Abstract
Background:
The selective serotonin reuptake inhibitors (SSRIs) citalopram, escitalopram, and sertraline are all metabolized by the cytochrome P-450 isoenzyme CYP2C19, which is inhibited by the proton pump inhibitors (PPIs) omeprazole, esomeprazole, lansoprazole, and pantoprazole. The aim of the present study was to evaluate the effect of these PPIs on the serum concentrations of citalopram, escitalopram, and sertraline.
Methods:
Serum concentrations from patients treated with citalopram, escitalopram, or sertraline were obtained from a routine therapeutic drug monitoring database, and samples from subjects concomitantly using PPIs were identified. Dose-adjusted SSRI serum concentrations were calculated to compare data from those treated and those not treated with PPIs.
Results:
Citalopram concentrations were significantly higher in patients treated with omeprazole (+35.3%; P < 0.001), esomeprazole (+32.8%; P < 0.001), and lansoprazole (+14.7%; P = 0.043). Escitalopram concentrations were significantly higher in patients treated with omeprazole (+93.9%; P < 0.001), esomeprazole (+81.8%; P < 0.001), lansoprazole (+20.1%; P = 0.008), and pantoprazole (+21.6%; P = 0.002). Sertraline concentrations were significantly higher in patients treated with esomeprazole (+38.5%; P = 0.0014).
Conclusions:
The effect of comedication with PPIs on the serum concentration of SSRIs is more pronounced for omeprazole and esomeprazole than for lansoprazole and pantoprazole, and escitalopram is affected to a greater extent than are citalopram and sertraline. When omeprazole or esomeprazole are used in combination with escitalopram, a 50% dose reduction of the latter should be considered.
Key Words: citalopram, escitalopram, sertraline, proton pump inhibitor, drug interactions
INTRODUCTION
The selective serotonin reuptake inhibitors (SSRIs) citalopram, escitalopram, and sertraline are all to some extent metabolized by the cytochrome P-450 isoenzyme CYP2C19. Citalopram, which is a racemic mixture of S-citalopram and R-citalopram, is predominantly metabolized to N-desmethylcitalopram via CYP2C19.1 Correspondingly, escitalopram (S-citalopram) is metabolized to N-desmethylescitalopram via CYP2C19. CYP2C19 seems to be even more important in the metabolism of S-citalopram than of R-citalopram2,3 possibly because CYP2C19 also catalyzes the metabolism from S-citalopram to the propionic acid metabolite.4 Sertraline is metabolized by CYP2C19 to N-desmethylsertraline and sertraline ketone, although other enzymes, such as CYP3A4 and CYP2B6, are involved as well.5–8
Proton pump inhibitors (PPIs) are widely used in the treatment of disorders related to gastric acid secretion. There is evidence from studies both in vitro and in humans that these drugs inhibit the activity of CYP2C19. In early studies using liver microsomes, relatively small differences were found in the inhibitory potencies between omeprazole, esomeprazole, and lansoprazole.9,10 However, in humans, omeprazole and esomeprazole have been found to be more potent inhibitors than lansoprazole,11 whereas pantoprazole does not seem to inhibit CYP2C19 to a clinically significant degree.12,13 These findings have more recently been confirmed in other in vitro studies, in which omeprazole and esomeprazole were the most powerful inhibitors, followed by lansoprazole and pantoprazole.14,15
Because citalopram, escitalopram, and sertraline are substrates of CYP2C19 and PPIs inhibit this enzyme, a theoretical rationale exists for a decreased metabolism of these SSRIs by concomitant treatment with PPIs. The impact of omeprazole on the metabolism of citalopram/escitalopram has been studied in humans in 2 previous studies.16,17 In the first of these, a mean of 51% increase in the plasma levels of escitalopram was seen after the administration of omeprazole to 16 healthy volunteers.16 In the second, about a 2-fold increase in the concentration of S-citalopram and a 25% increase in the concentration of R-citalopram were found in 9 healthy subjects.17 We have not identified any studies on the effect of other PPIs on the metabolism of citalopram or escitalopram, nor have we found any published clinical data on the effects of any PPIs on the metabolism of sertraline.
The aim of the present study was to evaluate the effect of the PPIs omeprazole, esomeprazole, lansoprazole, and pantoprazole on the serum concentrations of the SSRIs citalopram, escitalopram, and sertraline in the clinical setting, based on therapeutic drug monitoring data.
MATERIAL AND METHODS
In the period from January 2000 to December 2011, a total of 17,026 patient samples were sent to our laboratory for the analysis of citalopram, 21,258 samples were sent for the analysis of escitalopram, and 11,068 samples were sent for the analysis of sertraline. The results are stored in a database, which also includes information on daily dose, time of administration of the last dose, time of blood sampling, and concomitant medication. Of the samples analyzed for citalopram, there were 459 with information that the patient had been concomitantly treated with a PPI. The corresponding numbers for escitalopram and sertraline were 660 and 252, respectively.
Samples for which the request forms lacked information about daily dose, time of administration of the last dose, or time of blood sampling or those obtained less than 8 hours or more than 30 hours after the last dose were excluded. Moreover, samples for which there were no detectable serum concentrations of the SSRI, samples from patients younger than 16 years, samples with information of intake in overdose, and samples with alternating daily dosage were also excluded. Finally, samples where the patient was treated with known inducers and inhibitors of CYP2C19 or CYP3A418,19 were excluded.
On the basis of these criteria, 184 samples from patients with concomitant PPI treatment were excluded for citalopram, 253 were excluded for escitalopram, and 121 were excluded for sertraline. Thus, in the final material, 275 samples from patients with concomitant PPI treatment were included for citalopram, 407 for escitalopram, and 131 for sertraline.
The control group consisted of samples from patients not treated with a PPI, randomly picked from the database, applying the same exclusion criteria as above. Five control samples were chosen per PPI sample, rounded off to the nearest 500, but not less than a total of 1000. The number of controls was chosen because including more controls than cases in a study like ours reduces the standard error of the estimated coefficients and thus increases power. On the other hand, including much more than 4 controls per case is considered to be inefficient.20 We thus decided to use an approximate 5:1 ratio. The study was approved by the Regional Ethics Committee, northern Norway.
All substances were analyzed with liquid chromatography–mass spectrometry methods developed in our laboratory. Citalopram and escitalopram were extracted from 0.5 mL of serum with a 4-mL solution of hexane/butanol/acetonitrile (93/5/2) after the addition of the internal standard (50 μL of 10 μmol/L flurazepam in methanol) and alkalinization with 0.2 mL of 1 mol/L sodium carbonate. After mixing and centrifugation, the organic extract was evaporated to dryness with air, and the residue was reconstituted in 50-μL methanol, transferred to vials and injected on an Agilent MSD 1100 system (Agilent, Palo Alto, CA). Separation was performed on a Zorbax SB-C18 (30 × 4.6 mm) 3.5-μm column (Agilent). Methanol/ammonium acetate (60/40) was used as mobile phase. Citalopram and escitalopram were monitored after positive electrospray ionization at a mass-to-charge ratio of 325.1. The limit of quantitation was 10 nmol/L (3.25 ng/mL), and the method was linear at least up to 1000 nmol/L. The interassay coefficients of variation tested at different concentrations were less than 8.7%. The matrix effect was negligible, with responses in the range of 90%–100%. There was no indication of any analytical interference with the PPIs.
Sertraline was extracted from 1 mL of serum with 6-mL butyl chloride after the addition of the internal standard (50 μL of 25 μmol/L CP-53631) and alkalinization with 1 mL of 0.75 mol/L sodium carbonate. After mixing and centrifugation, the organic extract was evaporated to dryness with air, and the residue was reconstituted in 50-μL methanol, transferred to vials and injected on an Agilent MSD 1100 system (Agilent). Separation was performed on a Zorbax SB-C18 (150 × 4.6 mm) 15-μm column (Agilent). Acetonitrile/methanol/ammonium acetate (60/20/20) was used as mobile phase. Sertraline was monitored after positive electrospray ionization at a mass-to-charge ratio of 306.0. The limit of quantitation was 5 nmol/L (1.53 ng/mL), and the method was linear at least up to 500 nmol/L. The interassay coefficients of variation tested at different concentrations were less than 10.8%. The matrix effect was negligible, with responses in the range of 90%–100%. There was no indication of any analytical interference with the PPIs.
The primary target variable used is the SSRI serum concentration-to-dose ratio (C/D ratio), which is the serum concentration of the drug divided by its daily dose. The C/D ratio thus corresponds to the serum concentration per milligram of SSRI administered daily. To convert the unit from nmol/L to ng/mL, the concentrations should be divided by 3.08 for citalopram and escitalopram and by 3.27 for sertraline. To normalize the SSRI concentration for variations in time from the last dose to sampling, a noncompartment exponential model for the elimination phase of the SSRIs was assumed. The observed SSRI concentration at t hours since intake (Ct) was then converted to a standardized 12-hour concentration (C12) according to the following equation:
The rate constant k was set according to the equation k = loge 2/t1/2, using half-lives of 33 hours for citalopram, 30 hours for escitalopram, and 26 hours for sertraline. Specifically, the SSRI C/D ratio was calculated by dividing this standardized serum concentration by the total daily dose in milligrams. As the distributions of the C/D ratios were found to be heavily right skewed, the natural logarithm (loge) of the SSRI C/D ratios was employed as the outcome variable in the statistical model to achieve near normality.
As multiple samples from the same patient were allowed, a linear mixed model, allowing correlation between repeated observations, was used. This model assumes that each individual patient possesses a random intercept (ie, an individual “offset”) in addition to being affected by fixed factors. The factors included in the model for each of the 3 SSRIs were gender, age, and concomitant treatment with each of the 4 PPIs. Model parameters, including variance components, were estimated by the method of restricted maximum likelihood using the nlme package of the software R version 2.15, and R and GraphPad PRISM version 6.0 were used for graphical illustrations. Data are presented as means with 95% confidence intervals, or as medians with corresponding minimum and maximum values, as appropriate. P values less than 0.05 were considered statistically significant.
RESULTS
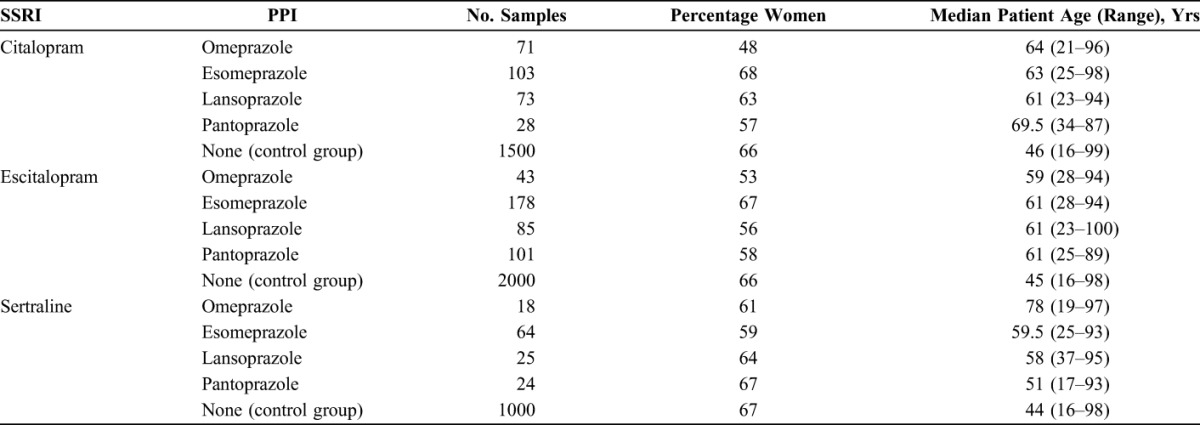
The final material consisted of 275 samples from 202 patients treated with citalopram and a PPI, 407 samples from 330 patients treated with escitalopram and a PPI, and 131 samples from 94 patients treated with sertraline and a PPI (Table 1). In addition, 1500 control samples from patients not treated with a PPI were included for citalopram, 2000 for escitalopram, and 1000 for sertraline (Table 1).
TABLE 1.
Descriptive Data of the Samples Included in the Study
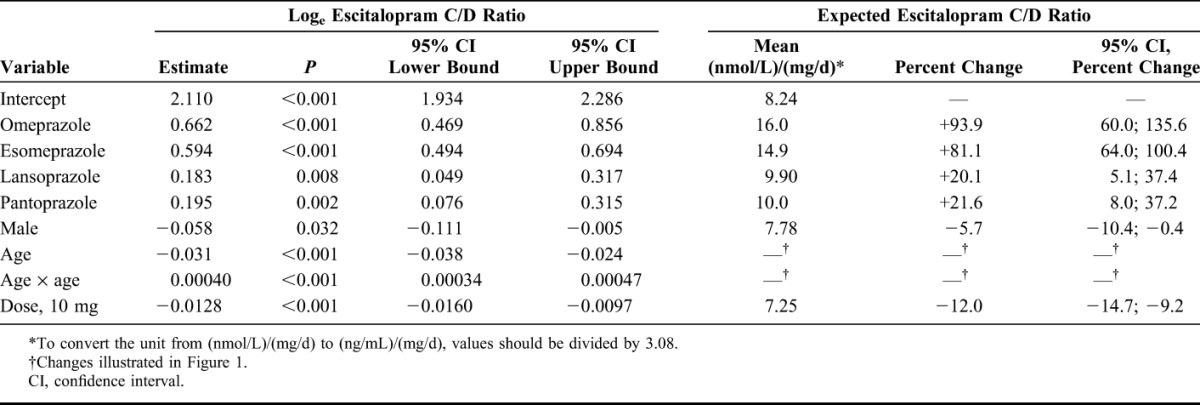
Factors affecting the C/D ratios for citalopram, escitalopram, and sertraline are shown in Tables 2–4. For citalopram, the C/D ratios were significantly higher in samples from patients treated with omeprazole (+35.3%; P < 0.001), esomeprazole (+32.8%; P < 0.001), and lansoprazole (+14.7%; P = 0.043) than in those in the control group. No significant effect was observed for pantoprazole. For escitalopram, the C/D ratios were significantly higher during comedication with all the 4 PPIs (+93.9%; P < 0.001 for omeprazole, +81.8%; P < 0.001 for esomeprazole, +20.1%; P = 0.008 for lansoprazole, and +21.6%; P = 0.002 for pantoprazole). For sertraline, a 38.5% increase in the C/D ratio was observed in combination with esomeprazole (P = 0.0014), whereas there were no significant differences in the C/D ratios in combination with omeprazole, lansoprazole, or pantoprazole.
TABLE 2.
Model Parameter Estimates Showing the Effect of the Explanatory Factors (One by One) on the Loge-Transformed Citalopram C/D Ratio
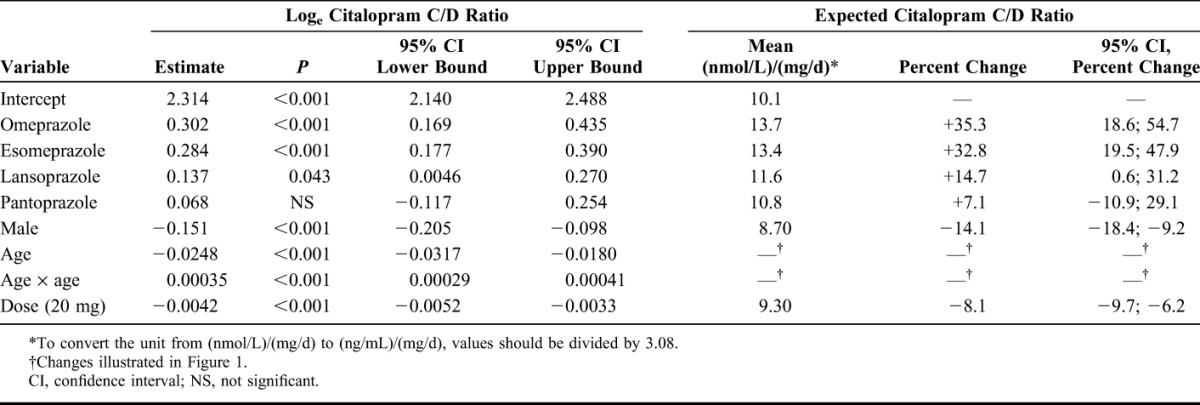
TABLE 4.
Model Parameter Estimates Showing the Effect of the Explanatory Factors (One by One) on the Loge-Transformed Sertraline C/D Ratio
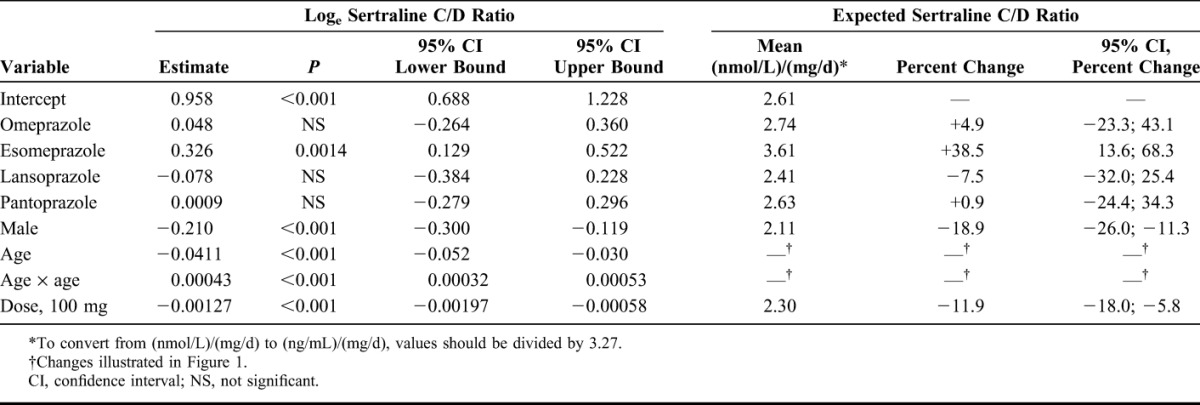
TABLE 3.
Model Parameter Estimates Showing the Effect of the Explanatory Factors (One by One) on the Loge-Transformed Escitalopram C/D Ratio
For all SSRIs, men had significantly lower C/D ratios than women (−14.1%; P < 0.001 for citalopram, −5.7%; P = 0.032 for escitalopram, and −18.9%; P < 0.001 for sertraline) (Tables 2–4). As illustrated in Figure 1, age affected the C/D ratios significantly for all the 3 SSRIs. As a comparison, also the effect of esomeprazole, which was the only PPI significantly affecting the C/D ratios for citalopram, escitalopram, and sertraline, is displayed in Figure 1. Some examples of expected mean serum concentrations related to gender, age, and concomitant PPI use are presented in Figure 2.
FIGURE 1.
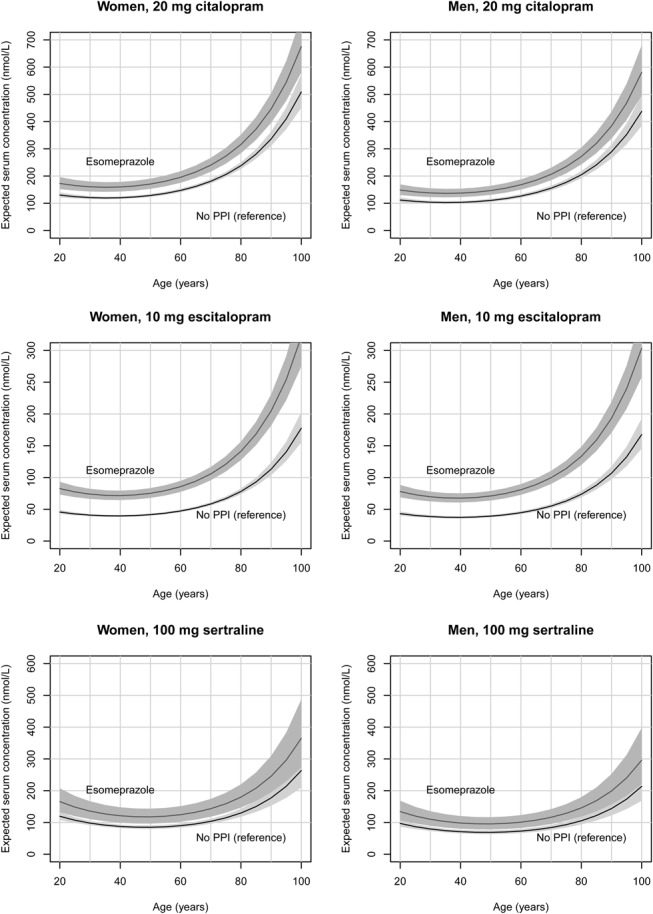
Expected serum concentrations of citalopram (top panel), escitalopram (middle panel), and sertraline (lower panel) in women (left part) and men (right part) related to age, applying the model presented in Tables 2–4. As a comparison, the effect of esomeprazole (which was the only PPI significantly affecting the C/D ratio for both citalopram, escitalopram, and sertraline) is also shown. The shaded areas represent 95% confidence intervals. For citalopram and escitalopram, 100 nmol/L correspond to 32.5 ng/mL. For sertraline, 100 nmol/L correspond to 30.6 ng/mL.
FIGURE 2.
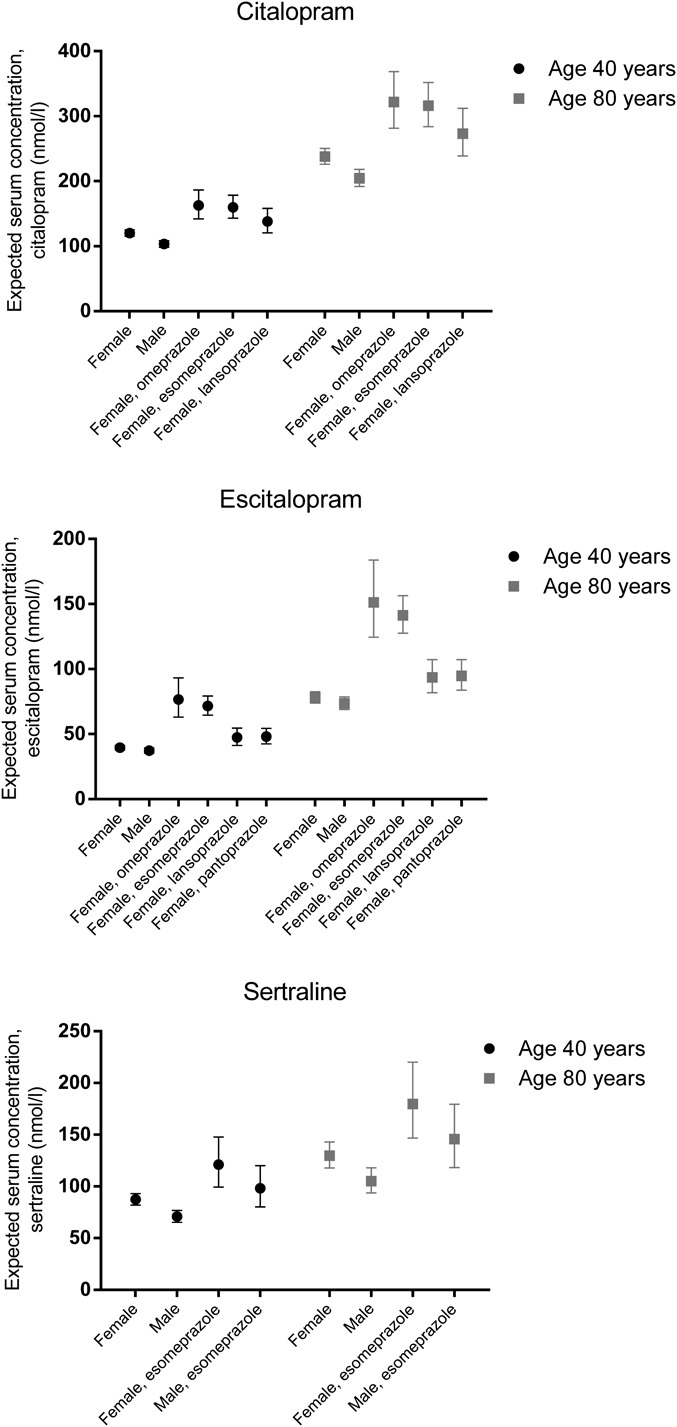
Expected serum concentrations of citalopram (top panel), escitalopram (middle panel), and sertraline (lower panel) with 95% confidence intervals in men and women at 2 different ages during monotherapy and during comedication with PPIs, applying the model presented in Tables 2–4. The doses are set to 20 mg/d for citalopram, 10 mg/d for escitalopram, and 100 mg/d for sertraline.
DISCUSSION
The principal finding in the present study is that comedication with PPIs to a varying extent increases the serum concentrations of citalopram, escitalopram, and sertraline. The effect is more pronounced for omeprazole and esomeprazole than for lansoprazole and pantoprazole, and escitalopram is affected to a greater extent than are citalopram and sertraline.
Our findings are largely in line with what could be expected from in vitro and in vivo data on which PPIs being the most potent inhibitors of CYP2C1911–15 and which SSRIs that to the largest extent are dependent on CYP2C19 in their metabolism.2–7 Specifically, omeprazole and esomeprazole have been found to be more potent CYP2C19 inhibitors than lansoprazole and pantoprazole,11–15 with esomeprazole and omeprazole having about the same inhibiting potential.11 The more pronounced effect on the C/D ratio for escitalopram than for citalopram is consistent with the fact that CYP2C19 seems to be even more important in the metabolism of S-citalopram than of R-citalopram.2,3 Our results also confirm and extend the findings from 2 previous studies in a total of 25 healthy volunteers, in which omeprazole increased the plasma concentration of escitalopram by a mean of approximately 50% and 100%, respectively.16,17 The corresponding increase in R-citalopram was a modest 25%,17 indicating an increase of approximately 45% for racemic citalopram. This is in accordance with the results from our study, in which the serum concentrations during omeprazole therapy were 93.9% higher for escitalopram and 35.3% higher for citalopram.
The effect of PPIs on the C/D ratios for sertraline is clearly lower than that for escitalopram and seems to be even lower than that for citalopram. It should however be noted that the results for sertraline are not as robust as for citalopram and escitalopram due to the lower number of samples in our material; for sertraline, there were only about 20 samples available in some of the PPI groups, whereas for escitalopram and citalopram, the corresponding numbers were closer to or above 100 samples (Table 1). We are not aware of any studies directly comparing the role of CYP2C19 in the metabolism of citalopram and sertraline, but the impact of CYP2C19 poor metabolism on the metabolism of sertraline was relatively low in a study in healthy volunteers.5 This finding and the results from the present study are consistent with the more extensive role of CYP3A4 and CYP2B6, which are not inhibited by PPIs, in the metabolism of sertraline.18,19,21,22
Age was associated with a significant increase in the C/D ratios for all the 3 SSRIs. The model that best fitted the original data was a combination of age as a linear function and a square function. By applying this combined model, the age effect was shown to be relatively low in the age range 20–60 years, whereas it increased considerably in the higher age groups (Fig. 1). For citalopram and escitalopram, the C/D ratios were roughly doubled in patients aged 80 years. For sertraline, the age effect was smaller. Due to the square function, the age effects after the age of 80 years become even more pronounced, although the results in these advanced age groups should be interpreted with caution due to the relatively low number of subjects included. Nevertheless, the age span of included subjects was considerable, with patients being up to 100 years old (Table 1). In a study where patients elder than and younger than 65 years were compared, the mean concentration of citalopram was 2.0-fold higher in those elder than 65 years.23 The corresponding ratios were 1.8 for escitalopram and 1.3 for sertraline. Our findings are thus in good agreement with those results. Unfortunately, patients of advanced age (eg, above 80 or 90 years) were not handled separately in that study.23 Our results are also in line with current dosage recommendations that elderly patients should be treated with a halved SSRI dose. In our study, patients using PPIs had a somewhat higher age (median of 51–78 years in the various subgroups) than those in the 3 control groups (median of 44–46 years) (Table 1). However, because we controlled for age in the statistical analysis, this factor has not influenced our results regarding the effect of the PPIs.
We found that men had 5%–20% lower C/D ratios than women, an effect that might be caused by a decreased CYP2C19 activity in women.24 However, such a gender-related difference has not been found in all studies, and most of the lower CYP2C19 activity seen in women could probably be attributed to an inhibitory effect of combined oral contraceptives on CYP2C19.24,25 As only a small proportion of all women in the present study used oral contraceptives, there are most likely other factors as well that cause this effect, although we cannot pinpoint these in further detail.
The most pronounced interaction was seen when omeprazole or esomeprazole was combined with escitalopram, causing an almost 2-fold increase in the escitalopram levels. This is of particular importance given the recent regulatory restrictions on the dosage of escitalopram (and citalopram) related to the risk of QT prolongation,26,27 although the extent of this increase is subject to controversy.28,29 The European Medicines Agency has stated that the maximum escitalopram dose should be 20 mg/d and 10 mg/d in the elderly.26 The US Food and Drug Administration has not given such advice, although a corresponding restriction has been issued for citalopram.27 On the basis of our data, using omeprazole or esomeprazole concomitantly with escitalopram 20 mg/d would cause an escitalopram concentration equivalent to taking an escitalopram dose of close to 40 mg/d, ie, above the recommended maximum daily dose. In contrast to the effects of omeprazole and esomeprazole on the concentration of escitalopram, the effects of the other combinations investigated in this study are so small that they could be considered clinically insignificant.
Pharmacokinetic enhancement, that is, exploiting a drug's inhibitory effect on the metabolism of another drug, is sometimes used in psychiatry. The most common situation is the addition of fluvoxamine to clozapine; then, the clozapine dose can be considerably decreased due to fluvoxamine's inhibitory effect of CYP1A2, which is the major enzyme involved in the metabolism of clozapine.30 Although the effect is less pronounced for the drug combinations in the present study, there could possibly be situations where adding, for example, omeprazole or esomeprazole to escitalopram could be advantageous, such as in subjects being CYP2C19 ultrarapid metabolizers. However, the procedure cannot be generally recommended due to the lack of clinical documentation, and it might in such cases probably be more convenient to switch to another SSRI not metabolized by CYP2C19.
This study has several limitations. First, it is unknown to which degree patients were adherent to the prescribed doses and medications. Thus, a differentiated degree of SSRI nonadherence related to comedication with PPIs would affect our finding. For example, if patients using PPIs were more adherent to the SSRIs than those in the control group, that would have explained our findings. On the other hand, if adherence to SSRIs was lower in the PPI group, our results would have underestimated the real effect. However, we consider it being unlikely that patients with gastritis, peptic ulcers, esophagitis, and the like would have an altered SSRI adherence compared with those without such conditions. Moreover, we consider it being highly unlikely that there should be any differences across the various PPIs with respect to SSRI adherence.
Second, it is unclear whether the subjects included in the present study are representative of the whole population of SSRI users. There might be specific reasons for why a blood sample for the analysis of the serum concentration of an SSRI is obtained, such as a suspicion of nonadherence, comedication with potentially interacting drugs, concomitant diseases, poor therapeutic response, adverse drug reactions, and so on. However, samples from patients using drugs with a known interaction potential with the SSRIs were excluded from the study, and although there are uncertainties with regard to the representativity of the material, we believe that a naturalistic study like the present, with more than 5000 subjects included in total, reflects the “real-life” situation more closely than, for example, pharmacokinetic studies in healthy volunteers or randomized clinical trials, which often exclude elderly patients, patients with comorbidities, and the like.
Third, we have not cross-checked against original data the information given on the request forms about doses, comedication, times of drug ingestion, and times of sampling. However, we consider that possible inaccuracies with this respect would be counterbalanced by the large number of samples included in the study. This assumption is also substantiated by the fact that for the drug combinations previously studied, the extent of the interactions was the same in our study as in previous studies.16,17
Fourth, relatively few samples were available for certain drug combinations, such as sertraline combined with omeprazole, lansoprazole, and pantoprazole. On the other hand, we were able to include more than 100 samples, for example, from subjects using esomeprazole combined with citalopram and escitalopram, which are considerably more than usually seen in more formal interaction studies. Moreover, we chose to include 5 control samples per PPI sample, thereby increasing power. Finally, by standardizing the time interval from ingestion of last dose to sampling to 12 hours and including gender and age in the statistical model, the variability in C/D ratios caused by these factors were eliminated. We also included the SSRI dose as a variable in the final analysis. This was done because we observed that the SSRI C/D ratios were clearly dependent on the dose. Although apparently illogical because these SSRIs do not exert dose-dependent kinetics, this effect is readily explained by the naturalistic design of the study: As several samples usually are obtained from most subjects, the SSRI dose will in many patients be gradually increased or decreased to achieve a serum concentration within the predefined reference range for the respective SSRI. Then, subjects who are relatively extensive metabolizers of the SSRIs, for example, due to genetic factors (and thereby have lower C/D ratios), will more often be treated with a higher SSRI dose, whereas those with relatively poor SSRI metabolism (ie, with higher C/D ratios) will more often be treated with a lower SSRI dose. In contrast to in randomized studies, the dose will thus be inversely related to the C/D ratio in a study like the present, and it would be proper to adjust for this effect.
In conclusion, in this study exploring the inhibiting potential of PPIs on the metabolism of some SSRIs in a naturalistic setting, the most pronounced effect was seen when omeprazole or esomeprazole was combined with escitalopram, causing an almost 2-fold increase in the escitalopram levels. Thus, when omeprazole or esomeprazole is combined with escitalopram, it should be considered to reduce the dose of escitalopram. In contrast, the effects of the other PPIs on escitalopram and of all PPIs on citalopram and sertraline were so small that they could be considered clinically insignificant.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Yu BN, Chen GL, He N, et al. Pharmacokinetics of citalopram in relation to genetic polymorphism. Drug Metab Dispos. 2003;31:1255–1259. [DOI] [PubMed] [Google Scholar]
- 2.von Moltke LL, Greenblatt DJ, Giancarlo GM, et al. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29:1102–1109. [PubMed] [Google Scholar]
- 3.Huezo-Diaz P, Perroud N, Spencer EP, et al. CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP. J Psychopharmacol. 2012;26:398–407. [DOI] [PubMed] [Google Scholar]
- 4.Rudberg I, Reubsaet JL, Hermann M, et al. Identification of a novel CYP2C19-mediated metabolic pathway of S-citalopram in vitro. Drug Metab Dispos. 2009;37:2340–2348. [DOI] [PubMed] [Google Scholar]
- 5.Wang JH, Liu ZQ, Wang W, et al. Pharmacokinetics of sertraline in relation to genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;70:42–47. [DOI] [PubMed] [Google Scholar]
- 6.Rudberg I, Hermann M, Refsum H, et al. Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. Eur J Clin Pharmacol. 2008;64:1181–1188. [DOI] [PubMed] [Google Scholar]
- 7.Ueda N, Yoshimura R, Umene-Nakano W, et al. Grapefruit juice alters plasma sertraline levels after single ingestion of sertraline in healthy volunteers. World J Biol Psychiatry. 2009;10:832–835. [DOI] [PubMed] [Google Scholar]
- 8.Obach RS, Cox LM, Tremaine LM. Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study. Drug Metab Dispos. 2005;33:262–270. [DOI] [PubMed] [Google Scholar]
- 9.Ko JW, Sukhova N, Thacker D, et al. Evaluation of omeprazole and lansoprazole as inhibitors of cytochrome P450 isoforms. Drug Metab Dispos. 1997;25:853–862. [PubMed] [Google Scholar]
- 10.Li XQ, Andersson TB, Ahlström M, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–827. [DOI] [PubMed] [Google Scholar]
- 11.Frelinger AL, Lee RD, Mulford DJ, et al. A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J Am Coll Cardiol. 2012;59:1304–1311. [DOI] [PubMed] [Google Scholar]
- 12.Gugler R, Hartmann M, Rudi J, et al. Lack of pharmacokinetic interaction of pantoprazole with diazepam in man. Br J Clin Pharmacol. 1996;42:249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juurlink DN, Gomes T, Ko DT, et al. A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ. 2009;180:713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zvyaga T, Chang SY, Chen C, et al. Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos. 2012;40:1698–1711. [DOI] [PubMed] [Google Scholar]
- 15.Ogilvie BW, Yerino P, Kazmi F, et al. The proton pump inhibitor, omeprazole, but not lansoprazole or pantoprazole, is a metabolism-dependent inhibitor of CYP2C19: implications for coadministration with clopidogrel. Drug Metab Dispos. 2011;39:2020–2033. [DOI] [PubMed] [Google Scholar]
- 16.Malling D, Poulsen MN, Søgaard B. The effect of cimetidine or omeprazole on the pharmacokinetics of escitalopram in healthy subjects. Br J Clin Pharmacol. 2005;60:287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha A, Coelho EB, Sampaio SA, et al. Omeprazole preferentially inhibits the metabolism of (+)-(S)-citalopram in healthy volunteers. Br J Clin Pharmacol. 2010;70:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Indiana University, School of Medicine, Division of Clinical Pharmacology. Cytochrome P450 drug interaction table. Available at: www.drug-interactions.com. Accessed January 3, 2014.
- 19.Baxter K, ed. Stockley's Drug Interactions. 9th ed London: The Pharmaceutical Press; 2010. [Google Scholar]
- 20.Machin D, Campbell MJ, Fayers PM, et al. Sample Size Tables for Clinical Studies, 2nd ed Oxford: Blackwell Science; 1997. [Google Scholar]
- 21.Walsky RL, Astuccio AV, Obach RS. Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J Clin Pharmacol. 2006;46:1426–1438. [DOI] [PubMed] [Google Scholar]
- 22.Castberg I, Skogvoll E, Spigset O. Quetiapine and drug interactions: evidence from a routine therapeutic drug monitoring service. J Clin Psychiatry 2007;68:1540–1545. [DOI] [PubMed] [Google Scholar]
- 23.Waade RB, Molden E, Refsum H, et al. Serum concentrations of antidepressants in the elderly. Ther Drug Monit. 2012;34:25–30. [DOI] [PubMed] [Google Scholar]
- 24.Tamminga WJ, Wemer J, Oosterhuis B, et al. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55:177–184. [DOI] [PubMed] [Google Scholar]
- 25.Hägg S, Spigset O, Dahlqvist R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol. 2001;51:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Escitalopram and risk of QT prolongation. Final SmPC and PL wording agreed by the PhVWP December 2011. Available at: http://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/Product_Information/PhVWP_Recommendations/CMDhPhVW0442012_January12.pdf. Accessed January 3, 2014.
- 27.U.S. Food and Drug Administration. FDA Drug Safety Communication: revised recommendations for celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. Available at: http://www.fda.gov/drugs/drugsafety/ucm297391.htm. Accessed January 3, 2014.
- 28.Vieweg WVR, Hasnain M, Howland RH, et al. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med. 2012;125:859–868. [DOI] [PubMed] [Google Scholar]
- 29.Hasnain M, Howland RH, Vieweg WVR. Escitalopram and QTc prolongation. J Psychiatry Neurosci. 2013;38:E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu ML, Lane HY, Chen KP, et al. Fluvoxamine reduces the clozapine dosage needed in refractory schizophrenic patients. J Clin Psychiatry. 2000;61:594–599. [DOI] [PubMed] [Google Scholar]


