Abstract
Spinal cord stimulation (SCS) is used to manage chronic intractable neuropathic pain. We examined parameters of SCS in rats with spared nerve injury by modulating frequency (4Hz vs. 60Hz), duration (30m vs. 6h), or intensity (50%, 75%, or 90% MT). To elucidate potential mechanisms modulated by SCS, we examined immunoreactivity glial markers in the spinal cord after SCS). An epidural SCS lead was implanted in the upper lumbar spinal cord. Animals were tested for mechanical withdrawal threshold (MWT) of the paw before and 2 weeks after SNI, before and after SCS daily for 4 days, and for 9 days after SCS. Seperate groups of animals were tested for glial immunoreactivity after 4 days of 6h SCS. All rats showed a decrease in MWT 2 weeks after nerve injury and an increase in glial activation. For frequency, 4Hz or 60Hz SCS reversed the MWT when compared to sham SCS. For duration, 6h of SCS showed a greater reduction in MWT when compared to 30 min. For intensity, 90% MT was greater than 75% MT and both were greater than 50% MT or sham SCS. SCS decreased glial activation (GFAP, MCP-1 and OX-42) in the spinal cord dorsal horn when compared to sham. In conclusion, 4Hz and 60Hz SCS for a 6h at 90% MT were the most effective parameters for reducing hyperalgesia, suggesting parameters of stimulation are important for effectiveness of SCS. SCS reduced glial activation at the level of the spinal cord suggesting reduction in central excitability.
Keywords: pain, neuropathic pain, electrical stimulation, glia, spinal cord
1. Introduction
Neuropathic pain is defined as pain resulting from damage or dysfunction of the peripheral nerves and more broadly, as a result of injury or disease of the somatosensory system. There are a number of diseases associated with neuropathic pain. Examples include autoimmune disease (e.g., multiple sclerosis), metabolic diseases (e.g., diabetic neuropathy), infection (e.g., shingles and the sequel, postherpetic neuralgia), vascular disease (stroke), trauma, and cancer. The study of neuropathic pain in experimental animals has been done with four models: partial sciatic ligation (PSL), chronic constrictive injury (CCI), spinal nerve ligation (SNL), and spared nerve injury (SNI) [2]. The anomalous pain states evoked by nerve injury in humans can be mimicked in spinal nerve injury (SNI) animal models of neuropathic pain [26].
Spinal cord glia, microglia and astrocytes, have important roles in the induction and maintenance of pain facilitation in animal models of chronic pain induced by peripheral nerve injury, inflammation and cancer [14,34,53,71–73,86]. Although, the specific mechanisms underlying neuropathic pain after peripheral nerve injury are not fully understood, recent research suggests that peripheral nerve injury evokes a time dependent re-organization of the central and peripheral nervous systems. Pain hypersensitivity was originally thought to result exclusively from altered neuronal activity in primary sensory and spinal cord neurons. It is now clear that glial cells also play a significant role in the pathogenesis of neuropathic pain [12,37,80–82]. In fact there is a time-dependent activation of both astrocytes and microglia after SNI in the spinal cord [17,45,46]. Further, inhibition of glial activation reduces mechanical hypersensitivity in animals with nerve injury [41,72,74,91].
Spinal cord stimulation (SCS) is used to manage chronic, neuropathic, intractable pain generally for the trunk and or limbs via delivery of electrical impulses to spinal segments [62]. SCS has been successful in providing analgesia, improving function, and enhancing quality of life for patients suffering from chronic pain [21]. In animals with nerve injury, SCS reduces mechanical hypersensitivity up to 40 min after 10 min SCS at 50 Hz [8,30,39]. Similarly, 4 Hz and 60 Hz SCS, but not 100 Hz SCS, reduces mechanical hypersensitivity of the paw and muscle up to 24 h after 30 min SCS [33]. As SCS is clinically used for longer durations, this study tested the effects of long-duration SCS (6h) and compared effects to short-duration SCS (30 min). As intensities vary between studies ranging from 50–90% motor threshold we tested the intensity-effect of SCS (50–90% motor threshold intensity). Lastly, we tested if if SCS reduces glial cell activation in the spinal cord after SCS as a potential underlying mechanism.
2. Methods
2.1. Experimental procedures
The experiments were performed on adult Sprague–Dawley rats, weighing 250–350 g and housed in transparent plastic cages with free access to food and water, in a 12 h light–dark cycle. All the experimental procedures were approved by the Animal Care and Use Committee at the University of Iowa.
2.1.2. Nerve Injury Model
All rats were anesthetized with 2–3% isoflurane. The tibial and common peroneal nerves on one limb were tightly ligated with 4–0 silk and the sural nerve was kept intact, as previously described [10]. The overlying muscle was sutured with 4–0 silk, and the skin was sutured closed with 3–0 silk.
2.1.3. Implantation of the electrode
After nerve injury, a small laminectomy was performed at the level of T13 vertebra which corresponds to the upper lumbar spinal cord region. The lead was inserted epidurally in the rostral direction. The lead was fixed with sutures to the muscle, the wound was sutured in layers, and the lead was tunneled to exit the skin at the base of the neck [33]. We utilized a spinal cord lead designed for use in rats (Medtronic, Minneapolis, MN) which is similar to that used in humans. The proximal end of the lead was tunneled outside the animal for later connection to an external neuro-stimulator (model # 37021) and programmer (model: #8840) (Medtronic Inc., Minneapolis, MN).
2.2. Behavior tests
Before surgery, rats were acclimated to the behavior room for thirty minutes followed by acclimation to the transparent plastic cubicles on elevated wire mesh floor for fifteen minutes. To test for mechanical withdrawal thresholds of the paw, calibrated von Frey filaments (10 filaments, 1, 13, 20, 36, 49, 67, 83, 105, 188 and 402) were applied to the lateral surface of both the ipsilateral and contralateral paw in the area innervated by the sural nerve. Each filament was applied for approximately 1 s with enough to bend the filaments. Each filament was applied twice and a positive response was one withdrawal. Once a positive response was found, the filament above and below the filament that caused a positive response was tested. Confirmation of withdrawal threshold was established if there was a positive withdrawal from the filament above and no withdrawal from the filament below. The lowest withdrawal force that produced a withdrawal was recorded as the threshold. A decrease in mechanical withdrawal threshold of the paw is interpreted as cutaneous hyperalgesia of the paw in this study. This is based on the fact that nociceptors are activated by von Frey filaments of 14 mN or greater, and that large diameter afferents are activated by less than 14 mN [29].
2.3 Immunohistochemistry
We tested for glial cell activation in the spinal cord using immunohistochemistry of astrocyte and microglial markers. Rats were anestheized with 100 mg/kg sodium pentobarbital and were perfused intracadially with a saline solution containing heparin (10U/ml) followed by 4% paraformaldehyde (PFA) with 15% piric acid in 0,1M phosphate buffer. The spinal cords were extracted, post fixed in 4% paraformaldehyde for 1 h, transferred to 30% sucrose solution for 24 hours, then frozen in dry-ice with tissuetek OCT. Tissue sections were cut on a cryostat at 40µM and placed on slides for future staining.
All sections were first blocked with 3% of normal goat serum for thirty minutes, followed by Avidin-Biotin Block (fifteen minutes each). For astrocyte staining, sections were incubated overnight with monoclonal anti-mouse anti-GFAP (Milipore - 1:5000, Cat.#MAB360). On the second day, the sections were incubated with biotinylated goat anti-mouse IgG (Invitrogen - 1:1000) for 1 hour followed by Strep-568 (Invitrogen - 1:1000) for 1 hour. We then incubated sections overnight in goat anti-rabbit MCP-1 (Milipore −1:500, Cat.#1834P). On the last day of immunostaining, the sections were incubated in biontinylated goat anti-rabbit IgG (Invitrogen- 1:1000) for 1 hour followed by Strep-488 (Invitrogen - 1:1000) for 1 hour. Slides were coverslipped with Vectashield. For microglia, we just changed the first primary antibody to OX-42 (AbD serotec, 1:2500, Cat.#MCA275G). In a seperate group, we tested for p-p-38 activation (Cell Signal, 1:500, Cat.#4631S). All the antibodies were diluted (100%+ 1% NGS+ 0.1% Triton-X 100+ 0.2% sodium azide).
Five spinal cord sections (L4- L5) were randomly chosen from each rat. The stained sections were examined with a Nikon TE-300 fluorescence microscope (Japan). The superficial laminas (I–II) and intermediate and deep dorsal horn (III–VI) were outlined, and the number of pixels occupied by immunoreactive cells was measured using Image J 1.24 software (NIH) [28,83]. Specifically, each tissue section was first converted to eight-bit gray scale, and then each tissue section was calibrated independently using the “uncalibrated OD” function with pixel values ranging from 0 to 255. The density values represent pixels per area. A background reading taken from the white matter of the dorsal column was subtracted from the density reading taken from the gray matter of the same tissue section. This controls for differences in nonspecific staining as a result of the DAB reaction [20].
2.4. Experimental protocol
2.4.1. Experiment 1
Experiment 1 tested for differences in analgesia by different durations of stimulation. Mechanical withdrawal thresholds were tested before and 2 weeks after SNI, and before and after daily application of SCS for 4 days. SCS was applied two weeks after SNI as follows. The lead was connected to a neurostimulator and SCS was applied at 4Hz or 60Hz frequencies. Stimulation amplitude was 90% of motor threshold (MT) and at an intensity of 0.35 volts for 4 days. Animals received either 30 min or 6h of SCS. All parameters of stimulation were programmed into the stimulator immediately, prior to the start of stimulation. All groups were tested after the stimulator turned off at days 5, 8 and 14 which corresponded to 1, 3, and 9 days after SCS. Animals were randomly assigned to 6 experimental groups, with two frequencies and two different durations of stimulation as follows: group 1 = 4 Hz with 30 minutes of SCS (n = 8), group 2 = 4 Hz with 6 hours of SCS (n = 6), group 3 = 60 Hz with 30 minutes of SCS (n = 8), group 4 = 60 Hz with 6 hours of SCS (n = 6), group 5 = Sham SCS for 30 minutes (n = 4) and Group 6 = Sham SCS for 6 hours (n=6).
2.4.2. Experiment 2
Experiment 2 tested for differences in analgesia produced by different intensities of stimulation. Mechanical withdrawal thresholds were tested before and 2 weeks after SNI, and before and after daily application of SCS for 4 days. SCS was applied two weeks after SNI as follows. Stimulation amplitude was 50 and 75% MT for 4 days and were compared to 90% MT intensity. All animals received and either 4 Hz or 60 Hz frequencies. All parameters of stimulation were programmed into the stimulator immediately prior to the start of stimulation. All groups were tested until day 5, 24h after stopping SCS. Animals were randomly assigned to 5 experimental groups, with two frequencies and two different amplitude doses of stimulation. The groups were divided into group 1 = 4 Hz with 50% MT (n = 8), group 2 = 4 Hz with 75% MT (n = 8), group 3 = 60 Hz with 50% MT (n = 8), group 4 = 60 Hz with 75% MT (n = 8), group 5 = Sham SCS for 6 hours (n=8). This data was compared to the data from Experiment 1 that received 90% MT to test for itensity-dependent effects. The sham animals were implanted with the neurostimulation hardware and were tethered to the neurostimulator system, but did not receive SCS.
2.4.3. Experiment 3
Experiment 3 tested for glial cell activation in the spinal cord using immunohistochemistry of astrocyte and microglial markers. Perfusion was done 7 days or 15 days after SNI, or after application of SCS for 4 days. SCS was applied two weeks after SNI as follows: amplitude was 90% MT, duration was 6 hours, and frequency was either 4 Hz or 60 Hz fequency. The groups were divided into group 1 = 7 days SNI p-p38 marker (n = 5), group 2 = naive control p-p38 and 0X-42 (n=5) or GFAP and MCP-1 (n = 5), group 3 = 60 Hz SCS OX-42 (n=5) or GFAP and MCP-1 (n = 5), group 4 = 4 Hz SCS OX-42 (n=5) or GFAP and MCP-1 (n = 5).
2.5 Statistical analysis
Analysis of the data was performed using SPSS 13.1 (Statistical Package for the Social Sciences). Data for mechanical withdrawal thresholds of the paw were presented as mean ± S.E.M‥ For tests, the differences between duration (sham, 30 minutes and 6 hours), amplitude-dose (sham, 50%, 75%, 90% MT), between frequency (4HZ, 60Hz) and side (right, left) before and after SCS were examined using a repeated measures ANOVA. Post hoc testing between different groups was performed with a Tukey’s test (parametric). A seperate analysis compared the percentage of responders and non-responders between durations and frequencies of stimulation. Responders and non-responsers were established based on the paw withdrawal threshold responses after SCS. Responders were defined as those with an increase in withdrawal thresholds during SCS compared to values prior to SCS. Density of immunostaining was analyzed using a one-way ANOVA and post hoc Tukey’s test (parametric). A p value <0.05 was considered significant.
3. Results
3.1 SNI model
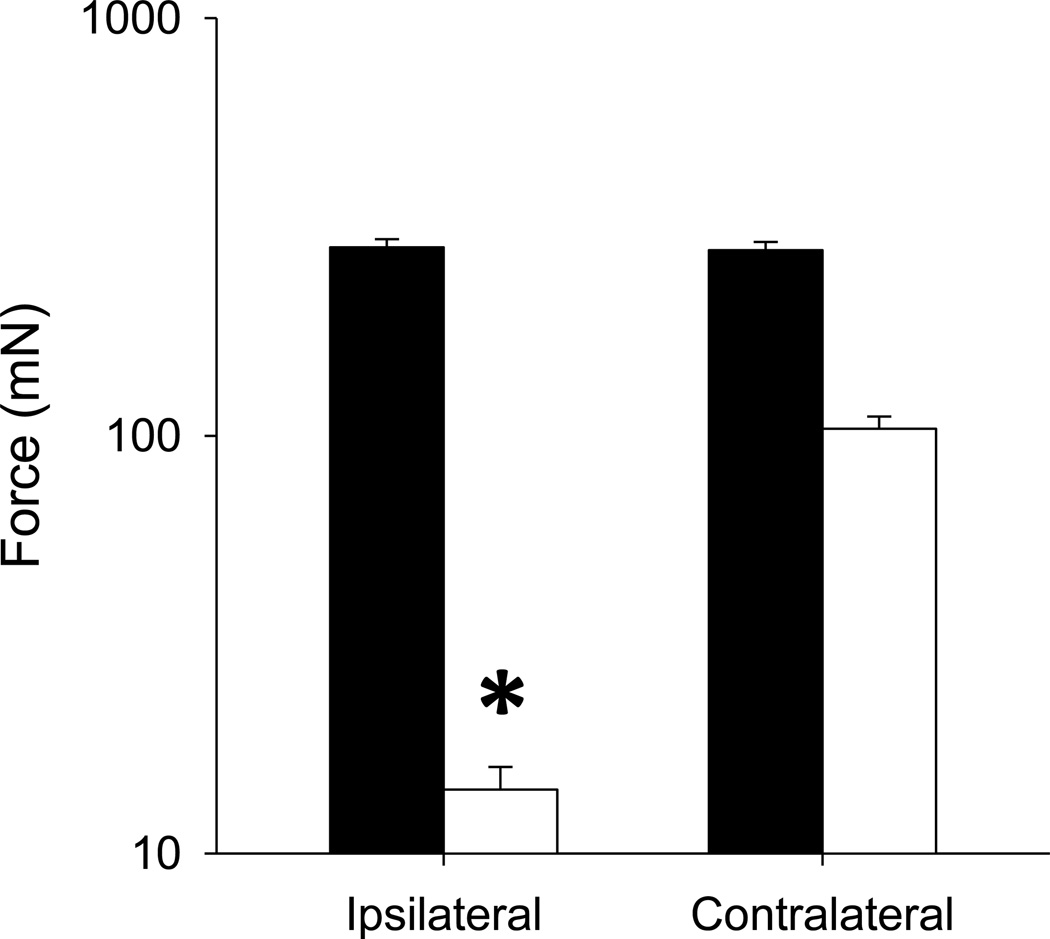
Before SNI, baseline withdrawal thresholds had on average of 295± 18. Two weeks after SNI, all groups showed a significant decrease in mechanical withdrawal thresholds ipsilaterally averaging 20 ± 3.2 mN. The contralateral side showed a decrease that averaged 89 ± 4.6 mN but was not statistically significant (Figure 1).
Figure 1.
Average withdrawal thresholds before (baseline) and 2 weeks SNI for the ipsilateral and contralateral sides. Data are man + S.E.M. *, p<0.05
3.1.1. Effects of SCS
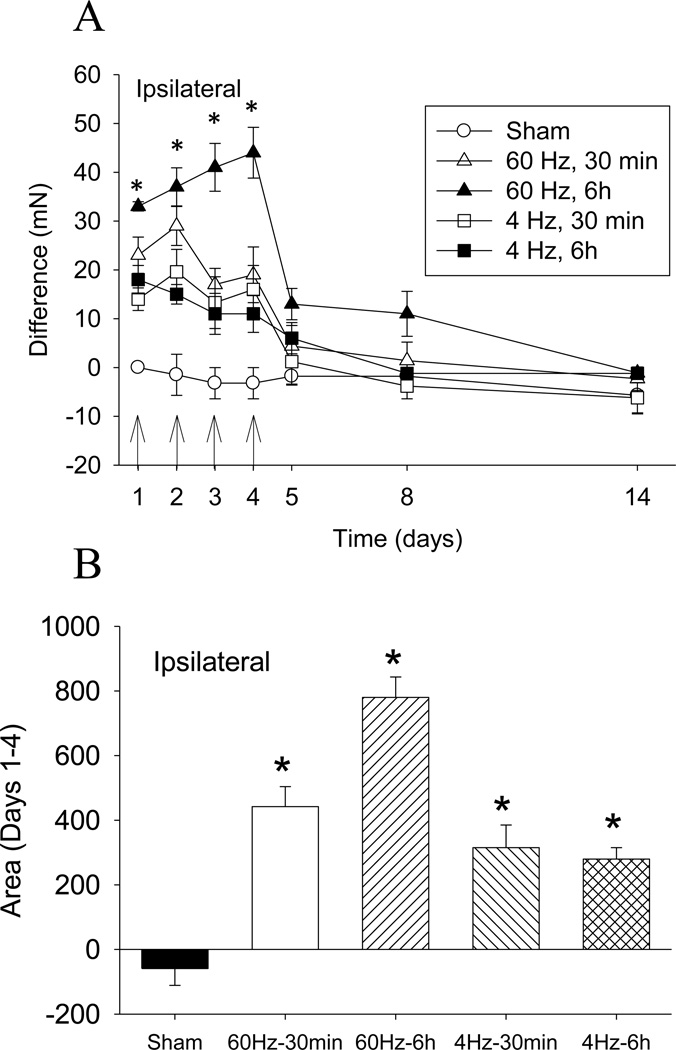
The paw withdrawal threshold singificantly increased ipsilaterally after treatment with either 60 Hz SCS or 4 Hz SCS (p=0.0001) when compared to sham SCS (p=0.0001). SCS for 6h showed significantly greater analgesia when compared to 30 min SCS (p=0.02) or sham (p=0.0001); 30 min SCS at 4 Hz was significantly greater than sham SCS (p=0.0001) (Figure 2A). Thus, the greatest reduction in withdrawal threhsold occured with 60 Hz SCS delivered for 6h at 90% MT. There is small but significant carryover effect 1 day and 3 days after SCS that is reversed 9 days after SCS. Figure 2B shows the area under the curve for the 4 groups on days 1–4. Again, the greatest reduction occurs at 60 Hz stimulation for 6 hours.
Figure 2.
A. Time course for changes in withdrawal thresholds after SNI and after SCS for up to 14 days. SCS significantly increased the mechanical withdrawal threshold bilaterally when compared to sham SCS. A greater effect was observed with 6h of SCS compared 30min SCS. The arrow shows the time of SCS treatment. B. Averege area under the curve for the changes in withdrawal thresholds during SCS compared to prior to SCS for all groups averaged over the first 4 days of SNI. Data are mean difference scores between after SCS compared to before SCS with S.E.M. * p<0.05, different from sham group.
A seperate analysis examined differences between responders and non-responders. On Day 1, the number of responders in the 60 Hz 6 hour SCS group was 100%, compared to 62% in the 60 Hz 30 min SCS group, 50% in the 4Hz 6h SCS group, and 17% in the 4Hz 30 min SCS group. After SCS, there was a significant difference when comparing 60Hz to 4Hz in the number of responders (p=0.013). When comparing duration of SCS for the number of responders there was no difference between durations of stimulation. However, 6h of stimulation was significantly different from sham (p=0.003).
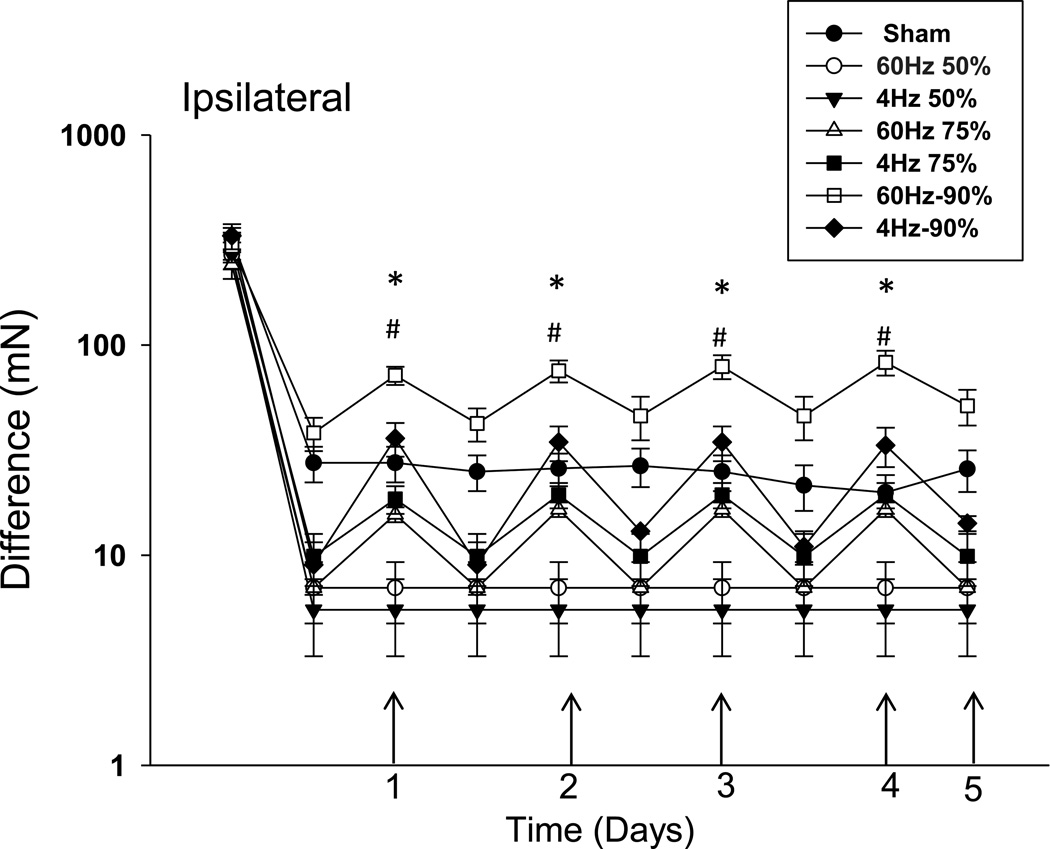
After SCS the greatest effect occurred with an intensity of 90% MT which was significantly different from 75% SCS (p=0.023), 50% SCS (p=0.019), or sham (p<0.0001). The changes in the ipsilateral paw withdrawal thresholds were significantly greater after 75% MT SCS when compared to 50% SCS (p=0.019) or sham SCS (p=0.0019). There was a significant difference when comparing the three intensities as follows: 90% MT > 75% MT > 50% MT = sham SCS (Figure 3).
Figure 3.
SCS at 90% MT and 75% MT significantly increased withdrawal thresholds of the paw. Notice a dose-response effect ipsilaterally with increasing intensity of SCS. The difference in paw withdrawal threshold after SCS when compared to pre-SCS values 2 weeks after SNI. * diference between 90% MT and others groups, # difference between 75% and 50% and sham.
3.1.2. SCS suppressed spinal glial activation in neuropathic pain
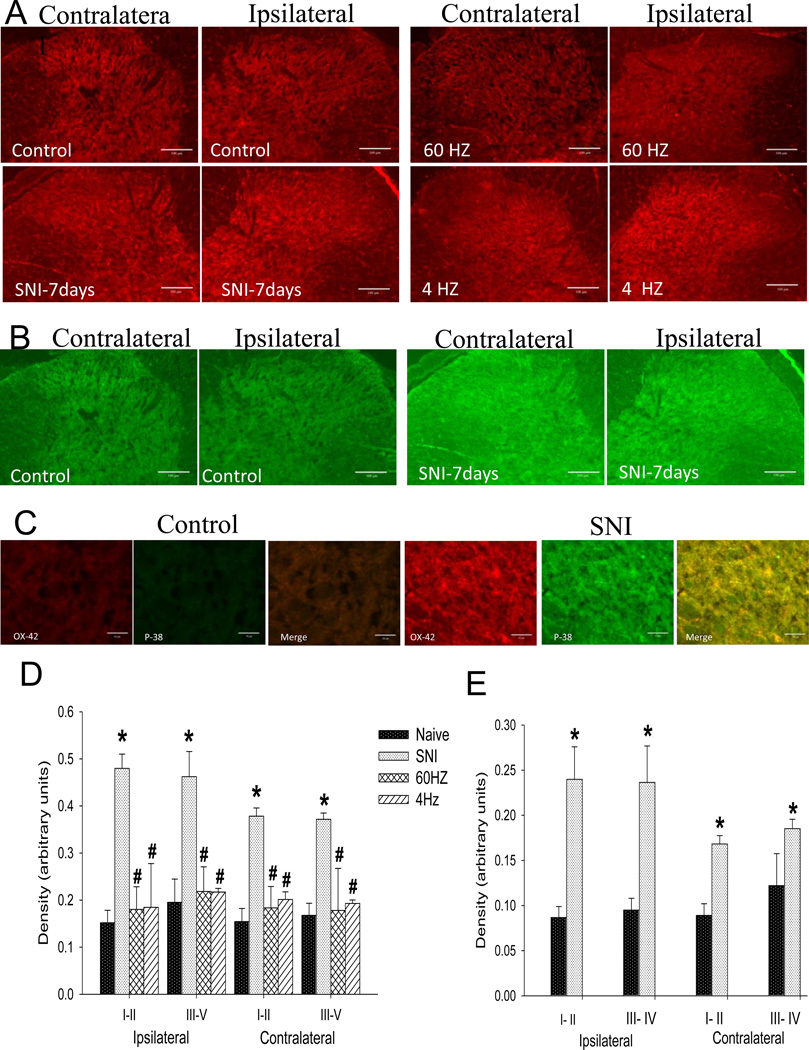
After SCS, immunohistochemistry was performed to determine whether SCS suppressed glial activation in the spinal dorsal horn in animals with neuropathic pain. Both microglial (OX-42 positive) (Figure 4-A) and astrocytic markers (GFAP positive and MCP-1 positive) (Figure 5-A and B) were significantly increased bilaterally 2 weeks after SNI. Both 4 days of 4 Hz and 60 Hz SCS significantly decreased OX-42, GFAP and MCP-1 immunoreactivity bilaterally when compared to sham SCS (Figure 4-A, 5 A and B). There were no changes in p-p38 15 days after SNI. However, as previuosly reported p-p38 is increased earlier after nerve injury, as early as 12 hours and peaked after 3 days [23] - we confirmed this increase 7 days after SNI (Figure 4-B and C). There was a significant increase in the density of p-p-38 staining 7 days after SNI in laminae I-II bilaterally (ipsilateral: p=0.004; contralateral p=0.001) and laminae III-V bilaterally ipsiletaral; p=0.010; contralateral; p=0.001)(Figure 4-E).
Figure 4.
A. Representative tissue sections for OX-42 immunostaining in the dorsal horn of naive control rats, SNI-7days, 60 Hz SCS and 4 Hz SCS. Bar =100µm. B. Representative tissue sections for p-p-38 immunostaining in the dorsal horn of naive control rats compared to 7 days after SNI. Bar =100µm. C. Representative tissue sections with high magnification (40X) for OX-42 in red, p-p-38 in green and merge in yellow of control rats and 7 days after SNI. Bar =50µm. D. There is a significant increase in the density of OX-42 staining bilaterally in the dorsal horn after SNI. Both 60 Hz SCS and 4 Hz SCS significantly decrease this staining. E. There is a significant increase in the density of staining bilaterally in the dorsal horn 7 days after SNI in p-p-38 staining. (*) compared with the naieve controls, (#) compared with the SNI, Data represent mean ≤ p=0.005.
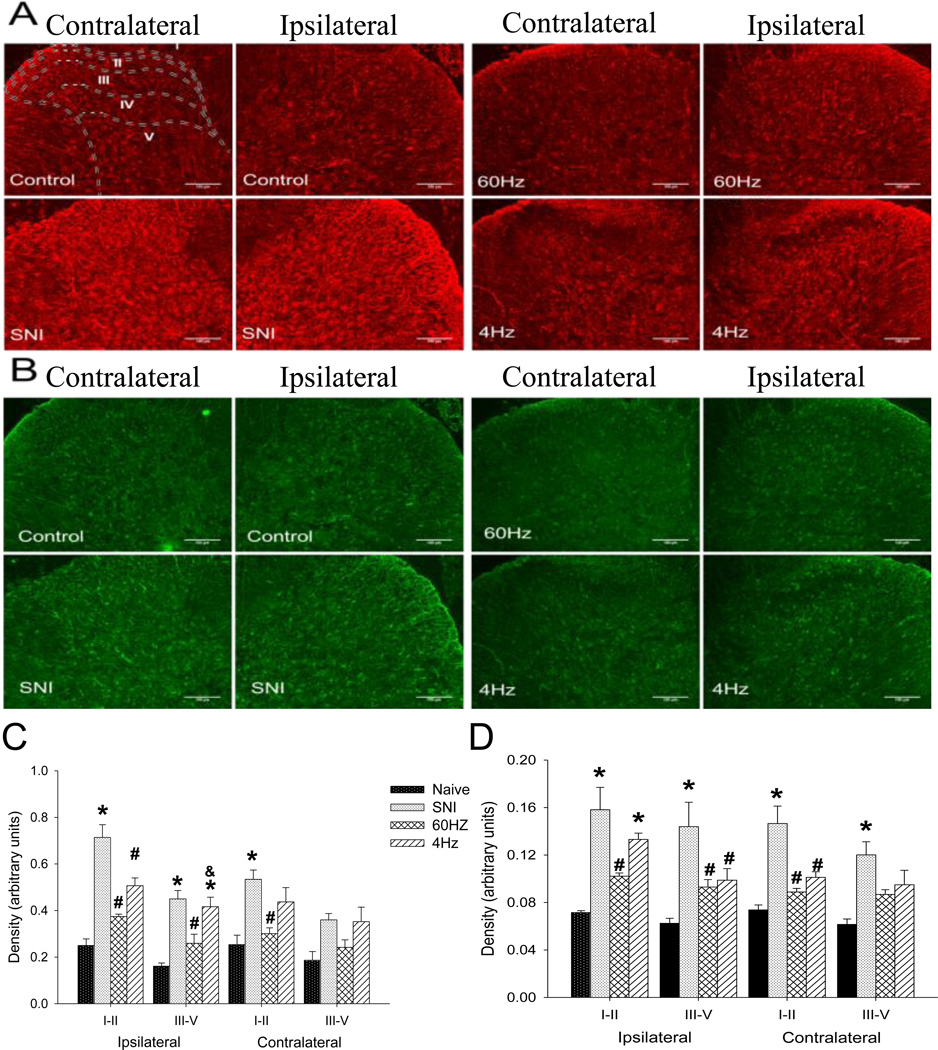
Figure 5.
A. Representative tissue sections for GFAP immunostaining in the dorsal horn of control rats, SNI, 60 Hz SCS and 4 Hz SCS. Sketches delineating boundaries of different laminae are superimposed over the spinal sections of control rats. B. Representative tissue sections for MCP-1 immunostaining in the dorsal horn of native control rats, SNI, 60 Hz SCS and 4 Hz SCS. C. There is a significant increase in the density of GFAP staining bilaterally in the dorsal horn after SNI. Both 60 Hz SCS and 4 Hz SCS significantly decrease this staining. D. There is a significant increase in the density of staining bilaterally in the dorsal horn after SNI. Both 60 Hz SCS and 4 Hz SCS significantly decrease MCP-1 staining. (*) compared with naive controls, (#) compared with the SNI, (&) compared with 60Hz SCS. Data represent mean ≤ p=0.005. Bar =100µm.
The density of the microglia marker OX-42 was increased bilaterally in laminae I-II 2 weeks after SNI when compared to naive controls (ipsilateral p=0.01; contralateral p=0.008). Both 60Hz and 4Hz SCS significantly decreased OX-42 staining in the superficial laminae bilaterally when compared to sham SCS: 60 Hz (ipsilateral p=0.0001; contralateral p=0.001) and 4Hz (ipsilateral p=0.0001; contralateral p=0.003). In laminae III-V the density of immunoreactivity for OX-42 was also increased after SNI both ipsilaterally (p=0.001) and contralaterally (p=0.001) when compared to naive controls. SCS signficantly decreased the OX-42 staining in the deep dorsal horn when compared to sham SCS: 60 Hz (ipsilateral p=0.005; contralateral p=0.004); 4 Hz group (ipsiletaral p=0.005; contralateral p=0.004) (Figure 4-D).
For the astrocyte markers we examined GFAP and MCP-1. Statistically significant increases in the density of immunoreactivity for GFAP and MCP-1 occured in laminae I-II for both the ipsilateral (GFAP p=0.0001; MCP-1 p=0.007) and the contralateral (GFAP p=0.001; MCP-1p=0.03) sides. SCS reduced the SNI-induced increased immunoreactivity for GFAP both ipsilaterally (60 Hz p=0.0001; 4Hz p=0.0001) and contralaterally (60 Hz p=0.002; 4 Hz p=0.0001) in the superficial laminae. Similarly, statistically significant increases in the density of GFAP and MCP-1 in laminae III-V after SNI occured after SNI and these increases were reduced by both 60 Hz and 4 Hz SCS ipsilaterally (GFAP: control vs SNI p=0.0001, SNI vs 60 Hz p=0.002, SNI vs 4Hz p=0.462; MCP-1: control vs. SNI p=0.007, SNI vs 60 Hz p=0.002; SNI vs 4Hz p=0.843) and contralaterally (GFAP: control vs SNI p=0.001; SNI vs. 60 Hz p=0.845; SNI vs. 4Hz p=0.123; MCP-1: control vs SNI p=0.003; SNI vs. 60 Hz p=0.720; SNI vs. 4Hz p=0.269) (Figure 5-C and D).
4. Discussion
In the present study, we demonstrated that long duration SCS (6 h) produces greater analgesia than short duration SCS (30 minutes); that 60 Hz SCS is more effective than 4 Hz SCS; and both frequencies of SCS are better than sham. Further both 60 Hz and 4 Hz SCS decreased activation of glial cells (microglia and astrocytes) in the spinal cord suggesting that SCS reduces central excitability.
4.1. Effect of SCS depends on intensity
SCS is generally delivered both clinically and in experimental studies below motor threshold [33,38,66,76] and the majority of these use intesnities around 2/3 of motor threshold to produce analgesia [32,89]. The current study showed that intensities at 75% of motor threshold were less effective than 90% and 50% had no effect. Similarly, intensity dependent reductions in inflammation-induced hypersensitivity are observed for transcutaneous electrical nerve stimulation (TENS) in both animal and human subjects [43,58]. Higher intensities of SCS produce a longer reduction in hyperaglesia and greater intensities of SCS correlatewith longer duration and magnitude of pain relief [38,39,90]. In parallel, Gerardini et al., 1999 [15] also showed that higher intensities of SCS (90% motor threshold) increased the survival rate of skin flaps rendered ischemic when compared to SCS at lower intensities (70% of motor threshold). Thus intensity-dependent effects occur for both SCS and TENS suggesting intensity is a key factor in stimulation-produced analgesia.
4.2. SCS is more effective with longer-duration stimualation
We showed a small, but significantly greater reduction in hypersensitivity with 6h of stimulation, particularly with 60 Hz SCS. Prior studies clearly show reductions in hypersensitivity with stimualtion durations as little as 5 minutes with the majority of studies showing good reductions in hypersensitivity with 30 min to 1h of SCS [1,33,50,63,65,90]. In animals, fifteen minutes of SCS inhibited evoked responses of WDR neurons to noxious mechanical stimuli in a model of neuropathic pain (SNL) [18]. In humans, the duration of SCS is more variable with reports of 6–8 sessions per day for 10–60 min each session [87] or for longer periods between 5 and 12 hours in a session [9,27]. The current study confirms that longer duration stimulation produces greater analgesia than shorter durations.
4.3. SCS effects can persist after stimulation
Prior work in a rat model of neuropathic pain (ligature of the sciatic nerve), 10 minutes of SCS attenuated hypersensitivity of the hindpaw for up to 40 minutes after cessation of the stimulation [39]. SCS also inhibits nociceptive discharges of dorsal horn neurons for approximately 30–40 min after cessation of SCS [31,54]. The current study extends these prior findings and shows a small but significant reduction in mechanical hypersensitivity with 60 Hz SCS for up to 3 days after cessation of stimulation. In humans, how long the pain relief endures after stimulation and which factors influence the duration of post-SCS pain relief is unknown [87]. Some authors explain that the carryover effect may involve a complex set of plastic changes and remodeling in spinal and supraspinal pain-processing structures [13,55,68] and repeated SCS in some studies shows a cumulative effect [33,90]. However, in the current and our prior study were were unable to show this cumulative inhibitory effect with repeated stimulation [58].
4.4. SCS reduces glial cell activation in the spinal cord
Glial cells have been implicated in producing hypersensitivity following nerve injury [42,56,60] with both spinal microglia and astrocytes activated after nerve injury [23,48,52,75]. In the current study, we showed activation of both microglia and astrocytes after nerve injury with early increases in phosphorylation of the mitogen activated kinase (MAPK) p-38 [23,78, 84]. However, our results showed the other microglial surface marker CD11b (OX-42) continued to show enhanced immunoreactivity and that microglial cells appeared to remain activated 10 days to 2 weeks after nerve injury [6,85]. The activation of astrocytes in the later phases also agrees with prior studies implicating these cells in the maintenance of mechanical allodynia in neuropathic pain [22,70].
The current study showed for the first time that both 4 Hz and 60 Hz SCS reduce microglia and astrocyte activation in the spinal cord. Similarly, other therapeutic treatments that use our endogenous analgesia system also reduce glial activation including electroacupunture, peripheral nerve stimulation, or joint mobilization [16,25,35,69]. SCS increases release of the inhibitory neurotransmitters GABA, serotonin, and opioids in the spinal dorsal horn [7,8,30,39,32,59,65,67-58] that could directly inhibit activation of glial cells. In fact both astrocytes and microglia express inhibitory neurotransmitter receptors including GABAergic, serotonergic and opioidergic receptors [11,19,24,36,44,47,49,51,52,61,64,67,79]. Further, activation of these inhibitory neurotransmitters in animals with neuropathic pain reduced glial cell activation [88]. Alternatively, neuronal release of excitatory neurotransmitters such as glutamate could activate microglia and increase release of more excitatory neurotransmitters from glial cells to perpetuate the nociceptive response [3,4,77]. Inhibitory neurotransmitters could indirectly reduce glial cell activity by reducing neuronal release of excitatory neurotransmitters [5]. It is likely that a combination of increases in inhibitory neurotransmitters and decreases in central exctiability result in the analgesia produced by SCS, and that the increase in inhibitory neurotransmitters contribute to the reduction in glial cell activity. Therefore, further studies examining the underlying mechanisms of the reduced glial cell activity are required.
The present study examined stimulation parameters and shows that the greatest analgesia produced by SCS occurs with 60 Hz SCS for 6 hour duration. We further show that SCS, both 4 Hz and 60 Hz, reduces glial cell activation of both astrocytes and microglia in the dorsal horn of the spinal cord. We suggest that SCS has the ability to modulate nociceptive input at the spinal cord using multiple inhibitory neurotransmitters that subsequently reduce glial cell activation.
Acknowledgements
This project was funded by a grant from Medtronic and AR052316 and AR061371.
Footnotes
Conflict of Interest Statement: There are no financial or other relationships that might lead to conflicts of interest.
References
- 1.Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saade NE. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 2012;215:196–208. doi: 10.1016/j.neuroscience.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 2.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casamenti F, Prosperi C, Scali C, Giovannelli L, Colivicchi MA, Faussone-Pellegrini MS, Pepeu G. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: implications for Alzheimer’s disease. Neuroscience. 1999;91:831–842. doi: 10.1016/s0306-4522(98)00680-0. [DOI] [PubMed] [Google Scholar]
- 4.Chen T, Koga K, Li XY, Zhuo M. Spinal microglial motility is independent of neuronal activity and plasticity in adult mice. Mol Pain. 2010;6:19. doi: 10.1186/1744-8069-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Willoughby KA, Ellis EF. Group I metabotropic receptor antagonism blocks depletion of calcium stores and reduces potentiated capacitative calcium entry in strain-injured neurons and astrocytes. J Neurotrauma. 2004;21:271–281. doi: 10.1089/089771504322972068. [DOI] [PubMed] [Google Scholar]
- 6.Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 7.Cui JG, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on touch-evoked allodynia involve GABAergic mechanisms. An experimental study in the mononeuropathic rat. Pain. 1996;66:287–295. doi: 10.1016/0304-3959(96)03069-2. [DOI] [PubMed] [Google Scholar]
- 8.Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 9.Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabet Med. 2005;22:393–398. doi: 10.1111/j.1464-5491.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 10.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 11.Deecher DC, Wilcox BD, Dave V, Rossman PA, Kimelberg HK. Detection of 5-hydroxytryptamine2 receptors by radioligand binding, northern blot analysis, and Ca2+ responses in rat primary astrocyte cultures. J Neurosci Res. 1993;35:246–256. doi: 10.1002/jnr.490350304. [DOI] [PubMed] [Google Scholar]
- 12.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 13.El-Khoury C, Hawwa N, Baliki M, Atweh SF, Jabbur SJ, Saade NE. Attenuation of neuropathic pain by segmental and supraspinal activation of the dorsal column system in awake rats. Neuroscience. 2002;112:541–553. doi: 10.1016/s0306-4522(02)00111-2. [DOI] [PubMed] [Google Scholar]
- 14.Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- 15.Gherardini G, Lundeberg T, Cui JG, Eriksson SV, Trubek S, Linderoth B. Spinal cord stimulation improves survival in ischemic skin flaps: an experimental study of the possible mediation by calcitonin gene-related peptide. Plast Reconstr Surg. 1999;103:1221–1228. doi: 10.1097/00006534-199904040-00018. [DOI] [PubMed] [Google Scholar]
- 16.Gim GT, Lee JH, Park E, Sung YH, Kim CJ, Hwang WW, Chu JP, Min BI. Electroacupuncture attenuates mechanical and warm allodynia through suppression of spinal glial activation in a rat model of neuropathic pain. Brain Res Bull. 2011;86:403–411. doi: 10.1016/j.brainresbull.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Giordano C, Siniscalco D, Melisi D, Luongo L, Curcio A, Soukupova M, Palazzo E, Marabese I, De Chiaro M, Rimoli MG, Rossi F, Maione S, de Novellis V. The galactosylation of N (omega)-nitro-L-arginine enhances its anti-nocifensive or anti-allodynic effects by targeting glia in healthy and neuropathic mice. Eur J Pharmacol. 2011;656:52–62. doi: 10.1016/j.ejphar.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–1405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- 19.Hirst WD, Price GW, Rattray M, Wilkin GP. Serotonin transporters in adult rat brain astrocytes revealed by [3H]5-HT uptake into glial plasmalemmal vesicles. Neurochem Int. 1998;33:11–22. doi: 10.1016/s0197-0186(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 20.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon Y, Huh BK. Spinal cord stimulation for chronic pain. Ann Acad Med Singapore. 2009;38:998–1003. [PubMed] [Google Scholar]
- 22.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–269. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. P38 Mitogen-Activated Protein Kinase is Activated After a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- 25.Kang JM, Park HJ, Choi YG, Choe IH, Park JH, Kim YS, Lim S. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 2007;1131:211–219. doi: 10.1016/j.brainres.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 27.Koeze TH, Williams AC, Reiman S. Spinal cord stimulation and the relief of chronic pain. J Neurol Neurosurg Psychiatry. 1987;50:1424–1429. doi: 10.1136/jnnp.50.11.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Guen S, Catheline G, Besson JM. Development of tolerance to the antinociceptive effect of systemic morphine at the lumbar spinal cord level: a c-Fos study in the rat. Brain Res. 1998;813:128–138. doi: 10.1016/s0006-8993(98)01012-9. [DOI] [PubMed] [Google Scholar]
- 29.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Yang H, Meyerson BA, Linderoth B. Response to spinal cord stimulation in variants of the spared nerve injury pain model. Neurosci Lett. 2006;400:115–120. doi: 10.1016/j.neulet.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 31.Lindblom U, Tapper DN, Wiesenfeld Z. The effect of dorsal column stimulation on the nociceptive response of dorsal horn cells and its relevance for pain suppression. Pain. 1977;4:133–144. doi: 10.1016/0304-3959(77)90127-0. [DOI] [PubMed] [Google Scholar]
- 32.Linderoth B, Gherardini G, Ren B, Lundeberg T. Preemptive spinal cord stimulation reduces ischemia in an animal model of vasospasm. Neurosurgery. 1995;37:266–271. doi: 10.1227/00006123-199508000-00011. discussion 271-2. [DOI] [PubMed] [Google Scholar]
- 33.Maeda Y, Wacnik PW, Sluka KA. Low frequencies, but not high frequencies of bi-polar spinal cord stimulation reduce cutaneous and muscle hyperalgesia induced by nerve injury. Pain. 2008;138:143–152. doi: 10.1016/j.pain.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:201–209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 35.Martins DF, Mazzardo-Martins L, Gadotti VM, Nascimento FP, Lima DA, Speckhann B, Favretto GA, Bobinski F, Cargnin-Ferreira E, Bressan E, Dutra RC, Calixto JB, Santos AR. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. Pain. 2011;152:2653–2661. doi: 10.1016/j.pain.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56:1127–1137. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- 37.Meller ST, Dykstra C, Grzybycki D, Murphy S, Gebhart GF. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 38.Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006;31:S6–S12. doi: 10.1016/j.jpainsymman.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Meyerson BA, Ren B, Herregodts P, Linderoth B. Spinal cord stimulation in animal models of mononeuropathy: effects on the withdrawal response and the flexor reflex. Pain. 1995;61:229–243. doi: 10.1016/0304-3959(94)00171-A. [DOI] [PubMed] [Google Scholar]
- 40.Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur J Pharmacol. 2007;560:142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Mika J, Osikowicz M, Rojewska E, Korostynski M, Wawrzczak-Bargiela A, Przewlocki R, Przewlocka B. Differential activation of spinal microglial and astroglial cells in a mouse model of peripheral neuropathic pain. Eur J Pharmacol. 2009;623:65–72. doi: 10.1016/j.ejphar.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 42.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum-Gardner E, Johnson MI, Rakel BA, Sluka KA, Walsh DM. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12:929–935. doi: 10.1016/j.jpain.2011.02.352. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson M, Eriksson PS, Ronnback L, Hansson E. GABA induces Ca2+ transients in astrocytes. Neuroscience. 1993;54:605–614. doi: 10.1016/0306-4522(93)90232-5. [DOI] [PubMed] [Google Scholar]
- 45.Obata H, Sakurazawa S, Kimura M, Saito S. Activation of astrocytes in the spinal cord contributes to the development of bilateral allodynia after peripheral nerve injury in rats. Brain Res. 2010;1363:72–80. doi: 10.1016/j.brainres.2010.09.105. [DOI] [PubMed] [Google Scholar]
- 46.Okubo M, Yamanaka H, Kobayashi K, Kanda H, Dai Y, Noguchi K. Up-regulation of platelet-activating factor synthases and its receptor in spinal cord contribute to development of neuropathic pain following peripheral nerve injury. Mol. Pain. 2012;8:8. doi: 10.1186/1744-8069-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pastor A, Chvatal A, Sykova E, Kettenmann H. Glycine- and GABA-activated currents in identified glial cells of the developing rat spinal cord slice. Eur. J. Neurosci. 1995;7:1188–1198. doi: 10.1111/j.1460-9568.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 48.Piao ZG, Cho IH, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain. 2006;121:219–231. doi: 10.1016/j.pain.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 49.Poblete JC, Azmitia EC. Activation of glycogen phosphorylase by serotonin and 3,4-methylenedioxymethamphetamine in astroglial-rich primary cultures: involvement of the 5-HT2A receptor. Brain Res. 1995;680:9–15. doi: 10.1016/0006-8993(95)00201-z. [DOI] [PubMed] [Google Scholar]
- 50.Qiu Y, Li T, Li H, Zuo Y. Continuous spinal cord stimulation reduced cardiac ischaemia/reperfusion injury in a rat model. Heart Lung Circ. 2012;21:564–571. doi: 10.1016/j.hlc.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 53.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 54.Rees H, Roberts MH. Antinociceptive effects of dorsal column stimulation in the rat: involvement of the anterior pretectal nucleus. J Physiol. 1989;417:375–388. doi: 10.1113/jphysiol.1989.sp017807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren B, Linderoth B, Meyerson BA. Effects of spinal cord stimulation on the flexor reflex and involvement of supraspinal mechanisms: an experimental study in mononeuropathic rats. J Neurosurg. 1996;84:244–249. doi: 10.3171/jns.1996.84.2.0244. [DOI] [PubMed] [Google Scholar]
- 56.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato KL, Sanada LS, Rakel BA, Sluka KA. Increasing Intensity of TENS Prevents Analgesic Tolerance in Rats. J Pain. 2012;13:884–890. doi: 10.1016/j.jpain.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato KL, King EW, Johanek LM, Sluka KA. Spinal cord stimulation reduces hypersensitivity trough activation of opioid receptors in frequency-dependent manner. Eur. J. Pain. 2012 doi: 10.1002/j.1532-2149.2012.00220.x. IN PRESS. [DOI] [PubMed] [Google Scholar]
- 59.Schechtmann G, Song Z, Ultenius C, Meyerson BA, Linderoth B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139:136–145. doi: 10.1016/j.pain.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 60.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 61.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shrivastav M, Musley S. Spinal cord stimulation for complex regional pain syndrome. Conf. Proc. IEEE. Eng. Med. Biol. Soc. 2009;2009:2033–2036. doi: 10.1109/IEMBS.2009.5334418. [DOI] [PubMed] [Google Scholar]
- 63.Smits H, van Kleef M, Holsheimer J, Joosten EA. Experimental Spinal Cord Stimulation and Neuropathic Pain: Mechanism of Action, Technical Aspects, and Effectiveness. Pain Pract. 2012 doi: 10.1111/j.1533-2500.2012.00579.x. [DOI] [PubMed] [Google Scholar]
- 64.Song P, Zhao ZQ. The involvement of glial cells in the development of morphine tolerance. Neurosci Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 65.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152:1666–1673. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Song Z, Ultenius C, Meyerson BA, Linderoth B. Pain relief by spinal cord stimulation involves serotonergic mechanisms: an experimental study in a rat model of mononeuropathy. Pain. 2009;147:241–248. doi: 10.1016/j.pain.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Stiller CO, Cui JG, O’Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39:367–374. doi: 10.1097/00006123-199608000-00026. discussion 374-5. [DOI] [PubMed] [Google Scholar]
- 68.Stiller CO, Linderoth B, O’Connor WT, Franck J, Falkenberg T, Ungerstedt U, Brodin E. Repeated spinal cord stimulation decreases the extracellular level of gamma-aminobutyric acid in the periaqueductal gray matter of freely moving rats. Brain Res. 1995;699:231–241. doi: 10.1016/0006-8993(95)00911-9. [DOI] [PubMed] [Google Scholar]
- 69.Sun S, Cao H, Han M, Li TT, Zhao ZQ, Zhang YQ. Evidence for suppression of electroacupuncture on spinal glial activation and behavioral hypersensitivity in a rat model of monoarthritis. Brain Res Bull. 2008;75:83–93. doi: 10.1016/j.brainresbull.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 70.Svensson CI, Brodin E. Spinal astrocytes in pain processing: non-neuronal cells as therapeutic targets. Mol. Interv. 2010;10:25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- 71.Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- 72.Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–1217. [PubMed] [Google Scholar]
- 73.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. 2007;21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Terayama R, Omura S, Fujisawa N, Yamaai T, Ichikawa H, Sugimoto T. Activation of microglia and p38 mitogen-activated protein kinase in the dorsal column nucleus contributes to tactile allodynia following peripheral nerve injury. Neuroscience. 2008;153:1245–1255. doi: 10.1016/j.neuroscience.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 76.Truin M, Janssen SP, van Kleef M, Joosten EA. Successful pain relief in non-responders to spinal cord stimulation: The combined use of ketamine and spinal cord stimulation. Eur. J Pain. 2011 doi: 10.1016/j.ejpain.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 77.Tsai RY, Tai YH, Tzeng JI, Cherng CH, Yeh CC, Wong CS. Ultra-low dose naloxone restores the antinociceptive effect of morphine in pertussis toxin-treated rats by reversing the coupling of mu-opioid receptors from Gs-protein to coupling to Gi-protein. Neuroscience. 2009;164:435–443. doi: 10.1016/j.neuroscience.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 78.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 79.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- 81.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 82.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 83.Wei LC, Shi M, Chen LW, Cao R, Zhang P, Chan YS. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Brain Res Dev Brain Res. 2002;139:9–17. doi: 10.1016/s0165-3806(02)00509-6. [DOI] [PubMed] [Google Scholar]
- 84.Wen YR, Suter MR, Kawasaki Y, Huang J, Pertin M, Kohno T, Berde CB, Decosterd I, Ji RR. Nerve conduction blockade in the sciatic nerve prevents but does not reverse the activation of p38 mitogen-activated protein kinase in spinal microglia in the rat spared nerve injury model. Anesthesiology. 2007;107:312–321. doi: 10.1097/01.anes.0000270759.11086.e7. [DOI] [PubMed] [Google Scholar]
- 85.Winkelstein BA, DeLeo JA. Nerve root injury severity differentially modulates spinal glial activation in a rat lumbar radiculopathy model: considerations for persistent pain. Brain Res. 2002;956:294–301. doi: 10.1016/s0006-8993(02)03560-6. [DOI] [PubMed] [Google Scholar]
- 86.Winkelstein BA, Rutkowski MD, Sweitzer SM, Pahl JL, DeLeo JA. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J Comp Neurol. 2001;439:127–139. [PubMed] [Google Scholar]
- 87.Wolter T, Kieselbach K. Spinal cord stimulation for Raynaud’s syndrome: long-term alleviation of bilateral pain with a single cervical lead. Neuromodulation. 2011;14:229–33. doi: 10.1111/j.1525-1403.2011.00332.x. discussion 233-4. [DOI] [PubMed] [Google Scholar]
- 88.Xie W, Strong JA, Zhang JM. Early blockade of injured primary sensory afferents reduces glial cell activation in two rat neuropathic pain models. Neuroscience. 2009;160:847–857. doi: 10.1016/j.neuroscience.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yakhnitsa V, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates dorsal horn neuronal hyperexcitability in a rat model of mononeuropathy. Pain. 1999;79:223–233. doi: 10.1016/s0304-3959(98)00169-9. [DOI] [PubMed] [Google Scholar]
- 90.Yang F, Carteret AF, Wacnik PW, Chung CY, Xing L, Dong X, Meyer RA, Raja SN, Guan Y. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–480. doi: 10.1016/j.neuroscience.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 91.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]