enrollees with type-2 diabetes. Journal of the National Medical Association. 2006;98:1071–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Huang ES, Brown S, Thakur N, Carlisle L, Foley E, Ewigman B, et al. Racial/ethnic differences in concerns about current and future medications among patients with type 2 diabetes. Diabetes care. 2009;32:311–6. doi: 10.2337/dc08-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aikens JE, Piette JD. Diabetic patients’ medication underuse, illness outcomes, and beliefs about antihyperglycemic and antihypertensive treatments. Diabetes care. 2009;32:19–24. doi: 10.2337/dc08-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skyler JS. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA diabetes trials: A position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association: Response to Lund and Vaag. Diabetes care. 2009;32 doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlamangla AS, Merkin SS, Crimmins EM, Seeman TE. Socioeconomic and Ethnic Disparities in Cardiovascular Risk In the United States, 2001-2006. Annals of Epidemiology. 2010;20:617–28. doi: 10.1016/j.annepidem.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. New England Journal of Medicine. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 31.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA : the journal of the American Medical Association. 2003;289:2083–93. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 32.Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Annals of Internal Medicine. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 33.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Medical care research and review : MCRR. 2000;57(Suppl 1):146–61. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- 34.Daly A, Michael P, Johnson EQ, Harrington CC, Patrick S, Bender T. Diabetes white paper: Defining the delivery of nutrition services in Medicare medical nutrition therapy vs Medicare diabetes self-management training programs. J Am Diet Assoc. 2009;109:528–39. doi: 10.1016/j.jada.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Wood JR, Kaminski BM, Kollman C, Beck RW, Hall CA, Yun JP, et al. Accuracy and precision of the Axis-Shield Afinion hemoglobin A1c measurement device. Journal of diabetes science and technology. 2012;6:380–6. doi: 10.1177/193229681200600224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos-Lozano A, Santin-Medeiros F, Cardon G, Torres-Luque G, Bailon R, Bergmeir C, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. International journal of sports medicine. 2013;34:975–82. doi: 10.1055/s-0033-1337945. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport. 2011;14:411–6. doi: 10.1016/j.jsams.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Aguilar-Farias N, Brown WJ, Peeters GM. ActiGraph GT3X+ cut-points for identifying sedentary behaviour in older adults in free-living environments. Journal of science and medicine in sport / Sports Medicine Australia. 2014;17:293–9. doi: 10.1016/j.jsams.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Shah M, Norwood CA, Farias S, Ibrahim S, Chong PH, Fogelfeld L. Diabetes transitional care from inpatient to outpatient setting: pharmacist discharge counseling. Journal of pharmacy practice. 2013;26:120–4. doi: 10.1177/0897190012451907. [DOI] [PubMed] [Google Scholar]
- 40.U. S. Department of Health and Human Services NIoH, National Cancer Institute. Theory at a Glance: A Guide for Health Promotion Practice. 2005 [Google Scholar]
- 41.Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, et al. National Standards for Diabetes Self-Management Education. Diabetes care. 2010;33:S89–S96. doi: 10.2337/dc10-S089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franz MJ, Powers MA, Leontos C, Holzmeister LA, Kulkarni K, Monk A, et al. The Evidence for Medical Nutrition Therapy for Type 1 and Type 2 Diabetes in Adults. Journal of the American Dietetic Association. 2010;110:1852–89. doi: 10.1016/j.jada.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Suppl 1):S120–43. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 44.Axelson ML, Brinberg D. The measurement and conceptualization of nutrition knowledge. Journal of Nutrition Education. 1992;24:239. [Google Scholar]
- 45.Connors M, Bisogni CA, Sobal J, Devine CM. Managing values in personal food systems. Appetite. 2001;36:189–200. doi: 10.1006/appe.2001.0400. [DOI] [PubMed] [Google Scholar]
- 46.Furst T, Connors M, Bisogni CA, Sobal J, Falk LW. Food choice: A conceptual model of the process. Appetite. 1996;26:247–65. doi: 10.1006/appe.1996.0019. [DOI] [PubMed] [Google Scholar]
- 47.Lynch EB, Fernandez A, Lighthouse N, Mendenhall E, Jacobs E. Concepts of diabetes self-management in Mexican American and African American low-income patients with diabetes. Health Education Research. 2012;27:814–24. doi: 10.1093/her/cys058. [DOI] [PubMed] [Google Scholar]
- 48.Lynch EB, Holmes S. Food group categories of low-income African American women. Journal of Nutrition Education and Behavior. 2011;43:157–64. doi: 10.1016/j.jneb.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Lynch EB, Holmes S, Keim K, Koneman SA. Concepts of healthful food among low-income African American women. Journal of Nutrition Education and Behavior. 2012;44:159. doi: 10.1016/j.jneb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Fischhoff B, Bostrom A, Quadrel MJ. Risk perception and communication. In: Detels RMJ, Beaglehole R, Tanaka H, editors. Oxford Textbook of Public Health. Oxford: Oxford University Press; 2002. pp. 1105–23. [Google Scholar]
- 51.Lynch EB, Liebman R, Ventrelle J, Avery EF, Richardson D. A self-management intervention for African Americans with comorbid diabetes and hypertension: a pilot randomized controlled trial. Prev Chronic Dis. 2014;11:E90. doi: 10.5888/pcd11.130349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolff K, Cavanaugh K, Malone R, Hawk V, Gregory BP, Davis D, et al. The Diabetes Literacy and Numeracy Education Toolkit (DLNET): Materials to facilitate diabetes education and management in patients with low literacy and numeracy skills. Diabetes Educator. 2009;35:233–45. doi: 10.1177/0145721709331945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harmer AR, Elkins MR. Amount and frequency of exercise affect glycaemic control more than exercise mode or intensity. British journal of sports medicine. 2014 doi: 10.1136/bjsports-2013-093225. [DOI] [PubMed] [Google Scholar]
- 54.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of Exercise on Glycemic Control and Body Mass in Type 2 Diabetes Mellitus: A Meta-analysis of Controlled Clinical Trials. JAMA: The Journal of the American Medical Association. 2001;286:1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 55.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: A consensus statement from the American Diabetes Association. Diabetes care. 2006;29:1433–8. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 56.Strom JL, Egede LE. The impact of social support on outcomes in adult patients with type 2 diabetes: a systematic review. Curr Diab Rep. 2012;12:769–81. doi: 10.1007/s11892-012-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raffel KE, Goddu AP, Peek ME. “I Kept Coming for the Love”: Enhancing the Retention of Urban African Americans in Diabetes Education. Diabetes Educ. 2014 doi: 10.1177/0145721714522861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osborn CY, Bains SS, Egede LE. Health literacy, diabetes self-care, and glycemic control in adults with type 2 diabetes. Diabetes technology & therapeutics. 2010;12:913–9. doi: 10.1089/dia.2010.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer Mentoring and Financial Incentives to Improve Glucose Control in African American VeteransA Randomized Trial. Annals of Internal Medicine. 2012;156:416–24. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rothschild SK, Martin MA, Swider SM, Tumialan Lynas CM, Janssen I, Avery EF, et al. Mexican American Trial of Community Health Workers: A Randomized Controlled Trial of a Community Health Worker Intervention for Mexican Americans With Type 2 Diabetes Mellitus. Am J Public Health. 2013 doi: 10.2105/AJPH.2013.301439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rothschild SK, Martin MA, Swider SM, Lynas CT, Avery EF, Janssen I, et al. The Mexican-American Trial of Community Health workers (MATCH): design and baseline characteristics of a randomized controlled trial testing a culturally tailored community diabetes self-management intervention. Contemp Clin Trials. 2012;33:369–77. doi: 10.1016/j.cct.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch E, Liebman R, Ventrelle J, Avery EF, Richardson D. A Self-Management Intervention for African Americans With Comorbid Diabetes and Hypertension: A Pilot Randomized Controlled Trial. Prev Chronic Dis. 2014;11:E90. doi: 10.5888/pcd11.130349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agurs-Collins TD, Kumanyika SK, Ten Have TR, Adams-Campbell LL. A randomized controlled trial of weight reduction and exercise for diabetes management in older African-American subjects. Diabetes Care. 1997;20:1503–11. doi: 10.2337/diacare.20.10.1503. [DOI] [PubMed] [Google Scholar]
- 64.Samuel-Hodge CD, Keyserling TC, Park S, Johnston LF, Gizlice Z, Bangdiwala SI. A randomized trial of a church-based diabetes self-management program for African Americans with type 2 diabetes. The Diabetes educator. 2009;35:439–54. doi: 10.1177/0145721709333270. [DOI] [PubMed] [Google Scholar]
- 65.Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–96. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes care. 2006;29:1675–88. doi: 10.2337/dc05-1942. [DOI] [PubMed] [Google Scholar]
- 68.Haas L, Maryniuk M, Beck J, Cox CE, Duker P, Edwards L, et al. National standards for diabetes self-management education and support. Diabetes Care. 2014;37(Suppl 1):S144–53. doi: 10.2337/dc14-S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


