Abstract
The 2 year survival rate of patients with breast cancer [BC] brain metastases is less than 2%. Treatment options for BC brain metastases are limited and there is an unmet need to identify novel therapies for this disease. Brain angiogenesis inhibitor 1 [BAI1] is a GPCR involved in tumor angiogenesis, invasion, phagocytosis, and synaptogenesis. For the first time, we identify BAI1 expression is significantly reduced in BC and higher expression is associated with better patient survival. Nestin is an intermediate filament whose expression is up-regulated in several cancers. We found higher Nestin expression significantly correlated with BC lung and brain metastases, suggesting both BAI1 and Nestin can be therapeutic targets for this disease. Here, we demonstrate the ability of an oncolytic virus, 34.5ENVE, to target and kill high nestin expressing cells and deliver Vstat120 [extracellular fragment of BAI1]. Finally, we created two orthotopic immune competent murine models of BC brain metastases and demonstrated 34.5ENVE extended the survival of immune competent mice bearing intracranial breast-cancer tumors.
Keywords: Breast Cancer Brain Metastases, Brain Angiogenesis Inhibitor 1, Nestin, Oncolytic Virus
Introduction
Breast cancer is one of the leading causes of brain metastases. The 2 year survival rate of patients with breast cancer [BC] brain metastases is less than 2% (1). Treatment options for patients refractory to standard surgery and radiation are limited. These tumors are frequently resistant to conventional chemotherapeutic drugs and antibody-based therapies which poorly penetrate the blood brain barrier (2, 3). There is an unmet need to identify novel, targeted strategies to treat this disease.
Vstat120 is a cleaved and secreted fragment of Brain Angiogenesis Inhibitor 1 [BAI1]. The loss of BAI1 expression is observed in several cancers including glioblastoma, colorectal cancer, gastric cancer, and renal cell carcinoma.(4) The re-expression of BAI1 or Vstat120 exerts potent anti-angiogenic and anti-tumor effects in animal models of glioblastoma and renal cell carcinoma (5, 6). Surprisingly, its expression and function has not been examined in BC. Nestin is an intermediate filament whose expression is upregulated in several cancers (7–9). Nestin is also expressed in cancer stem cells which are known to promote cancer resistance and progression (10). Here we determined the roles of BAI1 and Nestin gene expression in breast cancer metastases and patient survival. We found lower BAI1 expression correlates with poorer patient survival, and high Nestin expression is associated with an increased probability of metastases.
34.5ENVE is an oncolytic Herpes Simplex Virus which expresses Vstat120 and its replication is driven by a cancer stem cell specific Nestin promoter (11). 34.5ENVE has demonstrated unparalleled anti-tumor efficacy in murine models of glioblastoma (11). Given the roles of Nestin and BAI1 in BC brain metastases, we tested the ability of 34.5ENVE to target BC cells in vitro. In these studies, 34.5ENVE killed BC cells of varying molecular subtypes, including those known to frequently metastasize to the brain. To test the therapeutic efficacy of a Vstat120 expressing oncolytic virus [OV] in vivo, we created three new, orthotropic models of BC brain metastasis in immune-competent FVB/NJ mice. Two of these models recapitulated the biology of human BC brain metastases [BCBM]. In both of these models, we found 34.5ENVE treatment significantly improved the survival of mice with established BCBM.
Materials and Methods
Cell Lines and Viruses
Vero, DB-7, Met-1, U251-T3, MCF7, MDA-MB-231, and MDA-MB-468 cells were maintained in DMEM supplemented with 10% fetal bovine serum [FBS]. SKBR3 cells were maintained in McCoy’s 5A Medium supplemented with 10% FBS. All cells were incubated at 37°C in an atmosphere with 5% carbon dioxide and maintained with 100 units of penicillin/mL, and 0.1 mg of streptomycin/mL. U251 cells were obtained from Dr. Erwin G. Van Meir [Emory University, Atlanta, Georgia] and authenticated by us through the University of Arizona Genetics Core in July 2013. U251-T3 cells were created in our lab [May 2009] as a tumorigenic clone of U251 cells by serially passaging these cells three times in mice [these cells have not been separately authenticated]. DB-7, Met-1 [murine BC], MCF7, MDA-MB-231, SKBR3, and MDA-MB-468 [human BC] cells were obtained in December 2012 from Dr. Michael C. Ostrowski [Ohio State University, Columbus, OH] and have not been authenticated since receipt (12, 13). Monkey kidney epithelial derived Vero cells were obtained in April 2005 from Dr. E Antonio Chiocca [Ohio State University, Columbus, Ohio]. These cells have not been authenticated since receipt. All cells are routinely monitored for changes in morphology and growth rate. All cells were negative for mycoplasma. RAMBO and 34.5ENVE viruses were prepared and titered as previously described (11).
Cell Viability Assays
Cells were plated in 96 well plates and infected simultaneously with 2% FBS in DMEM containing 34.5ENVE at the indicated Multiplicity of Infection [MOI]. Viability was assessed as described previously using a standard MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (14).
Virus replication assay
500,000 cells plated in 6 well plates were infected with 34.5 ENVE at an MOI of 0.005. 3 days later cells and supernatants were harvested and the viral titers were determined via a standard plaque forming unit assay.
Animal surgery
All animal experiments were performed in accordance with the Subcommittee on Research Animal Care of The Ohio State University guidelines and were approved by the institutional review board. 6–8-week-old, female FVB/N mice [The Jackson Laboratory, Bar Harbor, Maine], were used for in vivo tumor studies. Intracranial surgeries were performed as previously described with stereotactic implantation of 100,000 DB-7, Met-1, or Mvt1 cells (11). Tumors were treated with HBSS or 34.5ENVE virus at the location of tumor implantation. Animals were euthanized when they showed signs of morbidity.
Immunohistochemistry/immunofluorescence
Mouse BCBM tumors were fixed in zinc formalin [Anatech Ltd] and paraffin embedded. Tumors were sectioned at 5 μm and stained using the following antibodies: anti-MECA32 [TROMA-1], anti-F4/80 [Invitrogen; MF48000], Alexa Fluor 594 [Invitrogen]. Human tumor immunohistochemistry was performed using antigen retrieval at pH 6.0. Tumors were stained with anti-CD163 [Leica Microsystems Novocastra], anti-CD31 [Dako; M0823], and a HRP-linked secondary antibody. Specimens were visualized with DAB.
Image Acquisition
Immunofluorescent images were acquired using a Nikon Eclipse E800 epifluorescence microscope equipped with a Photometrics Coolsnap camera and Nikon Plan Fluor objectives. MetaVue software [Molecular Devices] was used for image acquisition. Immunohistochemical staining was imaged using a Nikon Eclipse 50i microscope equipped with an Axiocam HRC camera [Zeiss] and Nikon Eclipse Ci microscope equipped with a DS-Fi2 camera system.
MRI Analysis
Mice-bearing intracranial tumors were treated with HBBS or virus. Anatomic imaging was performed on the days indicated using a Gadolinium enhanced T1-weighted imaging sequence. For data analysis, a region-of-interest [ROI] that included the tumor was manually outlined. Tumor volumes were calculated from ROIs as previously described.(11)
Statistical Analysis
Student’s t-test was used to analyze changes in cell killing, viral plaque forming assays, and tumor volume measurements. A P < 0.05 was considered statistically significant. In survival assays, Kaplan–Meier curves were plotted and the log rank test was utilized to determine statistical significance. All statistical analyses were performed with the use of Graph Pad Prism software [version 5.01].
Results
BAI1 expression in reduced in breast cancer and is associated with patient survival
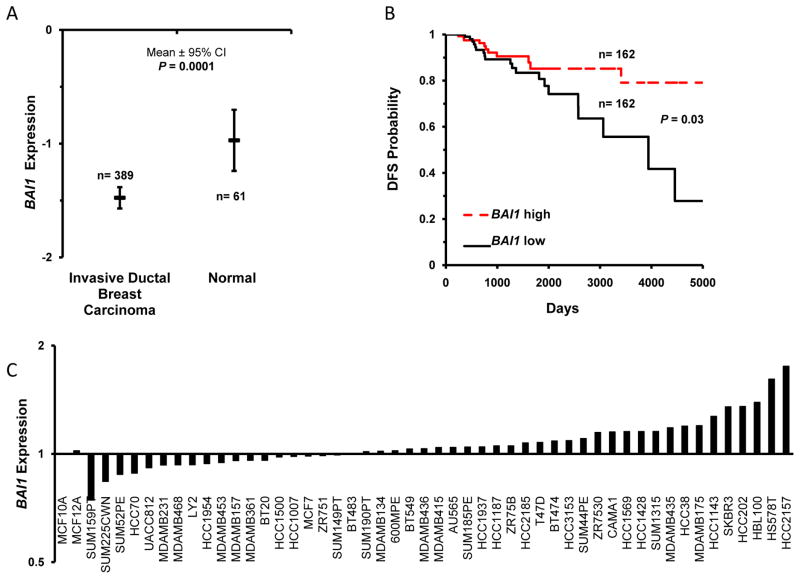
To determine the relevance of BAI1/Vstat120 in BC we analyzed patient derived gene expression data from The Cancer Genome Atlas [TCGA]. We observed a 52% reduction in BAI1 expression in invasive ductal breast carcinomas [n=389] compared to normal breast tissue [n=61; P<0.0001] [Figure 1A].(15) Further analysis revealed low BAI1 expression was also associated with decreased disease free survival [n=324; P<0.03] [Figure 1B]. An examination of BAI1 expression in 50 breast cancer cell lines from the Neve et al dataset showed BAI1 mRNA levels were reduced in 38% of breast cancer cell lines compared to the MCF-10A breast epithelial cell line [19 of 50 cell lines] [Figure 1C] (16). These results suggest the loss of BAI1 promotes BC tumorigenesis and the restoration of BAI1/Vstat120 may have therapeutic effects in BC.
Figure 1. Reduced BAI1 is associated with breast cancer patient survival.
A. Mean BAI1 expression in 61 normal mammary gland and 389 invasive ductal breast carcinoma samples in the TCGA data set [P = 0.000137896]. B. Kaplan Meier curves representing DFS in patients of the TCGA cohort with high [n = 162] or low [n = 162] BAI1 expression [P=0.03]. C. Analysis of BAI1 expression in the Neve et al. breast cancer cell line microarray database. Expression levels are normalized to the non-tumorigenic, epithelial cell line MCF-10A.
Nestin expression is up-regulated in breast cancer and is associated with metastases
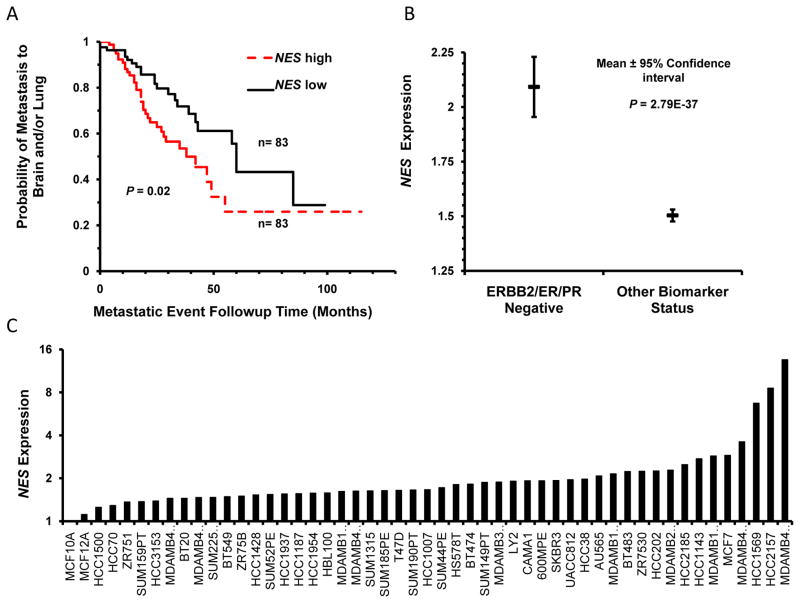
Nestin is up-regulated in several metastatic cancers, and its high expression correlates with reduced BC patient survival (7–9). Nestin is also expressed in cancer stem cells known to promote cancer resistance and progression (10). Analysis of a cohort of 166 patients stratified by median Nestin expression revealed a significant association between Nestin expression and incidence of brain and lung metastases [n=164; P<0.02] [Figure 2A] (17). Of the BC subtypes, we found high Nestin expression was most strongly associated with triple negative breast cancers [TNBC] which are highly prone to brain metastases [Figure 2B] (8). Additional analysis of the Neve et al microarray dataset showed Nestin was up-regulated in 100% of the BC cell lines examined [50 of 50] [Figure 2C] (16). These results suggest that Nestin may be a strong therapeutic target for aggressive and metastatic BCs.
Figure 2. Increased Nestin expression is associated breast cancer metastases.
A. The Bos et al cohort was stratified by median NES expression [83 NES high and 83 NES low patients] to examine the probability of brain and lung metastases in BC patients [P = 0.02]. B. NES expression correlates with TNBC status in the Curtis et al dataset. 1975 samples in the Curtis et al breast cancer patient cohort were stratified by TNBC status. There are 250 TN and 1725 patients with other biomarker status in this cohort. Mean NES expression is significantly higher [P = 2.79E-37] in TNBCs. C. Analysis of NES expression in the Neve et al. breast cancer cell line microarray database. Expression levels are normalized to the non-tumorigenic, epithelial cell line MCF-10A.
Oncolytic virus is cytotoxic in multiple human BC subtypes
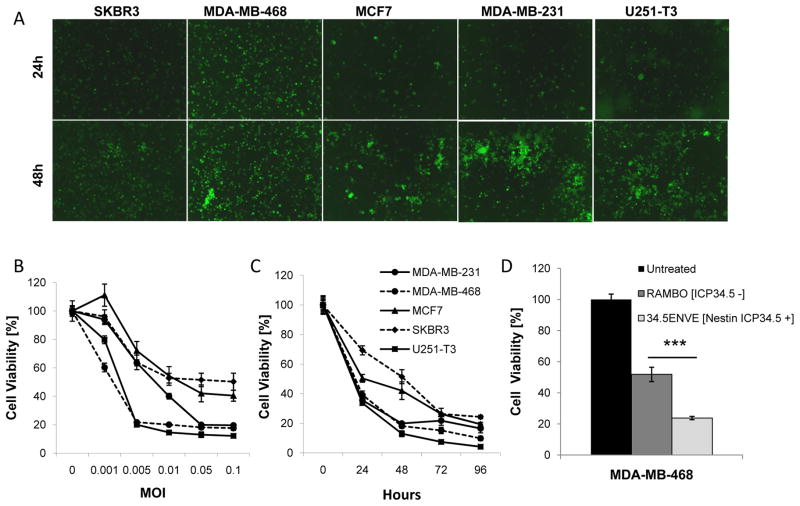
34.5ENVE expresses Vstat120 and its replication is driven by a Nestin promoter, thus we hypothesized 34.5ENVE might be therapeutically relevant for BC brain metastases. To test this hypothesis, we tested infection, replication, and cytotoxicity of 34.5ENVE in a variety of human BC cell subtypes [Table 1]. Over the course of 3 days, 34.5ENVE infected and replicated in human BC cells as determined by increasing virus encoded GFP expression [Figure 3A]. In vitro cytotoxicity of human BC cells to 34.5ENVE infection was dose dependent and increased with time [Figure 3B–C]. Four days after infection at an MOI of 0.05, we observed 83.4%, 75.7%, 80.6%, and 90.1% cell death in MDA-MB-231, SKBR3, MCF7, and MDA-MB-468 cells, respectively. Most BC treatments are targeted to particular subtypes, but 34.5ENVE killed BC cells across multiple subtypes. Importantly, we observed significant killing in the Her2+ and TNBC subtypes. TNBCs are notoriously resistant to conventional therapies, and Her2+ targeted antibody treatments poorly penetrate the blood-brain-barrier. As a result, 25–55% of these BC patients will develop brain metastases.(18) These results highlight the therapeutic potential of 34.5ENVE to treat BC brain metastases.
Table 1.
Description of BC molecular subtypes and Nestin expression in a panel of human BC cells.
| Cell Line | ER | PR | HER2 | Nestin |
|---|---|---|---|---|
| MDA-MB-231 | + | |||
| MDA-MB-468 | + | |||
| MCF7 | + | + | + | |
| SKBR3 | + | + |
ER, Estrogen Receptor; PR, Progesterone Receptor; HER2
Figure 3. Oncolytic HSV derived therapeutics target and kill multiple BC subtypes in vitro.
A. The ability of 34.5ENVE to infect and replicate in a panel of human BC cells was determined using 34.5ENVE directed GFP expression. The human glioma line, U251-T3, was used a positive control. Dose dependent [B.] and temporal [C.] viability of human BC cells treated with 34.5ENVE. Data shown are mean cell viability ± SD. D. Relative cytotoxicity of Nestin driven 34.5ENVE or control RAMBO virus at an MOI of 0.01 in MDA-MB-468 cells. Data shown are mean cell viability ± SD [P<0.001].
HSV-1 replication and cytotoxicity is enhanced by the viral neurovirulence gene ICP34.5 (11). To improve the safety and targeting of the 34.5ENVE virus, the expression of ICP34.5 is driven by a Nestin promoter. Nestin expression was increased in all 50 of the BC cell lines examined suggesting it is a relevant therapeutic target for BC [Figure 2C]. In order to determine the efficacy of Nestin driven ICP34.5 on tumor cell killing, we compared the cytotoxicity of 34.5ENVE with a similar virus lacking Nestin driven ICP34.5 expression [RAMBO] (11, 19). We observed 54.14% increased killing in the TNBC MDA-MB-468 cells in the Nestin-driven 34.5ENVE virus as compared to a virus without ICP34.5 [P<0.001] [Figure 3D]. These results further support the use of 34.5ENVE for the treatment of this disease. This is the first study to specifically use Nestin expression to target BC.
Syngeneic model of breast cancer brain metastases in an HSV-1 sensitive strain
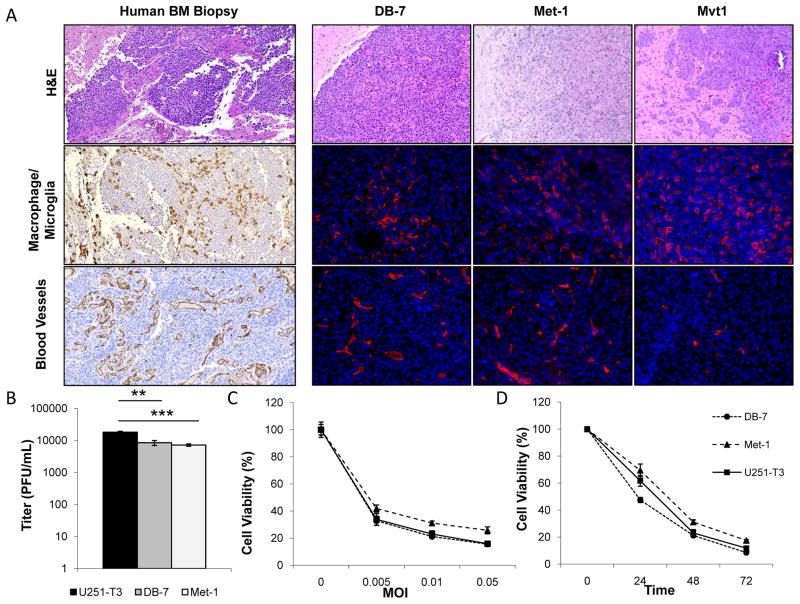
While there are several excellent models to study the biology of brain metastasis development in immune compromised mice, there are currently few immune competent models to test the safety and efficacy of its potential therapies.(1) Cody et al previously described an immune competent model of BCBM to test OVs, but the virus had limited anti-tumor efficacy in vitro and in vivo suggesting it was not an optimal model to evaluate oncolytic HSV derived therapeutics.(20) For these studies we characterized three novel, murine BC [DB-7, Met-1, and Mvt1] models. DB-7 and Met-1 cells are derived from transgenic FVB/N mice expressing polyoma virus middle T oncogene [PyVmT] under the control of a mammary epithelium promoter (12). PyVmT serves as a surrogate for activated receptor tyrosine kinase signaling pathways, such as Her2, commonly activated in BC (18). Mvt1 cells are derived from tumors of MMTV-c-myc/VEGF bitransgenic mice (21). In these studies, DB-7, Met-1, and Mvt1 BC cells were implanted intracranially into the brains of FVB/NJ mice. DB-7 and Met-1 tumor borders were generally demarcated from the normal brain parenchyma with localized invasion in the Met-1 tumors. Similar to patient specimens, these tumors contained significant tumoral vascularization as well as tumor associated microglia/macrophages [Figure 4A]. Mvt1 tumors were highly invasive and infiltrated into distant brain structures including the ventricles and meninges. This phenotype was characteristic of rare leptomingeal metastases and did not resemble the parenchymal brain metastases commonly observed in patients (22). Histological comparisons of patient BC brain metastases with all three murine tumors indicated DB-7 and Met-1 derived tumors most closely recapitulated the human CNS metastases and so these BCBM models were selected for further analysis [Figure 4A] (23).
Figure 4. Characterization of three murine models of BCBM for preclinical evaluation of oncolytic HSV-1 derived therapeutics.
A. Representative panel of DB-7, Met-1, Mvt1, and human breast cancer brain metastases tumors. Human biopsy sample was stained for H&E, macrophages [CD163], endothelial cells [CD31] [100x magnification]. Murine specimens were stained for H&E, macrophages [F4/80], and endothelial cells [MECA-32] [20X Magnification]. B. 72 hour viral titers of DB-7, Met-1, and U251-T3 cells were infected with 34.5ENVE [0.005 MOI]. Data shown are mean viral titers ± SD [U251-T3 to DB7 P<0.01; U251-T3 to Met-1 P<0.001]. C. 48 hour cell viability of BC cells treated with 34.5ENVE at the indicated MOIs. Data shown are mean cell viability ± SD. D. Temporal response of murine BC cells treated with 34.5ENVE at 0.01 MOI for 3 days. Data shown are mean cell viability ± SD.
34.5ENVE is cytotoxic to murine breast cancer cells in vitro
Human tropic viruses often replicate poorly in murine cells, so there are very few immune competent models of cancer to study OVs derived from HSV-1. To determine if we could evaluate the therapeutic effects of 34.5ENVE in this murine BCBM model, we examined the ability of the virus to infect and replicate in the DB-7 and Met-1 tumor derived cell lines. For these assays human glioma cells were used as a positive control. We observed an increase in virus encoded GFP expression over 48 hours following infection at a low MOI consistent with virus replication and spread in these cells [Supplemental Figure S1A]. Quantification of virus replication revealed that DB-7 and Met-1 murine cells supported replication at a similar rate compared to human glioma cells [Figure 4B]. 34.5ENVE killed tumor cells in a dose and time dependent manner at levels comparable to human glioma cells [Figure 4C–D]. Three days following infection at an MOI of 0.01, we observed 91.5%, 82.5%, and 88.2% cell death in DB-7, Met-1, and human glioma cells, respectively. We also verified these cells secreted virally expressed Vstat120 and demonstrated improved cytotoxicity of the ICP34.5 expressing 34.5ENVE virus [Supplemental Figure S1B–C]. The characterization of this HSV-1 sensitive murine model will aid in the future evaluation of preclinical toxicity and efficacy of novel, HSV-1 derived therapeutics.
34.5ENVE treatment extends survival in vivo
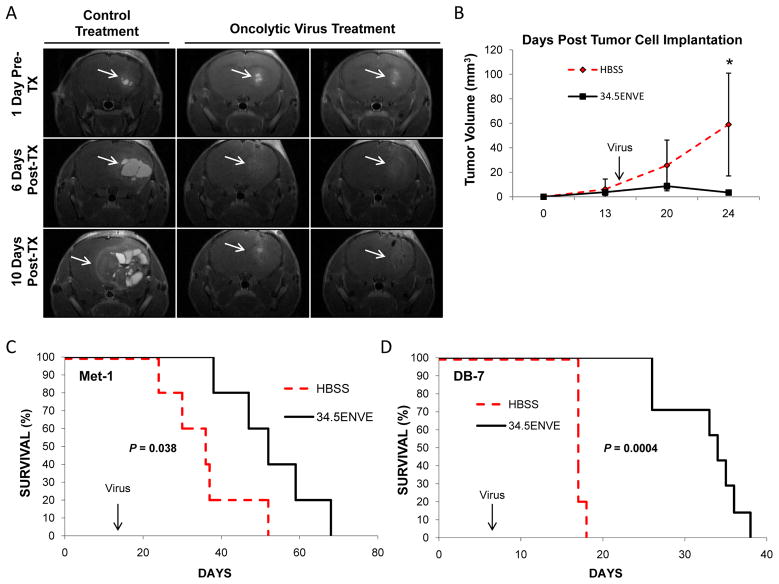
We utilized MRI to non-invasively evaluate the antitumor response of 34.5ENVE in mice with established Met-1 brain tumors. Mice were treated intratumorally with a single dose of HBSS or 34.5ENVE [n= 6/group] 14 days post-tumor cell implantation [average initial tumor volume 4.94 mm3]. Figure 5A shows representative coronal T1-weighted MRI images from mice treated with PBS or 34.5ENVE 1 day pretreatment and on days 6 and 10 post-treatment. The tumor volumes in mice treated with HBBS grew rapidly, and obtained an average tumor volume of 59.01 mm3 within 10 days of treatment [Figure 5B, Table 2]. Significantly, 34.5ENVE treated tumors showed substantial decreases in tumor volume [3.43 mm3 average tumor volume 10 days post viral therapy; P<0.02]. Interestingly, we observed initial pseudoprogression of tumors [by volume] in 34.5ENVE treated mice prior to tumor regression possibly due to tumor destruction and immune cell infiltration. Following these mice over time, we observed 34.5ENVE treatment significantly enhanced the survival of mice bearing Met-1 BC brain tumors. Control treated mice had a median survival of only 36 days, whereas mice receiving 34.5ENVE therapy survived significantly longer [median survival 52 days; P<0.038] [Figure 5C]. We next tested the antitumor effects of 34.5ENVE in the DB-7 BCBM model. Mice treated with 34.5ENVE showed a 100% increase in median survival compared to control mice with DB-7 tumors. HBSS [n= 5] and 34.5ENVE [n=7] treated mice showed median survival times of 17 and 34 days, respectively [Figure 5D] [P<0.0004].
Figure 5. Anti-tumor efficacy of 34.5ENVE in mice bearing established BCBM.
A. Representative T1-weighted MRI images of coronal sections of mice with Met-1 BM tumors treated with HBSS or 34.5ENVE. Scans were performed on the days indicated after treatment [TX] [Day 14 post tumor cell implantation]. White arrows indicate tumor location. B. Mean tumor volumes [mm3] ± SD of Met-1 BM tumors treated with HBSS or 34.5ENVE treated mice [n=6 mice/group]. C. Kaplan-Meir survival curve for A and B, mice treated on day 14 with HBSS or 2.0 × 105 pfu 34.5ENVE [n=6 mice/group, P=0.038]. D. Kaplan-Meir survival curve of DB-7 BM tumors treated on day 7 with HBSS or 2.5 × 105 pfu 34.5ENVE [n=5 HBSS; n=7 34.5ENVE, P=0.0004].
Table 2.
34.5ENVE treatment reduces tumor volumes in Met-1 BCBM tumors by MRI.
| Days Post Implantation | HBSS (mm3) | 34.5ENVE (mm3) | p-Value |
|---|---|---|---|
| 13 | 6.16 | 3.72 | 0.51 |
| 20 | 25.60 | 8.66 | 0.14 |
| 24 | 59.01 | 3.43 | 0.02 |
Data shown are mean tumor volumes [mm3] of Met-1 BM tumors treated with HBSS or 34.5ENVE from Figures 5A–C [n=6 mice/group].
Discussion
BC brain metastases continue to present a significant therapeutic challenge. A recent BC BM clinical trial with lapatinib and capecitabine noted that nearly a third of patients experienced at least one severe adverse event due to toxicity (24). Conversely, OV therapies which are currently in clinical trials for a variety of solid tumor malignancies including BC and brain tumors [NCT01656538, NCT02031965, NCT01174537, NCT00794131] have proven to be safe and well tolerated. In this study, we identified BAI1/Vstat120 and Nestin as novel therapeutic targets for BCBMs. We demonstrated an OV, 34.5ENVE, expressing anti-angiogenic Vstat120 and ICP34.5 under a Nestin promoter had significant cytotoxic effects in BC cells of varying molecular subtypes, including Her2+ and TNBC. Significantly, we also described two novel, immune competent murine models of BCBMs which closely recapitulates the human disease. Lastly, we demonstrated that a single, intratumoral dose of 34.5ENVE virus significantly enhanced the survival of mice with established metastatic BC brain tumors. The results of these studies warrant further investigation of BAI1 and Nestin dual targeted therapies to treat established BC brain metastases.
Supplementary Material
Acknowledgments
This work was supported in part by: NIH grants R01NS064607, R01CA150153, P30NS045758, and P01CA163205 to B. Kaur; NIH grant P01CA097189 to M.C. Ostrowski; NIH grant R01NS081125 to N.L. Lehman; NIH grant P30CA016058 to K. Powell; Pelotonia Fellowships to S. Dubin and A.C. Jaime-Ramirez; and with generous support from the OSUCCC Departments of Neurosurgery and Radiation Oncology to B. Kaur.
Footnotes
The authors declare they have no conflicts of interest
References
- 1.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. The American journal of pathology. 2005;167:913–20. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fidler IJ, Balasubramanian K, Lin Q, Kim SW, Kim SJ. The brain microenvironment and cancer metastasis. Mol Cells. 2010;30:93–8. doi: 10.1007/s10059-010-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Angulo AM, Hortobagyi GN. Brain metastases and breast cancer subtypes. Onkologie. 2010;33:143–4. doi: 10.1159/000296307. [DOI] [PubMed] [Google Scholar]
- 4.Cork SM, Van Meir EG. Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J Mol Med (Berl) 2011;89:743–52. doi: 10.1007/s00109-011-0759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur B, Cork SM, Sandberg EM, Devi NS, Zhang Z, Klenotic PA, et al. Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer research. 2009;69:1212–20. doi: 10.1158/0008-5472.CAN-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kudo S, Konda R, Obara W, Kudo D, Tani K, Nakamura Y, et al. Inhibition of tumor growth through suppression of angiogenesis by brain-specific angiogenesis inhibitor 1 gene transfer in murine renal cell carcinoma. Oncology reports. 2007;18:785–91. [PubMed] [Google Scholar]
- 7.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, et al. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer research. 2007;67:9199–206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Chen B, Zhu J, Zhang R, Yao F, Jin F, et al. Clinical implications for nestin protein expression in breast cancer. Cancer science. 2010;101:815–9. doi: 10.1111/j.1349-7006.2009.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolou P, Toloudi M, Chatziioannou M, Ioannou E, Papasotiriou I. Cancer stem cells stemness transcription factors expression correlates with breast cancer disease stage. Current stem cell research & therapy. 2012;7:415–9. doi: 10.2174/157488812804484639. [DOI] [PubMed] [Google Scholar]
- 10.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 11.Yoo JY, Haseley A, Bratasz A, Chiocca EA, Zhang J, Powell K, et al. Antitumor efficacy of 34.5ENVE: a transcriptionally retargeted and “Vstat120”-expressing oncolytic virus. Molecular therapy : the journal of the American Society of Gene Therapy. 2012;20:287–97. doi: 10.1038/mt.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borowsky AD, Namba R, Young LJ, Hunter KW, Hodgson JG, Tepper CG, et al. Syngeneic mouse mammary carcinoma cell lines: two closely related cell lines with divergent metastatic behavior. Clinical & experimental metastasis. 2005;22:47–59. doi: 10.1007/s10585-005-2908-5. [DOI] [PubMed] [Google Scholar]
- 13.Zabuawala T, Taffany DA, Sharma SM, Merchant A, Adair B, Srinivasan R, et al. An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer research. 2010;70:1323–33. doi: 10.1158/0008-5472.CAN-09-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojton J, Chu Z, Mathsyaraja H, Meisen WH, Denton N, Kwon CH, et al. Systemic delivery of SapC-DOPS has antiangiogenic and antitumor effects against glioblastoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21:1517–25. doi: 10.1038/mt.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6404–18. doi: 10.1158/1078-0432.CCR-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardcastle J, Kurozumi K, Dmitrieva N, Sayers MP, Ahmad S, Waterman P, et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18:285–94. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cody JJ, Scaturro P, Cantor AB, Yancey Gillespie G, Parker JN, Markert JM. Preclinical evaluation of oncolytic deltagamma(1)34.5 herpes simplex virus expressing interleukin-12 for therapy of breast cancer brain metastases. International journal of breast cancer. 2012;2012:628697. doi: 10.1155/2012/628697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei XF, Noble MS, Davoli MA, Rosfjord E, Tilli MT, Furth PA, et al. Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In vitro cellular & developmental biology Animal. 2004;40:14–21. doi: 10.1290/1543-706X(2004)40<14:ECOPMT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Scott BJ, Kesari S. Leptomeningeal metastases in breast cancer. American journal of cancer research. 2013;3:117–26. [PMC free article] [PubMed] [Google Scholar]
- 23.Pekmezci M, Perry A. Neuropathology of brain metastases. Surgical neurology international. 2013;4:S245–55. doi: 10.4103/2152-7806.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachelot T, Romieu G, Campone M, Dieras V, Cropet C, Dalenc F, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. The lancet oncology. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.