Abstract
Cerebral white matter lesions (WMLs) are considered a reflection of cerebral and systemic small vessel disease (SVD), and are associated with reductions in brain volume. Like the brain, the kidney is also sensitive to factors that affect vasculature. Glomerular dysfunction due to renal vascular damage can be measured with different biochemical parameters, such as creatinine or cystatin C, although cystatin C is considered to be more accurate than creatinine in the elderly. The purpose of the study was to determine whether manifestations of SVD in the kidney can predict SVD-based damage to the brain. We examined the relationship between glomerular dysfunction as a measure of SVD on WMLs, gray matter (GM) volume, and cognition in 735 cognitively normal participants from the Cardiovascular Health Study Cognition Study. The multivariate analyses controlled for demographic characteristics, hypertension, heart disease, diabetes, Apolipoprotein 4 allele, C reactive protein, lipids, physical activity, smoking, and body mass index (BMI). Elevated cystatin C levels were associated with lower neuropsychological test scores, the presence of MRI-identified brain infarcts, the severity of WMLs, and GM atrophy five years later. In adjusted models, GM volume was significantly associated with cystatin-C only until BMI and severity of WMLs were added to the model, meaning that the effect of SVD on GM volume is mediated by these two variables. These findings suggest that age-related SVD is a process that leads to altered brain structure, and creates a vulnerability state for cognitive decline.
Keywords: Cystatin C, gray matter volume, cognitive impairment, white matter lesions
Introduction
Cerebral white matter lesions (WMLs) are frequently seen in older adults, and they are associated with cerebral and systemic small vessel disease (SVD) [1–6], and with hypertension (HTN) [7–9]. They are associated with changes in brain white matter (WM) and gray matter (GM) integrity [10–13], as well as with brain functional changes [14], altered cognition, and dementia [13, 15–17].
The kidney is also exquisitely sensitive to factors that affect vascular integrity. Like the brain, the kidney is a low resistance end organ exposed to a high volume of blood flow [18].There is stiffening of the wall of large blood vessels with advancing age, which reduces the protective gradient between the heart and peripheral organs [18]. This leads to an excessive transfer of pulsatility to the microvasculature, especially in high flow organs [18]. In turn, this increases the microvascular resistance in the kidneys and brain, making them more likely to suffer vascular damage, especially in the small vessels [18–20].
Damage to the renal vasculature due to HTN is manifested by microalbuminuria, decreased estimated glomerular filtration rate (eGFR), and increased levels of serum creatinine and cystatin C. HTN-related damage to the cerebral vasculature is manifested by WMLs, lacunar infarcts and microhemorrhages [6]. Because kidney dysfunction secondary to SVD may be detected at the same time, or even before there is evidence of vascular damage in the brain, altered measures of kidney function may indicate an increased risk of subclinical cerebrovascular disease, even when WMLs are not evident [21].
Cystatin C is an inhibitor of cysteine proteases and is produced by nearly all human cells [22]. It may be a more sensitive measure of glomerular function in the elderly compared to creatinine (for example) because it is less influenced by muscle mass [23] which may decrease in older adults. Elevated cystatin C levels are associated with increased mortality, with cardiovascular and non-cardiovascular outcomes, [24–26], and with subclinical brain infarcts and WMLs [21, 27]. Non-demented elderly individuals with higher cystatin C levels are more likely to develop cognitive impairment [28]. However, there are no studies of the long-term relationships between cystatin C levels and brain structure. To the extent that elevated cystatin C reflects peripheral SVD, we would expect that this would be followed by increased levels of WMLs as a consequence of an active, pathological process in small vessels. In turn, this would result (via WMLs and related loss of WM and GM) in a vulnerability state for the clinical expression of neurodegenerative processes (e.g., Alzheimer's disease) or vascular cognitive impairment [29].
The purpose of this study was to examine whether manifestations of SVD in the kidney can predict SVD-based damage in the brain. We asked whether serum cystatin C levels in 1993–94 predicted brain SVD, regional GM volumes, and cognitive performance measured in 1998–99 in cognitively normal older adults. We hypothesized that in the context of multiple cerebral risk factors for SVD, kidney function (as assessed with cystatin C) would be a predictor of brain structure, as consequence of the same microvascular disease process that results in WMLs.
Materials/Methods
Participants
The study sample was drawn from participants of the Cardiovascular Health Study-Cognition Study (CHS-CS) [30, 31], which grew out of the larger Cardiovascular Health Study (CHS). The parent CHS recruited 5,201 individuals over the age of 65 from four communities (Pittsburgh, PA; Sacramento, CA; Winston-Salem, NC; and Hagerstown, MD) in 1989–90 [32]. In 1992–94, 3,608 participants had a magnetic resonance imaging (MRI) study of the brain, and second MRI was done in 2,101 participants in 1997–98. In 1998–99 the CHS attempted to identify all participants who were demented at the time of the first MRI (1992–94), or subsequently developed dementia [30, 31, 33]. A comparison of those who did and did not have an MRI in 1992–94 has previously been reported [17, 34].
In 1998–99, 1,574 of the 2,101 CHS participants with a second brain MRI were considered to be cognitively normal [35]. The demographic and clinical characteristics of all CHS participants with and without an MRI of the brain in 1998–99 are shown in Supplemental Table E-1. Due to the late inclusion of the 3-dimensional volumetric T1-weighted Spoiled Gradient Recall (SPGR) sequence into the scanning protocol not all of the participants had high-resolution anatomical imaging. Thus, we analyzed MRI data from 735 participants who met the following criteria: diagnosed as cognitively normal in 1998–99, had a high-resolution MRI scan, and had cystatin C levels taken in 1993–94 (See Figure 1). There were no differences between the participants whose data are included in this analysis and those who were not in terms of age, education, gender, race, WML, ventricular enlargement, presence of MRI-infarcts or Apolipoprotein E4 (ApoE-4) status (See Supplemental Table E-1). Those included were less likely to have HTN than those who were not [13].
Figure 1.
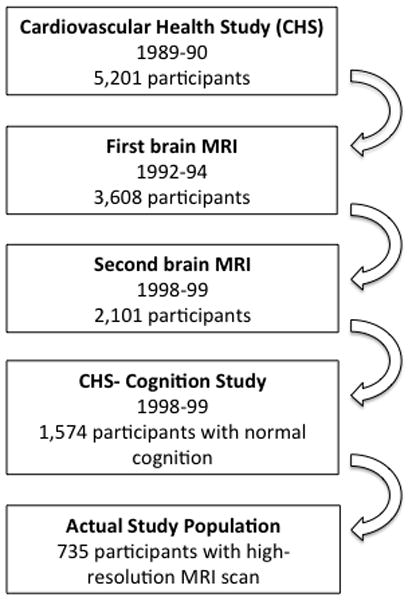
Schematic representation of the participants’ selection process.
Measures
Demographic variables included age, sex, education level and race. Medical conditions included having a history of heart disease, diabetes mellitus (classified by American Diabetic Association guidelines [36], HTN (and systolic and diastolic blood pressure (BP)), and the use of antihypertensive medications. Lifestyle factors included the number of blocks walked during the past week, weight, body mass index (BMI), waist circumference, smoking status (former and current smokers versus non-smokers), and systolic and diastolic BP. Cognitive function was assessed with the Modified Mini-Mental State Examination (3MSE) [37], the Digit Symbol Substitution Test (DSST) [38], and the Benton Visual Retention Test (BVRT) [39, 40]. Genetic predisposition to sporadic AD was assessed based on the presence of at least one copy of the ApoE*4 allele. Serum measures included cholesterol, C-reactive protein (CRP), and cystatin C. CRP was measured using a high-sensitivity immunoassay. Cystatin C was quantified from frozen samples using a BN II nephelometer (Siemens, Deerfield, IL). For the current analysis, we used medical and biochemical variables recorded in 1993–94, and cognitive measures and MRI scans obtained 1998–99.
MRI acquisition and measures
The MRI scanning was completed using 1.5 Tesla scanners as detailed elsewhere [34, 41]. Briefly, an SPGR sequence was obtained covering the whole brain (TE/TR = 5/25, flip angle = 40°, NEX = 1, slice thickness = 1.5 mm/0 mm interslice gap), with an in-plane acquisition matrix of 256 x 256x 124 image elements, a 250 x 250 mm field of view and an in-plane voxel size of 0.98 x .98 mm. WML burden was rated using a 10-point standardized CHS visual grading system, ranging from 0 (normal) to 9 (mostly abnormal), based on the total extent of the subcortical and periventricular hyperintensities on either axial T2-weighted or proton density images[1, 34, 41]. White matter lesion were considered present when the white matter grade scores were higher than 3[1, 13].
Voxel-level statistical analysis
The MRI scans were processed using Smallest Univalue Assimilating Nucleus from the fMRI Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl/) for 3-dimensional nonlinear noise reduction. The voxel-based morphometry (VBM2) script (http://dbm.neuro.uni-jena.de/vbm/vbm2-for-spm2/) was run in Statistical Parametric Mapping (SPM2) (http://www.fil.ion.ucl.ac.uk/spm/) using MATLAB v 7.4 (The MathWorks, Natick, MA). Images were normalized to the custom Pittsburgh Elderly Template [42] and then segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) based on the spatial prior probabilities from the template; a hidden Markov random field threshold of 0.3 was used in the segmentation step. Volumes for each tissue type were calculated by multiplying all voxels by the inverse of the Jacobian determinant of their spatial transformation matrix. The volumes of GM, WM, and CSF obtained from this step were summed to compute an estimate of the total intracranial volume (TIV). The images were smoothed using a 10mm (FWHM) isotropic Gaussian kernel and a mask was applied in all analyses to confine the statistical search space to GM voxels.
Statistical analysis
All other analyses were performed with the Statistical Package for the Social Sciences (IBM SPSS Statistics 18). Variables with non-normal distributions were transformed using a log10 transformation. Chi-squared tests were used to compare proportions, and t-tests and one-way ANOVAs were used to compare group means. The least significant difference (LSD) test was used (p<.05) following the one-way ANOVAs. We dichotomized serum cystatin C levels using a cutoff of 1.21 mg/dl; higher levels were considered abnormal [23].
In order to address study hypotheses, we proceeded in three steps. The first step was to identify predictors of serum cystatin C, which was our indicator variable of peripheral SVD. We first identified the variables that were significantly associated with cystatin C in unadjusted models. For the adjusted models we used linear regression to identify the variables associated with cystatin C (as a continuous dependent variable). The independent variables were entered into the model in three different blocks. The first block included factors of “no interest” such as demographic variables and ApoE4 status. The second block included variables that were hypothesized predictors including vascular risk factors, blocks walked per week, and coronary heart disease. We entered CRP in the third block in order to determine whether this inflammatory marker was linked to kidney function once the other factors had been accounted for. Within each block, the variables were entered with a forward stepwise method.
In the second step, we examined those factors associated with the presence of WMLs (grade ≤ 3 vs. >3) entering the independent variables in two different blocks. The first block included demographic variables, ApoE4 status, and cystatin C levels. The second block included vascular risk factors, blocks walked per week, and serum CRP levels. Within each block, the variables were entered with a forward stepwise method.
In the third step, we examined the effects of WMLs (as an independent variable) on brain volume. First, we created tertiles of GM volume (as a percentage of TIV (≤ 44.88%, 44.89 – 46.60%, and ≥ 46.61%)), and included the variables that were significantly associated with GM volume in the unadjusted models. Second, we regressed adjusted GM volume as a continuous variable on three blocks of data. The first block included demographic variables, ApoE4 status, and cystatin C levels. The second block included vascular risk factors. The third block included WMLs, the presence of infarcts on the brain MRI, and serum CRP levels. Within each block, the variables were entered with a forward stepwise method. We used both discrete and continuous measures of GM, because discrete variables are easier to understand clinically, but continuous variables provide more statistically powerful analyses.
Voxel-based statistical modeling
A multiple regression model in SPM2 was fitted to analyze the relationship between GM volume (at the voxel level) and cystatin C. The covariates of interest included age, sex, race, and MRI-identified infarcts. Other covariates were TIV, and indicator variables designating the site of the scan (to account for center-to-center variation). A second model included age, sex, race, WMLs, and BMI. A Family Wise Error threshold (p < 0.05) was applied to account for multiple comparisons, using an extent threshold of 100 voxels unless otherwise mentioned. Results were projected onto the standard single subject Montreal Neurological Institute (MNI) template for display purposes [43].
Results
Associations with Cystatin C
Table 1 shows the characteristic of the participants dichotomized by cystatin C level (±1.21 mg/l). The participants with high cystatin C levels were older, more likely to be Caucasian, had higher serum CRP levels, had more HTN, larger waist circumference, more heart disease and less physical activity compared to those with lower levels of cystatin C. Elevated cystatin C was also associated with greater prevalence of MRI-identified brain infarcts, WMLs, lower GM/TIV ratio and lower scores on the 3MSE, DSST, and BVRT. In the adjusted models (Table 2), older age, white race, greater waist size, heart disease, HTN, higher serum CRP and smoking all independently were associated with elevated cystatin C levels; exercise independently reduced risk.
Table 1.
Clinical characteristics and MRI findings of the studied population and by cystatin C groups.
| All Participants | Cystatin C ≤ 1.21 mg/l | Cystatin C > 1.21 mg/l | Test statistic and effect size a | |
|---|---|---|---|---|
| Participants | 735 | 534 | 201 | – |
| Age, years † | 73.1±3.8 | 72.7 ± 3.6 | 74.4 ± 4.1 | −5.58, 0.20* |
| Race, % white (n) | 91.3 (671) | 89.7 (479) | 95.5 (192) | 6.22, −0.09 * |
| Sex, % women (n) | 58.5 (430) | 60.7 (324) | 52.7 (106) | 3.79, 0.07 |
| Education level >12 years, % (n) | 48.7 (358) | 48.5 (259) | 49.3 (99) | 0.03, 0.007 |
| ApoE4 allele, % (n) | 23.8 (165) | 23.8 (121) | 23.7 (44) | 0.002, −0.002 |
| Cystatin C, mg/l † | 1.05±0.23 | - | - | - |
| CRP, mg/dl † | 4.77±9.39 | 4.4 ± 8.9 | 5.7 ±10.5 | −3.51, 0.13* |
| SBP, mmHg † | 130.0±18.5 | 129.3 ± 17.7 | 131.9 ± 20.4 | −1.69, 0.06 |
| DBP, mmHg † | 69.3±10.9 | 69.5 ± 10.4 | 68.6 ± 12.1 | 0.98, 0.03 |
| HTN, % (n) † | 32.7 (240) | 29.4 (157) | 41.3 (83) | 9.58, 0.11 * |
| Diabetes mellitus, % (n) † | 9.8 (72) | 10.1 (54) | 8.9 (18) | 0.22, −0.02 |
| Smoking, % (n) † | 50.3 (370) | 50.3 (269) | 50.2 (101) | 0.01, −0.005 |
| Total cholesterol, mg/dl † | 208.3±33.8 | 207.9 ± 33.0 | 209.4 ± 36.0 | −0.52, 0.02 |
| Lipid lowering medication, % (n) † | 8.3 (61) | 7.7 (41) | 10.0 (20) | 0.99, 0.03 |
| Aspirin intake, % (n) † | 43.5 (320) | 45.7 (244) | 37.8 (76) | 3.69, −0.07 |
| Blocks walked per week † | 38.6±54.6 | 41.8 ± 56.2 | 30.18 ± 48.9 | 3.27, 0.12 * |
| Body mass index, kg/m2 † | 26.8±4.2 | 26.6 ± 4.1 | 27.3 ± 4.5 | −1.85, 0.06 |
| Waist circumference, cm † | 97.1±12.9 | 94.5 ± 12.4 | 99.7 ± 13.4 | −4.86, 0.17 * |
| CHD, % (n) † | 15.4 (113) | 15.0 (80) | 16.4 (33) | 0.23, 0.02 |
| % GM/TIV ∫ | 45.5 ± 2.1 | 45.8 ± 2.1 | 45.2 ± 2.1 | 3.79, 0.13 * |
| WMG > 3, % (n) ∫ | 21.8 (160) | 19.1 (102) | 28.9 (58) | 8.16, 0.10 * |
| Infarcts on MRI, % (n) ∫ | 28.2 (207) | 25.8 (138) | 34.3 (69) | 5.19, 0.08 * |
| 3MSE § | 95.4±4.5 | 95.6 ± 4.3 | 94.8 ±5.0 | 2.13, 0.07 * |
| DSST § | 43.9±12.1 | 44.5 ± 12.2 | 42.3 ± 11.4 | 2.26, 0.08 * |
| BVRT § | 4.7±2.1 | 4.7 ± 2.1 | 4.4 ± 2.1 | 2.55, 0.09 * |
Continuous variables are represented as mean ± standard deviation.
Variables defined at the time of the blood sample (1993–94).
Variables defined at the time of the brain MRI scan (1997–98).
Variables defined at the last clinic evaluation (1998–99).
t and point biserial correlation for means, χ2 and φ for cross-tabulations.
indicates a p-value < 0.05.
3MSE: Modified Mini-Mental State Examination; ApoE4: Apolipoprotein Eε4; CHD: coronary heart disease; DBP: diastolic blood pressure; BVRT: Benton Visual Retention Test; CRP: C-reactive protein; DSST: Digit Symbol Substitution Test; GM: gray matter; MRI: magnetic resonance imaging; SBP: systolic blood pressure; TIV: total intracranial volume; WMG: white matter grade.
Table 2.
Standardized regression coefficients (β) from the models assessing the effect of measured variables on cystatin C levels.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Age, years | 0.231 | 0.227 | 0.231 |
| Race, white | −0.117 | −0.139 | −0.138 |
| Waist circumference, cm | - | 0.174 | 0.150 |
| Heart failure | - | 0.177 | 0.170 |
| HTN | - | 0.119 | 0.113 |
| Blocks walked per week | - | −0.107 | −0.102 |
| Smoking | - | 0.073 | 0.075 |
| C-reactive protein, mg/dl | - | - | 0.085 |
All variables were significant with a p-value < 0.05.
Model 1 a: adjusted for age, race, sex, and ApoE4 status.
Model 2 a: adjusted for age, race, sex, ApoE4 status, HTN, diabetes mellitus, total cholesterol, waist circumference, smoking, blocks walked by week, heart failure, and coronary heart disease.
Model 3 a: adjusted for age, race, sex, ApoE4 status, HTN, diabetes mellitus, total cholesterol, waist circumference, smoking, blocks walked by week, heart failure, coronary heart disease, and serum CRP levels.
These are the variables used in the different adjusted analyses; however, the table only includes those variables that were significant in each model.
Predictors of white matter lesions
Participants with WMLs were older, had higher cystatin C levels, had a greater prevalence of HTN, MRI-identified infarcts, lower GM volumes, and lower scores on the 3MS and the DSST compared with those without WMLs (Supplemental Table E-2). In the adjusted models, the presence of WMLs was predicted by age, HTN, and cystatin C (Table 3).
Table 3.
Standardized regression coefficients (β) from the models assessing the effect of measured variables on white matter lesions.
| Model 1 | Model 2 | |
|---|---|---|
| Age, years | 0.260 | 0.254 |
| Cystatin C, mg/l | 0.102 | 0.082 |
| HTN | - | 0.148 |
All variables were significant with a p-value < 0.05.
Model 1: adjusted for age, race, sex, ApoE4 status, and cystatin C levels.
Model 2: adjusted for age, race, sex, ApoE4 status, cystatin C levels, HTN, diabetes mellitus, total cholesterol, BMI, smoking, blocks walked by week, and serum CRP levels.
Consequences of white matter lesions on gray matter
The characteristics of the participants as a function of degree of GM atrophy are shown in Supplemental Table E-3. BMI was lower in the highest tertile compared to that in middle and lowest tertiles. SBP and cystatin C were lower in the highest tertile compared to the lowest. In the linear regression analysis (Table 4) in which GM/TIV was regressed on these factors, older age, gender, race (non-Caucasian), BMI and WMG were independently associated with volume. However, cystatin C levels were significantly associated with GM volume only until BMI and WMLs were added to the model.
Table 4.
Standardized regression coefficients (β) from the models assessing the effect of measured variables on gray matter volume (adjusted by total intracranial volume).
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Age, years | −0.304* | −0.327* | −0.254* |
| Sex | −0.184* | −0.191* | −0.205* |
| Race | −0.080* | −0.069 | −0.029 |
| Cystatin C, mg/l | −0.089* | −0.066 | −0.054 |
| Body mass index, kg/m2 | - | −0.143* | −0.162* |
| WML | - | −0.301* |
indicates a p-value < 0.05.
WML: white matter lesions measured as white matter grade (see test for details).
Model 1: adjusted by age, race, sex, educational level, ApoE4 status, and cystatin C levels.
Model 2: adjusted by age, race, sex, educational level, ApoE4 status, cystatin C levels, HTN, diabetes mellitus, total cholesterol, smoking, and BMI.
Model 3: adjusted by age, race, sex, educational level, ApoE4 status, cystatin C levels, HTN, diabetes mellitus, total cholesterol, smoking, BMI, WML, infarcts on MRI, and serum CRP levels.
The VBM analysis showed that higher cystatin C levels were associated with lower GM in the superior frontal gyrus bilaterally (Figure 2). However, this effect was no longer significant when BMI and WMLs were included in the SPM model. Age, WMLs and BMI had independent effects on GM volume (Figure 3). Older age was associated with lower GM volume in the superior temporal cortex (left > right), the hippocampus, the thalamus, and the posterior cingulate cortex. Higher WMLs were associated with lower GM mainly in the frontal cortex. Finally, higher BMI translated to lower GM volumes in the thalamus and the posterior cingulate cortex.
Figure 2.
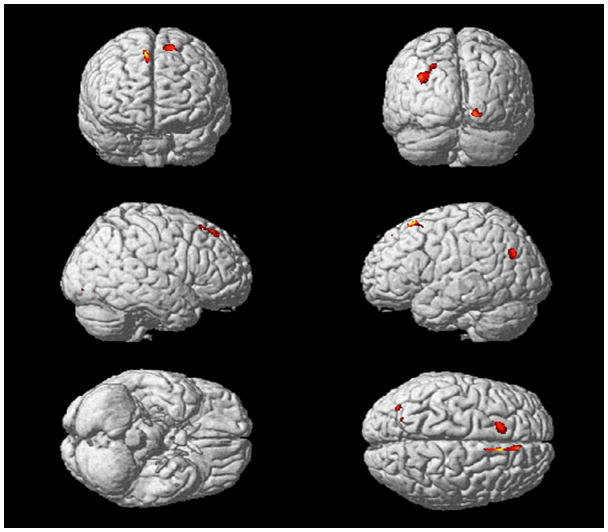
Main effect of cystatin C on regional gray matter volume projected onto the SPM single subject cortical surface. Higher cystatin C levels were associated with lower GM volumes most prominently in the superior frontal gyrus bilaterally, but also in the right lateral occipital cortex and in the left inferior parietal lobe. We applied a Family Wise Error Rate threshold (p < 0.05) with an extent threshold of 100 voxels.
Figure 3.
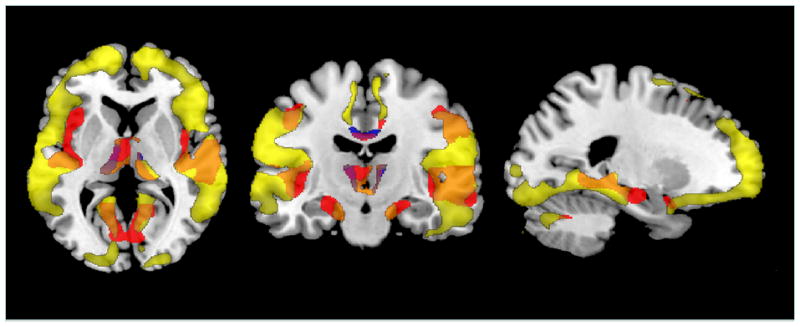
This figure shows the overlap of main effects of age (red), white matter grade (yellow) and body mass index (blue) on gray matter volume projected onto the Standard Single Subject MNI template (maps created using MRIcron, http://www.cabiatl.com/mricro/mricron/index.html).
Relationships among predictor variables
In order to better understand the relationships among the various predictor variables, and to attempt to provide a visual representation of those relationships, we completed a series of logistic regression analyses. Total GM volume was dichotomized at the lower 25th percentile (abnormal/normal) and simultaneously regressed on age (± 75 years), sex, race, ApoE*4 status, WMLs, Cystatin C (± 1.21), BMI (± 30), log10 blocks walked (± 0.47) and HTN. Of those predictors, WMLs, age and the presence of an ApoE*4 allele directly increased the risk of GM atrophy. We then regressed WMLs on the variables that were not associated with GM volume. We found that WMLs also were predicted by cystatin C, HTN and age. Finally, we regressed cystatin C levels on the remaining independent variables and found that an abnormal cystatin C level was associated with older age, male sex, Caucasian race and HTN.
Figure 4 shows the predictors and the consequences of WMLs; the numbers above the pathways are the Odds Ratios associated with each specific variable pair (p<.05). HTN, a risk factor for SVD (as indicated by cystatin C), predicted both WMLs and cystatin C levels, reflecting independent effects on cerebro- and renovasculature. HTN also had a mediated effect on WMLs through cystatin C.
Figure 4.
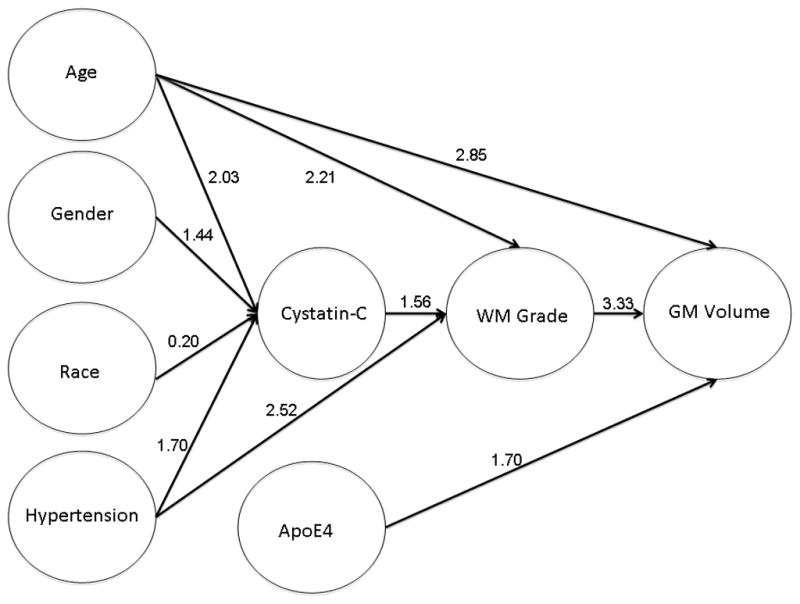
Graphic representation of the results of a series of logistic regression models. The arrows indicate the direction of the relationships, and the numbers are the odds ratios associated with each of the pathways. Age (± 75), BMI (± 30), GM volume (± 44.38%), log10 blocks walked (± 0.47) were dichotomized prior to the analysis; all variables were coded so that a value of ‘1’ was abnormal, and ‘0’ was considered normal. GM volume (normal vs. atrophic) was regressed (forward, stepwise, Wald criteria) on all of the variables shown in the figure, as well as blocks walked, and education. Only those variables that significantly altered the risk of atrophy were shown in the first level (i.e., age, ApoE4, and WML). WML was then regressed on the remaining variables, and age, Cystatin C, and HTN were significant. Finally, the factors associated with risk for elevated Cystatin C were assessed, and only age, race, sex and HTN remained in the model.
Discussion
This study showed that higher cystatin C levels were associated with more WMLs, lower GM volumes and poorer cognitive performance six years later, and that cystatin C independently predicted WMLs. However, when we included in the model multiple competing risk factors, the effect of serum cystatin C levels on GM volume was mediated by the presence of WMLs.
Previous studies have found that higher serum cystatin C levels are associated with lacunar infarcts and WMLs [21, 27], and that older adults with elevated cystatin C levels had lower 3MSE and DSST scores and were more likely to develop cognitive impairment after a 7-year follow-up [28]. Cross-sectional studies have also found that renal function, as measured by eGFR or microalbuminuria, is associated with brain atrophy [44, 45]. Our study replicates and extends these associations in the same sample of subjects, adding the inverse association between cystatin C and GM volume.
Cystatin C levels were significantly associated with GM volume only until BMI and WMLs were added to the model, meaning that the effect of cystatin C on GM volume is partially mediated by these two variables (Model 1 vs. Models 2 and 3) [46]. The same was found in the VBM analysis of GM; higher cystatin C levels were associated with lower GM volumes, only until BMI and WMLs were included in the model. The VBM model also found partially independent effects of age, WMLs and BMI on GM volume, demonstrating that “age-related” brain atrophy is not completely accounted for by chronological age, but also by other factors related to aging. Several studies have shown that WMLs correlate with reduced GM volume in cognitively normal older adults [12, 13]. On the other hand, obesity is a well-known risk factor for cognitive decline and dementia [47, 48] and numerous studies have suggested an association of obesity, measured by BMI, with lower total or regional brain volumes [49] [50–53]. The potential mechanism underlying the inverse association between BMI and brain volume is unknown, but it is unlikely to be direct. For example, obesity raises the risk of HTN and type 2 diabetes mellitus that themselves cause vascular changes and brain volume reduction [7, 54]. Other proposed mediators for the relationship between higher BMI and lower brain volume include inflammation [55], adipose tissue-derived hormones [56], and reduced exercise [57].
Our data replicate and extend prior findings of the effects of chronic HTN on peripheral small vessel disease, and the relationship with the cerebrovascular tone. Our analyses also suggest a functional pathway to explain these relationships, with chronic HTN causing damage to “unprotected” end organs (i.e., the kidney and brain) manifested as elevated levels of cystatin C and increased rate of WMLs.
Although our results are consistent with the idea that cystatin C levels are associated with WMLs due to a common SVD process, we cannot rule out the possibility that kidney dysfunction could directly lead to brain SVD [58]. Another intriguing finding was that the risk factors associated with elevated cystatin C and WMLs were different. We interpret this difference in the risk factors associated with cystatin C (i.e., age, race, waist circumference, heart disease, HTN, exercise, smoking, and CRP) and WMLs (i.e., age, HTN, and cystatin C) in the context of the regression-based model shown in Figure 4. That is, damage to the small vessels in the kidney precedes WMLs in the brain, and thus the predictor variables were more tightly linked to cystatin C, and act only indirectly on brain vasculature through WM volume. In fact, when we examined the effects of cystatin C and WMLs measured in 1992–94 on WMLs measured in 1998–99, we found that cystatin C levels predicted WMLs 6 years later even after controlling for the severity of WMLs determined in 1992–94. We speculate that SVD can be detected by cystatin C before WMLs. Whether this reflects a chronic vascular pathology that manifests itself first in the kidney, or whether measures of kidney function are simply more sensitive than measures of brain structure cannot be determined from our data.
Among the limitations of our study is the fact that we did not have anatomical brain images at the same time as we measured cystatin C. This was a consequence of several factors, including the fact that SPGR sequences were not available in 1992–94, logistical issues including subject burden, as well as financial constraints. Nevertheless, we feel that our data do allow us to report that SVD in the brain, manifested as WMLs is predicted by small vessel disease measured in the periphery. The principle correlate of WMLs is decreased GM volume in the brain in specific regions of interest, which has an impact on cognitive functions, and by inference on subsequent rates of dementia.
In conclusion, we observed that cystatin C levels in 1993–94 predicted brain SVD, regional GM volumes, and cognitive performance measured in 1998–99 in cognitively normal older adults in the CHS-CS. Furthermore, we tried to understand the relationships among these variables and we observed that cystatin C independently predicted WMLs, and the effect of cystatin C on GM volume was mediated by the presence of WMLs.
Supplementary Material
Supplemental Table E-1: Clinical characteristics of the Cardiovascular Health Study participants with and without MRI of the brain in 1998–99.
Supplemental Table E-2. Clinical characteristics and MRI findings by grades of white matter lesion.
Supplemental Table E-3. Clinical characteristics and MRI findings by tertile of gray matter volume adjusted by total intracranial volume.
Acknowledgments
The research reported in this article was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG023629, AG15928, AG20098, AG027002, AG05133, and AG027058 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. MR was supported by a grant from Fundación Caja Madrid (Spain).
Footnotes
Competing interest
The authors declare no competing interests.
References
- 1.Longstreth WT, Arnold AM, Manolio T, Burke G, Bryan N, Jungreis CA, O'Laery D. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3301 elderly people. The cardiovascular health study. Neuroepidemiology. 2000;19:30–42. doi: 10.1159/000026235. [DOI] [PubMed] [Google Scholar]
- 2.Longstreth WT, Arnold AM, Beauchamp NJ, Manolino TA, Lefkowitz D, Jungreis C, Hirsch CH, O'Leary DH, Furberg CD. Incidence, manifestations, and predictors of working white matter on serial cranial magnetic resonance imaging in teh elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 3.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler M. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam scan study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- 5.Manolio TA, Burke GL, O'Leary DH, Evans G, Beauchamp N, Knepper L, Ward B. Relationships of cerebral MRI findings to ultrasonographic carotid atherosclerosis in older adults : the Cardiovascular Health Study. CHS Collaborative Research Group. Arterioscler Thromb Vasc Biol. 1999;19:356–365. doi: 10.1161/01.atv.19.2.356. [DOI] [PubMed] [Google Scholar]
- 6.Mogi M, Horiuchi M. Clinical Interaction between Brain and Kidney in Small Vessel Disease. Cardiol Res Pract. 2011;2011:306189. doi: 10.4061/2011/306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 8.de Leeuw F-E, dr Groot JC, Oudkerk M, Witterman JCM, Hofman A, van Gjin J, Breteler MMB. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 9.Dufouil C, de Kersaint-Gilly A, Besancon V, Levy C, Auffray E, Brunnereau L, Alperovitch A, Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities. The EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 10.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Leeuw F-E, de Groot JC, Oudkerk M, Witeman JCM, Hofman A, van Gijn J, Breteler MMB. Aortic atherosclerosis at middle age predicts cerebral white matter lesions in the elderly. Stroke. 2000;31:425–429. doi: 10.1161/01.str.31.2.425. [DOI] [PubMed] [Google Scholar]
- 12.Wen W, Sachdev PS, Chen X, Anstey K. Gray matter reduction is correlated with white matter hyperintensity volume: a voxel-based morphometric study in a large epidemiological sample. Neuroimage. 2006;29:1031–1039. doi: 10.1016/j.neuroimage.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 13.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT, Jr, Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiology of aging. 2012;33:834 e837–816. doi: 10.1016/j.neurobiolaging.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29:193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Groot JC, de Leeuw F-E, Oudkerk M, van Gijn J, Hofman A, Jolles J, Brereler MMB. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 17.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R. Relationship between ApoE, MRI findings, and cognitive function in the cardiovascular health study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 21.Wada M, Nagasawa H, Kawanami T, Kurita K, Daimon M, Kubota I, Kayama T, Kato T. Cystatin C as an index of cerebral small vessel disease: results of a cross-sectional study in community-based Japanese elderly. Eur J Neurol. 2010;17:383–390. doi: 10.1111/j.1468-1331.2009.02809.x. [DOI] [PubMed] [Google Scholar]
- 22.van den Noortgate NJ, Janssens WH, Delanghe JR, Afschrift MB, Lameire NH. Serum cystatin concentration compared with other markers of glomercural filtration rate in the old. Am J Geriatr Psychiatry. 2002;50:1278–1282. doi: 10.1046/j.1532-5415.2002.50317.x. [DOI] [PubMed] [Google Scholar]
- 23.Madero M, Sarnak MJ. Association of cystatin C with adverse outcomes. Curr Opin Nephrol Hypertens. 2009;18:258–263. doi: 10.1097/mnh.0b013e328326f3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlipak MG, Fyr CLW, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–261. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman A, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Eng J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 26.Sarnak MJ, Katz R, Fried LF, Siscovick DS, Kestenbaum B, Seliger SL, Rifkin D, Tracy R, Newman A, Shlipak MG. Cystatin C and aging success. Arch Intern Med. 2008;168:147–153. doi: 10.1001/archinternmed.2007.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seliger SL, Longstreth WT, Jr, Katz R, Manolio T, Fried LF, Shlipak M, Stehman-Breen CO, Newman A, Sarnak M, Gillen DL, Bleyer A, Siscovick DS. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe K, Lindquist K, Shlipak MG, Simonsick E, Fried L, Rosano C, Satterfield S, Atkinson H, Windham BG, Kurella-Tamura M. Cystatin C as a marker of cognitive function in elders: findings from the health ABC study. Ann Neurol. 2008;63:798–802. doi: 10.1002/ana.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011 doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 31.Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: Design and Rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick AL, Kuller LH, Ives D, Lopez OL, Jagust W, Breitner J, Jones B, Lyketsos C, Dulberg C. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc. 2004;52:195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 34.Bryan RN, Manolio TA, Scertz LD, Jungreis C, Poirier VC, Elster AD, Kronmal RA. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson MC, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognitive Study Part 1. Arch Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 36.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. Report. [DOI] [PubMed] [Google Scholar]
- 37.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 38.Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- 39.Benton AL. Abbreviated versions of the Visual Retention Test. J Psychol. 1972;80:189–192. doi: 10.1080/00223980.1972.9924794. [DOI] [PubMed] [Google Scholar]
- 40.Benton AL, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment. A clinical manual. Oxford University Press; New York: 1983. [Google Scholar]
- 41.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Garden JM, Fried LP, Steinberg EP, Bryan RN. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 42.Spears JR, Greer PJ, Ziolko SK, Aizenstein HJ, Carmichael O, Becker JT, Meltzer CC. Evaluation of an age-specific neurological template. Presented at the Annual Meeting of the Organization of Human Brain Mapping; Toronto Ontario, Canada. June 2005.2005. [Google Scholar]
- 43.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 44.Knopman DS, Mosley TH, Jr, Bailey KR, Jack CR, Jr, Schwartz GL, Turner ST. Associations of microalbuminuria with brain atrophy and white matter hyperintensities in hypertensive sibships. J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakushiji Y, Nanri Y, Hirotsu T, Nishihara M, Hara M, Nakajima J, Eriguchi M, Nishiyama M, Hara H, Node K. Marked cerebral atrophy is correlated with kidney dysfunction in nondisabled adults. Hypertens Res. 2010;33:1232–1237. doi: 10.1038/hr.2010.171. [DOI] [PubMed] [Google Scholar]
- 46.Baron RM, Kenny DA. The Moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging 26 Suppl. 2005;1:11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 48.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 49.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004;63:1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 50.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31:1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 51.Taki Y, Kinomura S, Sato K, Inoue K, Goto R, Okada K, Uchida S, Kawashima R, Fukuda H. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16:119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 52.Ho AJ, Stein JL, Hua X, Lee S, Hibar DP, Leow AD, Dinov ID, Toga AW, Saykin AJ, Shen L, Foroud T, Pankratz N, Huentelman MJ, Craig DW, Gerber JD, Allen AN, Corneveaux JJ, Stephan DA, DeCarli CS, DeChairo BM, Potkin SG, Jack CR, Jr, Weiner MW, Raji CA, Lopez OL, Becker JT, Carmichael OT, Thompson PM. A commonly carried allele of the obesity-related FTO gene is associated with reduced brain volume in the healthy elderly. Proc Natl Acad Sci U S A. 2010;107:8404–8409. doi: 10.1073/pnas.0910878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raji CA, Ho AJ, Parikshak NN, Becker JT, Lopez OL, Kuller LH, Hua X, Leow AD, Toga AW, Thompson PM. Brain structure and obesity. Hum Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 55.van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112:900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- 56.Lieb W, Beiser A, Vasan RS, Tan ZS, Au R, Harris TR, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2562–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 58.Seliger SL, Longstreth WT., Jr Lessons about brain vascular disease from another pulsating organ, the kidney. Stroke. 2008;39:5–6. doi: 10.1161/STROKEAHA.107.496000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table E-1: Clinical characteristics of the Cardiovascular Health Study participants with and without MRI of the brain in 1998–99.
Supplemental Table E-2. Clinical characteristics and MRI findings by grades of white matter lesion.
Supplemental Table E-3. Clinical characteristics and MRI findings by tertile of gray matter volume adjusted by total intracranial volume.


