Abstract
Endogenous estrogens and estrogen metabolites (EM) in human peritoneal fluid may play an important role in health and disease, yet little is known regarding their types and levels present in human peritoneal fluid, primarily due to the lack of an analytical method that is capable of directly quantifying their absolute abundances. In this report, we describe the application of a capillary LC-MS/MS method for identifying and quantifying biologically active and total endogenous EM in human peritoneal fluid. The method requires only 50 μL of peritoneal fluid, yet can quantify 13 distinct EM. Calibration curves for each EM were linear over a 103-fold concentration range and the lower LOQ was 50 fg on-column. For a charcoal stripped human peritoneal fluid sample containing 10 pg/mL of each EM, accuracy ranged from 83 to 118%, and intrabatch precision ranged from 0.2 to 4.4% RSD and interbatch precision ranged from 5.5 to 15.5% RSD. The analyses of human female perito-neal fluid shows that at least 10 biologically active and 11 total endogenous EM can be positively identified and quantitatively measured. Many of the biologically active forms are present in high abundance and possess distinct biological activities which warrant further study. Although micellar EKC gave baseline separation of a standard mixture of 10 EM, the LOQs using UV detection were not suitable for the assay of the low level estrogens in biological samples.
Keywords: Capillary liquid chromatography, Electrospray ionization-tandem mass spectrometry, Endogenous estrogens and estrogen metabolites, Human peritoneal fluid, Selected reaction monitoring
1 Introduction
Peritoneal fluid, which exists under normal physiological condition in women, is rich in ovarian steroid hormones. Peritoneal fluid surrounds the Fallopian tube and oocyte immediately after ovulation, and may influence oocyte maturation, fertilization, and tube transport process. In addition, peritoneal fluid allows direct secretion of ovarian hormones into peritoneal and endometrial cavities, influencing the endometrial cycle [1–5]. Peritoneal fluid represents a suitable medium for studying estrogen metabolism in endometriosis, a disorder associated with pelvic pain and infertility which affects 2–10% of child-bearing age women [6]. It is believed that the parent estrogens (17β-estradiol and estrone) as well as some hydroxylated estrogen metabolites (EM), such as catechol estrogens, may contribute to the proliferative and inflammatory characteristics of endometriosis [6, 7]. In contrast, methylated catechol estrogens, such as 2-methoxy-17β-estradiol, a 2-O-methyl conjugate of 2-hydroxy-17β-estradiol formed via the action of catechol-O-methyl transferase (COMT), exhibit potent apoptotic, antiangiogenic, and antiproliferative activities [8].
Despite their potential relevance in disease mechanism, roles of peritoneal fluid estrogens and their metabolites have not been critically investigated due to the lack of appropriate analytical technologies that provide specific descriptive and quantitative information concerning each metabolite. To the best of our knowledge, data describing the estrogen content of peritoneal fluid is limited to RIA data acquired for 17β-estradiol and estrone [2–5]. Micellar EKC (MEKC) with UV detection was used in our laboratory for the separation of a standard mixture of estrone (E1), estradiol (E2), estriol (E3) and seven other metabolites [9]. Ji et al. [10] used MEKC for the analysis of E1, E2, and E3 in urine obtained from a pregnant woman, and they were only able to reliably quantify E1. Although CE provides high resolution and rapid separation which is ideal for analyzing isomers of endogenous estrogens and EM, the two previously reported MEKC-UV methods lack the required sensitivity for the quantitation of endogenous estrogens and their metabolites in biological samples [9, 10]. During the past several years our laboratory has been actively developing MS/MS-based methods for quantitatively measuring biologically active endogenous estrogens and EM in human urine and serum [11, 12]. The current manuscript details the use of a capillary LC-MS/MS method to accurately and precisely measure the absolute quantities of endogenous estrogens and their metabolites in as little as 50 μL of human peritoneal fluid. A comparison of the separation of standard mixture of 10 EM will highlight the differences between MEKC-UV/Vis and HPLC-MS/MS methods in terms of sensitivity, separation, experimental conditions, and time required to complete an analysis.
2 Materials and methods
2.1 Chemicals and reagents
Fifteen estrogens and EM (purity ≥ 98%) including E1, E2, E3, 16-epiestriol (16-epiE3), 17-epiestriol (17-epiE3), 16α-hydroxyestrone (α-OHE1), 16-ketoestradiol (16-ketoE2), 2-methoxyestrone (2-MeOE1), 4-methoxyestrone (4-MeOE1), 2-hydroxyestrone-3-methyl ether (3-MeOE1), 2-methoxyestradiol (2-MeOE2), 4-methoxyestradiol (4-MeOE2), 2-hydroxyestrone (2-OHE1), 4-hydroxyestrone (4-OHE1), and 2-hydroxyestradiol (2-OHE2) were obtained from Steraloids (Newport, RI). Deuterium-labeled estrogens and estrogen metabolites (d-EM; isotopic purity ≥ 98%), including estradiol-2,4,16,16-d4 (d4-E2), estriol-2,4,17-d3 (d3-E3), 2-hydroxyestradiol-1,4,16,16,17-d5 (d5-2-OHE2), and 2-methoxyestradiol-1,4,16,16,17-d5 (d5-2-MeOE2), were purchased from C/D/N Isotopes (Pointe-Claire, Quebec, Canada). The fifth d-EM 16-epiestriol-2,4,16 (d3-16-epiE3) was obtained from Medical Isotopes (Pelham, NH). Dichloromethane, methanol, and formic acid were obtained from EM Science (Gibbstown, NJ). Glacial acetic acid, sodium bicarbonate, and l-ascorbic acid were purchased from J. T. Baker (Phillipsburg, NJ). Sodium hydroxide and sodium acetate were purchased from Fisher Scientific (Fair Lawn, NJ). β-Glucuronidase/sulfatase (Helix pomatia, Type HP-2) and dextran coated charcoal were obtained from Sigma Chemical (St. Louis, MO). Dansyl chloride and acetone were purchased from Aldrich Chemical (Milwaukee, WI). All chemicals and solvents used in this study were HPLC or reagent grade.
2.2 Human peritoneal fluid collection
Human peritoneal fluid is aspirated from the vesico-uterine pouch and the pouch of Douglas from a premenopausal patient with possible endometriosis by laparoscopy using a 4-mm metal canula before any internal manipulations were done. Peritoneal fluid aspirates that were contaminated with blood were excluded. The fluid is put in a sterile tube and kept on ice until delivered to the laboratory within 30 min. The cellular constituents of the peritoneal fluid are removed by centrifugation at 2500×g for 10 min after which the supernatant was removed and stored in aliquots at −80°C until analysis. The procedure for human peritoneal fluid collection was approved by University of Texas Medical Branch Institutional Review Board.
2.3 Preparation of stock and working standard solutions
Stock solutions of EM and d-EM were each prepared at 80 μg/mL by dissolving 2 mg of the estrogen powders in methanol with 0.1% w/v l-ascorbic acid to a final volume of 25 mL in a volumetric flask. Stock solutions, which are stable for at least 2 months while stored at −20°C, were analyzed at the beginning of each batch to verify no degradation of the EM and d-EM standards had occurred. Working standards of EM (0.4 and 8 ng/mL) and d-EM (8 ng/mL) were prepared by dilutions of the stock solutions using methanol containing l-ascorbic acid (0.1% w/v).
2.4 Preparation of double charcoal stripped peritoneal fluid
Dextran coated charcoal was used to remove endogenous steroids from peritoneal fluid following the protocol described by Leake and co-workers [13]. Dextran coated charcoal was removed by centrifugation at 3000×g for 30 min. The supernatant was then passed through a 0.2 μm syringe filter. The procedure was repeated to prepare double charcoal stripped peritoneal fluid. The absence of detectable endogenous EM in the final product was confirmed using the analytical method described in this manuscript.
2.5 Calibration standards and quality control samples
Double charcoal stripped human peritoneal fluid, fortified with 0.1% w/v l-ascorbic acid and having no detectable levels of EM, was used to prepare calibration and quality control (QC) samples. Calibration standards were prepared by adding 4 μL of the d-EM working internal standard solution (32 pg d-EM) and various volumes of EM working standard solution, which typically contained 0.4–400 pg EM per 50 μL peritoneal fluid. Three separate QC samples were also prepared in double charcoal stripped human peritoneal fluid at 10, 200, and 2400 pg/mL of each EM.
2.6 Sample preparation procedure for HPLC-MS analysis
Separate sample preparation procedures were employed to target both unconjugated biologically active EM and total EM. l-Ascorbic acid was added to the samples to prevent possible degradation of EM during preparation. For measuring unconjugated biologically active EM, 4 μL of the d-EM working internal standard solution (32 pg d-EM) was added to 50 μL human peritoneal fluid aliquot followed by 950 μL of 0.15 M sodium acetate buffer (pH 4.6) containing 0.5% w/v l-ascorbic acid. Dichloromethane (6 mL) was added and the samples underwent slow inverse extraction at 8 rpm (RKVSD™, ATR, Laurel, MD) for 30 min. After extraction, the aqueous layer was discarded and the organic solvent portion was transferred into a clean glass tube and evaporated, under a stream of nitrogen gas at 60°C (Reacti-Vap III™, Pierce, Rockford, IL), to dryness. To the dried sample 40 μL of 0.1 M sodium bicarbonate buffer (pH 9.0) and 40 μL of dansyl chloride solution (1 mg/mL in acetone) were added. After vortexing, the sample was heated at 60°C (Reacti-Therm III™ Heating Module, Pierce) for 5 min to form the EM and d-EM dansyl derivatives (EM-Dansyl and d-EM-Dansyl, respectively) [11, 12, 14–16]. The purpose of dansylation is to improve MSionization efficiency of EMand improve the overall sensitivity of the method. This sample preparation procedure was carried out for all calibration standards, QC, and unknown peritoneal fluid samples. After derivatization, all samples were analyzed by capillary LC-ESI MS/MS. For measuring total EM, the same sample preparation was used except that 950 μL of 0.15 Msodiumacetate buffer (pH 4.6) containing both 0.5% w/v l-ascorbic acid and 5 μL β-glucuronidase/sulfa-tase was added to the sample, followed by 20 h incubation at 37°C [11, 12].
2.7 Capillary LC-ESI-MS/MS
Capillary LC-ESI-MS/MS analysis was performed using an Agilent 1200 series nanoflow LC system (Agilent Technologies, Palo Alto, CA) coupled to a Finnigan TSQ™ Quantum Discovery MAX triple quadrupole mass spectrometer (Thermo Electron, San Jose, CA). The LC separation was carried out on a 150 mm long×300 μm id column packed with 4 μm Synergi Hydro-RP particles (Phenomenex, Torrance, CA) and maintained at 40°C. A total of 8 μL of each sample was injected onto the column. The mobile phase, operating at a flow rate of 4 μL/min, consisted of methanol as solvent A and 0.1% v/v formic acid in water as solvent B. The capillary LC gradient and MS conditions were similar to the previously described [11].
2.8 MEKC analysis
All experiments were carried out using a Beckman P/ACE 2050 CE instrument. Separations were performed at 25°C and +17 kV. Fused-silica capillaries were purchased from Polymicro Technologies (Phoenix, AZ).
2.9 Ion suppression study
To screen for potential ion suppression, a dilute solution of the dansylated EM and d-EM mixture was infused at a constant rate into the effluent flowing from the LC system to the mass spectrometer to generate an elevated but constant baseline signal with ESI-MS/MS selected reaction monitoring (SRM). After obtaining a steady baseline, an 8 μL dansylated blank peritoneal fluid sample extract was injected onto the capillary column. Any eluted material from the injected peritoneal fluid sample extract that suppresses ionization in the mass spectrometer will cause a drop in the baseline intensity. The same system was also used to monitor any SRM intensity drop during the run of a blank injection.
2.10 Identification and quantitation of human peritoneal fluid EM
The identification of EM in the human peritoneal fluid was carried out by comparing the chromatographic retention time and mass spectral profile of specific EM in human peritoneal fluid with the 15 EM reference standards.
Quantitation of peritoneal fluid EM was carried out using Xcalibur™ Quan Browser (Thermo Electron) as previously described [11]. Calibration curves for the each EM were constructed by plotting EM-Dansyl/d-EM-Dansyl peak area ratios obtained from calibration standards versus amounts of EM that injected on column and fitting these data using linear regression with 1/X weighting. The amount of EM in peritoneal fluid samples was then interpolated using this linear function.
2.11 Absolute recovery of EM after extraction procedure
To one set of six 50-μL aliquots of the charcoal stripped human peritoneal fluid, 4 μL of the EM working standard solution (32 pg EM) was added, followed by hydrolysis with β-glucuronidase/sulfatase, and extraction as described above. A second set of six 50-μL aliquots of the double charcoal stripped human peritoneal fluid was treated identically, except that the EM was added after the hydrolysis and extraction procedure. Both sets of samples were derivatized and analyzed in consecutive LC-ESI-MS/MS analyses. The absolute recovery of EM after the hydrolysis and extraction procedure was calculated by dividing the mean EM-Dansyl peak area from the second set into that measured for the first set.
2.12 Accuracy and precision of the EM analysis
To assess the accuracy and precision of the method, four replicated 50-μL aliquots from each of three QC samples (containing 0.5, 10, and 120 pg each EM in 50-μL peritoneal fluid) were hydrolyzed, extracted, derivatized, and analyzed in four different batches. The accuracy was measured as the percent matching of calculated amount to the known amount of EM in QC samples [17–19]. The intra- and inter-batch precisions were measured by the percent RSDs at each QC level [17–19].
3 Results and discussion
Analysis of a standard mixture of estrogens and their metabolites was first carried out using HPLC-MS and MEKC/UV–Vis to determine the suitability of each method for the analysis of estrogens in peritoneal fluids. As can be seen from Table 1, HPLC with MS detection is much more sensitive than MEKC with UV–Vis detection. This increase in sensitivity is, due in part to the amount of sample that can be injected onto the respective columns, which is 1000-fold more for HPLC (μL vs. nL). The MEKC/UV–Vis method does, however, have some advantages over the HPLC method, mainly that no sample derivatization is required and the EM can be resolved in 10 min, whereas the HPLC-MS method requires approximately 100 min (Table 2). Since the level of endogenous estrogens and EM in biological samples was below the UV detection limits [10–12], the HPLC-MS method, because of its greater sensitivity, was selected for the quantitative analysis of estrogens and EM in 50 μL human peritoneal fluid.
Table 1.
Comparison of HPLC and MEKC experimental parameters for the analysis of estrogens and their metabolites
| HPLC | MEKC | |
|---|---|---|
| Detection method | MS | UV/Vis (200 nm) |
| Injection volume | 8 μL | 3nL |
| Solution concentration | 10 pg/mL | 2 μg/mL |
| Amount injected | 80 fg | 6 pg |
| LOD | 5fg | 2 pg |
| LOQ | 50 fg | 20 pg |
| Column dimensions | 15 cm ×300 μm | 47 cm × 50 μm |
| Column type | RP | Fused-silica capillary |
| Derivatization | Yes | No |
| Analysis time | 100 min | 10 min |
Table 2.
Comparison of migration times and retention times of standard mixture of estrogens by MEKC and HPLC
| Compound | Migration time (min) |
Retention time (min) | |
|---|---|---|---|
| Buffer 1a | Buffer 2b | ||
| E1 | 6.2 | 21.9 | 46.1 |
| E2 | 7.2 | 23.0 | 50.9 |
| E3 | 5.3 | 14.4 | 21.4 |
| 16-Keto-17β-estradiol | 6.8 | 15.6 | 21.2 |
| 2-MeOE2 | 8.7 | 23.8 | 45.7 |
| 2-OHE2 | 4.6 | 22.1 | 69.0 |
| 4-OHE1 | 4.4 | 18.8 | 69.3 |
| 2-MeOE1 | 9.2 | 22.8 | 40.6 |
| 16α-OHE1 | 6.5 | 16.0 | 22.2 |
| 4-Hydroxyestradiol | 4.3 | 19.0 | N.A. |
Buffer 1 composition: 10 mM sodium borate, pH 9.2, 50 mM SDS, and 20 mM λ-CD.
Buffer 2 composition: 10 mM sodium phosphate, pH 7.0, 50 mM SDS, and 20% methanol.
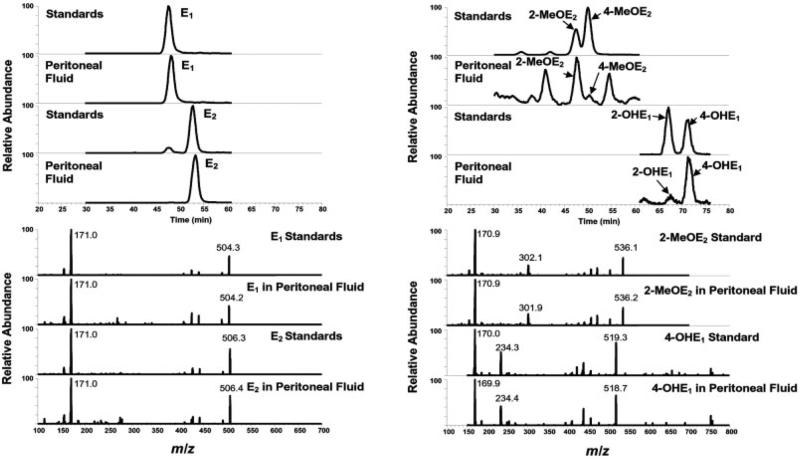
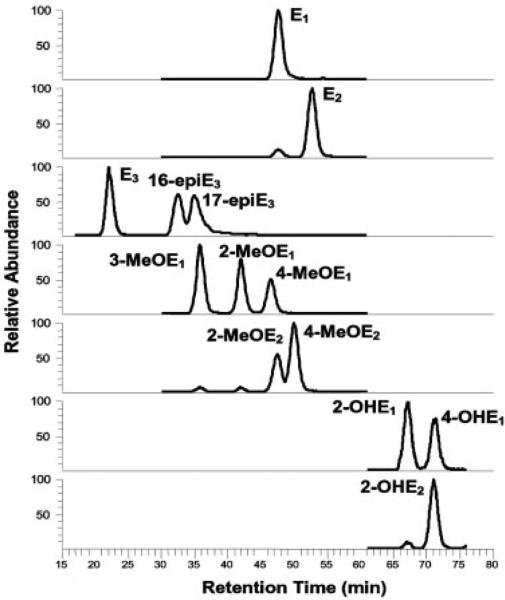
As with most biological fluids, very little information regarding the type and quantity of endogenous estrogens and their metabolites in human peritoneal fluid is known. By comparing the chromatographic retention time and mass spectral profile with those obtained from 15 EM reference standards, 11 EM were positively identified in the peritoneal fluid sample obtained from a premenopausal women with possible endometriosis. These EM included E1, E2, E3, 16-epiE3, 17-epiE3, 2-OHE1, 4-OHE1, 2-OHE2, 2-MeOE1, 2-MeOE2, and 4-MeOE2. Chromatograms and MS/MS spectra showing the identification of biologically active E1, E2, 2-MeOE2, and 4-OHE1 are presented in Fig. 1. Based on this knowledge, analytical parameters including SRM transitions for quantitative measurement of EM were established similar to our previously described methods for analyzing EM in urine and serum [11, 12]. The LC-ESI-MS/MS SRM profile of EM found in a spiked human peritoneal fluid sample is shown in Fig. 2. Using a simple methanol/water linear gradient, 13 of the EM could be resolved, with excellent peak shape, using RP C18 chromatography within a 75-min time range. No ion suppression or drop in the SRM intensities was observed in EM-dansyl and d-EM-dansyl eluted regions by human peritoneal fluid matrix under the LC-ESI-MS/MS SRM conditions used in this study.
Figure 1.
Chromatograms and tandem mass spectra showing the identification of biologically active E1, E2, 2-MeOE2, and 4-OHE1 in a human peritoneal fluid.
Figure 2.
Capillary LC-ESI-MS/MS SRM chromatographic profiles of a charcoal-stripped human peritoneal fluid sample spiked with the indicated EM to a final concentration of 40 pg/mL each.
Calibration curves for quantifying peritoneal fluid EM were prepared by spiking known amounts of EM standards into double-charcoal stripped human peritoneal fluid. These calibration curves were linear over a 103-fold concentration range with linear regression correlation coefficients ranging from 0.9985 to 0.9997. The standard error of the estimate (SEE) and the relative standard error of the estimate (RSEE) for the linear regression ranged from 0.0133 to 0.121 and from 1.43 to 4.66%, respectively, which indicated that confidence intervals of the slope were tight, and the intercept was close to zero [17]. The S/N obtained from analyzing 50 μL of the QC peritoneal fluid sample containing 10 pg/mL of each EM (representing 50 fg of each EM on-column) were greater than 80. The percent recovery of the known added amount (accuracy), intra-, and interbatch precision at this level were between 83–118%, 0.2–4.4%, and 5.5–15.5%, respectively. These values meet the criteria for an acceptable LOQ set by the FDA Guidance for Industry Bioanalytical Method Validation [19]. Therefore, the LOQ for each EM using this method is 50 fg on-column or 10 pg EM per mL peritoneal fluid.
The accuracy and precision values obtained for the QC human peritoneal fluid samples are provided in Tables 3 and 4. All samples shown in these tables were subjected to hydro-lysis, extraction, derivatization, and LC-ESI-MS/MS analysis. The percent recovery of a known added amount of each EM (accuracy) from the QC samples containing 10, 200, and 2400 pg EM/mL ranged from 83.3 to 117.7%, 90.2 to 105.3%, and 98.0 to 101.8%, respectively (Table 3). The intrabatch precision, as estimated by the percent RSD from four replicate analyses of the peritoneal fluid QC samples containing 10, 200, and 2400 pg EM/mL was 0.2–4.4%, 0.6–5.5%, and 0.4–2.8% RSD, respectively (Table 3). The interbatch precision for the complete analysis of peritoneal fluid EM (including hydrolysis, extraction, derivatization, and LC-ESIMS/MS steps) from four different batches ranged from 5.5 to 15.5%, 4.2 to 15.3%, and 1.6 to 7.9% RSD for the 10, 200, and 2400 pg EM/mL QC samples, respectively (Table 4). The absolute recoveries of EM from the human peritoneal fluid samples after the hydrolysis and extraction procedure ranged from 82.5 to 90.3%.
Table 3.
Accuracy and intrabatch precision observed for EM within the peritoneal fluid QC samplesa
| EM | 50 fg on column |
1000 fg on column |
12 000 fg on column |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | CV% | AC% | Mean | CV% | AC% | Mean | CV% | AC% | |
| E3 | 53.5 | 0.2 | 107.0 | 1053.2 | 2.2 | 105.3 | 11 833.7 | 0.6 | 98.6 |
| 16-epiE3 | 47.1 | 0.8 | 94.1 | 1003.2 | 2.7 | 100.3 | 11 971.2 | 0.6 | 99.8 |
| 17-epiE3 | 47.5 | 3.4 | 95.1 | 1013.4 | 1.6 | 101.3 | 11 977.3 | 0.5 | 99.8 |
| 3-MeOE1 | 41.7 | 0.7 | 83.3 | 1018.8 | 4.2 | 101.9 | 11 878.0 | 1.5 | 99.0 |
| 2-MeOE1 | 42.6 | 1.9 | 85.2 | 1000.3 | 4.0 | 100.0 | 11 838.4 | 0.4 | 98.7 |
| 4-MeOE1 | 44.0 | 1.0 | 88.0 | 988.7 | 2.0 | 98.9 | 12 051.8 | 0.8 | 100.4 |
| 2-MeOE2 | 47.9 | 1.4 | 95.9 | 1027.4 | 0.6 | 102.7 | 11 759.4 | 1.7 | 98.0 |
| E1 | 44.8 | 4.4 | 89.7 | 1046.5 | 5.5 | 104.6 | 11 902.6 | 1.1 | 99.2 |
| 4-MeOE2 | 45.6 | 3.3 | 91.3 | 1021.5 | 2.0 | 102.1 | 12 036.9 | 1.5 | 100.3 |
| E2 | 43.3 | 1.4 | 86.6 | 1006.8 | 1.3 | 100.7 | 12 066.5 | 1.3 | 100.6 |
| 2-OHE1 | 58.8 | 0.6 | 117.7 | 902.1 | 1.0 | 90.2 | 12 004.3 | 2.8 | 100.0 |
| 2-OHE2 | 49.9 | 1.8 | 99.7 | 980.8 | 3.3 | 98.1 | 12 160.4 | 1.3 | 101.3 |
| 4-OHE1 | 49.5 | 0.6 | 98.9 | 1004.9 | 2.4 | 100.5 | 12 214.3 | 0.7 | 101.8 |
Mean were expressed in fg (n = 4). Intrabatch precision (CV%) was measured as the percent RSDs. Accuracy (AC%) was measured as the percent matching of calculated amount to known amount of EM in QC human peritoneal fluid samples.
Table 4.
Interbatch precision of EM measurement in peritoneal fluid QC samplesa
| EM | 50 fg on column |
1000 fg on column |
12 000 fg on column |
|||
|---|---|---|---|---|---|---|
| Mean | CV% | Mean | CV% | Mean | CV% | |
| E3 | 54.2 | 5.5 | 1017.4 | 4.2 | 11 490.3 | 3.8 |
| 16-epiE3 | 50.6 | 7.6 | 970.8 | 10.3 | 11 716.6 | 3.8 |
| 17-epiE3 | 51.4 | 5.5 | 961.3 | 7.4 | 11 897.5 | 1.6 |
| 3-MeOE1 | 48.8 | 15.5 | 999.3 | 9.6 | 11 537.5 | 6.6 |
| 2-MeOE1 | 51.0 | 12.4 | 968.7 | 9.1 | 11 651.4 | 6.0 |
| 4-MeOE1 | 51.3 | 11.9 | 961.5 | 5.9 | 11 275.8 | 7.9 |
| 2-MeOE2 | 49.6 | 8.3 | 989.2 | 4.2 | 11 400.1 | 4.4 |
| E1 | 46.9 | 14.6 | 1033.9 | 6.0 | 11 611.4 | 5.1 |
| 4-MeOE2 | 50.3 | 10.9 | 945.1 | 5.8 | 11 490.9 | 4.2 |
| E2 | 49.5 | 10.0 | 1013.0 | 4.2 | 11 717.3 | 4.0 |
| 2-OHE1 | 57.4 | 8.5 | 827.1 | 15.3 | 12 127.9 | 5.6 |
| 2-OHE2 | 52.6 | 12.5 | 937.1 | 12.9 | 12 024.1 | 2.7 |
| 4-OHE1 | 54.9 | 11.8 | 975.9 | 14.5 | 11 784.6 | 4.0 |
Means were expressed in fg (n = 4). Interbatch precision (CV%) was measured as the percent RSDs.
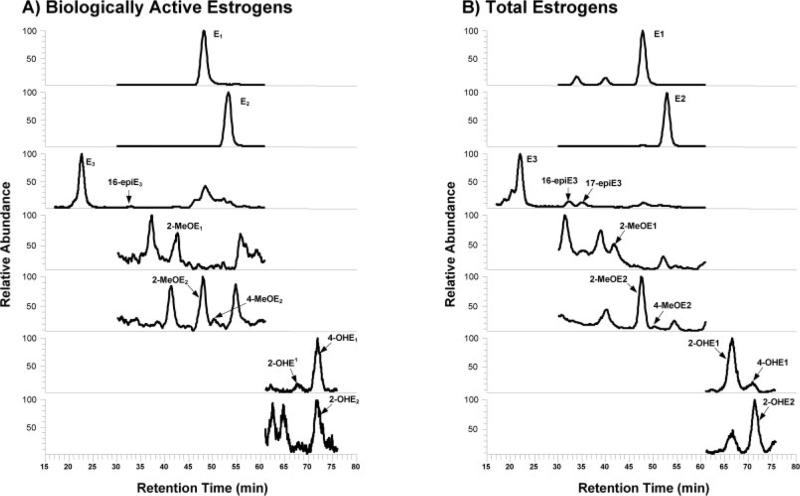
Once the LC and MS conditions necessary to resolve and quantify EM in spiked human peritoneal fluid samples had been established and validated using QC samples, this method was applied to measure the levels of biologically active and total EM in a series of human peritoneal fluid samples. To measure the biologically active EM, the hydro-lysis step is excluded from the sample preparation. Exclusion of this step leaves all conjugated (e.g., sulfated, glucuronidated, etc.) metabolites in their native form. The measurement of total EM includes the hydrolysis step, thereby converting all of the variably conjugated structures of each specific metabolite into a single unconjugated form. The LC-ESI-MS/ MS SRM profile of biologically active and total EM in a perito-neal fluid sample acquired from an actual patient is shown in Figs. 3A and B. The quantitative data for both biologically active and total EM are presented in Table 5. The means and SD for each of the EM was calculated from eight completely separate analyses of aliquots of the peritoneal fluid sample. In a recent study conducted in our laboratory, only five biologically active EM could be observed in human sera [12], whilein this study ten were found in peritoneal fluid. While the peritoneal fluid and serum samples did not originate from the same patient, the results suggest that a higher concentration of biologically active EM exist in peritoneal fluid than serum. Two recent studies conducted in our laboratory have shown that 15 total EM could be observed in human urine and serum samples [11, 12] when conjugation is removed using hydrolysis. In the analysis of peritoneal fluid, however, only 11 total EM were found after hydrolysis (Table 5). In peritoneal fluid, the most potent endogenous estrogen E2 was by far the most abundant one found in both biologically active and total EM (Table 5). In contrast, the less potent estrogen E1 was the most abundant total EM found in urine and serum [11, 12]. The extensive metabolism by peripheral organs such as liver is likely responsible for the greater number of EM and higher abundance of less potent estrogen E1 observed in urine and serum samples. On the other hand, the aberrant expression of aromatase (enzyme responsible for synthesis of E2) and deficiency of 17β-hydroxysteroid dehydrogenase type 2 (enzyme responsible for converting E2 to E1) due to endometriosis itself may contribute to the higher biologically active and total E2 observed in human peritoneal fluid [6]. Given these differences in observed EM profiles among urine, serum, and peritoneal fluid samples, the ability to directly and quantitatively measure both biologically active and total EM in human peritoneal fluid has a number of potential applications in the study of diseases such as endometriosis.
Figure 3.
Capillary LC-ESI-MS/MS SRM chromatographic profiles of (A) biologically active and (B) total EM observed in peritoneal fluid obtained from a premenopausal women.
Table 5.
Endogenous estrogen and EM concentrations (pg/mL) in peritoneal fluid acquired from a premenopausal womana
| EM | Biologically active EM |
Total EM |
||||
|---|---|---|---|---|---|---|
| Mean | SD | CV% | Mean | SD | CV% | |
| E3 | 172.6 | 4.4 | 2.5 | 186.6 | 2.3 | 1.2 |
| 16-epiE3 | 38.4 | 0.8 | 2.0 | 41.3 | 0.4 | 1.0 |
| 17-epiE3 | NF | NF | NF | 39.8 | 0.5 | 1.3 |
| E1 | 493.3 | 17.3 | 3.5 | 1308.1 | 17.9 | 1.4 |
| E2 | 5993.2 | 108.2 | 1.8 | 7980.3 | 76.0 | 1.0 |
| 2-OHE1 | 16.5 | 1.6 | 9.9 | 1043.3 | 20.4 | 2.0 |
| 4-OHE1 | 84.4 | 2.6 | 3.1 | 490.4 | 43.3 | 8.8 |
| 2-OHE2 | 16.8 | 0.7 | 4.2 | 216.1 | 5.3 | 2.5 |
| 2-MeOE1 | 17.0 | 0.6 | 3.6 | 55.5 | 2.0 | 3.7 |
| 2-MeOE2 | 34.7 | 1.5 | 4.3 | 170.7 | 2.5 | 1.5 |
| 4-MeOE2 | NF | NF | NF | 10.6 | 0.3 | 2.7 |
Mean and standard derivation (SD) were expressed in pg/mL (n = 8). Intrabatch precision (CV%) was measured as the percent RSDs. NF = not found above the LOQ.
4 Concluding remarks
Peritoneal fluid endogenous estrogens and EM play an important role in reproductive physiology and may be involved in the mechanism of estrogen-related diseases in endometrium, ovaries, and cervix. The analytical technologies detailed in this manuscript provides essential tools for critical investigation of such roles of endogenous estrogens and EM in clinical and translation research. One area we are actively pursuing is to increase the throughput of the overall assay. The obvious area that could be improved is in the separation prior to MS analysis. The present RP chromatography approach requires approximately 80 min due to the need to sufficiently separate the EM; many of which are very similar in structure. Due to the nature of high resolution and rapid separation of CE and the high specificity and sensitivity of MS/MS, combining CE with MS/MS for measuring endogenous estrogens and EM in biological samples could improve the throughput of our current capillary LC-MS/MS method. Even a two-fold improvement in throughput would have a dramatic effect on the ability to conduct large epidemiology studies using this method.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the United States Government.
Abbreviations
- 16-epiE3
16-epiestriol
- 17-epiE3
17-epiestriol
- 2-MeOE1
2-methoxyestrone
- 2-MeOE2
2-methoxyestradiol
- 2-OHE1
2-hydroxyestrone
- 2-OHE2
2-hydroxyestradiol
- 3-MeOE1
2-hydroxyestrone-3-methyl ether
- 4-MeOE1
4-methoxyestrone
- 4-MeOE2
4-methoxyestradiol
- 4-OHE1
4-hydroxyestrone
- d-EM
deuterated estrogen metabolites
- E1
estrone
- E2
estradiol
- E3
estriol
- EM
estrogen metabolites
- QC
quality control
- SRM
selected reaction monitoring
Footnotes
The authors have declared no conflict of interest.
References
- 1.Foster EA. Med. Hypotheses. 2003;60:845–848. doi: 10.1016/s0306-9877(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 2.Maathuis JB, Van Look PF, Michie EA, Endocrinol J. 1978;76:123–133. doi: 10.1677/joe.0.0760123. [DOI] [PubMed] [Google Scholar]
- 3.Koninckx PR, Renaer M, Brosens IA. Br. J. Obstet. Gynaecol. 1980;87:177–183. doi: 10.1111/j.1471-0528.1980.tb04514.x. [DOI] [PubMed] [Google Scholar]
- 4.Koninckx PR, Heyns W, Verhoeven G, Van Baelen H, et al. J. Clin. Endocrinol. Metab. 1980;51:1239–1244. doi: 10.1210/jcem-51-6-1239. [DOI] [PubMed] [Google Scholar]
- 5.Scheenjes E, Thijssen JH, te Velde ER, Blankenstein MA, Kremer J. Gynecol. Endocrinol. 1991;5:157–166. doi: 10.3109/09513599109028437. [DOI] [PubMed] [Google Scholar]
- 6.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, et al. Ann. N. Y. Acad. Sci. 2002;955:75–85. doi: 10.1111/j.1749-6632.2002.tb02767.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhu BT, Conney AH. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Lakhani NJ, Sarkar MA, Venitz J, Figg WD. Pharmacotherapy. 2003;23:165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 9.Chan KC, Muschik GM, Issaq HJ, Siiteri PK, Chromatogr J. A. 1995;690:149–154. doi: 10.1016/0021-9673(94)00990-q. [DOI] [PubMed] [Google Scholar]
- 10.Ji AJ, Nunez MF, Machacek D, Ferguson JE, et al. J. Chromatogr. B. 1995;669:15–26. doi: 10.1016/0378-4347(95)00143-7. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Veenstra TD, Fox SD, Roman JM, et al. Anal. Chem. 2005;77:6646–6654. doi: 10.1021/ac050697c. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Roman JM, Issaq HJ, Keefer LK, et al. Anal. Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 13.Cook B, Beastall G. In: Steroid Hormones: A Practical Approach. Green B, Leake RE, editors. IRL Press; Oxford, Washington, DC: 1987. pp. 1–65. [Google Scholar]
- 14.Frei-Häusler M, Frei RW. J. Chromatogr. 1973;79:209–216. doi: 10.1016/s0021-9673(01)85290-0. [DOI] [PubMed] [Google Scholar]
- 15.Quirke JME, Adams CL, Van Berkel GJ. Anal. Chem. 1994;66:1302–1315. [Google Scholar]
- 16.Anari MR, Bakhtiar R, Zhu B, Huskey S, et al. Anal. Chem. 2002;74:4136–4144. doi: 10.1021/ac025712h. [DOI] [PubMed] [Google Scholar]
- 17.Duncan MW, Gale PJ, Yergey AL, editors. Principles of Quantitative Mass Spectrometry. 1st Edn. Rockpool Press; Danver, CO: 2002. pp. 40–75. [Google Scholar]
- 18.Swartz M, Krull IS, editors. Analytical Method Development and Validation. 1st Edn. Marcel Dekker, Inc.; New York, NY: 1997. pp. 53–68. [Google Scholar]
- 19.U.S. Food and Drug Administration, Centre for Drug Evaluation and Research, Guidance for Industry Bioanalytical Method Validation. 2001:4–11. www.fda.gov/cder/guidance/4252fnl.htm.