Abstract
Alternative pathways to the vascular endothelial grow factor (VEGF), such as hepatocyte growth factor or HGF/c-met, are emerging as key players in tumor angiogenesis and resistance to anti-VEGF therapies. The aim of this study was to assess the effects of a combination strategy targeting VEGF and c-met pathway in clear cell renal cell carcinoma (ccRCC) models. Male SCID mice (8/group) were implanted with 786-O tumor pieces and treated with either a selective VEGF receptor tyrosine kinase inhibitor, axitinib (36 mg/kg, 2×/day), a c-met inhibitor, crizotinib (25 mg/kg, 1×/day), or combination. We further tested this drug combination in a human ccRCC patient derived xenograft, RP-R-01, in both VEGF-targeted therapy sensitive and resistant models. To evaluate the resistance phenotype, we established RP-R-01 sunitinib resistant model by continuous sunitinib treatment (60 mg/kg, 1×/day) of RP-R-01 bearing-mice. Treatment with single agent crizotinib reduced tumor vascularization but failed to inhibit tumor growth in either model, despite also a significant increase of c-met expression and phosphorylation in the sunitinib resistant tumors. In contrast, axitinib treatment was effective in inhibiting angiogenesis and tumor growth in both models, with its anti-tumor effect significantly increased by the combined treatment with crizotinib, independently from c-met expression. Combination treatment also induced prolonged survival and significant tumor growth inhibition in the 786-O human RCC model. Overall, our results support the rationale for the clinical testing of combined VEGF and HGF/c-met pathway blockade in the treatment of ccRCC, both in first and second line setting.
Keywords: RCC, angiogenesis, VEGFR, c-met, RTKi
Introduction
Renal cell carcinoma (RCC) strikes approximately >64,000 people and causes >13,000 deaths year in the United States (1). Approximately 80% of RCC cases are diagnosed as clear cell (cc) RCC and the majority of them are sporadic tumors with acquired defects in both alleles of VHL tumor suppressor gene, resulting in von Hippel-Landau (VHL) protein dysregulation (2). This defective protein is unable to bind under hypoxic conditions, and trigger proteasome-mediated degradation of hypoxia-inducible transcription factor (HIF). The subsequent transcriptional hyper-activation of HIF targeted genes, such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), transforming growth factor alpha (TGF-α), hepatocyte growth factor (HGF) and mesenchymal-epithelial transition factor (MET), drives tumor progression and hyper-vascularization (3–5).
Anti-VEGF drugs have been shown great therapeutic benefit in ccRCC patients. The VEGF pathway does play a pivotal role in tumor angiogenesis and its over-activation is often associated with tumor growth and metastases (6). Among the VEGF-targeted therapies FDA-approved as frontline treatment for advanced RCC, tyrosine kinase inhibitors (TKIs) represent the most common choice (7). Due to their mechanism of action at the ATP-binding site, TKIs are selective rather than specific for a single kinase. Sunitinib, in particular, has been shown to inhibit platelet derived growth factor receptor (PDGFR), v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (c-kit) and VEGF receptors 1 and 2 (8). Albeit these multi TKIs can be highly effective by targeting more than one oncogenic pathway, a selective and potent VEGFR TKI may improve effectiveness and decrease the adverse events often observed in patients treated with multi target small molecules. Axitinib (former AG-013736) is a potent small molecule TKI, highly selective for VEGF receptor 1, 2 and 3 has been approved as a second line treatment for RCC and is currently being tested in phase II/III clinical trials for the treatment of solid tumors (9,10). Axitinib advantages include a well-tolerated clinical safety profile and a relative short half-life (2–5 hours) that allows dose adjustment/titration (11).
VEGF-targeted therapies elicit survival benefit in RCC, but fail to produce enduring clinical responses in most patients. Indeed, inevitably, disease progresses following a transient 9–11 month period of clinical benefit. Among the different mechanisms of evasive resistance to anti-angiogenic therapies, the upregulation of alternative pro-angiogenic signals and an increase of the invasive and metastatic behavior of tumor cells have been reported to play an important role (12,13).
The hepatocyte growth factor (HGF)/mesenchymal–epithelial transition factor (c-met) pathway has been shown to be relevant in acquired drug resistance as well as in tumor vascularization, epithelial to mesenchymal transition, and metastases (14). C-met is one of the most deregulated RTK in advanced cancers and MET activating mutations are the genetic cause of hereditary papillary type I RCC and other cancers (15). Intriguingly, c-met is transcriptionally activated by hypoxia and acts as mediator of anti-angiogenic therapy resistance in models of glioblastoma multiforme (16,17) and other solid tumors (18). Crizotinib (also known as PF-2341066) is an orally available, potent, and selective dual inhibitor of anaplastic lymphoma kinase (ALK) and c-met kinase that has been approved for the treatment of ALK-positive non-small cell lung cancers (19,20).
The aim of this study was to test the anti-tumor efficacy of axitinib and crizotinib combination in ccRCC models. The data suggest that combination with crizotinib increases axitinib induced anti-angiogenic and anti-tumor activity in both TKIs sensitive and TKIs resistant models.
Materials and Methods
Compounds
Axitinib (AG013736 or Inlyta®), crizotinib (PF-02341066 or Xalkori®), and sunitinib (Sutent®) were provided by Pfizer (New York, NY). For in vivo formulations, axitinib was prepared in 5% carboxymethylcellulose solution and crizotinib was dissolved in water by pH adjustment to a value between 3.5 and 4. Drugs were administered by oral gavage (per os or PO). The experimental groups were the following: vehicle (5% carboxymethylcellulose, 2×/day, 5×/week, PO), axitinib (36 mg/kg, 2×/day, 5×/week, PO), crizotinib (25 mg/kg, 1×/day, 5×/week, PO), axitinib plus crizotinib combination (same schedule and concentration as in single agent groups). Treatments were administered as follows: four weeks (786-O one month endpoint), six weeks (RP-R-01 sunitinib resistant), ten weeks (RP-R-01 sunitinib sensitive) or up to 15 weeks (786-O survival). Mouse body weight and tumor caliper measurements were taken weekly. No overt signs of toxicity were observed in any treatment group (i.e. significant weight loss or diarrhea).
Xenograft models and treatment protocol
Immunodeficient SCID male mice purchased from Roswell Park Cancer Institute (RPCI) were utilized for these studies and all procedures were approved by the Institute Animal Care and Use Committee (IACUC). Mice were kept in a temperature controlled room on a 12/12 hours light/dark schedule with food and water ad libitum. Collection of tumor samples was obtained via regulatory approval at the institution.
786-O cells were purchased from ATCC. Mice (8/group) were implanted under the right kidney capsule with ~1 mm3 size tumor pieces derived from previously orthotopically implanted, untreated 786-O tumors. Treatments began approximately 5 weeks later, when tumors were detectable by palpation, and followed the schedule described above. 786-O survival study: 32 mice (8/group) were implanted and treated as described above. Animals were monitored twice daily for health issues, moribund mice were euthanized by CO2 asphyxiation and deaths were recorded for each mouse. Each animal found dead or euthanized was necropsied. Criteria for euthanasia were based on an independent assessment by a veterinarian according to AAALAC guidelines and only cases where the conditions of the animal were considered incompatible with life were reported as deaths. As a control of good surgical procedure, we performed necropsies on mice survived until the end of treatment and tumors were found to be present under the right kidney capsule in all of the cases.
RP-R-01 is a patient-derived xenograft model developed from a skin metastasis of a patient with sporadic ccRCC VHL−/− that developed while on sunitinib treatment, as previously described (21). This model was propagated in vivo only in order to maintain the heterogeneity of the primary tumor. Short term study: mice (3/group) were implanted subcutaneously in the flank area with ~4 mm2 size RP-R-01 tumor pieces. Treatment started when average tumor dimension reached ~35 mm2: mice were randomized in the above-mentioned experimental groups and treated for either 2 or 7 days. Since previous studies performed in our lab showed good anti-tumor efficacy of sunitinib in this model, we implanted mice (8/group) subcutaneously with RP-R-01 tumor pieces, as described above, as models of sunitinib sensitive human ccRCC. Treatment started when average tumor dimension reached ~50 mm2. In order to establish a sunitinib resistant model, we implanted 35 mice subcutaneously in the flank area with ~4–5 mm2 size RP-R-01 tumor pieces and, approximately 6 weeks later, when tumors reached an average size of ~25 mm2, mice were treated with sunitinib (60 mg/kg, 5×/week, PO). We defined resistant tumors when they reached doubled size upon treatment (~50 mm2). Thereafter, mice were divided into homogenous groups (7 mice/group) as determined by caliper measurements and randomized to the above-mentioned experimental groups. Mice in all experiments have been sacrificed between 12 and 18 hours post last treatment.
mmunohistochemistry
Tissues were fixed for 24 hours in 10% neutral buffered formalin (c-met E-cadherin and Ki67) or zinc fixative (CD31), paraffin embedded and cut at 4 µm, placed on charged slides, and dried at 60°C for one hour. Slides were cooled to room temperature, deparaffinized in xylene, and rehydrated using graded alcohols. Antigen unmasking was heat-mediated, in citrate buffer (pH 6.0) and followed by a 20 minutes cool down. Endogenous peroxidases were quenched with 3% H2O2 for 10 minutes and washed with PBS-Tween20 0.1%. Slides were then blocked for one hour with PBS 1% BSA (bovine serum albumin) and incubated overnight in primary antibodies: mouse CD31 (1:100, 550274, BD Pharmingen), c-met (1:300, 8198, Cell Signaling), E-cadherin (1:400, 3195, Cell Signaling) or Ki67 (1:500, Thermo Scientific RM-9106). Sections were then incubated in horseradish-conjugated anti-rabbit (E-cadherin, c-met and Ki67) or anti-rat (CD31) antibody according to manufacturer protocol (Vector Laboratories) followed by enzymatic development in diaminobenzidine (DAB). Slides were finally counterstained with hematoxylin, dehydrated, and mounted with cytoseal 60 (Thermo Scientific). Quantification of the staining was performed by using ImageJ software in a blinded fashion by analyzing four randomly selected fields per tissue of 6 to 8 samples per treatment. CD31, E-cadherin and c-met results are expressed as the average % positive area per treatment ± S.E; Ki67 as % positive nuclei per treatment ± S.E calculated by Immunoratio plugin for ImageJ (22).
Immunofluorescence
Tissues were snap frozen and stored at −80°C, 10 µm thick sections were cut with a cryostat and placed in positively charged slides. Sections were then fixed for 10 minutes at −20°C in PBS-4% paraformaldehyde solution and washed-permeabilized in PBS 0.3% Triton X-100. Phosphorylated c-met staining was adapted from protocol described by Sennino et al (23): slides were blocked for 1 hour with immunomix (PBS 0.3% Triton X-100, 5% normal horse serum, 0.2% BSA) and incubated in primary c-met phosphorylation-specific antibody overnight at room temperature (pYpYpY1230/1234/1235, 1:250, 44888G, Invitrogen). Following primary incubation, sections were incubated with FITC-conjugated anti-rabbit antibody for one hour at room temperature in a humidified dark chamber. Cy3 goat anti rat (Invitrogen) was used to detect the anti-CD31 antibody in the dual color fluorescence experiments. Immuno-complexes were then briefly fixed for 5 minutes in 1% paraformaldehyde, nuclei stained with DAPI and slides mounted with vectashield mounting medium (Vector Laboratories). The number of phosphorylated c-met positive cells was counted in a blinded fashion by analyzing at least six randomly selected 40× fields per tissue of six samples per treatment.
Intratumoral hypoxia detection
At the end of treatment, mice in the RP-R-01 short term experiment were injected intraperitoneally with 60 mg/Kg pimonidazole hydrochloride (Hypoxiprobe plus kit), and 1 hour later, mice were euthanized. In order to stain hypoxic areas, we followed the protocol described for immunofluorescence, using 4.3.11.3 mouse-FITC MAb1 according to the manufacturer. The percentage of hypoxic area was counted in a blinded fashion by analyzing at least six randomly selected 10× fields per tissue of three samples per treatment.
Statistical Analysis
Differences among experimental groups were tested by either Student’s t test or for variances by ANOVA. P<0.05 was considered statistically significant. The difference in tumor weight between treatment groups was statistically evaluated by non-parametric Mann-Whitney U test.
Results
Anti-tumor effect of axitinib and crizotinib in the 786-O orthotopic model
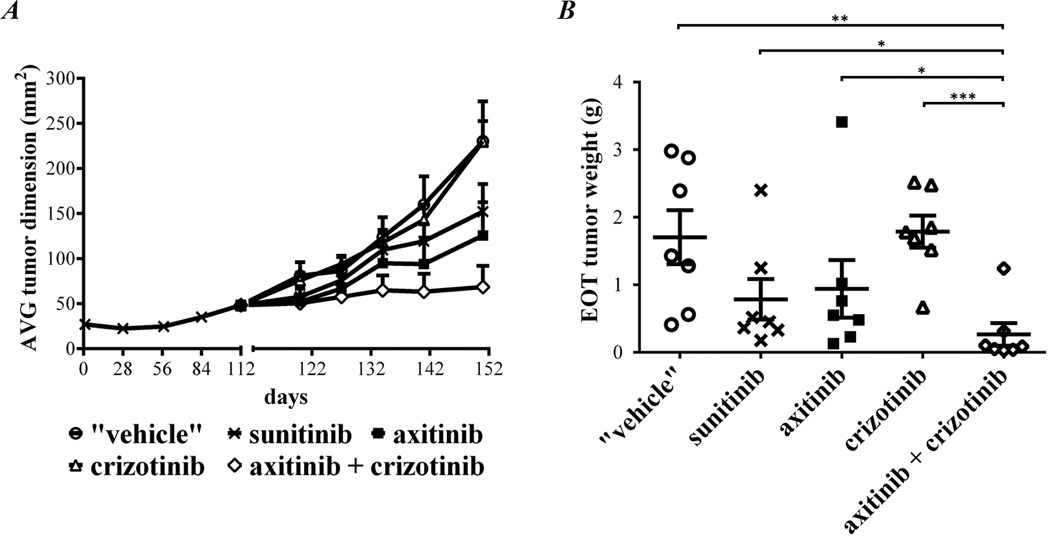
To examine the therapeutic effect of axitinib and crizotinib in the high c-met expressing 786-O model (Supplementary Fig. 1A), male SCID mice were orthotopically implanted under the kidney capsule with tumor tissues. When tumors became detectable by palpation, mice were randomized to treatment with either vehicle, axitinib, crizotinib or combination. After four weeks, mice were sacrificed and tumors were excised and weighed. Although tumor weights in both single agent groups were smaller than in vehicle treated mice (17% overall reduction in axitinib and 10% in crizotinib), no statistical differences were observed. However, the average tumor weight in the combination group was significantly smaller as compared to single agent groups (p= 0.0292 vs. axitinib and p= 0.0321 vs. crizotinib) and vehicle group (p= 0.0004), showing a 76% overall reduction as compared to vehicle group (Fig. 1A). Ki67 staining did not show significant differences in the proliferative index among groups in this highly proliferative model (data not shown). Microvessel density analysis by CD31 staining revealed, as expected, a reduction in tumor vascularization following treatment with either axitinib (p< 0.0001) or crizotinib (Fig. 1C and D). Noteworthy, tumor vascularization was further reduced following combined treatment (p= 0.0040 vs. axitinib and p< 0.0001 vs. both vehicle and crizotinib single agent).
Figure 1. Anti-tumor effect of axitinib and crizotinib in the 786-O orthotopic model.
Mice orthotopically implanted with 786-O tumor pieces (8 mice/group) were treated for four weeks with vehicle, axitinib (36 mg/kg, 2×/day, 5×/week), crizotinib (25 mg/kg, 1×/day, 5×/week) or combination. (A) End point tumor weight: each point represents one tumor, bars represent average) of each treatment group ± S.E. * p <0.05, *** p <0.001, as compared to combination group, using two tailed t-test analysis. (B) Kaplan-Meier survival curve of mice orthotopically implanted with 786-O tumor pieces and treated as described above. The vertical ticks represent censoring times. ** p <0.01 calculated by log-rank test. (C) Tumors from mice treated as in (A) were harvested, processed, and tissue sections were stained for CD31 for visualization of endothelial cells. (D) Blinded quantitative analysis of CD31, expressed as mean percentage positive stained area ± S.E. * p <0.05, **** p <0.0001, as compared to combination group, using two tailed t-test analysis.
Then, in view of the combinatorial anti-tumor effect of axitinib and crizotinib we conducted a survival experiment involving 786-O tumors orthotopically implanted in mice treated as described above. Kaplan-Meier survival curves show an increase in median survival in both single agent treated mice as compared to vehicle (42.5 days in vehicle, 77.5 in axitinib, 57 in crizotinib) (Fig. 1B). At end of the ~ 4 month experiment combination treatment resulted in a statistically significant improvement and extension of survival (median survival: 107 days; log-rank test: p= 0.0045).
Axitinib and crizotinib short term treatment in the sunitinib sensitive RP-R-01 PDX model
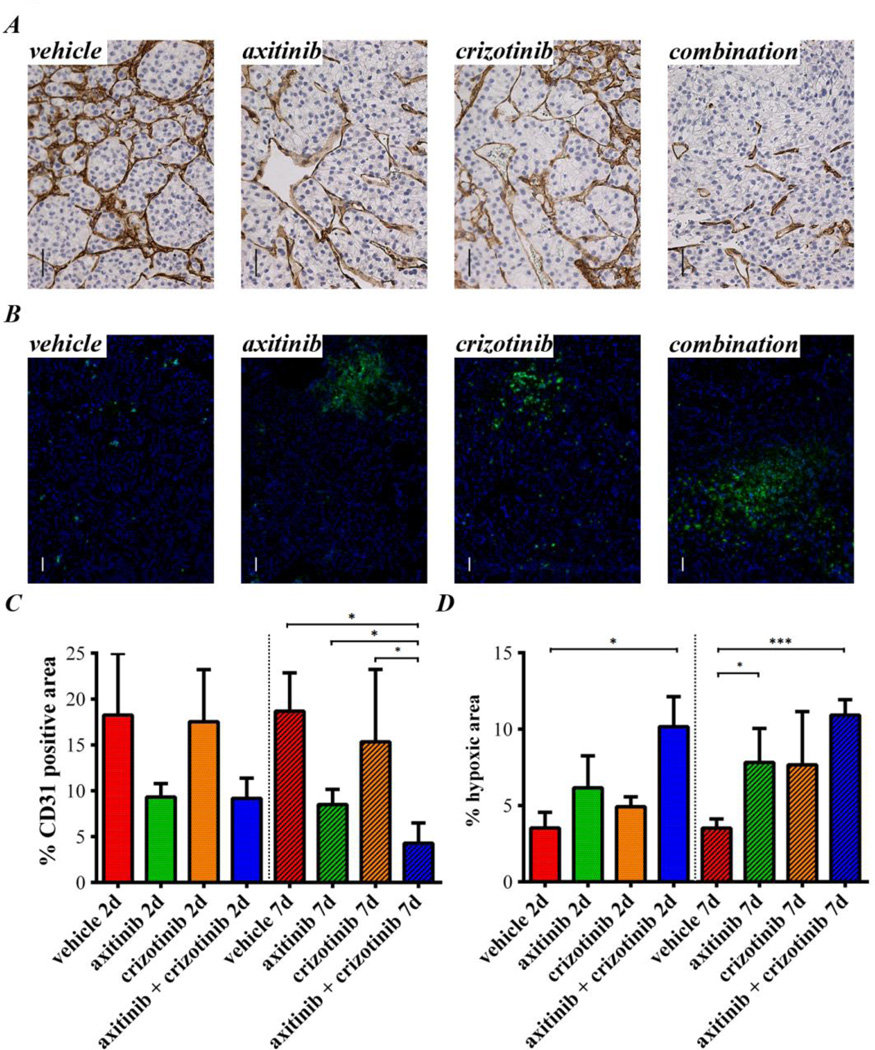
To evaluate the efficacy of axitinib and crizotinib in another sunitinib sensitive but low c-met expression model (Supplementary Fig. 1A), SCID mice were implanted with the ccRCC PDX RP-R-01. Tumors were measured weekly with a caliper and, when the average tumor dimension reached ~35 mm2, mice were randomized into 8 groups and treated with either vehicle, single agents or combination for either 2 or 7 days. At either time points, average tumor dimension was not significantly different among groups (Supplementary Fig. 1B and C). Despite homogenous tumor dimension among groups, tumor vascularization was already reduced after 2 days of axitinib treatment (Fig. 2A). Crizotinib single agent treatment did not affect blood vessel density, but combination treatment displayed a stronger reduction of CD31 positive area, significantly lower than each other group after 7 days of treatment (p= 0.0159 vs. vehicle, p= 0.0404 vs. axitinib and p= 0.0357 vs. crizotinib, Fig. 2C). In agreement with this significant reduction in tumor vascularization, pimonidazole staining (Fig. 2B) displayed a statistically significant increase in intra-tumor hypoxia in the combination group already at day 2 (p= 0.0400 vs. vehicle, Fig. 2D). Furthermore, at day 7, also axitinib (p= 0.0394) and crizotinib (p= 0.0642) single agents showed an increased intra-tumor hypoxia compared to vehicle group but, again, also at this time point the extent of combined treatment-induced hypoxia was greater (p= 0.0009). As expected, the immuno-detection of the phosphorylated c-met (Tyr1230/1234/1235) displayed a substantial reduction in the number of positive cells in the tumors treated with crizotinib (Supplementary Fig. 1D and E). CD31-pimonidazole dual-color immunofluorescence highlighted an obvious induction of hypoxia in all three treated groups, originating surprisingly from the blood vessels (with an overall more robust effect in the combination group, Supplementary Fig. 2A). As shown in Supplementary Fig. 2B, endothelial cells of the tumor blood vessels display phosphorylated c-met staining at least equal to RP-R-01 cancer cells, making also them a putative crizotinib target.
Figure 2. Axitinib and crizotinib short term treatment in the sunitinib sensitive RP-R-01 PDX model.
Subcutaneous RP-R-01 bearing mice (3/group) were treated with vehicle, axitinib (36 mg/kg, 2×/day), crizotinib (25 mg/kg, 1×/day) or combination for either 2 (labeled as 2d) or 7 days (7d). Tumors were then harvested, processed, and tissue sections were stained by immunohistochemistry for (A) CD31, to display endothelial cells and (B) pimonidazole by immunofluorescence (green), to assess intra-tumor hypoxia (DAPI counterstain marks nuclei in blue). Scale bar, 50µm. Blinded quantitative analysis of (C) tumor vascularization and (D) intra-tumor hypoxia, expressed as mean percentage positive stained area ± S.E,. * p <0.05, ***p <0.001, using two tailed t-test analysis (the vertical spotted lines highlights the difference in time among groups).
Anti-tumor effect of axitinib and crizotinib in the sunitinib sensitive RP-R-01 PDX model
To assess the anti-tumor efficacy of axitinib and crizotinib in RP-R-01, we performed also a long term treatment experiment. When the average tumor dimension reached ~50 mm2, mice were randomized into 4 groups and treated with either vehicle, single agents or combination. Tumor growth in mice treated with either crizotinib or vehicle was similar and mice were sacrificed after 40 days of treatment (Fig. 3A). Treatment with axitinib single agent significantly decreased the growth of tumors, but combination with crizotinib further enhanced axitinib anti-tumor efficacy. After 70 days of treatment, tumors in the combination group were significantly smaller than in the axitinib group (p= 0.0474 for tumor dimension and p= 0.0490 for tumor weight; Fig. 3B). Ki67 staining confirmed the inhibition of tumor proliferation in both axitinib and crizotinib groups (p< 0.0001 vs. vehicle; Fig. 3C and E). Figure 3D shows representative CD31 staining of tumor tissues. We observed a significant reduction in tumor vascularization in both axitinib and combination groups (p< 0.0001 vs. vehicle), although comparison between these two groups was not significant. Blood vessel reduction in crizotinib treated tumors as compared to vehicle group was modest and not statistically significant (Fig. 3F). Immunohistochemistry for c-met did not show any significant difference in expression among the experimental groups (Supplementary Fig. 3A and C). Furthermore, neither c-met phosphorylation (Tyr1230/1234/1235) increase in axitinib-treated group and decrease in crizotinib-treated group were statistically significant (Supplementary Fig. 3B and D).
Figure 3. Anti-tumor effect of axitinib and crizotinib in RP-R-01 ccRCC PDX (TKI sensitive model).
Subcutaneous RP-R-01 bearing mice (8 mice/group) were treated with vehicle, axitinib (36 mg/kg, 2×/day, 5×/week), crizotinib (25 mg/kg, 1×/day, 5×/week) or combination. (A) Tumor growth curve: each line represents the average tumor size (mm2) of each treatment group ± S.E. (B) End point tumor weights (the vertical spotted line highlights the difference in time among groups). Tumors from treated mice were then harvested, processed, and tissue sections were stained for (C) Ki67 to evaluate proliferation and (D) CD31 for visualization of endothelial cells. (E) Blind quantitative analysis of Ki67, expressed as mean percentage positive stained nuclei ± S.E and (F) CD31, expressed as mean percentage positive stained area ± S.E. * p <0.05, **p <0.01, ***p <0.001, **** p <0.0001, as compared to combination group, using two tailed t-test analysis.
Establishing a sunitinib resistant RP-R-01 ccRCC PDX model
To mimic a TKIs resistant scenario, SCID mice were implanted with RP-R-01 tumor tissues. Sunitinib treatment (60 mg/Kg, 1×/day, 5×/week, PO) started when tumors were detectable by caliper measurement (~25 mm2) and continued until the average tumor dimension doubled (~50 mm2), around day 112. At that time, we defined tumors as sunitinib resistant and mice were then randomly distributed into five experimental groups: either released from treatment ("vehicle"), maintained on sunitinib, switched to axitinib, to crizotinib or to axitinib plus crizotinib. Immunohistochemical comparison of sunitinib sensitive and resistant RP-R-01 tumors (harvested at day 112) indicated an increase in c-met expression (Fig. 4A and B) and a decrease in E-cadherin expression (Fig. 4F and G), suggesting a role of HGF/c-met pathway in sunitinib acquired resistance and a potential switch to a more mesenchymal phenotype. Increased c-met expression was paired with an increased activity of c-met RTK in the sunitinib resistant tumors as suggested by immunofluorescence detection of the phosphorylated Tyr1230/1234/1235 in the c-met activation loop (p= 0.0453, Fig. 4C and D). Western blot analysis performed on three representative tumors per group confirmed the increase of c-met phosphorylation in the sunitinib resistant experimental setting, and suggested not only an increase in the number of positive cells but also an overall increase of c-met activation (Fig. 4E).
Figure 4. RP-R-01 sunitinib sensitive (SS) and resistant (SR) histological comparison.
(A) Immunohistochemistry staining of RP-R-01 sunitinib sensitive and sunitinib resistant (at day 112) tumor pieces with c-met. Scale bar, 50µm. (B) Blind quantitative analysis of c-met, expressed as mean percentage positive stained area ± S.E. (C) Immunofluorescence staining of phosphorylated c-met (green), DAPI counterstain marks nuclei in blue. Scale bar, 20µm. (D) Blinded quantitative analysis of phosphorylated c-met, expressed as mean percentage positive cells ± S.E. (E) Western blot analysis of c-met expression and phosphorylation in lysates from three randomly selected RP-R-01 sunitinib sensitive and resistant tumor pieces. Numbers above bands represent signal intensity normalized to respective loading control (β-actin). (F) Immunohistochemistry staining of RP-R-01 sunitinib sensitive and sunitinib resistant tumor pieces with E-cadherin. Scale bar, 50µm. (G) Blinded quantitative analysis of E-cadherin, expressed as mean percentage positive stained area ± S.E. * p <0.05, **p <0.01 using two tailed t-test analysis.
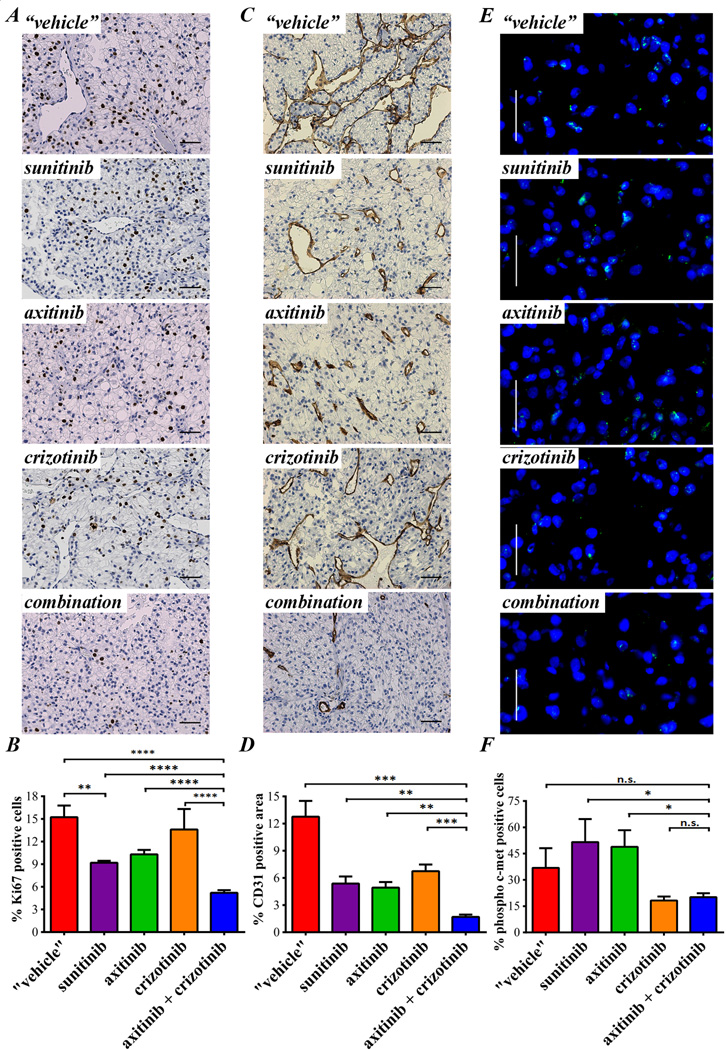
Anti-tumor effect of axitinib and crizotinib in the sunitinib resistant RP-R-01 PDX model
Despite an increase in c-met expression and phosphorylation was observed, treatment with crizotinib did not result in tumor growth inhibition (Fig. 5A). On the other hand, mice treated with axitinib or maintained on sunitinib, showed significant inhibition of tumor growth as compared with mice taken off treatment (tumor weight at day 151, end of treatment: p= 0.0247 vs. "vehicle" both axitinib or sunitinib, Fig. 5B). Once again, axitinib plus crizotinib combined treatment was more effective than continuous sunitinib treatment or switching to axitinib mono-therapy (end of treatment tumor weight: p= 0.0277 vs. sunitinib and p= 0.0133 vs. axitinib). Ki67 immunostaining showed a strong increase of resistant tumor proliferation in mice taken off treatment as compared to tumors from mice maintained on sunitinib (p= 0.0023, Fig. 6A and B). Despite crizotinib treatment did not show any significant decrease in Ki67 staining, combination with axitinib decreased the percentage of proliferative nuclei to less than 5% (p< 0.0001). Figure 6C shows tumor blood vessel staining with CD31. Tumors released from sunitinib treatment were hyper-vascularized (p= 0.0057 "vehicle" vs. sunitinib) and this effect was reverted by both axitinib and crizotinib treatment (p= 0.0035 and p= 0.0096 vs. "vehicle", respectively). Combination treatment reduced tumor vascularization even more (p =0.0013 vs. axitinib and p= 0.0002 vs. crizotinib, Fig. 6D). C-met expression by immunohistochemistry did not show significant differences among experimental groups (data not shown). Though, the strong increase in c-met phosphorylation (Tyr1230/1234/1235) in sunitinib and axitinib treated tumors was dramatically reduced by concomitant crizotinib treatment (p= 0.0399 vs. sunitinib and p= 0.0149 vs. axitinib single agent, Fig. 6E and F). Interestingly, single agent crizotinib treatment was able to significantly reduce phosphorylated c-met expression, but this biological effect was not associated with tumor growth inhibition. In accordance with the inhibition of the c-met pathway activity, crizotinib also induced a decrease in E-cadherin staining. However, this reduction was not associated with significant morphological changes (Supplementary Fig. 4).
Figure 5. Anti-tumor effect of axitinib and crizotinib in the sunitinib resistant RP-R-01 ccRCC PDX.
Subcutaneous RP-R-01 bearing mice (8 mice/group) were treated with sunitinib (60 mg/Kg, 1</day, 5</week) until tumor dimension doubled in size. Then, mice bearing tumors defined as sunitinib resistant were randomly distributed into five experimental groups: released from treatment ("vehicle"), maintained in sunitinib, axitinib (36 mg/kg, 2×/day, 5×/week), crizotinib (25 mg/kg, 1×/day, 5×/week) or axitinib plus crizotinib. (A) Tumor growth curve: each line represents the average tumor size (mm2) of each treatment group ± S.E. (B) End point tumor weights (the vertical spotted line highlights the difference in time among groups). * p <0.05, **p <0.01, and *** p <0.001, as compared to combination group, using two tailed t-test analysis.
Figure 6. Molecular effects of axitinib and crizotinib in the sunitinib resistant RP-R-01 ccRCC PDX.
Sunitinib resistant RP-R-01 bearing mice treated as in figure 4 were sacrificed after about 5 total months of treatment, tumors harvested, processed, and tissue sections were stained for (A) Ki67 to evaluate proliferation, and (C) CD31 for visualization of endothelial cells. (B) Blinded quantitative analysis of Ki67, expressed as mean percentage positive stained nuclei ± S.E and (D) CD31, expressed as mean percentage positive stained area ± S.E. Scale bar, 50µm. (E) Immunofluorescence staining of phosphorylated c-met (green), DAPI counterstain marks nuclei in blue. Scale bar, 20µm. (F) Blind quantitative analysis of phosphorylated c-met, expressed as mean percentage positive cells ± S.E. * p <0.05, **p <0.01, *** p <0.001, **** p <0.0001 as compared to all other groups, using two tailed t-test analysis.
Discussion
Treatment of ccRCC patients with TKIs, such as sunitinib, has been shown to induce significant clinical benefit in ccRCC patients and represents standard of care. However, inevitably tumor develops drug resistance and additional treatments are needed. Axitinib is a small molecule that inhibits VEGFRs activity with high specificity. In a phase 3 clinical trial axitinib has been reported to significantly improve progression free survival compared to sorafenib and has been approved as second line treatment in advanced renal cell carcinoma patients (9,10). In our RP-R-01 sunitinib resistant model, the comparison between sunitinib and axitinib treatment did not show significant differences in terms of tumor growth and vascularization. This observation suggests that, at least in this model, inhibition of additional kinases (such as PDGFR and c-kit) may not provide advantage in tumor growth inhibition as compared with more selective VEGFR inhibition. On the other hand, we observed early onset of tumor resistance to anti-VEGF targeted therapy in the 786-O model as previously reported (24). Interestingly, axitinib treatment in the 786-O orthotopic model inhibited tumor vascularization but not tumor growth, and did not induce significant improvement in survival compared to vehicle. However, combination with crizotinib not only increased axitinib inhibition of tumor microvessel density, but also tumor growth and it improved survival. In this rapidly highly proliferative model, only when c-met inhibition and VEGFR blockade occurred concomitantly, the treatment led to tumor growth inhibition and improved survival.
The patient derived xenograft developed in our laboratory, RP-R-01 (21) is a clinically relevant model of ccRCC since these tumors, by beg passage only in vivo, likely maintain the original heterogeneity that is often lost in tumor cell lines, and, more importantly, retain the clear cell morphology. In the short term treatment experiment performed with this model, we noticed a significant reduction of blood vessels in axitinib treated tumors in the absence of significant response in tumor growth. Blood vessels reduction in axitinib plus crizotinib group was even more significant, leading to a striking early induction of hypoxia following only two days of treatment. Moreover, a substantial increase in intra-tumor hypoxia in single agent groups was detectable following one week treatment. HIF-1α Western blot analysis also showed increased levels in the combination group following either short or long term treatment in the sunitinib sensitive model but significant decrease in the sunitinib-resistant tumors following long term treatment (Supplementary Fig. 5-1-C). Interestingly, pimonidazole-CD31 dual staining pointed that treatment-induced hypoxia originates from the endothelial cells, suggesting a direct drug effect on the blood vessels. Furthermore, RP-R-01 tumors showed a strong response to VEGF-targeted therapies, and TKI resistance is a true acquired event that occurs after months of sunitinib treatment instead of the relatively short period, as displayed by tumor cell lines. In order to establish a TKI resistant model, we treated RP-R-01 bearing mice with sunitinib (60 mg/Kg, 1×/day, 5×/week) until, following a period of stabilization, the average tumor size doubled from baseline. In these sunitinib resistant tumors, we observed an increase in c-met expression and activation as reported by other groups in glioblastoma, pancreatic neuroendocrine tumors and other solid tumors (16–18,23). In contrast to the sunitinib sensitive model, in the resistant RP-R-01 tumors we noticed a significant decrease in tumor vascularization in the combination group as compared to the axitinib treated group, suggesting a role of c-met in blood vessels homeostasis. However, regardless of c-met expression and activity, crizotinib was not effective as single agent at the dose utilized, but it was able to significantly improve axitinib anti-tumor activity in both models. Furthermore, regardless of the inhibition of c-met phosphorylation following crizotinib treatment, tumor growth rate was similar to vehicle treated tumors, suggesting a possible "rebound" effect when anti-VEGF therapy is halted (25), and the potential benefit of continuing VEGF blockade in patients despite radiological signs of disease progression on initial TKIs. Overall, our data suggest that c-met inhibition is therapeutically effective in the setting of concomitant inhibition of VEGF in RCC models, without significant toxicity (Supplementary Fig 6).
The molecular mechanisms responsible for c-met capability of compensating VEGF inhibition in RCC remain to be elucidated. One possibility is that the c-met/HGF pathway has a stronger role in tumor endothelial and stromal cells, where it acts as a potent pro-angiogenic trigger, supporting tumor growth. In fact, HGF is a well-known inducer of endothelial cell proliferation, survival and migration, and a chemoattractant for pro-angiogenic bone marrow derived progenitor cells (26). These changes in the tumor microenvironment may foster angiogenesis, leading to tumor growth regardless of the status of c-met expression in the tumor. It has been shown that treatment with a decoy c-met not only delays the growth of c-met positive xenografts, but also the growth of c-met negative tumors (27). This observation could explain our findings showing the lack of association between the effect of crizotinib and c-met expression in cancer cells. A second hypothesis is that anti-VEGF therapies eliminate basal c-met inhibition as demonstrated by Lu et al (16). A model of glioblastoma multiforme showed a direct VEGFR2 physical association with c-met that led to its post-translational inactivation. In this context, VEGF blockade abrogated the suppression of c-met phosphorylation, activating the c-met/HGF pathway directly in cancer cells. Finally, c-met transcriptional activation could directly lead to survival benefit and prevent from apoptosis in a VEGF inhibition context, as demonstrated following epidermal growth factor (EGFR) inhibition by gefitinib (28).
There are emerging clinical data suggesting that c-met represents a potential target for therapeutic intervention. In a recent Phase I clinical trial treatment with cabozantinib, a dual c-met and VEGFR inhibitor, has been to shown to induce a 30% objective response rate and a progression-free survival of 14.7 months in 25 patients with prior VEGF or mTOR inhibitors (29). These promising results have led to the further clinical development of cabozantinib in patients with recurrent renal cell carcinoma both in first-line setting and following TKIs. Our preclinical data did not identify an optimal setting (TKI sensitive vs. TKI resistant disease) for the introduction of c-met inhibition. The challenge is represented by the fact that, at least under our experimental conditions, tumor c-met expression does not seem to be predictive of response to a selective inhibitor. Future preclinical and clinical testing of c-met inhibitors will define the role of these agents in the armamentarium available to effectively treat recurrent renal cell carcinoma.
In conclusion, to our knowledge, this study is the first preclinical evidence of the key role of c-met in response to anti-VEGF therapy in ccRCC by using different models. Overall, our results highlight the potential therapeutic combination of VEGF and HGF/c-met pathway inhibition in the treatment of ccRCC, both in the first and second line setting and independently from constitutively overexpressed c-met in tumor cells.
Supplementary Material
Acknowledgements
We would like to thank the MTMR and Pathology Core Facilities at Roswell Park Cancer Institute for animal handling and processing the tissue samples. This research was supported in part by the National Cancer Institute, National Institutes of Health (P30CA016056) (RP), and by a research grant from Pfizer (RP).
The abbreviations used are
- ccRCC
clear cell renal cell carcinoma
- PDX
patient-derived xenograft
- TK
tyrosine kinase
- TKI
tyrosine kinase inhibitor
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- HGF
hepatocyte growth factor
- VHL
von Hippel-Lindau
Footnotes
Conflict of interest: Roberto Pili is a paid consultant and has received research funding from Pfizer.
This study was previously presented at the 2013 American Association for Cancer Research Annual Meeting
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 3.Baldewijns MM, van Vlodrop IJH, Vermeulen PB, Soetekouw PMMB, van Engeland M, de Bruïne AP. VHL and HIF signalling in renal cell carcinogenesis. J of Path. 2010;221:125–138. doi: 10.1002/path.2689. [DOI] [PubMed] [Google Scholar]
- 4.Oh RR, Park JY, Lee JH, Shin MS, Kim HS, Lee SK, et al. Expression of HGF/SF and Met protein is associated with genetic alterations of VHL gene in primary renal cell carcinomas. APMIS: Acta Pathologica, Microbiologica, et Immunologica Scandinavica. 2002;110:229–238. doi: 10.1034/j.1600-0463.2002.100305.x. [DOI] [PubMed] [Google Scholar]
- 5.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 6.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 8.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nature Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 9.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. The Lancet Oncol. 2013;14:552–562. doi: 10.1016/S1470-2045(13)70093-7. [DOI] [PubMed] [Google Scholar]
- 11.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 12.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nature Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nature Rev Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nature Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 15.Danilkovitch-miagkova A, Zbar B. Dysregulation of Met receptor tyrosine kinase activity in invasive tumors. J Clin Invest. 2002;109:863–867. doi: 10.1172/JCI15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahangiri A, De Lay M, Miller LM, Carbonell WS, Hu Y-L, Lu K, et al. Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res. 2013;19:1773–1783. doi: 10.1158/1078-0432.CCR-12-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee Pa, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 19.Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 20.Shaw AT, Kim D-W, Nakagawa K, Seto T, Crinó L, Ahn M-J, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. NEJM. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 21.Hammers HJ, Verheul HM, Salumbides B, Sharma R, Rudek M, Jaspers J, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9:1525–1535. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuominen VJ, Ruotoistenmäki S, Viitanen A, Jumppanen M, Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast cancer research: BCR. 2010;12:R56. doi: 10.1186/bcr2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, et al. Suppression of Tumor Invasion and Metastasis by Concurrent Inhibition of c-Met and VEGF Signaling in Pancreatic Neuroendocrine Tumors. Cancer Disc. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatt RS, Wang X, Zhang L, Collins MP, Signoretti S, Alsop DC, et al. Renal cancer resistance to antiangiogenic therapy is delayed by restoration of angiostatic signaling. Mol Cancer Ther. 2010;9:2793–2802. doi: 10.1158/1535-7163.MCT-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michieli P, Mazzone M, Basilico C, Cavassa S, Sottile A, Naldini L, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73. doi: 10.1016/j.ccr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 28.Jun HJ, Acquaviva J, Chi D, Lessard J, Zhu H, Woolfenden S, et al. Acquired MET expression confers resistance to EGFR inhibition in a mouse model of glioblastoma multiforme. Oncogene. 2012;31:3039–3050. doi: 10.1038/onc.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choueiri T, Pal SK, McDermott DF, Morrissey S, Ferguson KC, Holland J, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014;8:1603–1608. doi: 10.1093/annonc/mdu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.