Abstract
Integration of diverse synaptic inputs is a basic neuronal operation that relies on many neurocomputational principles, one of which is neural summation. However, we lack empirical understanding of neuronal summation in the human brains in vivo. Here we explored the effect of neural summation in the motor cortex using two subthreshold pulses of transcranial magnetic stimulation (TMS), each with intensities ranging from 60% - 95% of the resting motor threshold (RMT) and interstimulus intervals (ISI) varying from 1 – 25 ms. We found that two subthreshold TMS pulses can produce supra threshold motor response when ISIs were less than 10 ms, most prominent at 1, 1.5 and 3 ms. This facilitatory, above threshold response was evident when the intensity of the subthreshold pulses were above 80% of RMT but was absent as the intensity was 70% or below. Modeling of the summation data across intensity suggested that they followed an exponential function with excellent model fitting. Understanding the constraints for inducing summation of subthreshold stimulations to generate above threshold response may have implications in modeling neural operations and potential clinical applications.
Keywords: subthreshold, summation, TMS, paired pulse, M1
Introduction
Integration of diverse synaptic inputs is a basic neuronal operation that relies on many neurocomputational principles, one of which is neural summation. Early theories suggested that two inputs onto the same dendritic branch would cancel out or attenuate each other; while inputs onto different branches would summate (Rall 1964). Using modern microiontophretic techniques, it was found that linear summation is the dominant effect in excitatory inputs to pyramidal neurons in spatial and temporal summations (Cash and Yuste 1998). Attenuation is less common, but does occur locally in some specific dendritic configurations, although linear summation remains the overall outcome in complex neuronal dendritic configurations (Cash and Yuste 1999). More detailed mechanisms relating to the plasticity of dendritic integration have recently emerged. For example, in response to paired-pulse stimuli, the decay of the voltage response determines the temporal summation of the repeated input, a process that is regulated mainly by G-protein-activated inward rectifier K+ currents (Makara and Magee 2013). Linking such summation effects to the human brain may permit modeling studies in clinical populations. However, summation has been tested in humans primarily in the visual and auditory systems using sensory and behavioral stimuli (e.g., Plack et al. 2006; Morris et al. 2010) that are significantly more complex than the paired current pulses used in basic neuroscience experiments, making it more difficult to translate findings to basic cellular mechanisms.
Safe, localized stimuli to cortical areas can be clinically achieved by transcranial magnetic stimulation (TMS) pulses that generate cortical electric current perpendicular to the magnetic pulse (Hallett 2007). The magnetic field from the TMS coil impresses on electrically conductive brain tissue to produce secondary electrical currents and excites local neural tissue (Barker et al. 1985). Paired pulse TMS has been widely used for research and provides a paradigm that bears close resemblance to paired-pulse based summation experiments in animals. Surprisingly, to our knowledge, there has not been a systematic exploration of the summation mechanism in humans using this approach.
Three early TMS studies have implied a summation effect using a mixture of sub and suprathreshold TMS that included subthreshold-subthreshold testing (Tokimura et al. 1996; Ziemann et al. 1998; Ilic et al. 2002), suggesting that it is feasible to use this approach to study neural summation in humans in vivo. However, neither of these studies systematically studied the summation responses produced by paired presentation of subthreshold stimuli nor have introduced the concept. In the present study, we tested the neural summation effects in human motor cortex by delivering a series of paired subthreshold pulses. We used the data to model the likelihood of subthreshold intensity needed to generate a MEP response. We set the intensity of both first pulse (S1) and second pulse (S2) to same subthreshold levels which ranged from 60 to 95% of resting motor threshold which was defined according to conventional criteria as the minimum intensity needed to elicit a MEP of > 50 μV in at least 5 out of 10 consecutive stimuli (Rossini et al. 1994). Meanwhile, a wide range of interstimulus interval (ISIs; from 1 to 25 ms) was adopted to characterize the time course of the summation effects.
Materials and Methods
Participants
Nine right-handed medically and psychiatrically healthy volunteers (mean age 31.9 ± 10.2 years, range 21 – 48 years; 6 males and 3 females) participated in the study. All subjects were interviewed with the Structured Clinical Interview for DSM-IV to exclude psychiatric and substance abuse diagnosis. Major medical and neurological illnesses were exclusionary. TMS screening interviews confirmed that none of the subjects had contraindications for TMS (Rossi et al. 2009). All subjects gave their written informed consent approved by the University of Maryland Baltimore Institutional Review Board.
Electromyographic recordings
Surface electromyography (EMG) was recorded from the right index finger (first dorsal interosseous (FDI) muscle) with Ag/AgCl disc electrodes (CareFusion Inc., WI, USA) placed in a tendon-belly montage. A ground electrode was placed over the right ulnar styloid. The EMG signal was recorded in DC mode with NeuroScan Synamp2 amplifier (Charlotte, NC), amplified (gain of 10) and digitized at 5k Hz with a 60 Hz notch filter and stored for offline analysis (Darling et al. 2006; Sommer et al. 2002). The offline analysis was conducted by using Scan 4.3 software (Neurosoft, Inc., EI Paso, TX) and MATLAB (MathWorks, Inc., Natick, MA).
Transcranial magnetic stimulation
Focal magnetic stimuli were given over the left primary motor cortex (M1) through a figure-of-eight coil (70 mm outer diameter of each wing) using Magstim 200 Magnetic stimulators with a monophasic current waveform (Magstim Co., Whitland, UK). Prior to the start of the experiment each subject underwent an anatomical MRI scan using T1-weighted MP-RAGE sequences. Imaging data were collected using a Siemens 3T Trio scanner and a 32 channel head coil located at the Maryland Psychiatric Research Center. The structural images were imported into Brainsight™ TMS Frameless Navigation system (Rogue Research Inc, Montreal, Canada) to allow for online control of precise coil positioning (Du et al. 2012). The stimulus target for each participant was the scalp position above the left hemisphere where TMS induced the maximum peak-to-peak MEP amplitude from the right first dorsal interosseous muscle (averaged MNI stereotaxic coordinates: -43, -12, 63). The coil was held by a mechanical arm with the coil handle pointing backward and rotated 45° away from the midline to induce currents that traveled in a posterior-to-anterior direction across the central sulcus (Brasil-Neto et al. 1992; Werhahn et al. 1994; Kammer et al. 2001). The participant was sitting in an upright position with chin rest and two head supports to stabilize the head during TMS. They were also instructed to remain relaxed throughout the application of TMS, while their muscle was monitored for relaxation, confirmed by visual inspection of the EMG. Resting motor threshold (RMT) was defined according to conventional criteria as the minimum intensity needed to elicit a MEP of > 50 μV in at least 5 out of 10 consecutive stimuli (Rossini et al. 1994).
The pulse of each TMS pair (S1 and S2) was delivered with same subthreshold intensity. There were six different intensities of 95%, 90%, 85%, 80%, 70% and 60%. The lower end of the intensities were selected based on initial testing showing no summation when stimulus was below 60 to 70% RMT. For each intensity, there were 13 different ISIs: 1, 1.5, 2, 2.5, 3, 5, 7.5, 10, 12.5, 15, 17.5, 20 and 25 ms. The ppTMS at each ISI and the subthreshold single pulse in the same session were delivered in a pseudoramdomised order to avoid anticipation. Specifically, thirteen different ISIs and two subthreshold single pulses were randomized and constituted a block. Then, eight different blocks were concatenated to form a session. The six different intensities of RMT were tested in six separate sessions. The sessions were in pseudorandomised order, balanced across subjects. The intersession interval ranged from 10 to 20 minutes.
In addition, based on previously published results of finer changes in facilitation occurring at 1 to 2 ms ISI (e.g., Ziemann et al. 1998), a more detailed temporal ISI mapping was created for the 95% RMT subthreshold ppTMS in a separate session. In this session, single subthreshold stimulation and ppTMS with ISIs from 1 to 2.2 ms with 0.1 ms step were tested.
Eight repetitions for each ISI and 16 repetitions for single subthreshold stimulation were evaluated in each session. Therefore, there are 120 trials (ppTMS or single pulse TMS) for each participant within a session. In addition, 24 single pulses at RMT were recorded before and after the testing sessions. These at-threshold single pulses were compared to paired subthreshold ppTMS as a control.
Intertrial intervals ranged from 5 to 10 s within all sessions. Peak-to-peak amplitude of the MEPs was measured. The root mean squared (rms) amplitude of MEP in the period 75 ms to 5 ms prior to the delivery of S1 was obtained. Only trials in which rms of MEP was less than 10 μV were included for further analysis.
Data analysis
Summation effects were examined in two ways. First, we compared amplitudes of paired subthreshold stimuli across different stimulus intensities. Second, we compared subthreshold-subthreshold stimuli with the at-threshold stimulus. In the first approach, the impact of intensity and ISI effects on ppTMS summation effect was evaluated using a 6 × 13 repeated measures analysis of variance (ANOVA) with intensity (95%, 90%, 85%,80%, 70% and 60% RMT) and ISI (1, 1.5, 2, 2.5, 3, 5, 7.5, 10, 12.5, 15, 17.5, 20 and 25ms ISIs) as within-subject factors. For ppTMS with ISIs from 1 to 2.2 ms, a one-way repeated measures ANOVA was conducted (only intensity at 95% was used here). For further comparisons with the at-threshold single pulse (at RMT) control, single stimulation was compared with ppTMS at each ISI by using paired-sample t test. We then modeled the relationship between the subthreshold pulse strength (x) on the activated MEP (y) in those ISIs that showed significant summation effects using an exponential model with equation of y = β0 × eβ1x. β0 and β1 are constant values to be determined by fitting the model to the data. Mean MEP amplitudes across participants were fitted in the model. All tests were two-tailed.
Results
There was no difference between peak-to-peak amplitudes induced by single threshold stimulations at the beginning and the end of the experiment (115.7 ± 77.3 μV vs. 133.7 ± 75.1 μV, respectively, t(8) = .84, p = .42). Therefore, these two amplitudes were averaged to represent the response to threshold stimulation. Single subthreshold pulses across all intensities confirmed responses from the subthreshold stimuli were absent in most and none were above the response amplitude from single at-threshold pulse. The time courses of paired-subthreshold TMS effect at each intensity level are illustrated in Figure 1. There were significant main effects of intensity (F(5,40) = 23.60, p = 0.0003) and ISI (F(12,96) = 10.70, p = .001), and an intensity by ISI interaction (F(60,480) = 6.85, p = .001). Post-hoc analysis using repeated measures ANOVA between pairs of neighboring intensity (e.g., comparing 70% vs. 80%) across all ISIs indicated that there was no significant difference between 70% and 60% RMT stimulations (p = .22) but from 70% to 95%, each increase in intensity (comparing 70% with 80%, 80% with 85%, 85% with 90%, and 90% with 95%) differed significantly from the intensity below it (p values ranged from .004 to .012; FDR corrected), consistent with an intensity effect. This test suggested that a summation was generated by paired subthreshold stimuli at 80% RMT or above. This effect occurred primarily within 10 ms ISI although some residual summation effect was observed in response to ISIs as long as 15 ms at 95% RMT (Figure 1).
Fig. 1.
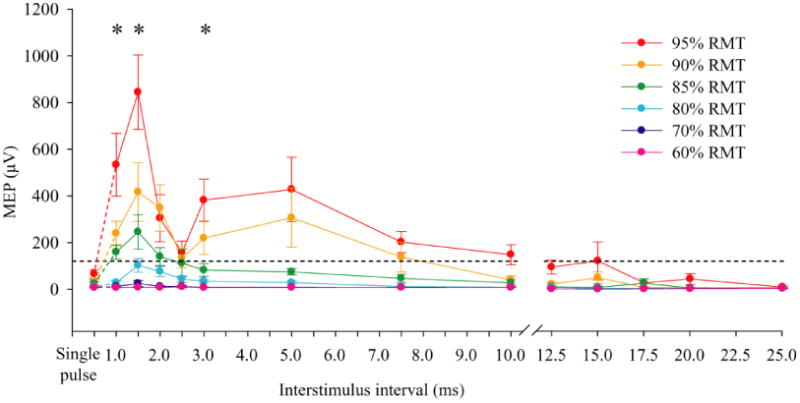
Effect of interstimulus interval (ISI) and intensity of subthreshold stimulus on motor evoked potential (MEP). The peak-to-peak amplitudes of MEP were shown. Dotted horizontal line marks the amplitude of MEP with at-threshold single pulse stimulation. The facilitation occurred as a function of ISI and intensity of the subthreshold stimuli. Compared with non-response at 70% resting motor threshold (RMT), some summation effects can been seen at ISI as far apart as 15 ms. However, compared with at-threshold single pulse stimulaiton, summation occured mainly at 1, 1.5 and 3 ms ISIs with the higher subthreshold stimulation, marked with ‘*’ (p<.05). Error bar indicates SE
Giving the intensity by ISI interaction, additional post-hoc tests focused on individual ISI. Summation effect here was defined as statistically significantly higher response amplitude by paired subthreshold pulses compared with at-threshold single pulse response amplitude. Compared with single pulses, paired subthreshold pulses had significantly higher amplitudes at 1 ms (t(8) = 3.50, p = .008), 1.5 ms (t(8) = 4.80, p = .001) and 3 ms (t(8) = 3.43, p = .009) at 95% RMT. For 90% RMT stimulations, summation effects were shown at 1, 1.5 and 2 ms ISIs (all ps < .05). No summation effects occurred with 60%, 70%, 80% and 85% RMT using this statistical definition.
The same 6 × 13 repeated measure ANOVA was applied to the latencies of the positive peak and negative peak of MEP, respectively. No significant interaction or main effects were found on latency of positive peak (all ps > 0.05) or negative peak (all ps > 0.05). Also, there was no significant difference between the latencies of subthreshold ppTMS and at threshold single pulse TMS (MT).
The ANOVA for ISIs from 1 to 2.2 ms showed a significant effect of ISI (F(13,65) = 4.25, p = .024). Significant summation effects were observed at 1 – 2.1ms ISIs (all ps < .05) compared with at-threshold single pulse (Figure 2).
Fig. 2.
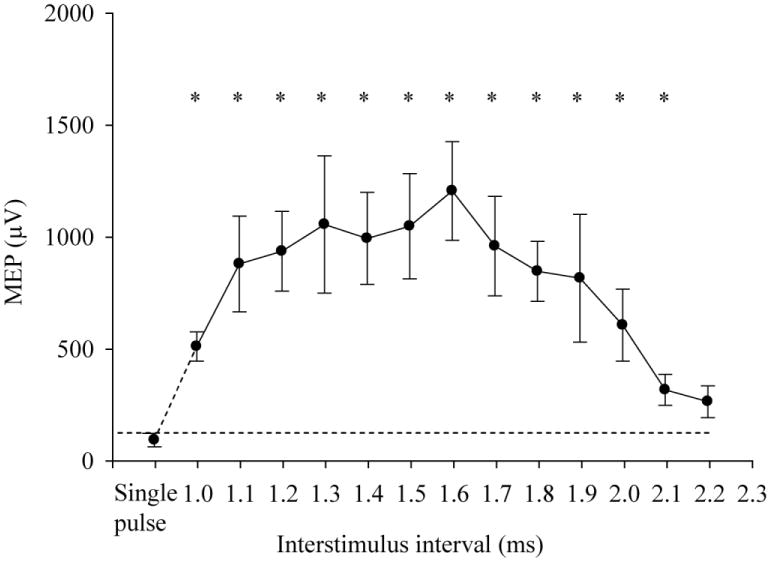
Effect of interstimulus interval (ISI) from 1.0 to 2.2 ms at 95% of resting motor threshold (RMT) on motor evoked potential (MEP). Dotted line indicates amplitude of MEP with at-threshold single stimulation. ‘*’ indicates p<.05. Error bar indicates SE
To further model the nature of the summation effect, we examined the relationship between intensity of the subthreshold stimuli and amplitude of MEP at ISIs that showed the most consistent summation effects (i.e., 1, 1.5 and 3 ms ISIs) (Figure 3).The amplitudes of MEP were well predicted by the strength of subthreshold ppTMS using an exponential model. For 1 ms ISI, 92.8% amplitude variance can be explained by the exponential model (adjusted R2 = 92.8%, F = 65.87, p = .001; y = .0009e13.78x).Similar results were observed for 1.5 ms (adjusted R2 = 99.7%, F = 1916.30, p < .001; y = .0009e13.97x) and 3 ms ISIs (adjusted R2 = 94.2%, F = 81.98, p = .001; y = .0009e13.10x).When narrowing the intensity range to the intensities with significant summation effect (e.g., 80%-95% RMT), the relationship between MEP size and intensity was linear (adjusted R2 = .89 for 1 and 1.5 ms ISIs; adjusted R2 = .93 for 3ms ISI).
Fig. 3.
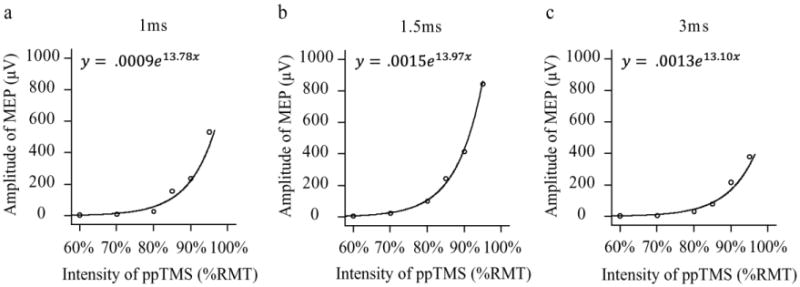
Subthreshold summation effects followed a similar exponential function across different ISI. The exponential equation and curve-fit were shown for each ISI that exhibited summation effect (a, b and c). Although the amplitudes were quite different in different ISIs, the fits were excellent and the parameters of the exponential functions were similar
Discussion
Although we cannot easily assess the neural input-output function in humans in dendritic and neuronal levels, TMS offers a way to examine the input summation in local neural assemblies. We found that TMS can reliably reproduce neural summation-like phenomenon out of paired subthreshold stimuli in the human motor cortex. Paired-pulse subthreshold TMS can produce above-threshold MEP as a function of intensity and ISI. As expected, the higher the subthreshold intensity, the stronger the summation. The summation effect was the most robust at ISI 1.3 to 1.6 ms (Figure 1 and 2). At intensities below 80% of RMT (based on testing using 60% and 70% RMT), essentially no summation effect could be observed. No summation was obtained with subthreshold ppTMS at ISIs of 15 ms or longer at any subthreshold stimulus pairs up to 95% RMT.
The ppTMS with 95% RMT at ISIs of 1 to 2.2 ms replicated the findings when ppTMS was given with S1 above or around RMT and S2 is at RMT (Tokimura et al. 1996; Ziemann et al. 1998; Di Lazzaro et al. 2000; Shirota et al. 2010). Specifically, ppTMS with ISIs of 1 – 2 ms generated much larger EMG responses than the response to baseline. This suggests that setting both S1 and S2 to same subthreshold intensity does not differ from the paradigms in which S1 and S2 are assigned different intensities as long as they are close to motor threshold.
We consider the subthreshold-subthreshold testing as “summation” rather than “facilitation” in the sense that facilitation implies a response that is enhanced by another event. In contrast, summation refers to two no-response subthreshold stimuli, which when combined generate a response that is at or above the response amplitude of an at-threshold stimulus. When the intensity was varied from 60% to 95% RMT, summation was shown only at 80% or higher RMT. The summation was ‘intensity dependent’, but following an exponential rather than linear function (Figure 3), at least with a 1, 1.5, and 3 ms ISIs. This ISI range is within the known ISI to generate SICF (Tokimura et al. 1996; Ziemann et al. 1998; Di Lazzaro et al. 2000; Shirota et al. 2010), with the only difference here that both stimuli were subthreshold. When both stimuli were set to same subthreshold intensity, the minimum intensity required to generate a significant summation appears to be at 80%, or between 70% to 80% of RMT. This is lower than what was described previously (Tokimura et al. 1996; Ziemann et al. 1998; Di Lazzaro et al. 2000; Shirota et al. 2010) and is likely due to more comprehensive search for the minimum threshold conducted in this experiment.
Intracortical I-wave has been used to explain SICF. It has been proposed that the discrete ISIs associated with SICF reflect the periodicity of I-waves induced by S1 (Tokimura et al. 1996; Di Lazzaro et al. 1999; Hanajima et al. 2002). Single high intensity TMS pulse can elicit a group descending volley in the pyramidal tracts including both D wave and I waves (Amassian et al. 1990; Rothwell et al. 1994; Kaneko et al. 1996). However, when the intensity of TMS was reduced, the number of elicited I waves was also decreased (Kaneko et al. 1996). Following this proposed mechanism, it is likely that in the current study, when the intensity of S1 was lower than 70 to 80% RMT, S1 did not evoke enough I-waves (e.g., I2 and I3-waves) to influence the following S2. Others have modeled the effect of subthreshold S1 as primarily to activate inhibitory interneurons, which successful simulated SICI and ICF effects (Rusu et al. 2014). However, subthreshold-subthreshold stimuli were not simulated; it may seem that inhibitory interneuron activation alone would not easily explain a summation effect from two subthreshold stimuli.
Beside the I-wave based explanation, other potential mechanisms should also be considered. For example, the short latency potentiation observed with ISIs in the 1.0 – 1.75 ms range could be due to a passive summation that effectively increases the duration of the stimulus pulse. At these short intervals, the time constant of the neuronal membrane may not be fast enough to result in two discrete depolarizations in response to their application. Rather, the membranes could respond as if the duration of the pulse had been widened to include both pulses. The net effect would be to increase the overall response to TMS. As the interval between the pulses is increased beyond 1.75 ms, the membrane may begin to partially recover between the pulses and by 2.5 ms the sequential subthreshold stimuli are encoded as two separate events and the response again approaches control values, explaining a dip in the summation effect graph (Figure 1). As the interval between pulses is increased and the interval between the two pulses is long enough to elicit discrete synaptic responses, a form of synaptic potentiation may begin to develop. Therefore, it is possible that the very short ISI summation may have a different underlying mechanism compared with the summation occurring later. However, the data that do not directly support this hypothesis are from model fitting in Figure 3, which suggests that the response curves for 1 and 1.5 ms (before 2.5 ms) and the response curve for 3 ms (after 2.5 ms) were similar, suggesting a common mechanism. Invasive animal studies may be required to fully test these hypotheses.
The MEP size to paired subthrehold ppTMS can be predicted by the intensity of stimulation using a non-linear exponential model. However, it is also plausible that the exponential model observed here is the lower half of a typical, intensity-response sigmoid function. Indeed, with single pulse TMS, the input-output relationship between MEP size and stimulus intensity is sigmoidal (Ginanneschi et al. 2005). In this context, we should point out that the summation of paired subthreshold stimulations occurred with intensity from 80% to 95% RMT is likely linear because in this range of intensity, the linear model between ppTMS response and intensity fit the data well (adjusted R2= .89 - .93). This is consistent with the fact that at the cellular level, summation of two subthreshold stimulations is nearly uniformly linear and supported by several active conductances to ensure the arithmatic summation of multiple dendritic inputs (Cash and Yuste 1999). Considering the differences between TMS stimulation and cellular level stimulation, further studies are needed to clarify the neural mechanism of paired subthreshold ppTMS summation.
We extended the ISI range to 25 ms and found no paired pulse summation effects beyond 10 to 15 ms ISI at any of the tested subthreshold intensities. This suggests that the summation effect described here may be different from intracortical facilitation (ICF) where S1 was subthreshold and S2 was suprathreshold, and facilitation was shown at ISIs from 9 to 21 ms (Kujirai et al. 1993; Maeda et al. 2002; Rosenkranz and Rothwell 2003; McClintock et al. 2011; Du et al. 2014). This result indicates that subthreshold-subthreshold summation may have a separate underlying mechanism compared to that for ICF. In summary, we clarified the subthreshold intensity and ISI constraints for inducing paired-subthreshold summation. Future studies are needed to determine the potential biological and behavioral implications of subthreshold summation in human motor cortex, and whether this phenomenon is generalizable to other cortical areas of the brain.
Acknowledgments
This research was supported in part by National Institutes of Health (NIH) grants MH085646, DA027680, MH049826, and MH077852.
Footnotes
Conflict of Interest: All authors report no conflict of interests.
References
- Amassian VE, Quirk GJ, Stewart M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electroencephalogr Clin Neurophysiol. 1990;77:390–401. doi: 10.1016/0168-5597(90)90061-h. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: Factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Cash S, Yuste R. Input summation by cultured pyramidal neurons is linear and position-independent. J Neurosci. 1998;18:10–15. doi: 10.1523/JNEUROSCI.18-01-00010.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron. 1999;22:383–394. doi: 10.1016/s0896-6273(00)81098-3. [DOI] [PubMed] [Google Scholar]
- Darling WG, Wolf SL, Butler AJ. Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res. 2006;174:376–385. doi: 10.1007/s00221-006-0468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Du X, Chen L, Zhou K. The role of the left posterior parietal lobule in top -down modulation on space-based attention: A transcranial magnetic stimulation study. Hum Brain Mapp. 2012;33:2477–2486. doi: 10.1002/hbm.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Summerfelt A, Chiappelli J, Holcomb HH, Hong LE. Individualized brain inhibition and excitation profile in response to paired-pulse TMS. J Mot Behav. 2014;46:39–48. doi: 10.1080/00222895.2013.850401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginanneschi F, Del Santo F, Dominici F, Gelli F, Mazzocchio R, Rossi A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res. 2005;161:374–382. doi: 10.1007/s00221-004-2084-x. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: A primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Enomoto H, Shiio Y, Mochizuki H, Furubayashi T, Uesugi H, Iwata NK, Kanazawa I. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J Physiol. 2002;538:253–261. doi: 10.1113/jphysiol.2001.013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer T, Beck S, Thielscher A, Laubis-Herrmann U, Topka H. Motor thresholds in humans: A transcranial magnetic stimulation study comparing different pulse waveforms, current directions and stimulator types. Clin Neurophysiol. 2001;112:250–258. doi: 10.1016/s1388-2457(00)00513-7. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Gangitano M, Thall M, Pascual-Leone A. Inter- and intra-individual variability of paired-pulse curves with transcranial magnetic stimulation (TMS) Clin Neurophysiol. 2002;113:376–382. doi: 10.1016/s1388-2457(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Makara JK, Magee JC. Variable dendritic integration in hippocampal CA3 pyramidal neurons. Neuron. 2013;80:1438–1450. doi: 10.1016/j.neuron.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: A neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Liu CC, Cropper SJ, Forte JD, Krekelberg B, Mattingley JB. Summation of visual motion across eye movements reflects a nonspatial decision mechanism. J Neurosci. 2010;30:9821–9830. doi: 10.1523/JNEUROSCI.1705-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack CJ, Oxenham AJ, Drga V. Masking by inaudible sounds and the linearity of temporal summation. J Neurosci. 2006;26:8767–8773. doi: 10.1523/JNEUROSCI.1134-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Theoretical significance of dendritic trees for neuronal input-output relations. In: Reiss RF, editor. Neural Theory and Modeling. Stanford Univ. Press; 1964. pp. 73–97. [Google Scholar]
- Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol. 2003;551:649–660. doi: 10.1113/jphysiol.2003.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini P, Barker A, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: Further evidence for the site of activation. J Physiol. 1994;481:243–250. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu CV, Murakami M, Ziemann U, Triesch J. A model of TMS-induced I-waves in motor cortex. Brain Stimul. 2014;7:401–414. doi: 10.1016/j.brs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Hamada M, Terao Y, Matsumoto H, Ohminami S, Furubayashi T, Nakatani-Enomoto S, Ugawa Y, Hanajima R. Influence of short-interval intracortical inhibition on short-interval intracortical facilitation in human primary motor cortex. J Neurophysiol. 2010;104:1382–1391. doi: 10.1152/jn.00164.2010. [DOI] [PubMed] [Google Scholar]
- Sommer M, Wu T, Tergau F, Paulus W. Intra- and interindividual variability of motor responses to repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2002;113:265–269. doi: 10.1016/s1388-2457(01)00726-x. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Ridding M, Tokimura Y, Amassian V, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Werhahn K, Fong J, Meyer B, Priori A, Rothwell J, Day B, Thompson P. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


