Abstract
Introduction
Human injury or infection induces systemic inflammation with characteristic neuro-endocrine responses. Fluctuations in autonomic function during inflammation are reflected by beat-to-beat variation in heart rate, termed heart rate variability (HRV). In the present study, we determine threshold doses of endotoxin needed to induce observable changes in markers of systemic inflammation, we investigate whether metrics of HRV exhibit a differing threshold dose from other inflammatory markers, and we investigate the size of data sets required for meaningful use of multi-scale entropy (MSE) analysis of HRV.
Methods
Healthy human volunteers (n=25) were randomized to receive placebo (normal saline) or endotoxin/lipopolysaccharide (LPS): 0.1, 0.25, 0.5, 1.0, or 2.0 ng/kg administered intravenously. Vital signs were recorded every 30 minutes for 6 hours and then at 9, 12, and 24 hours after LPS. Blood samples were drawn at specific time points for cytokine measurements. HRV analysis was performed using EKG epochs of 5 minutes. MSE for HRV was calculated for all dose groups to scale factor 40.
Results
The lowest significant threshold dose was noted in core temperature at 0.25ng/kg. Endogenous TNF-α and IL-6 were significantly responsive at the next dosage level (0.5ng/kg) along with elevations in circulating leukocytes and heart rate. Responses were exaggerated at higher doses (1 and 2 ng/kg). Time domain and frequency domain HRV metrics similarly suggested a threshold dose, differing from placebo at 1.0 and 2.0 ng/kg, below which no clear pattern in response was evident. By applying repeated-measures ANOVA across scale factors, a significant decrease in MSE was seen at 1.0 and 2.0 ng/kg by 2 hours post exposure to LPS. While not statistically significant below 1.0 ng/kg, MSE unexpectedly decreased across all groups in an orderly dose-response pattern not seen in the other outcomes.
Conclusions
By usingrANOVA across scale factors, MSE can detect autonomic change after LPS challenge in a group of 25 subjects using EKG epochs of only 5 minutes and entropy analysis to scale factor of only 40, potentially facilitating MSE’s wider use as a research tool or bedside monitor. Traditional markers of inflammation generally exhibit threshold dose behavior. In contrast, MSE’s apparent continuous dose-response pattern, while not statistically verifiable in this study, suggests a potential subclinical harbinger of infectious or other insult. The possible derangement of autonomic complexity prior to or independent of the cytokine surge cannot be ruled out. Future investigation should focus on confirmation of overt inflammation following observed decreases in MSE in a clinical setting.
Keywords: Heart rate variability, lipopolysaccharide, multi-scale entropy, systemic inflammatory response syndrome, cytokines, human
Introduction
Human injury or infection induces a systemic inflammatory phenotype that is mechanistically linked both with the activation of innate immunity and with characteristic neuro-endocrine responses. The specific agents causing changes in readily measurable clinical parameters of systemic inflammation, such as vital signs and circulating analytes, may not always be identifiable. However, acute sterile or infectious insult does result in several common immune cell and systemic tissue function themes (1, 2). Among these responses, altered autonomicsignals– both sympathetic and parasympathetic - are frequently seen to coincide with the phenotypic manifestations of inflammation (3, 4). Although administration of an immune activating ligand such as lipopolysaccharide (LPS) [TLR4 ligand] induces both inflammatory mediator production and consequent systemic manifestations of inflammation in a dose-response manner (5), the precise dose response relationship between autonomic signaling and systemic inflammatory responses has not yet been established.
The controlled administration of endotoxin derived from E.Coli and Salmonella Typhi species has been used to model the physiologic effects of acute inflammation in humans at doses that achieve robust systemic responses (6). Yet there have to date been only limited investigations of systemic and organ-specific responses to very low levels of such ligand challenges. These studies of the effects seen with lower doses of E. coli endotoxin (0.06-0.20 ng/kg) have included investigations of various interventions on low-grade systemic inflammation (5, 7, 8), aspects of neuropsychological function, cellular ATP levels and levels of protein expression in leukocytes, and sleep (9).
Mammalian heart rate varies virtually continuously. Even during periods where the human heart rate appears grossly unchanged, measuring the intervals between, e.g., each successive R-wave on an electrocardiogram (EKG) will show that this “R-R interval” almost always changes by at least a few milliseconds (ms). Furthermore, since heart rate is really a frequency, and frequency = (the number of events ÷ time), an instantaneous HR can be calculated from any 2 successive QRS complexes. Therefore, because length of time between successive heartbeats changes from one beat to the next, an apparently steady HR of 60 will be seen to vary, e.g. 60.11, 59.78, 59.92, 60.44, etc. The pattern of how it varies isa characteristic distinct from the HR itself -- an HR of 120 and an HR of 40 could have the same variability if in both cases the intervals between successive beats were seen to grow and shrink in an identical pattern. The various patterns of this instantaneous, beat-to-beat variation are termed heart rate variability (HRV).
The relationship between autonomic function and HRV has been studied for well over a decade (10, 11). Briefly, the Vagus nerve (CN X) synapses on many organ systems, including both the intrinsic cardiac pacemaker and the reticulo-endothelial system residing largely in the spleen and responsible for much of the acute inflammatory response. Cytokines released as a part of this response affect both sympathetic and parasympathetic function, creating a complex layering of effects on cardiac activity (4, 12). Yet because of the relatively direct effects of CN X on both the cardiac pacemaker and the reticulo-endothelial system, instantaneous variation in heart rate serves as an indirect assay for changes in overall autonomic function (11, 13). Higher variability appears to be generally beneficial. As described below, studies in many settings show that systemic inflammation, acute injury, and weakened health states are associated with lower HRV as measured by several distinct methods.
Many exposures and outcomes are associated with alterations in straightforward statistical aspects of HRV known as time domain measures. These include the percentage of times where the interval between two beats is more than 50ms longer or shorter than the previous interval (PNN50), the standard deviation of the intervals (SDNN) (14), and the value obtained by taking the difference between each interval and the one before it and squaring that number (to remove the negative value that will be present in half, since the values being subtracted from each other rise and fall), taking the average of all these, and then taking the square root of that average (RMSSD) (10).
Moreover, the variation itself varies in certain patterns. One such “oscillation” is the familiar acceleration and deceleration of pulse with normal breathing, termed the respiratory sinus arrhythmia. In recent years, a growing body of work has discovered other differences between HRV amongst different subjects or settings, and in response to certain exposures. A plot of the full spectrum of the frequencies reflected in HRV can be constructed to reflect the proportion of all the heartbeat intervals whose length corresponds to that frequency. The HRV measures termed frequency domain include measures of the density of HRV oscillations falling within certain narrow ranges of frequency along this spectrum, called bands, as well as the ratio between activity levels in different bands. Frequency domain measures have been shown to vary in settings where time domain measures do not, demonstrating an additional layer of HRV complexity. They are believed to reflect aspects of autonomic functioning such as the ratio of sympathetic to parasympathetic signaling (10).
An emerging area of study uses a comparatively novel mathematical tool to detect otherwise hidden patterns in HRV. Belonging to the same mathematical family as the better known field of chaos, this tool analyzes what is termed the multi-scale entropy (MSE) of HRV, and the term MSE is often used loosely to refer both to the mathematical process and to the underlying pattern-attribute the process reveals (15). Mathematicians term this attribute “complexity,” which in this setting is a term of art. Its difference from the colloquial meaning is discussed more fully in the online supplemental materials. (see Supplement) Broadly speaking, this complexity is at odds both with randomness and with regularity.
MSE of HRV is important because it often exhibits a marked decrease where conventional HRV measures do not. For example, it has been shown that MSE varies with states of health such as age, sleep, progression of gestation, and CHF (15); that lower MSE is a predictor for worse outcomes in the injured and critically ill (16, 17); and that MSE decreases following exposure to inflammatory stressors (15, 17). This evidence suggests that MSE’s measurement of complexity provides a window onto a previously unobservable autonomic attribute which is both beneficial and vulnerable to insult.
Heretofore, most studies of human MSE have used large sets of data, with periods (epochs) of EKG recording several hours long, analyzed to scale factors (see Materials and Methods, below) as high as 200 (16, 17). With current software, computing MSE from such large data sets requires lengthy computing times for current office or laboratory computers or for potential bedside monitors. Advances in software and hardware will of course eventually shorten these computing times, but more recent work has suggested that MSE might be successfully employed with shorter epochs or lower ranges of scale factors that could dramatically reduce the size of the data sets involved (18, 19).
We therefore hypothesized that in human subjects given an LPS challenge, MSE of HRV would detect inflammatory stress using EKG epochs of 5 minutes analyzed to scale factor 40. We also designed this study to investigate the threshold doses of E.Coli endotoxin needed to induce observable changes in common markers of systemic and tissue activation, as well as to investigate whether commonly applied metrics of HRV exhibit a differing threshold of endotoxin dose-responsiveness from other markers of inflammation. In order to maximize the chance of identifying the lowest threshold dose of LPS, we included the very low doses of 0.1 and 0.25 ng/kg, which are an order of magnitude lower than conventionally used experimental doses. The study was powered to detect significant alterations in the listed laboratory and clinical markers and HRV parameters, including MSE analysis using short epochs and low scale factors, with six equal sized groups of 4-5 subjects, corresponding to placebo and the dose groups described.
Materials and Methods
Adult subjects (n=25; 8=female, 17=male; age range= 19 to 31) were screened for normal health as outpatients prior to admission to the Center for Translational and Clinical Research at UMDNJ-Robert Wood Johnson Medical School (UMDNJ-RWJMS), now Rutgers-RWJMS. All subjects received written, informed consent under guidelines established by the Institutional Review Board of UMDNJ-RWJMS. Upon admission the day prior to the study, in-patient examination confirmed that no changes in health status had occurred since initial recruitment. Female subjects also underwent a urine test to exclude pregnancy. Subjects consumed oral diet upon admission and then were placed NPO from midnight of the admission day and were administered intravenous fluids (5% dextrose and 0.45% sodium chloride; 1 ml/kg/hr) via a peripheral venous catheter.
As previously described (15, 17), a radial arterial catheter was placed the morning of the study day (07:00) and utilized for continuous monitoring of heart rate and blood pressure as well as periodic blood sampling for up to six hours after LPS challenge. A rectal thermometer was placed for continuous monitoring of core body temperature over the same time frame. All subjects were randomized to receive placebo (normal saline) or a one-time dose of Clinical Center Reference Endotoxin, NIH Bethesda, USA (CC-RE, Lot #2) at a dose of 0.1, 0.25, 0.5, 1.0, or 2.0 ng/kg administered over a one minute-period via peripheral intravenous catheter at 09:00 on study day 1(considered time point 0).
Clinical monitoring
Vital signs, including heart rate and mean arterial blood pressure (MAP), were recorded every 30 minutes from the arterial monitoring system for the first 6 hours (09:00-15:00 hours) and then taken manually at 9, 12, and 24 hours after LPS administration. Core temperatures were recorded every 30 minutes through +6 hours via rectal thermometer, then orally at 9, 12, and 24 hours after LPS administration. At 6 hours after LPS bolus, the arterial catheter and rectal thermometer were removed. The peripheral intravenous catheter infusing saline solution was removed once each subject tolerated a regular diet. Subjects remained in the study unit overnight and were discharged to home the following morning after the 24 hour post-LPS study determinations were obtained.
Assessment of heart rate variability
A base-line determination of HRV was obtained at the time of admission as well as hourly from 0 to +6 hours following endotoxin challenge and at +9 and +24 hours after LPS. Each recording interval consisted of two consecutive 5-minute periods, conventionally termed epochs. During such determinations, heart rate was monitored using a continuous EKG technique with three standard limb leads and CardioPro® 2.0 software with one Infiniti and one Procomp Plus® recorder (Thought Technology, Ltd., Montreal, P.Q., Canada). HRV parameters as well as interbeat intervals were collected using EKG data at a rate of 256 samples/second as previously described (20). In a continuous EKG record, each QRS complex was detected and the “normal-to-normal” (NN) intervals (all intervals between adjacent QRS complexes resulting from sinus node depolarization) were tabulated, thus providing a record of instantaneous heart rate. For each epoch, noise artifact and irregular heartbeats were manually edited by visual inspection and interpolation prior to calculation of interbeat intervals using CardioPro software. We analyzed each epoch and excluded complete measurement epochs where events such as extra systolic heartbeats, skipped beats, and other arrhythmias comprised greater than 10% of the total epoch. The power spectral density then was calculated using a Fast Fourier transformation algorithm. All signals were exported in standard ASCII format to Excel and EAS 9.0 for analysis and graphics.
Parameters of HRV were analyzed for both time domain and frequency domain measures. Time domain measures included 1) the standard deviation of the average length of interval between each successive heartbeat over a 5 minute period (SDNN), a measure of total heart rate variability, and 2) the percentage of interval differences of successive intervals between heartbeats greater than 50 ms (pNN50). Frequency domain measures included 1) high frequency variability (HF) [0.15-0.4Hz] that correlates with parasympathetic and vagal tone, and 2) the low frequency/ high frequency ratio (LF/HF) that is hypothesized to be associated with sympathetic:/parasympathetic balance (10). For each subject, HRV results for a given parameter were averaged across each epoch to provide a single value.
Analysis of entropy
An adequate discussion of the mathematical fundamentals of MSE is beyond the scope of this paper. Therefore, in addition to referring the reader to the excellent explanations by Costa, et al. (16), an introduction for physicians and other biomedical researchers is included as online supplemental material (see Supplement). To summarize the MSE process briefly, cardiac activity is recorded continuously by EKG for a specified period of time called an epochas with other HRV parameters. An initial plot is then constructed containing the beat numbers on the x-axis while the y-axis contains the time interval between that beat and the previous one. For any two consecutive plot points, there will be several other instances on that plot where a different consecutive pair have similar values, in the same order. Extending each of these matching pairs into a 3-point sequence will produce some 3-point matches as well. Compared to the total number of 2-point matches in a series, the proportion of times where a 2-point match is actually the beginning of a 3-point match is the basis of the sampled entropy of that series.
The whole series is then made what mathematicians term “coarser” (and also shorter) by a process known as scale factoring. At each scale factor step, increasingly large subsets of consecutive points in the original series are averaged to create a new set of plot points. This new series is then analyzed for sampled entropy, and the ratio of 3-point matches to 2-point matches described above may be quite different. The scale factor is the number of original points that get averaged together in each subset, for that step. MSE is plotted with scale factor on the x-axis and sampled entropy for the modified series on the y-axis. Time is not plotted directly in MSE; an MSE plot will be for all the data from a given epoch, regardless of how long the epoch lasted or when it began. Assessing change in MSE “over time” requires separate analyses of epochs taken at different times after, e.g., exposure to LPS.
We performed entropy analysis using this multi-scale approach described by Costa et al., shown as sample entropy over increasing scale factors (15). MSE was analyzed for each 5-minute epoch for scale factors 1 through 40 using methods and software previously reported by our group (21). The trends of MSE change from baseline at 1- and 2- hours post exposure, for all doses and for placebo, were compared by repeated-measures analysis of variance with the dependent variable being scale factor from 1 through 40.
Analysis of blood samples
Blood samples were collected at time points -24, 0, 0.5, 1, 1.5, 2, 3, 4, 6, and 24 hours in relation to endotoxin administration. Standard laboratory assessment for complete blood count, including a WBC count, was performed at each time period. Blood-derived plasma was then analyzed by ELISA for measurement of the soluble inflammatory markers TNFa and IL-6. The peak value of these cytokines was determined for each individual.
Statistical analysis
Analysis of HRV parameters
HRV was measured and compared across dose groups by two-way analysis of variance with repeated measures on scale factor for the MSE analysis and on time for the other analyses using Statistica version 6.1 (StatSoft, Inc., Tulsa, OK). P-values less than 0.05 were considered to be statistically significant. The Pearson product-moment correlation coefficient was calculated to measure the association between baseline parameters of HRV and maximum recorded plasma TNFa and IL-6 levels.
Results
Vital Signs
Temperature
Neither the placebo group nor the group receiving endotoxin at 0.1 ng/kg showed a significant change in temperature after exposure. In contrast, the groups receiving doses of 0.25 ng/kg and 0.5 ng/kg showed a significant rise above baseline, of 0.7±0.4 (p<0.02) and 0.8±0.3 °C (p<0.02) respectively, by 3 hours after exposure. Subjects receiving endotoxin doses of 1ng/kg and 2 ng/kg, however, demonstrated rises in temperature that differed significantly from placebo nearly twice as quickly -- as early as 1.5 hours after exposure. Moreover, the 1 and 2 ng/kg groups’ temperature rises, 1.6±0.1 (p<0.02) and 1.6±0.2 °C (p<0.02) respectively, were about twice as high as the rises for the 0.25 and 0.5ng/kg groups. Only at the 1 and 2 ng/kg doses did temperature response become “clinically significant” by meeting criteria for the systemic inflammatory response syndrome (SIRS) >38°C. (Figure 1A)
Figure 1.
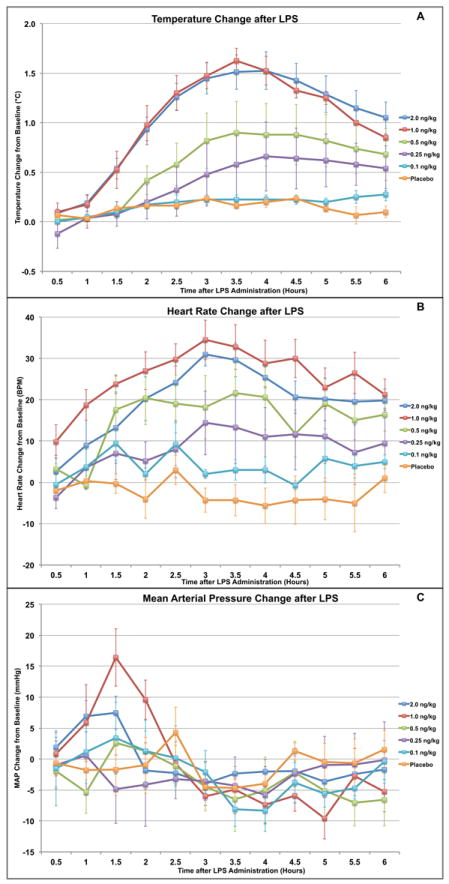
Observed changes in vital signs after endotoxin administration in human volunteers. A dose-dependent relationship is observed with numerically significant differences (p<0.05) from placebo with larger doses endotoxin for (A) Temperature (>0.25 ng/kg) and (B) Heart Rate (>0.5 ng/kg) response. Only at the 1 and 2 ng/kg doses did changes occur in temperature and heart rate which would consistently meet SIRS criteria. For Mean Arterial Pressure (C), no consistent or significant dose-response pattern was discernable after endotoxin.
Heart Rate
By two hours post exposure, groups receiving endotoxin doses of 0.5 ng/kg and higher showed a rise in heart rate significantly above both baseline and the placebo group. This rise eventually peaked at 42±1 (p<0.05) and 35±5bpm (p<0.05) above baseline for the 1 and 2 ng/kg groups respectively, with the 0.5 ng/kg group showing a lower peak of 23±7bpm (p<0.02) above baseline and returning to baseline sooner. Again only at the 1 and 2 ng/kg doses did changes occur which would consistently meet SIRS criteria. In contrast, the changes for groups receiving doses of 0.25 and 0.1 ng/kg showed essentially the same pattern as the placebo group throughout the course of the study, with a small variation at 3-4 hours post exposure. (Figure 1B)
Mean Arterial Pressure
In these young, healthy subjects there was no significant numerical or clinical difference in the pattern of change in MAP between any of the dose groups and the placebo group. (Figure 1C)
White Blood Cell Count
Groups receiving doses of 0.5 ng/kg and higher showed a significant dose-dependent rise in WBC count by 6 hours post exposure ranging from an increase of 4±0.7 ×109 cells/L (p<0.05) for the 0.5 ng/kg group through an increase of 6±0.8 ×109 cells/L (p<0.02) for the 2.0 ng/kg group (data not shown). Similar to vital sign changes, only at the 1 and 2 ng/kg doses did changes occur which would meet SIRS criteria. In contrast, the placebo group and the 0.1 and 0.25 ng/kg groups showed no significant change in WBC count (data not shown).
Pro-Inflammatory Cytokines
Interleukin-6
Groups receiving endotoxin doses of 0.1 and 0.25 ng/kg exhibited no significant rise in serum levels of IL-6. In contrast, the 0.5ng/kg group showed an average peak rise of 82pg/ml (p<0.02) and the 1 and 2 ng/kg groups each showed average peak rises of nearly 300 pg/ml (p<0.02). (Figure 2A)
Figure 2.
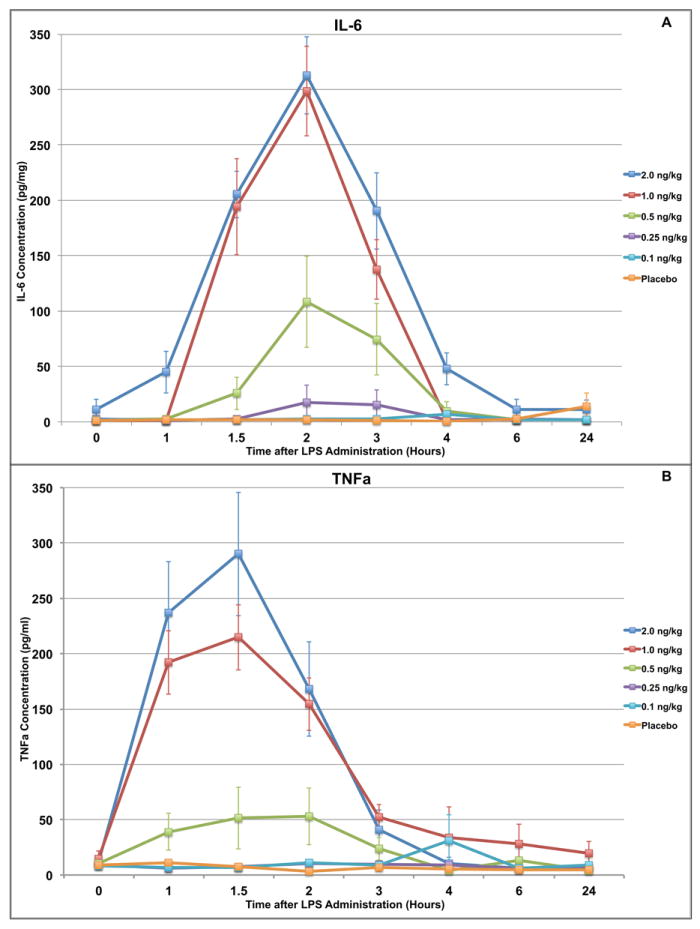
In vivo changes in cytokine levels after endotoxin administration in human volunteers. (A) A predictable dose-dependent relationship is identified for serum IL-6 levels. Lower doses (0.1 and 0.25 ng/kg) do not generate a response while the response to 0.5ng/kg is significant (p<0.05) compared to placebo but mild in degree. Higher doses (≥1 ng/kg) generate a more robust response peaking at 2 hours post-exposure. (B) Similarly, a predictable dose-dependent relationship is identified for serum TNFa levels. There is no response to lower doses, a mild but significant response to the 0.5ng/kg dose, and a highly amplified response to the larger doses (≥1 ng/kg).
Tumor Necrosis Factor Alpha
Groups receiving endotoxin doses of 0.5, 1 and 2 ng/kg showed a significant rise in TNFa, peaking at 53±18pg/ml, and 215±29pg/ml, and 290±56 pg/ml above baseline (p<0.01) respectively, by 1.5 hours post administration. The placebo group and the groups receiving endotoxin doses of 0.25 ng/kg or less showed no significant change in TNFa from baseline across the course of the study. (Figure 2B)
Heart Rate Variability
SDNN
Only the groups receiving an endotoxin dose of 2 ng/kg showed a significantly decreased SDNN compared to the placebo group by rANOVA over the total time period from 1 to 6 hours after exposure (p<0.02). The 1 ng/kg group had a larger maximum decrease (69 vs. 55 at 3 hours post exposure), but because of high variability in the data the rANOVA compared to placebo was not statistically significant. Neither the 0.1 ng/kg group nor the 0.5 ng/kg group showed changes differing significantly from the placebo group. (Figure 3A)
Figure 3.
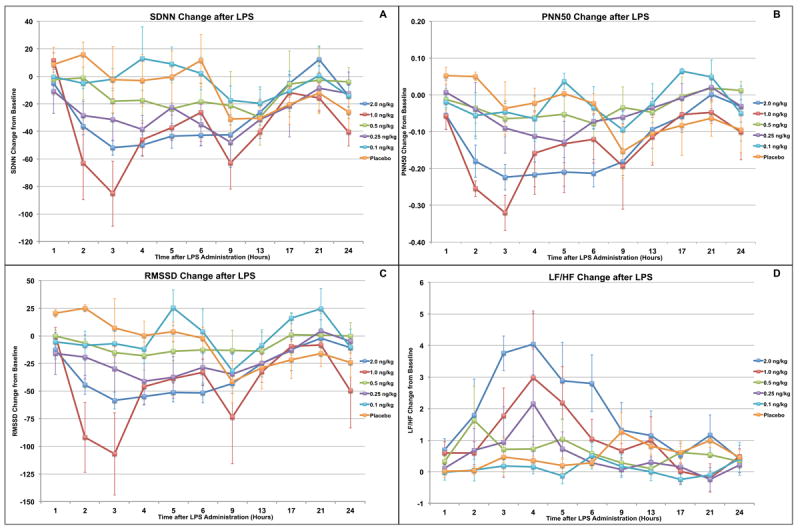
In vivo changes to traditional heart rate variability (HRV) measurements after endotoxin administration in human volunteers. (A) Only subjects receiving the highest endotoxin dose (2 ng/kg) had a significantly decreased SDNN (p<0.02) compared to placebo. For PNN50 (B) and RMSSD (C), significant decreases were seen only with the 1 and 2 ng/kg doses (p<0.03). LF/HF (D) was significantly decreased only in subjects receiving the 2 ng/kg dose (p<0.03).
PNN50
Only the groups receiving endotoxin doses of 1 or 2 ng/kg showed post-exposure decreases in PNN50 that were significantly lower than the placebo group by rANOVA over 1 through 6 hours post exposure compared to placebo (p<0.03). (Figure 3B)
RMSSD
Only the groups receiving endotoxin doses of 1 or 2 ng/kg showed post-exposure decreases in RMSSD that were significantly lower than placebo by rANOVA from 1 to 6 hours after exposure (p<0.04). (Figure 3C)
LF/HF
Only the group receiving the highest endotoxin dose, 2 ng/kg, showed rises in LF/HF which were significantly above the placebo group by rANOVA from 1 to 6 hours after exposure (p<0.03). (Figure 3D)
Multiscale Entropy
Repeated-measures ANOVA using scale factor as the dependent variable and endotoxin dose as the independent variable at 2 hours after exposure showed the MSE curves significantly decreased compared to placebo for LPS doses of1 and 2 ng/kg (p<0.05). While not statistically significant at lower doses, MSE exhibited a regular dose-response pattern proportional to endotoxin dose across all groups. This contrasted with the pattern seen in the other outcomes, including the other HRV parameters, which rarely displayed any evident pattern below the lowest dose required for statistical significance.
Discussion
There is evidence that hospitalized patients who persistently manifest the traditional criteria of a systemic inflammatory response syndrome (SIRS) are at increased risk of multi-system complications and mortality (22). Although it is known that these inflammatory manifestations can be elicited by either sterile or infectious insults, some uncertainty remains as to what systemic and tissue mechanisms initiate and sustain these manifestations. Preclinical models have defined the dynamic nature of tissue and cellular responses to inflammatory insults over the course of injury and recovery, whereas investigations in humans with severe systemic inflammation are challenged by patient-specific co-morbidities and treatment interactions. These confounding variables have, in part, limited the emergence of consensus regarding mechanistic, therapeutic, and prognostic profiles although there is general consensus that SIRS is a highly multifactorial condition.
We, and others, have previously documented that administration of LPS, a Toll-like receptor-4 specific ligand, at higher doses (≥2 ng/kg), is sufficient to induce robust manifestations of systemic inflammation, reproducible patterns of inflammatory mediator and acute phase reactant appearance, and dynamic regulation of immune cell gene transcription (5, 20, 21, 23). At these higher doses, LPS also transiently influences the function of solid organ and endothelial systems as well as efferent sympathetic and parasympathetic signaling in humans (4). In the present study, we sought to determine if a threshold effect among the varying components of the traditional SIRS response as well as several other known systemic and tissue-specific responses to LPS-induced TLR4 activation could be detected.
Among the additional LPS responsive indices, we also derived the information embedded within time and frequency domains of electrocardiographic recordings commonly referred to as heart rate variability (10, 18, 24). These determinations are of increasing interest in the management of acute sterile and infectious inflammatory conditions, where altered variability of beat-to-beat heart rate intervals are assumed to reflect the influence of autonomic outputs (11, 18, 21, 24-26). The derived metrics of HRV assess both sympathetic and vagal/parasympathetic activities, either of which may acutely influence the production of ligand-induced pro-inflammatory mediators (16-18, 24, 25, 27-29) and, by extension, the manifestations of systemic inflammation.
In the present study, we have undertaken a human LPS dose-response analysis that defines the relative threshold for measurable clinical, laboratory, and autonomic outflow responses to systemic LPS/TLR4 activation. These in vivo LPS-induced dose-response relationships are documented for the parameters of the systemic inflammatory response as well as for several immune activation biomarkers. However, it is unknown if efferent autonomic signaling, as assessed by metrics of HRV, also exhibits a similar dose-responsiveness in humans. Importantly, it is also unknown if measurably overt LPS/TLR4 activation of the immune system occurs over a differing dose-range and time frame than do changes in autonomic signaling. The data also permit an assessment of any dose-response relationship between traditional parameters ascribed to the systemic inflammatory response phenotype to other markers of LPS/TLR4 activation, including efferent autonomic function. Given the growing interest in utilization of HRV metrics as a research and clinical tool to assess inflammatory risk and therapeutic intervention, an understanding of these relationships will also inform our understanding of the value of existing methodologies.
We have previously demonstrated that the basal (pre-LPS) metrics of vagal/parasympathetic activity positively correlates with the LPS-induced generation of pro-inflammatory cytokines in this human model. Although this finding may seem contrary to current hypotheses regarding the importance of vagally mediated signals on inflammatory mediator production (4), it does suggest that other individualized, subject factors, including age, fitness level (30), and, perhaps, gender (31) affect the inflammatory response among overtly healthy individuals.
Across the range of LPS doses utilized in this study, there was evidence of a hierarchy of systemic manifestations and immune cell activation markers. The earliest overt endogenous response was evident in core temperature that was significantly increased at the 0.25 ng/kg dose and exhibited ever more significant changes during dose escalation (0.5-2.0 ng/kg). Although the mechanism whereby intravascular LPS is recognized by the central sensor(s) is presently unknown, the low threshold for body temperature change to LPS stimulation, also noted by others (5, 9), underscores the potential importance of peripheral ligand recognition mechanisms during the initial detection of inflammatory signals. One such pathway involves vagal afferent signaling and it remains to be determined if this recognition pathway is influenced by dysfunction of the vagal circuitry that may arise during severe sterile or infectious challenges (28).
The potential identification of a threshold E.Coli LPS dose of 0.5 ng/kg may allow endotoxin researchers to reduce the doses routinely used, if it is confirmed by future work in other settings and with different species and batches of LPS. Endotoxin research now commonly uses doses ranging up to 2 ng/kg in North America and 4 ng/kg in Europe. In appropriate settings, lowering routine study doses to a clearly reliable threshold should ameliorate discomfort in subjects and conserve supplies of the drug, with less concern that robustness of physiologic response will be unacceptably compromised. Endogenous TNFa (p<0.01) and IL-6 (p<0.02) appearance were responsive to LPS/TLR4 agonists at the next dosage level (0.5 ng/kg level) where significant changes in circulating leukocyte count (P<0.02) and increases in heart rate (p<0.05) were also observed. Consequently, three of the traditional parameters of the SIRS response were influenced at this dosage level, although the absolute magnitude of each parameter usually did not fulfill the traditional criteria (32) at this dose and it has been reported previously that SIRS criteria are fulfilled at higher doses. Such a finding is consistent with the evolution of inflammatory responses secondary to either sterile or infectious challenges, whereas the manifestations of respiratory compromise may be delayed relative to other systemic metrics. Importantly, no subject experienced any respiratory complaints during these studies and at the lower dosages (< 1.0 ng/kg) the spontaneous respiratory rate remained less than 20/minute throughout the study period.
The novel use of rANOVA across scale factor (as opposed to the more usual axis of time used for the other HRV parameters) seems to greatly reduce the data needed for significance with MSE, and it was only by doing so that significance could be demonstrated for MSE using short epochs and low scale factors in a group this small. For example, using a scale factor limit of 40 as opposed to 200 reduced the x-axis by 80%, and accordingly reduced the area under the curve so dramatically that significance was not reached using that measure. Future investigations will hopefully be able to use rANOVA across scale factor to confirm significant MSE responses in additional settings. This method and the lower data sets it allows should encourage MSE of HRV research by a wider range of investigators.
Limitations
The present study was performed in relatively younger patients without pre-existing acute or chronic illnesses that may influence LPS responses, including autonomic function. In ageing populations or those with disease co-morbidities autonomic balance may be altered in such a manner that vagal activity exerts less anti-inflammatory influence (33). While previous studies have confirmed a sustained innate immune and systemic inflammatory response to LPS among healthy older subjects,(27) it is also known that socio-economic status and ethnicity influence autonomic balance in older subjects (34). Both fitness and gender influence the balance of autonomic signals across the ageing spectrum (35) and physically-fit individuals of both genders exhibit increased basal vagal output. Although our subjects were of normal body mass and most undertook some modest, intermittent exercise, none were trained athletes. Hence, the current data may reflect a distinct population and the overall results should be extended to other Western populations with some caution. It will be of future interest to determine the relationship of responses to TLR4 agonists across a wider spectrum of physical fitness.
As anticipated for a study designed to identify a threshold response, the lowest doses of LPS did not exhibit statistically significant changes in any outcomes studied. Yet in contrast to both the threshold response seen in most markers, as well as our demonstration that MSE can be useful with common LPS doses but comparatively small data sets, the suggestion of an MSE dose-response to LPS was entirely serenditpitous. The study was designed and powered to determine a threshold effect, rather than to investigate a proportional dose-response effect, and by its nature the design specifically sought -- via much shorter epochs and much lower scale factors --to use MSE data sets roughly 80% smaller than prior work has used. Not surprisingly, then, the number of subjects in each group, including the lowest, is insufficient to investigate the unanticipated effect arising from doses more than an order of magnitude lower than those commonly used, and having a correspondingly lower magnitude of impact. Grossly it would seem that the pattern displayed in Fig. 4 is extremely unlikely to be the result of random chance and that it probably reflects a real effect of LPS on MSE that is genuinely occurring even at very low doses. Unfortunately, we are unable to identify any statistical method capable of formally confirming the reliability of a gradual dose response patternseen in outcomes which must by definition be plotted curves.
Figure 4.
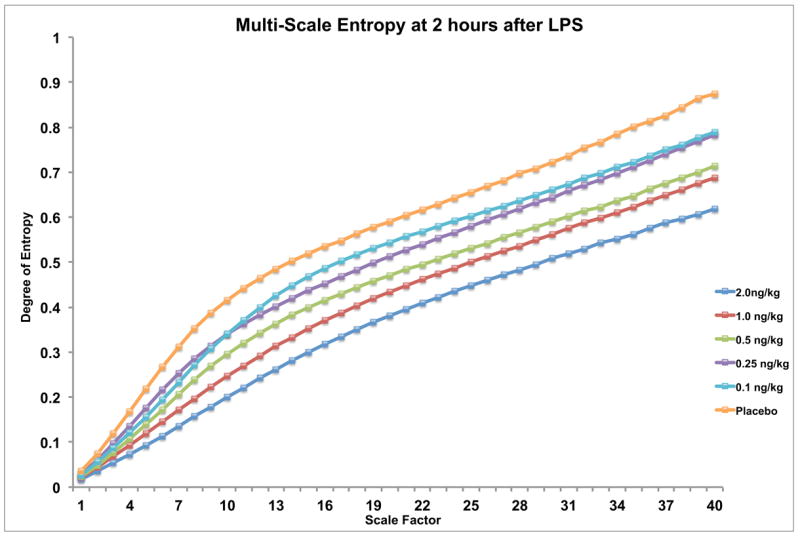
Multi-scale entropy of heart rate variability at 2 hours after endotoxin administration in human volunteers. By applying repeated-measures ANOVA using scale factor as the dependent variable and endotoxin dose as the independent variable, significant decreases in entropy are seen at 2 hours in subjects receiving the 1 and 2 ng/kg doses (p<0.05). Interestingly, while not statistically significant at the lower doses, the figure suggests a regular dose-response pattern proportional to endotoxin concentration which continues smoothly through even the lowest dose (0.1 ng/kg). This contrasts with the threshold-dose effect seen in other outcomes in Figs. 1-3, where clear patterns are rarely evident below the dose required for significance.
Future work may establish MSE’s sensitivity by repeating our design but with larger numbers of subjects. Far more practicable, however, would be a study designed to identify reduction of MSE by very low doses of LPS using longer epochs and higher scale factors to achieve the necessary robustness. Alternatively, it would be reasonable to analyze data collected from actual ICU patients using rANOVA across scale factors to evaluate the positive predictive value of a decrease in MSE for subsequent development of SIRS criteria, positive cultures, elevated levels of troponinI, etc.
Conclusions
If MSE is eventually found to be more sensitive than current clinical markers, and if software and hardware power continue to advance at historical rates, MSE may prove a fruitful component of a bedside monitor to detect inflammatory insults such as infection or ischemia far earlier than is currently possible. Apart from opening whole new frontiers in our understanding of the fabric of human physiology, such detection would allow intervention during the subclinical phase of a second-hit insult, with obvious potential for meaningful reductions in morbidity and mortality.
Supplementary Material
Acknowledgments
All work was performed in the Center for Translational and Clinical Research at UMDNJ-Robert Wood Johnson Medical School (UMDNJ-RWJMS), now Rutgers-RWJMS
Supported by grant RO1 GM-034695 from the National Institutes of Health (to S. F. L.)
References
- 1.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haimovich B, Reddell MT, Calvano JE, Calvano SE, Macor MA, Coyle SM, Lowry SF. A novel model of common Toll-like receptor 4- and injury-induced transcriptional themes in human leukocytes. Crit Care. 2010;14(5):R177. doi: 10.1186/cc9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheff JD, Mavroudis PD, Foteinou PT, Calvano SE, Androulakis IP. Modeling physiologic variability in human endotoxemia. Crit Rev Biomed Eng. 2012;40(4):313–322. doi: 10.1615/critrevbiomedeng.v40.i4.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 5.Vedder H, Schreiber W, Yassouridis A, Gudewill S, Galanos C, Pollmacher T. Dose-dependence of bacterial lipopolysaccharide (LPS) effects on peak response and time course of the immune-endocrine host response in humans. Inflamm Res. 1999;48(2):67–74. doi: 10.1007/s000110050408. [DOI] [PubMed] [Google Scholar]
- 6.Bahador M, Cross AS. From therapy to experimental model: a hundred years of endotoxin administration to human subjects. J Endotoxin Res. 2007;13(5):251–279. doi: 10.1177/0968051907085986. [DOI] [PubMed] [Google Scholar]
- 7.Heffron S, Mehta N, Llera-Moya M, Terembula K, Hinkle C, Wolfe M, Reilly M. Very-low dose endotoxemia induces high density lipoprotein remodeling and reduces cholesterol efflux in the absence of a clinical inflammatory response. Circulation. 2007;116(II) [Google Scholar]
- 8.Krogh-Madsen R, Moller K, Dela F, Kronborg G, Jauffred S, Pedersen BK. Effect of hyperglycemia and hyperinsulinemia on the response of IL-6, TNF-alpha, and FFAs to low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab. 2004;286(5):E766–772. doi: 10.1152/ajpendo.00468.2003. [DOI] [PubMed] [Google Scholar]
- 9.Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005;19(5):453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 11.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002;8(4):311–315. doi: 10.1097/00075198-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Huston JM. The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis. Surg Infect. 2012;13(4):187–193. doi: 10.1089/sur.2012.126. [DOI] [PubMed] [Google Scholar]
- 13.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 14.Scheff JD, Mavroudis PD, Calvano SE, Lowry SF, Androulakis IP. Modeling autonomic regulation of cardiac function and heart rate variability in human endotoxemia. PhysiolGenomics. 2011;43(16):951–964. doi: 10.1152/physiolgenomics.00040.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71(2 Pt 1):021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 16.Morris JA, Jr, Norris PR, Waitman LR, Ozdas A, Guillamondegui OD, Jenkins JM. Adrenal insufficiency, heart rate variability, and complex biologic systems: a study of 1,871 critically ill trauma patients. J Am Coll Surg. 2007;204(5):885–892. doi: 10.1016/j.jamcollsurg.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Norris PR, Stein PK, Morris JA., Jr Reduced heart rate multiscale entropy predicts death in critical illness: a study of physiologic complexity in 285 trauma patients. J Crit Care. 2008;23(3):399–405. doi: 10.1016/j.jcrc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, McIntyre L, Sundaresan SR, Maziak DE, Shamji FM, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One. 2009;4(8):e6642. doi: 10.1371/journal.pone.0006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Gao J, Tung WW, Cao Y. Multiscale analysis of heart rate variability: a comparison of different complexity measures. Ann Biomed Eng. 2010;38(3):854–864. doi: 10.1007/s10439-009-9863-2. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez SM, Katsamanis Karavidas M, Coyle SM, Lu SE, Macor M, Oikawa LO, Lehrer PM, Calvano SE, Lowry SF. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res. 2007;13(6):358–368. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]
- 21.Jan BU, Coyle SM, Macor MA, Reddell M, Calvano SE, Lowry SF. Relationship of basal heart rate variability to in vivo cytokine responses after endotoxin exposure. Shock. 2010;33(4):363–368. doi: 10.1097/SHK.0b013e3181b66bf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barie PS, Hydo LJ, Pieracci FM, Shou J, Eachempati SR. Multiple organ dysfunction syndrome in critical surgical illness. Surg Infect. 2009;10(5):369–377. doi: 10.1089/sur.2009.9935. [DOI] [PubMed] [Google Scholar]
- 23.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, et al. A network-based analysis of systemic inflammation in humans. Nature. 2005;437(7061):1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad S, Tejuja A, Newman KD, Zarychanski R, Seely AJ. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care. 2009;13(6):232. doi: 10.1186/cc8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seely AJ. Heart rate variability and infection: diagnosis, prognosis, and prediction. J Crit Care. 2006;21(3):286–289. doi: 10.1016/j.jcrc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Dick TE, Molkov YI, Nieman G, Hsieh YH, Jacono FJ, Doyle J, Scheff JD, Calvano SE, Androulakis IP, An G, et al. Linking Inflammation, Cardiorespiratory Variability, and Neural Control in Acute Inflammation via Computational Modeling. Front Physiol. 2012;3:222. doi: 10.3389/fphys.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofek K, Krabbe KS, Evron T, Debecco M, Nielsen AR, Brunnsgaad H, Yirmiya R, Soreq H, Pedersen BK. Cholinergic status modulations in human volunteers under acute inflammation. J Mol Med. 2007;85(11):1239–1251. doi: 10.1007/s00109-007-0226-x. [DOI] [PubMed] [Google Scholar]
- 28.van Westerloo DJ, Giebelen IA, Florquin S, Daalhuisen J, Bruno MJ, de Vos AF, Tracey KJ, van der Poll T. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191(12):2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 29.van Westerloo D, van der Poll T. Acute vagotomy activates the cholinergic anti-inflammatory pathway. Am J Physiol Heart Circ Physiol. 2005;288(2):H977–978. doi: 10.1152/ajpheart.00837.2004. [DOI] [PubMed] [Google Scholar]
- 30.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31(5):1001–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- 31.Prather AA, Carroll JE, Fury JM, McDade KK, Ross D, Marsland AL. Gender differences in stimulated cytokine production following acute psychological stress. Brain Behav Immun. 2009;23(5):622–628. doi: 10.1016/j.bbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 33.McKinley PS, King AR, Shapiro PA, Slavov I, Fang Y, Chen IS, Jamner LD, Sloan RP. The impact of menstrual cycle phase on cardiac autonomic regulation. Psychophysiology. 2009;46(4):904–911. doi: 10.1111/j.1469-8986.2009.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen KL, Marsland AL, Flory J, Votruba-Drzal E, Muldoon MF, Manuck SB. Community socioeconomic status is associated with circulating interleukin-6 and C-reactive protein. Psychosom Med. 2008;70(6):646–652. doi: 10.1097/PSY.0b013e31817b8ee4. [DOI] [PubMed] [Google Scholar]
- 35.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


