Abstract
Background
We evaluated the use and efficacy of adjuvant chemotherapy after resection of T1-2N1M0 non-small cell lung cancer (NSCLC) in elderly patients.
Methods
Factors associated with the use of adjuvant chemotherapy in patients older than 65 years of age who underwent surgical resection of T1-2N1M0 NSCLC without induction chemotherapy or radiation in the Surveillance, Epidemiology, and End Results-Medicare database from 1992 to 2006 were assessed using a multivariable logistic regression model that included treatment, patient, tumor, and census tract characteristics. Overall survival (OS) was analyzed using the Kaplan–Meier approach and inverse probability weight-adjusted Cox proportional hazard models.
Results
Overall, 2,781 patients who underwent surgical resection as the initial treatment for T1-2N1M0 NSCLC and survived at least 31 days after surgery were identified, with adjuvant chemotherapy given to 784 patients (28.2 %). Factors that predicted adjuvant chemotherapy use were younger age and higher T status. The 5-year OS was significantly better for patients who received adjuvant chemotherapy compared with patients not given adjuvant chemotherapy: 35.8 % (95 % confidence interval [CI] 31.9–39.6) vs. 28.0 % (95 % CI 25.9–30.0) (p = 0.008). In the inverse probability weight-adjusted Cox proportional hazard regression model, adjuvant chemotherapy use predicted significantly improved survival (hazard ratio 0.84; 95 % CI 0.76–0.92; p = 0.0002).
Conclusions
Adjuvant chemotherapy after resection of T1-2N1M0 NSCLC is associated with significantly improved survival in patients older than 65 years. These data can be used to provide elderly patients with realistic expectations of the potential benefits when considering adjuvant chemotherapy in this setting.
Several randomized trials and meta-analyses have shown that adjuvant chemotherapy after resection of stages II–IIIA non-small cell lung cancer (NSCLC) improves survival.1–6 Currently, both the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) recommend adjuvant chemotherapy for patients with completely resected stage II or IIIA NSCLC.7,8 However, pooled data for 4,584 patients from five large trials of cisplatinum-based adjuvant chemotherapy demonstrated a relatively modest 5-year absolute overall survival (OS) benefit of 5.4 %.5 These randomized adjuvant chemotherapy studies also generally enrolled selected participants with relatively heterogeneous stages, good functional statuses, and a low number of comorbidities, and either excluded or tended to have a limited number of older participants.1–3,9,10 Therefore, advising individual older patients about the potential benefits of adjuvant chemotherapy after NSCLC resection in routine clinical practice can be difficult despite the availability of the data from these trials. This study was undertaken to improve the level of evidence available to guide therapy for elderly patients after surgical resection of stage II NSCLC due to N1 nodal disease by specifically examining the use and efficacy of adjuvant chemotherapy using the Surveillance, Epidemiology, and End Results (SEER)–Medicare database.
MATERIALS AND METHODS
This study was performed with approval from the Duke University Institutional Review Board. A retrospective cohort study of patients diagnosed with NSCLC was conducted using the SEER–Medicare database, which brings together Medicare administrative claims data with detailed clinical tumor registry data in a representative sample of the US population across a wide geographic variation.11 From the entire lung cancer cohort, patients who were definitively identified as having stage T1-2N1M0 NSCLC between 1992 and 2006 were selected. Staging was based on the 6th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Individual T, N, and M statuses were recorded in the SEER database starting in 2004 and, therefore, T1-2N1M0 patients diagnosed with lung cancer between 2004 and 2007 were directly identified using these variables. Patients diagnosed with lung cancer before 2004 do not have individual T, N, and M statuses recorded in the SEER and, therefore, T, N, and M statuses were derived from other SEER variables. The T status was derived from the ‘Extent of Disease (EOD) 10—Tumor Size (1988–2003)’ and the ‘EOD 10—Tumor Extent (1988–2003)’ SEER variables. The N status was derived from the ‘EOD 10—Nodes (1988–2003)’ SEER variable, while the M status was derived from the overall AJCC stage SEER variable.
Because the main aim of the study was to evaluate the use and impact of adjuvant chemotherapy after surgical resection, only patients who underwent surgical resection without having received radiation or chemotherapy prior to surgery were included in the analysis. All stages in the study are pathologic because SEER reports the pathologic stage for patients who are not given any pre-resection treatment. Patients were identified as having received surgery, radiation, and/or chemotherapy if there was at least one indicator of treatment within 6 months of diagnosis in the Medicare Provider Analysis and Review (MEDPAR), Outpatient Claims, Durable Medical Equipment (DME) and Carrier Claims Medicare files using Health Care Common Procedure Coding System (HCPCS) codes, International Classification of Diseases, 9th Revision (ICD-9) diagnosis and procedure codes, Current Procedural Terminology (CPT) codes, Revenue Center Codes (RCC), and Diagnostic-related group (DRG) codes as previously described.12–16 The date of onset of chemotherapy was identified using methods originally developed for date of disease onset.17 Only patients whose extent of resection was sublobar, lobar, or a pneumonectomy were included. Patients who died within 31 days of surgery would not have been candidates for adjuvant chemotherapy and were therefore excluded to reduce potential bias in favor of adjuvant chemotherapy.
Patients younger than 65 years of age were excluded because they are eligible for Medicare by reason of disability or end-stage renal disease. Only patients who had both continuous Part A and Part B Medicare coverage with no health maintenance organization enrollment between 1 year prior to and 6 months after diagnosis, or until the month of death for patients who died within 6 months of diagnosis, were included in the analysis to minimize the chance that our analysis could fail to capture treatment due to non-Medicare coverage. This requirement for continuous Medicare coverage for 1 year prior to diagnosis effectively meant that only patients aged 66 years or older at the time of diagnosis were included in the analysis. In addition, patients who did not have a lung cancer diagnosis code in their Medicare claims within 2 months prior to and 3 months after the date of their lung cancer diagnosis in SEER were excluded due to concerns about discrepancies between Medicare records and SEER data. Each patient’s Charlson Comorbidity Index was calculated at the date of diagnosis using Medicare records during a year prior to the date of diagnosis.18,19
Both univariate and multivariable adjusted logistic regression analyses were performed relating adjuvant chemotherapy use to the following patient characteristics: age, T status, sex, race (Black vs. others), extent of surgical resection (lobar, sublobar, pneumonectomy), and Charlson Comorbidity Index, as well as the following information from the patient’s census tract based on the 1990 or 2000 census bureau survey depending on the patient’s year of diagnosis: percentage of Black patients, percentage of persons 25 years of age or older with at least 4 years of college education, and percentage of residents living below the poverty level.
OS analyses were performed both with the Kaplan–Meier method comparing survival curves with the log-rank test, and with an unadjusted Cox proportional hazard model that included age, T status, Charlson Comorbidity Index, extent of surgical resection, adjuvant chemotherapy, and adjuvant radiation. Adjusted survival analysis was also performed using inverse probability weighting to create pseudo-randomization. For each patient, a propensity score for getting adjuvant chemotherapy was calculated using a logistic regression model, with adjuvant chemotherapy as the outcome and the following covariates as predictors: age, extent of surgical resection, T status, Charlson Comorbidity Index, sex, and race, as well as the following census tract information: percentage of people with 4 years of college education, percentage of Black patients, and percentage of people living below the poverty line. An inverse probability weight for each patient was then created as the reciprocal of the probability of having been given adjuvant chemotherapy (calculated propensity score). An adjusted Cox proportional hazard model for survival was then created including this inverse probability weight in addition to age, extent of surgical resection, T status, Charlson Comorbidity Index, adjuvant chemotherapy, and adjuvant radiation.
Unpaired Student’s t tests were used to compare continuous data, and Chi square test for categorical variables. A two-tailed p value of less than 0.05 was considered significant. Data are presented as number (percentage), mean (standard deviation), odds ratios (OR), or hazard ratio (HR) (95 % confidence interval [CI]) where appropriate. The SAS 9.2 statistical package (SAS Institute, Cary, NC, USA) was used for statistical analyses.
RESULTS
Initially, 413,776 individuals with lung cancer were identified in the database, of whom 222,524 were 65 years of age or older with non-small cell cancer histology diagnosed from 1992 to 2006. Of these patients, 2,781 (1.2 %) with T1-2N1M0 disease treated with surgical resection without first being treated with chemotherapy or radiation therapy, survived at least 31 days after surgery, and who met all other inclusion criteria were identified. Of these patients, 784 (28.2 %) were treated with adjuvant chemotherapy and 1,997 (71.8 %) were not given adjuvant chemotherapy. The use of adjuvant chemotherapy over time is shown in Fig. 1. As shown in the plot, the use of chemotherapy appeared to increase in 2003 and 2004, which would have corresponded to completion of the randomized trials that demonstrated the benefit of adjuvant chemotherapy.1,3 A platinum-based regimen was given to 593 (76 %) of the 784 chemotherapy patients: 67 (11 %) one cycle; 63 (11 %) two cycles; 100 (17 %) three cycles; 363 (61 %) four or more cycles.
FIG. 1.
The use of adjuvant chemotherapy after resection of T1-2N1M0 non-small cell lung cancer over the study time period
The characteristics of the patients who were given or were not given adjuvant chemotherapy after surgery are listed in Table 1. In univariate analysis, there were no significant differences in extent of surgical resection, T status, sex, race, Charlson Comorbidity Index, and any of the census tract variables between patients given and not given adjuvant chemotherapy. The percentage of patients younger than 70 years of age was higher in the group who were given chemotherapy, while the percentage of patients who were 75 years of age or older was higher in the group that did not receive chemotherapy. In addition, patients who were given adjuvant chemotherapy were more likely to have also been given adjuvant radiation therapy. After multivariate adjustment, only younger age and higher T status were significantly associated with adjuvant chemotherapy use after surgical resection (Table 2).
TABLE 1.
Specific characteristics of 2,781 patients with T1-2N1M0 NSCLC with surgical resection as the initial treatment in the SEER–Medicare database from 1992 to 2006 stratified by whether adjuvant chemotherapy was used or not
| Variable | Adjuvant chemotherapy (n = 784) (%) | No adjuvant chemotherapy (n = 1,997) (%) | p value |
|---|---|---|---|
| Extent of surgery | 0.76 | ||
| Sublobar | 35 (4.5) | 85 (4.3) | |
| Lobar | 650 (82.9) | 1,639 (82.1) | |
| Pneumonectomy | 99 (12.6) | 273 (13.7) | |
| Age (years) | < 0.0001 | ||
| 66–69 | 281 (35.8) | 480 (24.0) | |
| 70–74 | 262 (33.4) | 639 (32.0) | |
| 75–79 | 188 (24.0) | 540 (27.0) | |
| 80–84 | 48 (6.1) | 269 (13.5) | |
| 85+ | 5 (0.6) | 69 (3.5) | |
| T stage | 0.16 | ||
| T1 | 220 (28.1) | 615 (30.8) | |
| T2 | 564 (71.9) | 1,382 (69.2) | |
| Adjuvant radiation | < 0.0001 | ||
| Yes | 326 (41.6) | 589 (29.5) | |
| No | 458 (58.4) | 1,408 (70.5) | |
| Sex | 0.61 | ||
| Male | 431 (55.0) | 1,119 (56.0) | |
| Female | 353 (45.0) | 878 (44.0) | |
| Race | 0.59 | ||
| Black | 37 (4.7) | 85 (4.3) | |
| Non-Black | 747 (95.3) | 1,912 (95.7) | |
| Charlson comorbidity index | 0.69 | ||
| 0 | 199 (25.4) | 488 (24.4) | |
| 1 | 205 (26.1) | 516 (25.8) | |
| 2 | 150 (19.1) | 370 (18.5) | |
| 3 | 106 (13.5) | 260 (13.0) | |
| 4+ | 124 (15.8) | 363 (18.2) | |
| Census tract percentage of Black patients | 7.4 ± 16.8 | 7.0 ± 16.6 | 0.62 |
| Census tract percentage of people with 4 years of college education | 26.0 ± 16.7 | 25.7 ± 16.6 | 0.69 |
| Census tract percentage of people living below the poverty line | 9.9 ± 8.8 | 10.0 ± 8.0 | 0.71 |
Continuous data ares presented as mean ± SD
NSCLS non-small cell lung cancer, SEER Surveillance, Epidemiology, and End Results
TABLE 2.
Multivariable model of the use of adjuvant chemotherapy for patients with T1-2N1M0 NSCLC with surgical resection as the initial treatment in the SEER–Medicare database from 1992 to 2006
| Predictor | OR | 95 % CI | p value |
|---|---|---|---|
| Extent of surgery | |||
| Sublobar vs. lobar | 1.03 | 0.67–1.57 | 0.90 |
| Pneumonectomy vs. lobar | 0.82 | 0.63–1.06 | 0.12 |
| Age (years) | |||
| 70–74 vs. 66–69 | 0.68 | 0.55–0.84 | 0.0003 |
| 75–79 vs. 66–69 | 0.58 | 0.46–0.73 | < 0.0001 |
| 80–84 vs. 66–69 | 0.30 | 0.21–0.42 | < 0.0001 |
| 85+ vs. 66–69 | 0.12 | 0.05–0.29 | < 0.0001 |
| T stage (T2 vs. T1) | 1.22 | 1.01–1.47 | 0.04 |
| Census tract percentage of people with 4 years of college education | 1.00 | 1.00–1.01 | 0.36 |
| Census tract percentage of Black patients | 1.00 | 1.00–1.01 | 0.75 |
| Charlson comorbidity index | |||
| 1 vs. 0 | 1.02 | 0.80–1.29 | 0.88 |
| 2 vs. 0 | 1.06 | 0.81–1.37 | 0.68 |
| 3 vs. 0 | 1.06 | 0.80–1.42 | 0.68 |
| 4+ vs. 0 | 0.92 | 0.70–1.21 | 0.54 |
| Race (Black vs. non-Black) | 1.04 | 0.61–1.78 | 0.88 |
| Sex (male vs. female) | 0.96 | 0.80–1.14 | 0.60 |
| Census tract percentage of people living below the poverty line | 1.00 | 0.99–1.01 | 0.66 |
NSCLC non-small cell lung cancer, SEER Surveillance, Epidemiology, and End Results, OR odds ratio, CI confidence interval
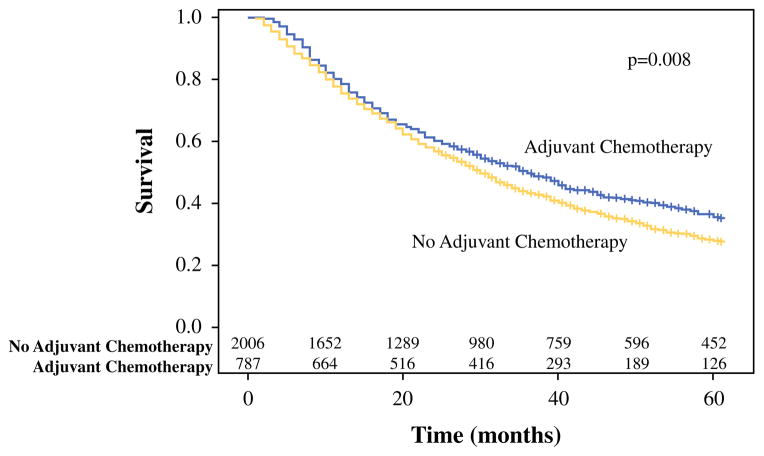
The 5-year OS was significantly better for patients who received adjuvant chemotherapy compared with patients not given adjuvant chemotherapy (35.6 % [95 % CI 31.8–39.4] vs. 27.9 % [95 % CI 25.9–29.9]; p = 0.008) (Fig. 2). In the multivariate-adjusted Cox proportional hazard regression model including inverse probability weight, adjuvant chemotherapy use was the only factor that predicted significantly improved survival (HR 0.84; 95 % CI 0.76–00.92; p = 0.0002) (Table 3). Factors that predicted significantly worse survival included adjuvant radiation use, sublobar instead of lobar resection, age 75 years or older compared with age younger than 70 years, higher T status, and Charlson Comorbidity Index of 3 or higher compared with an index of zero.
FIG. 2.
Survival curve for all patients after resection of T1-2N1M0 non-small cell lung cancer, stratified by whether or not adjuvant chemotherapy was given
TABLE 3.
Unadjusted and inverse probability weight-adjusted Cox proportional hazard regression model of survival for patients with T1-2N1M0 NSCLC with surgical resection as the initial treatment in the SEER-Medicare database from 1992 to 2006
| Predictor | Unadjusted
|
Adjusted
|
||
|---|---|---|---|---|
| HR | p value | HR | p value | |
| Adjuvant chemotherapy (yes vs. no) | 0.83 | 0.0008 | 0.84 | 0.0002 |
| Adjuvant radiation (yes vs. no) | 1.50 | < 0.0001 | 1.59 | < 0.0001 |
| Extent of surgery | ||||
| Sublobar vs. lobar | 1.19 | 0.11 | 1.27 | 0.04 |
| Pneumonectomy vs. lobar | 1.16 | 0.03 | 1.12 | 0.11 |
| Age, years | ||||
| 70–74 vs. 66–69 | 0.99 | 0.83 | 0.98 | 0.73 |
| 75–79 vs. 66–69 | 1.16 | 0.02 | 1.15 | 0.03 |
| 80–84 vs. 66–69 | 1.27 | 0.004 | 1.44 | < 0.0001 |
| 85+ vs. 66–69 | 1.72 | < 0.0001 | 1.92 | < 0.0001 |
| T stage (T2 vs. T1) | 1.38 | < 0.0001 | 1.35 | < 0.0001 |
| Charlson comobidity index | ||||
| 1 vs. 0 | 1.07 | 0.35 | 1.05 | 0.45 |
| 2 vs. 0 | 1.16 | 0.04 | 1.11 | 0.15 |
| 3 vs. 0 | 1.40 | < 0.0001 | 1.35 | 0.0002 |
| 4+ vs. 0 | 1.40 | < 0.0001 | 1.31 | 0.0003 |
NSCLC non-small cell lung cancer, SEER Surveillance, Epidemiology, and End Results, HR hazard ratio
DISCUSSION
In this study, we demonstrated that in the SEER–Medicare database adjuvant chemotherapy was associated with significantly better survival of elderly patients after resection of T1-2N1M0 NSCLC. Adjuvant chemotherapy was used in the minority of patients but the use appeared to increase corresponding to the completion of the phase III trials demonstrating the benefits of this therapy.1,3,4 The use of adjuvant chemotherapy was dependent on age (more often used in younger patients) and T status (more often used for T2 than T1 tumors). Independent of other factors, adjuvant chemotherapy predicted improved survival in inverse probability weight-adjusted multivariable survival analysis.
These data can be used to provide elderly patients and providers with realistic expectations of the potential benefits when considering adjuvant chemotherapy in this setting. Although several recent randomized studies and meta-analyses have demonstrated a survival benefit for adjuvant chemotherapy, guideline adherence for adjuvant chemotherapy after NSCLC resection has been shown to be only 61 %.20 Barriers to the use of adjuvant chemotherapy in non-trial settings likely include opinions of both physician and patient regarding the ability to tolerate chemotherapy and whether the potential benefits of adjuvant therapy outweigh the risks, particularly considering that the benefits of adjuvant chemotherapy are generally relatively modest.5 Counseling patients on the potential benefits for their specific situation can be somewhat difficult as the phase III studies included a relatively heterogeneous group of stages.1–3 Moreover, counseling elderly patients may be even more difficult as most of the randomized adjuvant chemotherapy studies either excluded older participants or enrolled very limited numbers.1–3,9,10 Even though NSCLC is generally a disease of the elderly and the median age at diagnosis is 70 years, only 9 % of patients in the meta-analysis of the randomized trials were older than 70 years.21,22 In the meta-analysis of the randomized trials, patients older than 70 years of age were shown to have had a survival benefit from adjuvant chemotherapy similar to younger patients, despite receiving lower doses and fewer cycles, having lower performance status, and having more non-lung cancer-related causes of death.22 Other retrospective analyses of both randomized study data and registry data have also demonstrated benefits of adjuvant chemotherapy use in patients older than 65 years.23,24 The current study adds to this literature by allowing accurate estimates of the potential benefits of adjuvant chemotherapy use for the specific situation of elderly patients who have undergone resection of NSCLC which is stage II due to N1 nodal involvement in a non-trial setting.
Although the results of this study showed that younger age and a higher T status predicted the use of adjuvant chemotherapy, a study limitation is that clinical information regarding the decision to use or not use adjuvant chemotherapy is not available in the dataset. SEER–Medicare does not contain information on important clinical variables, such as a patient’s overall functional status, pulmonary function data, smoking status, and surgical margin status. Adjuvant chemotherapy may have been preferentially selected for patients who did well after surgery or who had better functional status, better pulmonary function, and less significant current and past smoking use, which are all factors that can impact both treatment selection as well as outcomes such as survival. Another limitation is that we do not know how many patients were evaluated for chemotherapy and declined recommended therapy. Previous studies have shown that as many as 26 % of patients decline further treatment after surgery.25 It is likely that some patients did not feel the risks of adjuvant chemotherapy were worth the potentially increased chance of long-term survival. Future studies should focus the patient–physician decision-making process in this situation, including analysis of patients’ comprehension of options, risks, and benefits, and individual goals of therapy and preferences.
This study has other limitations due to its retrospective nature and reliance on an administrative database in which some data may be missing. Also, the analysis cannot control for specific pathologic details regarding nodal involvement that are not available in the SEER–Medicare database but could impact the adjuvant chemotherapy decision. Even though the study sample is limited to patients with N1 nodal disease, the extent of nodal involvement in this situation can vary from microscopic disease recognized on pathologic examination after resection to bulky, clinically positive disease. In addition, all patients in this study had insurance coverage via Medicare, and therefore the results are not necessarily generalizable to a population of patients that includes uninsured or underinsured patients. However, use of the population-based SEER–Medicare database has the significant advantage of allowing evaluation of a large number of patients in a specific disease stage subset. It is very unlikely that a prospective study that includes a similar number of elderly patients with this specific tumor stage could ever be performed.
Disparities in overall lung cancer treatment and prognosis are known to exist for race, socioeconomic status, and educational status.26–32 However, this study did not find any disparities in treatment due to variables associated with these factors. Importantly, only the clinical variables of patient age and tumor T status appeared to impact whether or not a patient was given adjuvant chemotherapy. It is possible that once patients have access to and agree to a treatment such as surgery for lung cancer, any subsequent disparities in treatment are unlikely.
CONCLUSIONS
Adjuvant chemotherapy use after resection of T1-2N1M0 NSCLC in elderly patients declines with increasing age. However, adjuvant chemotherapy is associated with significantly better survival in this patient population. Patient and providers should carefully consider this potential benefit against the risks of adjuvant chemotherapy, and age alone should not likely preclude treatment that could improve a patient’s long-term prognosis.
Acknowledgments
This work was supported by the National Institute of Health funded Cardiothoracic Surgical Trials Network (5U01HL088953-05: MFB) and by the National Institute on Aging (1R21AG045245-01: MFB, IA).
Footnotes
DISCLOSURE None.
References
- 1.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–27. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 3.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–97. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 4.Strauss GM, Herndon JE, 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–51. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 6.Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–77. doi: 10.1016/S0140-6736(10)60059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:1236–71. doi: 10.6004/jnccn.2012.0130. [DOI] [PubMed] [Google Scholar]
- 8.Pisters KM, Evans WK, Azzoli CG, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I-IIIA resectable non small-cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. doi: 10.1200/JCO.2007.14.1226. [DOI] [PubMed] [Google Scholar]
- 9.Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell lung cancer. J Natl Cancer Inst. 2003;95:1453–61. doi: 10.1093/jnci/djg059. [DOI] [PubMed] [Google Scholar]
- 10.Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–82. doi: 10.1016/j.ejcts.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 12.Berry MF, Worni M, Pietrobon R, D’Amico TA, Akushevich I. Variability in the treatment of elderly patients with stage IIIA (N2) non-small-cell lung cancer. J Thorac Oncol. 2013;8:744–752. doi: 10.1097/JTO.0b013e31828916aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farjah F, Wood DE, Yanez D, 3rd, Symons RG, Krishnadasan B, Flum DR. Temporal trends in the management of potentially resectable lung cancer. Ann Thorac Surg. 2008;85:1850–5. doi: 10.1016/j.athoracsur.2007.12.081. [DOI] [PubMed] [Google Scholar]
- 14.Farjah F, Flum DR, Ramsey SD, Heagerty PJ, Symons RG, Wood DE. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol. 2009;4:355–63. doi: 10.1097/JTO.0b013e318197f4d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund JL, Stürmer T, Harlan LC, Sanoff HK, Sandler RS, Brookhart MA, et al. Identifying specific chemotherapeutic agents in Medicare data: a validation study. Med Care. 2013;51:e27–34. doi: 10.1097/MLR.0b013e31823ab60f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamont EB, Herndon JE, 2nd, Weeks JC, Henderson IC, Lilenbaum R, Schilsky RL, et al. Criterion validity of Medicare chemotherapy claims in Cancer and Leukemia Group B breast and lung cancer trial participants. J Natl Cancer Inst. 2005;97:1080–3. doi: 10.1093/jnci/dji189. [DOI] [PubMed] [Google Scholar]
- 17.Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K, Yashin AI. Age patterns of incidence of geriatric disease in the U.S. elderly population: Medicare-based analysis. J Am Geriatr Soc. 2012;60:323–7. doi: 10.1111/j.1532-5415.2011.03786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic co-morbidity in longitudinal-studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Askamit I, Tuscher L, Bergstrom K. Rates of guideline adherence among US community oncologists treating NSCLC. Am J Manag Care. 2013;19:1854–192. [PubMed] [Google Scholar]
- 21.Howlader N, Noone AM, Krapcho M, et al., editors. SEER cancer statistics review, 1975–2010. Bethesda (MD): National Cancer Institute; Apr, 2013. Available from: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site. [Google Scholar]
- 22.Fruh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non–small-cell lung cancer. J Clin Oncol. 2008;26:3573–81. doi: 10.1200/JCO.2008.16.2727. [DOI] [PubMed] [Google Scholar]
- 23.Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007;25:1553–1561. doi: 10.1200/JCO.2006.09.5570. [DOI] [PubMed] [Google Scholar]
- 24.Wisnivesky JP, Smith CB, Packer S, Strauss GM, Lurslurchachai L, Federman A, et al. Survival and risk of adverse events in older patients receiving postoperative adjuvant chemotherapy for resected stages II–IIIA lung cancer: observational cohort study. BMJ. 2011;343:d4013. doi: 10.1136/bmj.d4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zornosa C, Mamet R, Reid ME, et al. Utilization of adjuvant therapy among completely resected non-small cell lung cancer (NSCLC) patients in the National Comprehensive Cancer Network (NCCN) Outcomes Database Project [abstract no. 7017] J Clin Oncol. 2010;28(15 Suppl) [Google Scholar]
- 26.Gadgeel SM, Kalemkerian DP. Racial differences in lung cancer. Cancer Metastasis Rev. 2003;22:39–46. doi: 10.1023/a:1022207917249. [DOI] [PubMed] [Google Scholar]
- 27.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 28.McCann J, Artinian V, Duhaime L, Lewis JW, Jr, Kvale PA, DiGiovine B. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–6. doi: 10.1378/chest.128.5.3440. [DOI] [PubMed] [Google Scholar]
- 29.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–76. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clegg LX, Reichman ME, Miller BA, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the Surveillance, Epidemiology, and End Results. National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–35. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu KC, Miller BA, Springfield SA. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc. 2007;99:1092–100. [PMC free article] [PubMed] [Google Scholar]
- 32.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–94. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]