Abstract
Background:
Heroin abuse is a significant public health issue and is on the rise because of the unintended consequences of strengthening controls for nonmedical use of prescription pain killers. Included in this trend is an increase in opiate exposed newborns that are particularly vulnerable to a number of negative health outcomes.
Methods:
After presenting a fully validated liquid chromatography–tandem mass spectrometric method for codeine, morphine, 6-monoacetylmorphine, and meconin, a metabolite of the heroin contaminant noscapine, we compared the outcome of 46 authentic umbilical specimens with the results generated using a previous less sensitive method that did not include meconin. Additionally, we provided a summary of opiate finding from a year-long survey of specimens received into a commercial reference laboratory.
Results:
The limits of detection for all 4 compounds were 0.1 ng/g, the limit of quantitation was 0.2 ng/g, and the assay was linear from 0.2 to 10.0 ng/g. Of the 46 comparative specimens, this method improved the identification of heroin exposure from 2 to 5, and the year-long survey identified 86 heroin-exposed newborns with 11 of them identified by the sole identification of meconin.
Conclusions:
This study demonstrated that a more sensitive analytical platform and the inclusion of meconin in the opiates assay improved the ability to distinguish between in utero heroin exposure and maternal administration of codeine or morphine.
Key Words: heroin, diacetylmorphine, 6-monoacetylmorphine, meconin, umbilical cord
INTRODUCTION
From 1992 to 2010, the proportion of admissions into substance abuse treatment facilities because of prescription opiate addiction has risen from 1% to 8.6% of total admissions, whereas the proportion of cocaine and methamphetamine-based admissions have dropped or remained steady.1 The Center for Substance Abuse Research further noted that during the same time period there were statistically significant increases of self-reported chronic nonmedical use of prescription pain relievers.2 In response to those trends, measures to contain the prescription opiate epidemic have been implemented but have led to the unintentional consequence of diverting this opiate-addicted population to a low cost and readily available supply of heroin.3 One study demonstrated that since the introduction of extended-release oxycodone (an abuse-deterrent formulation strategy embraced by the FDA), the abuse of oxycodone decreased but was unfortunately accompanied with a 42% increase of heroin use in the study population.4 Included in this dangerous trend is an increase of opiate exposed newborns that exhibit a wide array of negative signs and symptoms called Neonatal Abstinence Syndrome (NAS).5
NAS, although implicated from the abuse of other drugs, is typically associated with the sudden neonatal withdrawal from opiates. Symptoms include irritability, hypertonia, tremors, feeding intolerance, emesis, watery stools, seizures, and respiratory distress (Hudak et al6). Between the years 2000 and 2009, the incidence of NAS has risen from 1.2 to 3.4 per 1000 births.5 Patrick et al5 noted that the average length of stay for all births during the study period was approximately 3 days, whereas the newborns identified with NAS had an average length of stay of 16 days creating added burden on hospital resources that are disproportionally covered by State programs.
Screening newborns for drugs of abuse has historically been accomplished using maternal self-report and analysis of neonatal biological specimens such as urine, meconium, and umbilical cord. Maternal self-report may be unreliable because of deception and the stigma of illicit drug use and urine testing has a limited detection window of only a couple of days.7,8 Meconium, the first fecal matter produced by the newborn, has been used for the past 20 years for drugs of abuse testing because of its relative availability (78%–90%) and its long detection window (up to 20 weeks).7 Umbilical cord testing has rapidly replaced meconium over the past 5 years as the newborn toxicology gold standard because of its universal availability (every baby has sufficient umbilical cord for testing), shortened turnaround time (umbilical cord is collected immediately after birth while meconium may take several days to pass), and improved chain of custody integrity (meconium requires multiple collections by multiple collectors over multiple shifts, whereas umbilical cord is a single-step collection).9,10
The first report of using umbilical cord segments to screen newborns for opiates exposure was given by Montgomery et al,9 where they reported finding opiates in matched pairs of meconium and umbilical cord with a 95% agreement between the 2 specimen types. They used immunoassay methods (ELISA), and these results were verified using a modified meconium gas chromatography–mass spectrometric method.9 A large trial of 498 umbilical cords was used to survey 2 populations (Newark, NJ and Salt Lake City, UT), and these data were used to evaluate the sensitivity (91%) and specificity (98%) of the immunoassay compared with the modified gas chromatography–mass spectrometric method.10 The following year, a more sensitive liquid chromatography–tandem mass spectrometry method was published by the Chemistry and Metabolism group at the National Institute on Drug Abuse with a reported limit of detection (LOD) of 1.0 ng/g for 6-monoacetylmorphine (6MAM) and 2.5 ng/g for codeine and morphine but they did not report finding any unique heroin metabolites in authentic umbilical cord specimens.11 Our laboratory used a slightly modified version of this method until October 2012, and feedback from the field suggested that improvements in sensitivity were needed for identifying heroin-exposed newborns.
Heroin (diacetylmorphine) is rapidly metabolized to 6MAM which is then quickly hydrolyzed to morphine.12 In urine, the detection window for 6MAM is less than 1 day with morphine persisting for several days afterward.12 The presence of 6MAM is unique to heroin but morphine may be present because of the ingestion of morphine or codeine.13 Because of the rapid elimination of 6MAM, routinely only morphine is detected.13 Additionally, in some instances, mothers are administered morphine during labor and delivery, which confounds interpretations in the absence of a unique heroin metabolite.
A similar situation exists in several European countries where pharmaceutical grade diacetylmorphine is commonly used.14 In that environment, 6MAM is not unique for illicit heroin use. Morley et al14 demonstrated that the detection of meconin, a metabolite of the illicit heroin contaminant noscapine, was an effective strategy to discriminate illicit heroin use from pharmaceutical grade diacetylmorphine use. Unexpectedly, the detection of meconin outperformed the detection of 6MAM by 69% in a survey of 300 urine specimens from known illicit heroin users.14 The findings of Morley et al prompted the question, would the inclusion of meconin in the umbilical cord assay improve the identification of in utero heroin exposure?
We propose that improvements of the analytical sensitivity and the inclusion of meconin in the LC-MS/MS opiates assay will augment our efforts to identify heroin-exposed newborns. We will present a fully validated LC-MS/MS method for the analysis of codeine, morphine, 6MAM, and meconin in umbilical cord and report here for the first time the presence of unique heroin metabolites in authentic umbilical cord specimens. We will analyze a selection of authentic umbilical cord specimens to compare this method to the previous method. Finally, we will summarize our findings for the specimens received by our laboratory for 1 year using the new method.
EXPERIMENTAL METHODS
Ethics
The UC specimens used in this study were referred to our laboratory for routine analysis. Opiates analyses were performed on de-identified aliquots of specimens remaining after the original intended analysis. These aliquots were considered to be waste and did not require an ethics review.
Subjects
Forty-six consecutive specimens that tested positive for morphine were selected, de-identified, and a 1-g segment was stored in a 5-mL conical bottom screw top polypropylene tube at −20°C. The year-long survey of opiate findings was summarized from the results of umbilical specimens from high-risk births routinely received into a commercial reference laboratory for newborn forensic toxicology analysis.
Chemicals, Reagents, and Materials
Meconin-d3 and meconin were purchased from Cayman Chemical (Ann Arbor, MI) as solids and codeine, codeine-d3, morphine, morphine-d3, 6-monoacetylmorphine-d3 (MAM-d3), and 6MAM were purchased from Cerilliant (Round Rock, TX) as 1 mg/mL ampoules. All reagents (ACS grade) and all solvents (HPLC grade) were purchased from Thermo-Fisher Scientific (Hanover Park, IL). Solid-phase extraction (SPE) cartridges (ZSDAU020, 200-mg bed, 10 mL reservoir) were purchased from United Chemical Technologies (Bristol, PA). Extracts were evaporated in a TurboVap LV II purchased from Biotage (Charlotte, NC) under a stream of nitrogen at 60°C. Umbilical cord tissue aliquots were homogenized in a Next Advance Bullet Blender (Averill Park, NY) using 3 stainless steel wood screws.
Preparation of Calibration Standards and Quality Control Samples
The meconin and meconin-d3 reference standards were made by dissolving 50 mg of each solid with 50 mL of methanol (1 mg/mL). The codeine, codeine-d3, morphine, morphine-d3, meconin, and meconin-d3 stock standards (100 mcg/mL) were prepared by the appropriate dilution of each reference standard with methanol or acetonitrile. Calibrator and control cocktails (10 ng/mL) were independently prepared by appropriate dilution of codeine, morphine, 6MAM, and meconin with acetonitrile. Internal standard spiking solution was prepared by an appropriate dilution of codeine-d3, morphine-d3, 6MAM-d3, and meconin-d3 (10 ng/mL) in acetonitrile. An unextracted standard was prepared by adding 50 μL of calibrator spiking solution and 50 μL of internal standard spiking solution to a 13 × 100-mm glass tube, evaporating under a stream of nitrogen, and reconstituting in 100 μL of mobile phase A (10 mM ammonium acetate/0.1% formic acid). A single point calibrator (0.5 ng/g) was prepared by adding 50 μL of calibrator spiking solution to 1.0 g of certified negative umbilical cord in a labeled 5-mL conical polypropylene tube. The negative, low (0.2 ng/g), mid (0.625 ng/g), and high (4.0 ng/g) controls were prepared by the addition of 0, 20, 62.5, and 400 μL of control spiking solution to certified negative umbilical cord aliquots, respectively.
Specimen Preparation
Accurately weighed portions between 0.1 and 1.0 g of the umbilical cord specimens were transferred to labeled 5-mL conical polypropylene tubes with screw top caps. To each calibrator, control, and specimen, 50 μL of internal standard and 3 mL of acetonitrile were added with vortex mixing. After the addition of 3 stainless steel wood screws to each tube, the capped tubes were placed in a Bullet Blender for approximately 5 minutes at setting 7. The extracts were centrifuged for 5 minutes at 980g and decanted into clean 13 × 100-mm glass tubes. The extracts were evaporated to dryness at 60°C under a stream of nitrogen, and the residues were reconstituted in 3 mL of 0.1 M phosphate buffer (pH 6).
Solid-Phase Extraction
SPE cartridges were fitted on a vacuum-equipped manifold and prewashed with 3 mL of methylene chloride:isopropanol:ammonium hydroxide (80:20:2). The cartridges were dried by applying full vacuum for approximately 5 minutes. Each cartridge was conditioned with 3 mL of methanol, 3 mL of deionized water, and 3 mL 0.1 M phosphate buffer (pH 6). The specimen extracts were poured onto the cartridges and allowed to flow through freely under the force of gravity. The cartridges were dried for 1 minute with vacuum. The cartridges were rinsed with 3 mL of deionized water and 1 mL of 0.1 M HCl followed by 5 minutes of drying using full vacuum. Meconin was eluted from the cartridges with 3 mL of methylene chloride:isopropanol (80:20) followed by 3 mL of methylene chloride:isopropanol:ammonium hydroxide (80:20:2) to elute the remainder of the opiates. The combined eluates were evaporated under a stream of nitrogen at 60°C, and the residues were reconstituted in 500 μL of mobile phase A (10 mM ammonium acetate/0.1% formic acid). The extracts were transferred to 2 mL vials fitted with 250 μL glass inserts.
LC-MS/MS Conditions
The specimens were analyzed using an Agilent Technologies 1200 HPLC system that consisted of G1367D autosampler, a G1379B degasser, a G1312B binary pump, and a G1310A isocratic pump (Wilmington, DE). Separation was achieved using a Synergi Hydro RP (50 mm × 2.0 with 2 μm particle size) C-18 column (Phenomenex, Torrence, CA). The column was held at 40°C in a G1316B thermostated column compartment (Wilmington, DE). The solvent system consisted of A (10 mm ammonium acetate/0.1% formic acid) and B (acetonitrile/0.1% formic acid) with a flow rate of 0.250 mL/min. The solvent program held B at 4.0% from 0.0 minutes to 7.0 minutes, increased to 40.0% from 7.0 minutes to 7.1 minutes, and decreased to 4% from 7.1 minutes to 11 minutes.
The detector was an AB Sciex 5500 mass spectrometer equipped with electrospray ionization in the positive mode. The ion spray voltage was set at 5500 V, and the source temperature was 650°C. The curtain gas and collision gas was nitrogen and was held at 30 psi and 5 psi, respectively. The transition parameters are listed in Table 1. All data were processed using Analyst 1.5.1 (Foster City, CA).
TABLE 1.
Parameters for MS/MS Transitions
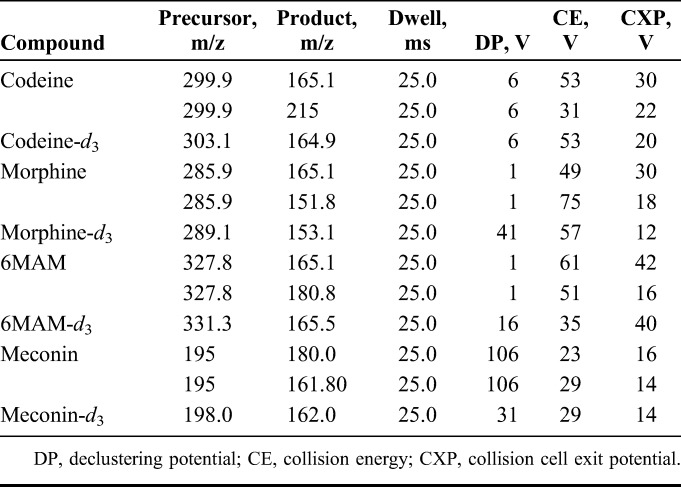
Identification Criteria
The identification criteria used for this method included 4 components: retention time, signal to noise, baseline, resolution, and relative ion intensity. The retention time of each analyte was required to be within 0.2 minutes of the calibrator. A signal to noise of greater than 3:1 ratio was required of each ion chromatogram. A minimum of 90% return to baseline was required to consider a peak to be effectively resolved form a co-eluting peak. The relative ion intensity of the product ions for each analyte (mass ratio) was required to be within 30% of the corresponding ion intensity of the calibrator.
Method Validation
The method was validated according to the recommendations of commonly accepted guidelines.15–17 The following specifications were evaluated for the assay: LOD, limit of quantitation (LOQ), linear range, carryover potential, specificity, selectivity, accuracy, precision, extraction efficiency, matrix effect, stability of extracts on the autosampler, and stability of specimens during freeze-thaw conditions.
The LOD and LOQ for each assay were decided by analyzing a series of controls. The LOQ was the lowest point where the mean of the measured concentrations was within 20% of target value and satisfied all identification criteria. The LOD was the lowest triplicate that satisfied all identification criteria without concern of the measured concentration. The concentrations attempted were 0.5, 0.4, 0.2, and 0.1 ng/g.
Linearity for each analyte was determined by the analysis of a series of fortified negative umbilical cords in replicates of four. Calibration curves were prepared using analyte to internal standard area response ratios. A weighted (1/x) least squares linear regression was used to mitigate heteroscedasticity. The means and SDs of the calibration curve slopes and intercepts were calculated. The concentrations used were 0.2, 0.4, 0.5, 2.5, 5.0, and 10.0 ng/g.
The possibility of carryover for each assay was evaluated by analyzing a known negative control after a control containing 10.0 ng/g. A successful carryover challenge must be less than the LOD.
The analysis of 6 negative controls spiked with a cocktail of potentially interfering substances (Table 2) was used to evaluate the specificity of the assay. Analyte must not be detected at a level equal to or greater than the LOD. Analyzing 6 LOQ controls prepared with a cocktail of potentially interfering compounds challenged the selectivity of the method. All 6 replicates must satisfy the identification criteria and the measured concentrations must be within 20% of the target value.
TABLE 2.
List of Potentially Interfering Substances

Accuracy and precision of the assay was determined by analyzing prepared controls at 3 different concentrations, replicates of 5 over 4 different days. The 4 concentrations chosen for this challenge were 0.5, 1.0, 5.0, and 10.0 ng/g. The accuracy and precision challenge was considered to be successful if each intra- and the interassay mean were within 15% of the target value and the maximum intra- and interassay variance must be less than 20%, respectively.
The extraction efficiency and matrix effect were determined using procedures defined by Matuszewski.17 Three sets of controls were arranged over 4 concentrations with 5 replicates each. The first set was unextracted controls reconstituted in mobile phase A. The second set was negative umbilical cord extracts fortified with an appropriate amount of spiking solution following the SPE procedure. The third set was negative umbilical cord controls obtained from 5 different sources fortified with the 4 opiates and then subjected to the extraction procedure. The extraction efficiency for each analyte is expressed as the ratio of the average peak area in set 3 to set 2. The matrix effect for each analyte is defined as the ratio of the mean peak area of set 2 to set 1. The total recovery is defined as the ratio of the mean peak area of set 3 to set 1.
The stability of prepared extracts was reviewed by the reanalysis of a control set from the precision and accuracy experiment that had been stored at room temperature for 5 days. The stability was written as a ratio of the results of the incubate controls and the original measured concentrations. The stability to freeze-thaw conditions were evaluated by subjecting a control set from the precision and accuracy experiment to 3 daily freeze-thaw cycles. Freeze-thaw stability was written as a ratio of the experimental means versus the respective target concentration.
Application of Method to Authentic Specimens
This method was applied to 46 authentic umbilical cord specimens that were received by our laboratory for routine forensic toxicological analysis using LC-MS instruments. These specimens have been previously identified as positive for morphine using a slightly modified version of a previously published method. Additionally, this method has been in routine use at our laboratory for more than 1 year, and a histogram of the observed concentrations for codeine, morphine, 6MAM, and meconin was presented.
Statistical Analysis
Statistical analysis was accomplished using IBM SPSS Statistics version 21. Pearson correlation coefficients were used to evaluate the association of 6MAM and meconin concentrations observed in umbilical cord and to compare the 2 methods.
RESULTS
Validation Results
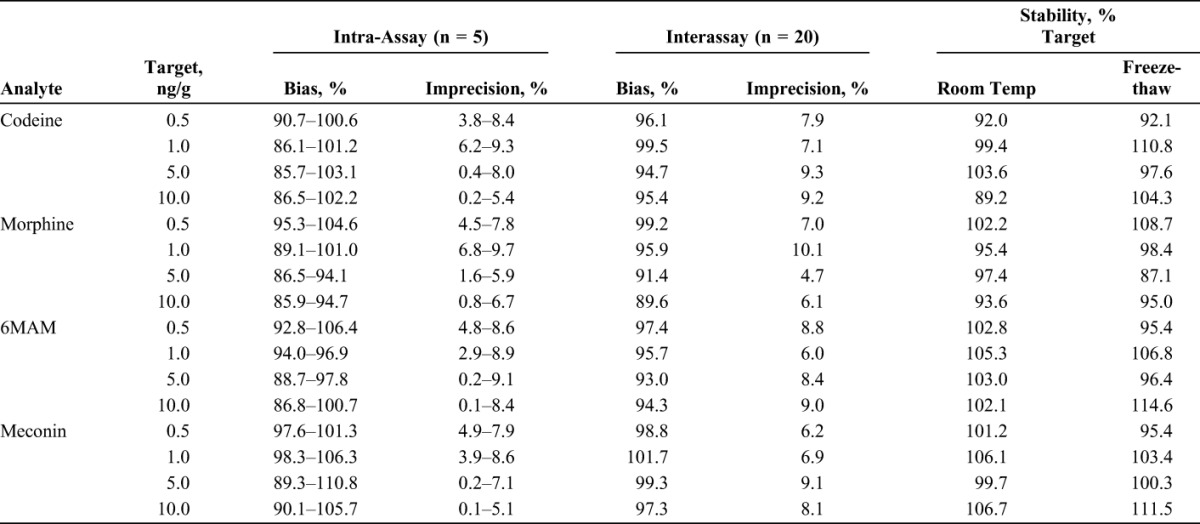
The extraction procedure and LC-MS/MS parameters used for this method are very similar to previously published procedures and proved to be clean, robust, and stable throughout the validation study. The LOD, LOQ, linearity, extraction efficiency, matrix effect, and total recovery results are presented in Table 3. The bias, imprecision, and stability results are presented in Table 4. No carryover was observed in a negative control analyzed immediately after a high control (10.0 ng/g) fortified. The specificity and selectivity of the assay was adequate because the 6 negative controls spiked with the cocktail of interfering substances did not produce in any detectable analyte and the 6 LOQ controls were properly identified within 20% of target value.
TABLE 3.
The LOD, LOQ, Linearity, Extraction Efficiency, Matrix Effects, and Total Recovery of Codeine, Morphine, 6MAM, and Meconin in Umbilical Cord
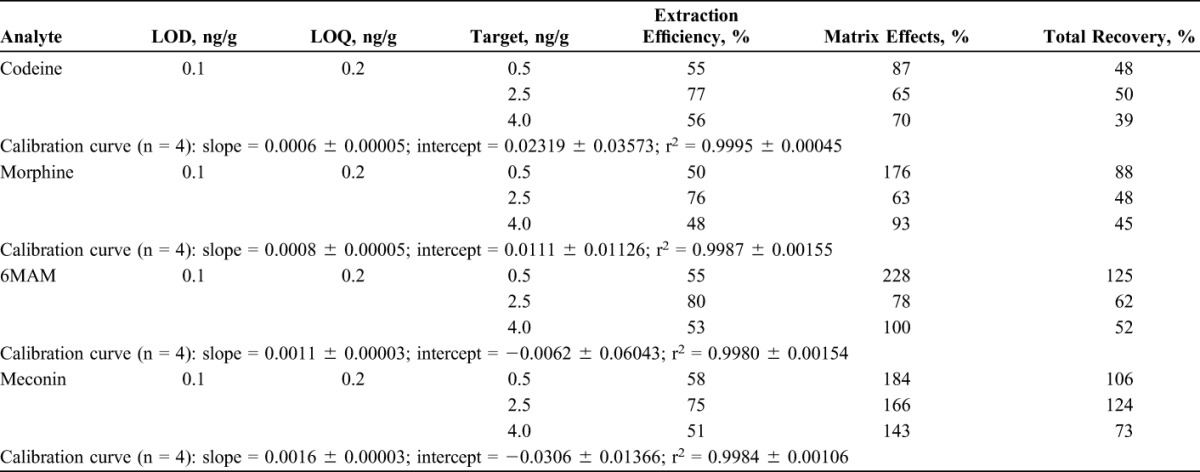
TABLE 4.
The Bias and Imprecision of Codeine, Morphine, 6MAM, and Meconin in Umbilical Cord
Application of Method
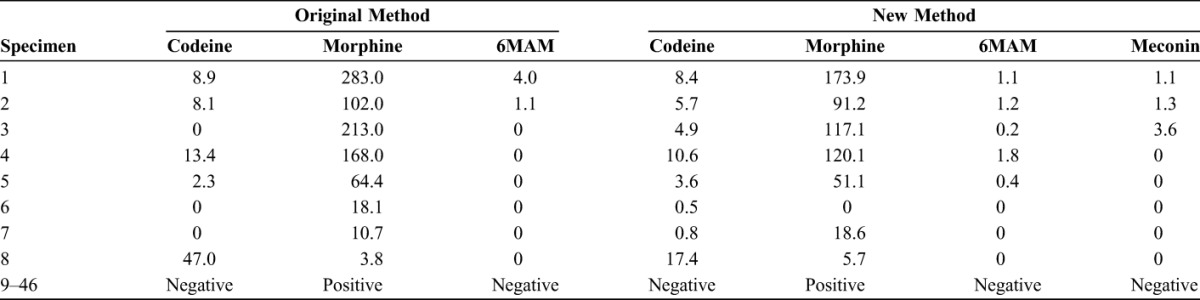
Forty-six previously analyzed morphine positive umbilical cord specimens (mean = 3.5 ± 64.4 ng/g; median = 7.5 ng/g) were retested using the newly validated method for codeine, morphine, 6MAM, and meconin. The average morphine finding was 25.7% lower using the new method (mean = 20.9 ± 41.1 ng/g; median = 5.7 ng/g). There were too few positive results in this subset to statistically compare codeine, 6MAM, and meconin. Codeine, 6MAM, and meconin were detected in 10, 5, and 4 of the morphine positive specimens, respectively. Using the new method, 5 specimens were identified as containing heroin metabolites in contrast to only 2 of these specimens being reported to contain heroin-based compounds using the previous method. The original and retest results for these umbilical cord specimens are listed in Table 5.
TABLE 5.
Results of Codeine, Morphine, 6MAM, and Meconin Compared With Results From the Original Method
The new method has been in routine use at our laboratory for more than 1 year at the time of writing this manuscript. From October 2012 through September 2013, this laboratory received 23,271 umbilical cord specimens from a nationwide hospital client base for forensic newborn toxicology analysis and 1773 (7.6%) of those specimens were identified as morphine positive (mean = 23.1 ± 65.0 ng/g; median = 5.74 ng/g). Of those 1773 morphine-positive specimens, we identified 86 specimens (4.9% of the morphine-positive specimens or 0.4% of all specimens) that were positive due to in utero heroin exposure defined as 6MAM positive (mean = 5.03 ± 19.48 ng/g; median = 1.22 ng/g) and/or meconin positive (mean = 0.99 ± 0.9 ng/g; median = 0.6 ng/g). There were 9 specimens that were positive for both 6MAM and meconin, 11 specimens positive for meconin only, and 66 specimens that were positive for 6MAM only. Histograms of the observed concentrations of the 4 opiates are presented in Figure 1.
FIGURE 1.
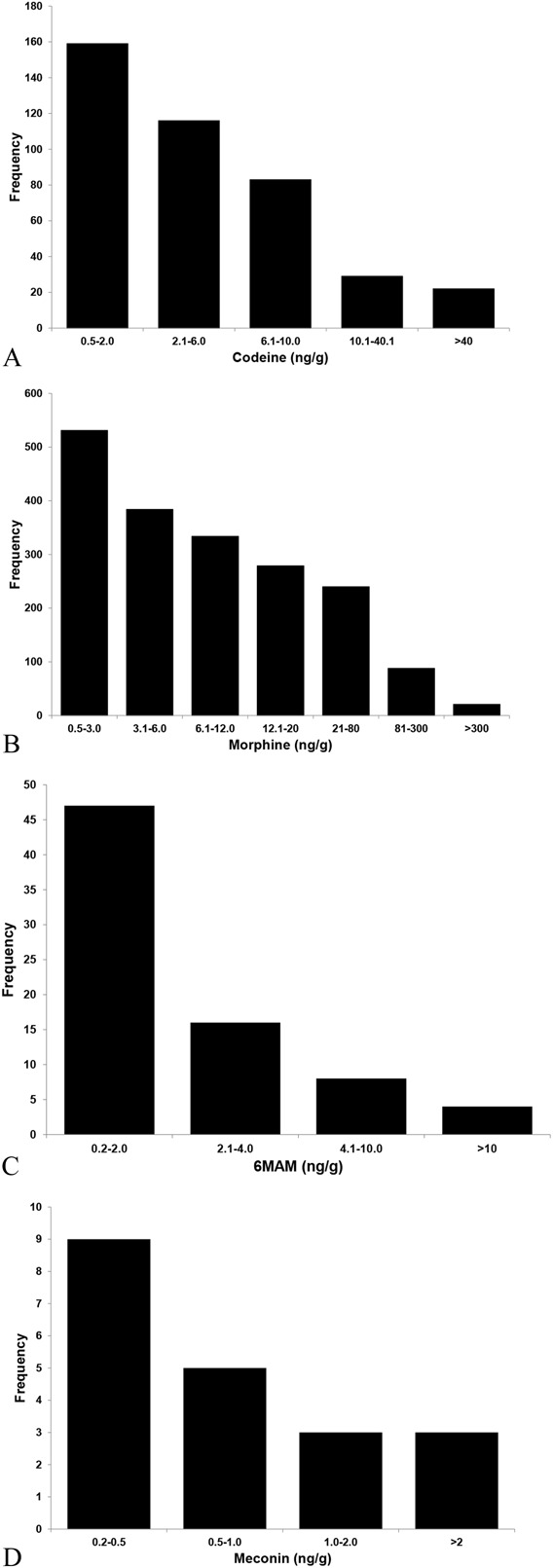
Four histograms depicting the frequencies of the observed concentrations for codeine (A), morphine (B), 6MAM (C), and meconin (D) during a year-long survey of umbilical cords received in a commercial reference laboratory for drugs of abuse analysis.
DISCUSSION
A fully validated method for the detection of codeine, morphine, 6MAM, and meconin in umbilical cord has been presented. The laboratory received and analyzed 46 previously identified morphine-positive umbilical cords and presented our observations from more than 23,000 analyses over a period of 1 year. The improvements made in sensitivity (inclusion of meconin and migrating to a more sensitive analytical platform) allowed for increasing the number of heroin exposure interpretations from 2 to 5 in the analysis comparisons. The inclusion of meconin in the analysis allowed for an additional 11 heroin exposure interpretations, which increased the number of heroin exposure interpretations from 75 to 86 newborns.
The measured concentrations obtained for morphine using the new method were compared with the concentrations observed from the previous method. A Bland–Altman chart was constructed (not depicted here) and it revealed that the new method was biased by −25.7%. Two reasonable explanations for this bias may be age of specimens at the time of analysis and differences in the analytical measurement range (AMR) of these 2 assays. For the original analysis, the specimens were only a couple of days old and were stored at −20°C for approximately 3 months before analysis using the new method. The AMR of the previous method was 1.0–40.0 ng/g and the AMR for the new method was 0.1–10.0 ng/g.
Between October 2012 and September 2013, our laboratory analyzed 23,271 umbilical cord specimens for the presence of drugs of abuse. We found that 1773 specimens were positive for morphine (7.6%) and 410 were also positive for codeine (mean = 11.2 ± 29.1 ng/g; median = 3.2 ng/g). Maternal ingestion of heroin is one reasonable explanation for the presence of morphine and/or codeine but they are not unique to heroin ingestion and may also be explained by the ingestion of codeine and/or morphine legitimately provided to the mother.
Eighty-six of these specimens did contain compounds unique to heroin, 6MAM (mean = 5.03 ± 19.48 ng/g; median = 1.22 ng/g), and/or meconin positive (mean = 0.9 ± 0.9 ng/g; median = 0.6 ng/g). The measured concentrations of 6MAM and meconin were not associated (r = −0.1; P = 0.903) which may be explained by the fact that 6MAM is a direct metabolite of heroin and meconin is a metabolite of contaminant of heroin production, noscapine, which may vary with poppy source and production methods.14
Meconin, a noscapine metabolite, was originally suggested by Morley et al14 as an improved means to discriminate between illicit and pharmaceutical-grade heroin, which is commonly prescribed in Europe. Pharmaceutical-grade heroin is of high purity and does not contain impurities such as noscapine. Their data suggested that meconin may improve the detection of heroin use presumably because of higher stability of meconin than 6MAM in urine. Their survey of 300 known illicit heroin abusers, where all 300 contained morphine as the principal opiate detected, revealed only 168 that contained detectable levels of 6MAM. Meconin, however, was found in 284 of these cases thus improving illicit heroin detection by 69%. Our study identified 11 (14.7%) of 86 heroin-exposed newborns based solely on the presence of meconin in the umbilical cord. Although not the 69% improvement noted by Morley et al, it is our opinion that it would be unethical to knowingly misclassify almost 15% of our heroin-exposed newborns.
During the process of attempting to improve detection of in utero heroin exposure, the laboratory's previous cutoff for codeine, morphine, and 6MAM of 2 ng/g, which was similar to the reported LOQ of de Castro et al (2.5 ng/g), proved to lack the required analytical sensitivity. By migrating from an Applied Biosystems 3200 liquid chromatography–tandem mass spectrometry to a more sensitive platform, an AB Sciex 5500, we were able to lower our codeine/morphine cutoff to 0.5 ng/g and the cutoff for 6MAM/meconin to 0.2 ng/g. Another suggested cutoff in use today is 6 ng/g for codeine and 4 ng/g for morphine and 6MAM.18 Using the quantitative findings of a year-long survey, a comparison of the 2 cutoffs revealed that the higher cutoffs fail to identify 276 (67%) of the 410 codeine positives, 690 (39%) of the 1774 morphine positives, and 63 (84%) of the 75 6MAM-positive specimens (Fig. 1). It is self-evident from these data that the higher cutoffs suggested significantly reduce the value of using umbilical cord as a newborn toxicology specimen. Future research in this area includes further improvements in analytical sensitivity and evaluation of other unique heroin-based compounds.
There were limitations of this study that limit the generalizability of our findings that should be addressed here. The results used for method comparison purposes were obtained from analytical platforms with very different analytical sensitivities and because of production constraints of a high-throughput commercial laboratory those analyses were not performed contemporaneously. Additionally, the specimens used in this study were a convenience sampling of high-risk specimens received by our laboratory for routine analysis for drugs of abuse, and for obvious ethical reasons, a random controlled trial was not feasible for this type of study.
CONCLUSIONS
This project has been a part of an ongoing effort at our laboratory to improve the ability to detect in utero heroin exposure and provide evidence that discriminated heroin exposure from pharmaceuticals prescribed or administered to the mother during gestation or labor. This study demonstrated that umbilical cord tissue is a suitable specimen type for the detection of codeine, morphine, 6MAM, and meconin. Codeine and morphine may be present in umbilical cord tissue because of maternal consumption of controlled pharmaceutical preparations but 6MAM and meconin are specific and unique to heroin. This assay represents one of the many improvements over previous methods for determining fetal heroin exposure. This assay will be used to provide health professionals evidence of fetal heroin exposure to facilitate referral to the proper agencies as mandated by each State.
Footnotes
Supported by United States Drug Testing Laboratories.
The authors of this manuscript are employees of United States Drug Testing Laboratories, a privately owned commercial reference laboratory.
The funding source had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Center for Substance Abuse Research (CESAR). Chronic nonmedical use of prescription opioid pain relievers nearly doubles since 2002. CESAR Fax. 2012;21:1. [Google Scholar]
- 2.Center for Substance Abuse Research (CESAR). National treatment admissions for opiates other than heroin continue to increase; now surpass cocaine and methamphetamine. CESAR Fax. 2012;21:1. [Google Scholar]
- 3.Schatman M, Darnall B. A practical and ethical solution to the opioid scheduling conundrum. J Pain Res. 2014;7:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coplan P, Kale H, Sandstrom L, et al. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release of oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol Drug Saf. 2013;22:1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrick S, Schumacher R, Benneyworth B, et al. Neonatal abstinence syndrome and associated health Care Expenditures United States, 2000-2009. JAMA. 2012;307:1934–1940. [DOI] [PubMed] [Google Scholar]
- 6.Hudak M, Tan R, Frattarelli D, et al. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–e560. [DOI] [PubMed] [Google Scholar]
- 7.Ostrea E, Brady M, Gause S, et al. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89:107–113. [PubMed] [Google Scholar]
- 8.Schutzman D, Frankenfield-Chernicoff M, Clatterbaugh H, et al. Incidence of intrauterine cocaine exposure in a suburban setting. Pediatrics. 1991;88:825–827. [PubMed] [Google Scholar]
- 9.Montgomery D, Plate C, Alder S, et al. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. 2005;26:11–14. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery D, Plate C, Jones M. Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey. J Perinatol. 2008;28:750–753. [DOI] [PubMed] [Google Scholar]
- 11.de Castro A, Concheiro M, Shakleya D, et al. Development and validation of a liquid chromatography mass spectrometry assay for the simultaneous quantification of methadone, cocaine, opiates and metabolites in human umbilical cord. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3065–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh S, Gorodetzky C, McQuinn R. Urinary excretion of heroin and its metabolites in man. J Pharmacol Exp Ther. 1976;196:249–256. [PubMed] [Google Scholar]
- 13.Fehn J, Megges G. Detection of O6-monoacetylmorphine in urine samples by GC/MS as evidence for heroin use. J Anal Toxicol. 1985;9:134–138. [DOI] [PubMed] [Google Scholar]
- 14.Morley S, Forrest A, Galloway J. Validation of meconin as a marker for illicit opiate use. J Anal Toxicol. 2007;31:105–108. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration, Center for Drug Evaluation Research, Center for Veterinary Medicine. Guidance for Industry: bioanalytical method validation. 2011. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed July 5, 2009.
- 16.The Commission of European Communities. J Eur Communities L. 2002;221:8. [Google Scholar]
- 17.Matuszewski B, Constanzer M, Chavez–Eng C. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. [DOI] [PubMed] [Google Scholar]
- 18.Marin S, Metcalf A, Krasowski M, et al. Detection of neonatal drug exposure using umbilical cord tissue and liquid chromatography time-of-Flight mass spectrometry. Ther Drug Monit. 2014;36:119–124. [DOI] [PubMed] [Google Scholar]


