Abstract
The vascular endothelial growth factor receptor-2 (VEGFR-2) belongs to the family of receptor tyrosine kinases (RTKs) and is a key player in vasculogenesis and pathological angiogenesis. An emerging picture of posttranslational modifications (PTMs) of VEGFR-2 suggests that they play central roles in generating a highly dynamic and complex signaling system that regulates key angiogenic responses ranging from endothelial cell differentiation, proliferation, migration to permeability. Recent mass spectrometry analysis of VEGFR-2 uncovered previously unrecognized PTMs on VEGFR-2 with a distinct function. The ligand binding extracellular domain of VEGFR-2 is composed of seven immunoglobulin-like domains highly decorated with N-glycosylation, while its cytoplasmic domain is subject to multiple PTMs including Tyr, Ser/Thr phosphorylation, Arg and Lys methylation, acetylation and ubiquitination. Here we review the PTMs on VEGFR-2, their importance in angiogenic signaling relays and possible novel therapeutic potentials.
Life and waves have this in common
they carry some things in
and flush some things away
and with the coming of the tide
the waves flush the sandcastles
but push in a piece of wood
so the humble man
may fix the roof
of his hutMargot Bickel (translated from German by Katharine Fournier)
Introduction
Increased angiogenesis, formation of new blood vessels, plays a prominent role in the progression of cancer, neovascular ocular diseases including age-related macular degeneration (AMD), and other diseases [1, 2]. Conversely, insufficient angiogenesis also is linked to coronary heart diseases, stroke and delayed wound healing [2]. Although major progress toward anti-angiogenesis therapy for treatment of cancer and AMD has been achieved, translating the pre-clinical successes into the clinic has been more challenging than anticipated.
The effects of current FDA-approved anti-angiogenic drugs such as bevacizumab/Avastin (a recombinant humanized monoclonal antibody directed against the vascular endothelial growth factor (VEGF) binds to VEGF and inhibits VEGF receptor binding), sorafenib (a small molecule multi-kinase inhibitor that, inhibits the VEGFR-2, PDGFR and Raf kinase) and sunitinib (a small molecule multi-kinase inhibitor that blocks the tyrosine kinase activities of VEGFR2, PDGFR, FLT3, and c-kit) are all short-lived, and patients often develop resistance, respond poorly or are refractory to anti-angiogenic drugs altogether [3–5]. In certain cases, anti-angiogenesis drugs even promote tumor metastasis [6–8], raising serious questions about the benefit of anti-angiogenesis agents in the treatment of cancer.
The refractoriness and acquired resistance to anti-angiogenesis drugs represent major challenges to effective cancer therapies. By design, these anti-angiogenesis agents either target the function of VEGFR-2by blocking the VEGF ligands binding to VEGFR-2 or inhibit the kinase activity of VEGFR-2, as is the case with the small molecule kinase inhibitors [8]. VEGFR-2 is one of the most important receptor tyrosine kinases (RTK) among the VEGF receptor subfamily, and its pivotal role is well recognized in diseases ranging from cancer to age-related macular degeneration and inflammation. Consequently, VEGFR-2 has been a topic of immense interest for drug targeting and the current anti-angiogenesis FDA approved drugs Bevacizumab, Sunitinib, Sorafenib and Pegaptanib. Pegaptanib is a pegylated anti-VEGF aptamer which blocks VEGF binding to VEGFR-2 and is FDA approved for treatment of AMD [8].
VEGFR-2 expression and function are highly regulated at the transcriptional, post-transcriptional and posttranslational (PTM) levels. VEGFR-2 undergoes multiple co- and post- translational modifications, such as glycosylation, methylation and phosphorylation. In general, some modifications such as N- and O-linked glycosylation take place in the rough endoplasmic reticulum either during synthesis (co-translational), whereas other, post-translational, modifications such as phosphorylation, acetylation, ubiquitination, remodeling of N-linked glycans and O-linked glycosylation take place after the initial synthetic step has been completed Post-translational modifications can take place in different cellular compartment such as the Golgi, endosomes, nucleus and secretory vesicles and at the plasma membrane. To move forward and to develop effective angiogenesis-based medicine, we need answers to the questions such as the following: (1) what are the posttranslational modifications (PTMs) governing activation and inhibition of angiogenic signaling in normal and pathological conditions, (2) how do they interact in normal and pathological circumstances, (3) how and with what intensity does their interaction continue to influence overall angiogenic events and the disease processes? The use of advanced proteomics and microsequencing technologies, coupled with the analysis of large repositories of cancer patient samples could permit visualization of the disease processes at the molecular level, and this may lead to better understanding and development of new strategies to treat cancer and other debilitating human diseases.
The human genome encodes for 58 receptor tyrosine kinases (RTKs). VEGF receptors, including VEGFR-1 (FLT-1), VEGFR-2 (FLK1/KDR) and VEGFR-3 (FLT4), represent one of the 20 subfamilies of RTKs [9]. All RTKs have a similar molecular design: an extracellular domain with ligand binding domains, a single transmembrane domain, and a cytoplasmic region encompassing a juxtamembrane (JM), a tyrosine kinase (TK) domain, and a regulatory carboxyl terminal. Prior to ligand binding RTKs are mostly present as monomers and are inactive; in the presence of specific ligands, RTKs undergo dimerization followed by increase in catalytic activity and trans-phosphorylation [9]. VEGFR-2 undergoes extensive PTMs, including N-glycosylation, Tyr, Ser/Thr phosphorylation, Arg and Lys methylation, acetylation and ubiquitination (Figure 1).
Figure 1.
Schematic of major PTMs on VEGFR-2. VEGFR-2 is heavily N-glycosylated on the extracellular domain. Glycan symbols are those recommended by the Consortium for Functional Glycomics (www.functionalglycomics.org): Gal, yellow circle; GalNAc, yellow square; GlcNAc, blue square; Glu, blue circle; Man, green circle. VEGFR-2 is phosphorylated on multiple serine, threonine and tyrosine residues (see text). Phosphorylation of VEGFR-2 is catalyzed by VEGFR-2 itself and other kinases. Multiple Lys and Arg residues on the cytoplasmic region of VEGFR-2 are methylated (see the text). VEGFR-2 undergoes ligand-induced ubiquitination. P, phosphorylation; Me, methylation; Ub, ubiquitination; SAM, S-adenosylmethionine; ATP, Adenosine triphosphate.
Glycosylation of VEGFR-2
N-linked glycosylation is the most common co- or post-translational modification found in eukaryotic cells, involving cell surface and secreted soluble proteins. VEGFR-2, like most membrane proteins that translocate into the ER, is co-translationally modified with an N-linked oligosaccharide that is added onto an Asn in a consensus Asn-X-Ser/Thr motif (in which X represents any amino acid except Pro). The subsequent trimming of glucose residues by glucosidases, and the assistance of lectin-like chaperones is thought to be required for membrane proteins to exit the ER and avoid ER-associated degradation (ERAD) [10]. Subsequent actions by mannosidases and glycosyltransferases can remodel the initial high mannose oligosaccharide structure into hybrid and complex forms (Figure 1). The extracellular region of VEGFR-2 is composed of 762 amino acids folded into seven immunoglobulin domains [11]. There are numerous N-linked glycosylation sites on the extracellular domain of VEGFR-2. Preventing N-glycosylation of VEGFR-2 with treatment of endothelial cells with agents such as Tunicamycin, an inhibitor of N-glycosylation, renders VEGFR-2 unstable and, as a result, it is rapidly degraded (unpublished data). Furthermore, Itraconazole, an antifungal drug that interferes with N-glycosylation processes, also blocks VEGFR-2 trafficking and its cell surface expression [12]. Glycosylation of VEGFR-2 is required for its ability to bind and undergo VEGF ligand-dependent stimulation [13], underscoring the role of glycosylation in its expression and appropriate signaling. In addition, the binding of the cytoplasmic domain of VEGFR-2 to chaperone proteins also protects VEGFR-2 from ERAD. A recent study through a yeast two-hybrid binding assay, identified phosducin-like 3 (PDCL3), as a chaperone protein involved in maturation of VEGFR-2 [14]. De-coupling of the binding of VEGFR-2 from PDCL3 promoted its ubiquitination and degradation [14].
Tyrosine phosphorylation of VEGFR-2
There are at least 19 possible tyrosine phosphorylation residues on the cytoplasmic domain of VEGFR-2 and all are conserved in humans and mice. Figure 2A summarizes the phosphorylation sites on VEGFR-2 and association of some key signaling proteins with VEGFR-2. Phosphorylation of tyrosines 1052 and 1057 on mouse VEGFR-2 (corresponding to Y1054 and Y1059 on human VEGFR-2), which are situated in the kinase domain, is involved in the kinase activity of VEGFR-2 [15–17]. Phosphorylation of Y1052 and Y1057 of VEGFR-2 was confirmed by various studies using phospho-specific antibodies [18–20]. Our recent mass spectrometry analysis of VEGFR-2, performed with an LTQ-Orbitrap XL instrument (ThermoFisher, San Jose, CA) extended the earlier antibody-based observations by providing site localizations for the post-translational modifications. Figure 2B shows the ESI-CID tandem mass spectrum of the [M + 2H]2+ m/z 722.2837 (calc.) of the tryptic peptide DIpYKDPDpYVR that contains the phosphorylated residues Y1052 and Y1057 was observed in the Orbitrap with Δ −0.58 ppm. This measurement accuracy permits distinction between phosphate (79.9663 u) and sulfate (79.9568 u) modifications that have the same nominal mass. The 243.0-u intervals measured in the quadrupole ion trap between b2 and b3 and between y7 and y8 for Y1052 and between b7 and b8 and between y2 and y3 for Y1057 define these residues as the phosphorylation sites (pY calc. 243.03 u).
Figure 2.
Outline of phosphorylation sites and major signaling pathways associated with VEGFR-2. (A) VEGFR-2 undergoes ligand-induced dimerization (not shown) and phosphorylation at various tyrosine and serine/threonine residues (see the text). (B) CID tandem mass spectrum of VEGFR-2 phosphorylated at Tyr1052 and Tyr 1507 [M + H]2+ m/z 722.2837. JM, juxtamembrane; KD, kinase domain; KI, kinase insert; PEST, The PEST motif (rich in proline [P], glutamic acid [E], serine [S], and threonine [T]), CT, carboxyl terminus. VEGFR-2 was immunoprecipitated with anti–VEGFR-2 antibody from PAE cells ectopically expressing VEGFR-2. The immunoprecipitated proteins were separated on the SDS-PAGE gel and selected spots from the gel were subjected to proteolytic digestion (incubated with trypsin at 37 °C for 4 h). LC/MS/MS analysis was performed by Nextgensceinces (Ann Arbor, MI). Gel digests were pooled and aliquots were analyzed directly using LC/MS/MS. The remaining sample was enriched for phosphopeptides using TiO2 beads (GL Biosciences) and analyzed with a 30-min gradient on a ThermoFisher LTQ Orbitrap XL mass spectrometer. The mass spectrometer was operated in the data-dependent mode; the six most abundant ions were selected for MS/MS. The Orbitrap MS scan was performed at 60,000 FWHM resolution at m/z 400. Product ion data were searched against the concatenated forward and reverse IPI Mouse database using the Mascot search engine. (N. Rahimi et al., unpublished results) Assignments were manually verified at BUSM.
Single mutations of either Y1052 or Y1057 to F did not interfere significantly with the kinase activation of VEGFR-2. However, mutation of both Y1052 and Y1057 rendered VEGFR-2 into an inactive kinase [16]. In addition to their central role in the kinase activation of VEGFR-2, phosphorylated Y1052 and Y1057 also interact with c-Cbl ubiquitin E3 ligase [18] and the SH2 domain (Src homology domain-2) of Src family kinases [16]. c-Src appears to be recruited to VEGFR-2 also indirectly through association with TSAd [21]. After c-Src is recruited to VEGFR-2, in addition to other cellular effects in endothelial cells, it phosphorylates Y1173 (corresponding to Y1175 on human VEGFR-2) on VEGFR-2 [16, 22], in addition to other cellular effects in endothelial cells.it.
Phosphorylation of Y1173 plays a key role in recruitment of key signaling substrates to VEGFR-2 and thus its phosphorylation is critically important for VEGFR-2 induced angiogenic signaling pathways in endothelial cells [23]. Recruitment of multiple signaling proteins to VEGFR-2 such as phospholipase Cγ1 (PLCγ1), PI3kinase, and Shb adaptor protein [24–28] all are established through phosphorylated Y1173. PI3kinase also associates with VEGFR-2 through Y799 (corresponding to Y801 on human VEGFR-2) [24]. Binding of PI3kinase to VEGFR-2 could be established directly [24] or indirectly through Shb adaptor protein [25]. Since multiple substrates bind to VEGFR-2 via Y1173, it is likely that factors that alter expression or their function could skew the angiogenic signaling of VEGFR-2 resulting in hyper- or hypo-activation of a specific signaling pathway. Further research is needed to systematically establish the role of other potentially phosphorylated tyrosine residues on VEGFR-2 and their engagement in the recruitment of signaling proteins to VEGFR-2. Also, given that tyrosine phosphorylation is a spatially and temporally regulated PTM, to fully harness tyrosine phosphorylations of VEGFR-2 and elucidate their roles relative to angiogenic events, its highly important to understand the molecular mechanisms governing spatial and temporal tyrosine phosphorylation of VEGFR-2 in normal and pathological angiogenesis.
Serine and threonine phosphorylation of VEGFR-2
In addition to extensive tyrosine phosphorylation, VEGFR-2 also undergoes multiple serine and threonine phosphorylations. A recent study has identified a PEST motif on the C-terminus of VEGFR-2 [29]. The PEST motif (rich in proline [P], glutamic acid [E], serine [S], and threonine [T]) is a signature of short-lived proteins degraded by the ubiquitin pathway. Phosphorylated Ser and Thr in the PEST domain serve as “phosphodegron” motif for the recruitment of F-box-containing ubiquitin E3 ligase, β-TRCP that leads to ubiquitination and degradation of VEGFR-2 [29]. In addition, multiple serine and threonine residues close to the C-terminus of VEGFR-2 are also phosphorylated, possibly by Casein kinase 1 (CKI) and are involved in the recruitment of β-TRCP to VEGFR-2[30]. Our recent mass spectrometry analysis of VEGFR-2 also identified at least 10 possible serine and threonine phosphorylation residues on VEGFR-2 (Figure 3A), making VEGFR-2 among the most highly phosphorylated proteins.
Figure 3.
Summary of serine and threonine phosphorylation of VEGFR-2. (A) Table shows the phosphorylated serine and threonine residues on mouse VEGFR-2 identified by LC-MS/MS analysis. Predicated kinases involved in the phosphorylation of these sites are shown (GPS 2.1-Kinase-specific Phosphorylation Site Prediction was used). (B) CID tandem mass spectrum of the peptide NKLpSPSFGGM[O]MPSK containing VEGFR-2 phosphorylated at Ser1277, [M + 2H]2+ m/z 788.8490; see caption of Figure 2 for experimental details. (N. Rahimi et al., unpublished results)
The ESI-CID tandem mass spectrum of the tryptic peptide NKLpSPSFGGM[O]MPSK containing phospho-Ser1277 and oxidation at Met183, for which the [M + 2H]2+ m/z 788.8490 (calc.) was observed in the Orbitrap with Δ +0.62 ppm, is shown in Figure 3B. The accurate mass and the facile loss of 98 Da from the precursor indicate that the modification is phosphorylation and not sulfation. The CID spectrum recorded in the quadrupole ion trap contains a complete set of b fragments and a nearly complete set of y-type fragment ions, as well as numerous lower abundance fragments that correspond to water losses from the members of these series; the measured intervals of 167.0 u between b3 and b4 and between y10 and y11 indicate that the residue in position 1277 is phosphoSer (calc. 167.0 u) and the interval of 147.0 u observed between b9 and b10 indicates that the Met oxidation occurred primarily on M183 rather than M184.
Different Ser/Thr kinases are predicted to phosphorylate these residues (Figure 3A), suggesting that phosphorylation of these residues on VEGFR-2 could project diverse effects on VEGFR-2 function and angiogenesis. Of these Ser/Thr phosphorylated residues, phosphorylation of Ser1277, possibly by p38 MAPK, could significantly affect VEGFR-2 stability. Activation of VEGFR-2 stimulates phosphorylation of p38 MAPK [29, 31] resulting in the activation of p38, which generates diverse endothelial cell responses including inhibition of endothelial cell proliferation, migration [31] and tube formation [32]. Conversely, its activation also stimulates endothelial cell survival [33]. A recent study demonstrates that activation of p38 also targets VEGFR-2 and inhibits ligand-induced VEGFR-2 downregulation [29]. It is plausible that p38, in part by phosphorylating Ser1277, inhibits VEGF-induced downregulation of VEGFR-2.
Protein arginine and lysine methylation
In recent years, it has become evident that protein methylation, like protein phosphorylation, is a major PTM controlling the function of a broad class of proteins beyond histones [34, 35]. Methylation is a PTM that occurs on the side chains of Lys and Arg residues of proteins and can regulate protein-protein interactions and protein functions. The transfer of methyl groups from S-adenosylmethionine (SAM), a universal methyl donor, to the side-chain nitrogen atoms of Arg and Lys residues is catalyzed by protein arginine methyltransferases (PRMTs) and lysine methyltransferases (KMTs), respectively [36]. Currently, 10 Protein arginine methyltransferases (PRMT) have been identified in mammalian cells; based on their enzymatic activities, these are classified into three types [34]. PRMTs contain a set of four conserved sequence motifs (I, post-I, II, and III) and a THW loop which constitute the SAM-binding pocket [37] and are known to catalyze the formation of mono-methylarginine (MMA), asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA) (Figure 4). Besides these conserved sequence motifs and a THW loop, most PRMTs also contain some other defined protein domain [38], which may contribute to their cellular localization, interaction with other proteins or provide additional function. It is thought all PRMTs (type I, II & III) could catalyze MMA, whereas type I PRMTs catalyze ADMA and type II PRMTs catalyze SDMA [34].
Figure 4.
Outline of arginine and lysine methylation pathways. PRMTs are responsible for methylation of arginine, whereas KMTs are responsible for catalyzing methylation of lysine residues. PRMT, protein arginine methyltransferases, lysine methyltransferases (KMT), MMA, Mono-methylarginine; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
KMTs catalyze the formation of mono-, di-, or tri-methylation on the ε-amino group of lysine residues on proteins (Figure 4). Methylation of lysine in proteins increases hydrophobicity and steric bulk and hence can influence local hydrogen bond formation and protein-protein interactions. All the currently known KMTs contain SET (Su(var)3–9, Enhancer of Zeste, Trithorax) domain except DOT1/DOT1L[39, 40]. A recent study suggests that the human genome might encode for more than 200 KMTs [41], significantly increasing the number of known SET domain and non-SET domain containing KMTs.
Role of protein methylation in signal transduction and VEGF receptors activation
Similar to protein phosphorylation, emerging evidence now demonstrates that methylation modulates protein function at multiple levels including protein-protein interaction, enzymatic activation of RTKs, and protein phosphorylation (Figure 5). Methylated Arg and Lys can create a new binding site on a protein. For example, the chromo domain (chromatin organization modifier) [42], PHD (Plant Homeo domain) domain [43], Tudor domain (a conserved domain originally found in Tudor protein encoded in Drosophila)[44], MBT (Malignant Brain Tumor) domain [44, 45], and WD40 (beta-transducin repeat) domain [46] all interact with their partner proteins through Arg and Lys-methylation. Methylation also can inhibit protein-protein interactions. The binding of Sam68 (Src-associated substrate in Mitosis of 68 kDa) with Src family kinases is mediated through SH3 (Src homology domain 3) of Src and the proline-rich domain of Sam68 is inhibited by methylation of a distal Arg residue [47]. Similarly, the interaction of STAT1 with PIAS1 [48] and the binding of p53 with its associated genes [49] are inhibited by methylation.
Figure 5.
Outline of Arg/Lys-methylation in protein-protein interaction and cross-talk with protein phosphorylation. (A) Tyrosine phosphorylation creates binding with SH2 domain containing proteins, whereas Lys/Arg methylation creates binding for proteins with Chromo, PHD, Tudor, MBT and WD40 domains. (B) Protein methylation in some cases inhibits protein-protein interaction (see the text). (C) Cross-talk between protein methylation and tyrosine phosphorylation.
There is also cross-talk between methylation and phosphorylation pathways. Arginine methylation of the FOXO1 transcription factor at conserved Arg248 and Arg250 by PRMT1 inhibits Akt/PKB-mediated phosphorylation of FOXO1 [50]. Methylation of EGFR at Arg1175 increases the association of EGFR with a tyrosine phosphatase, SHP1, leading to dephosphorylation of Tyr1173, which is involved in the activation of MAPK [51, 52].
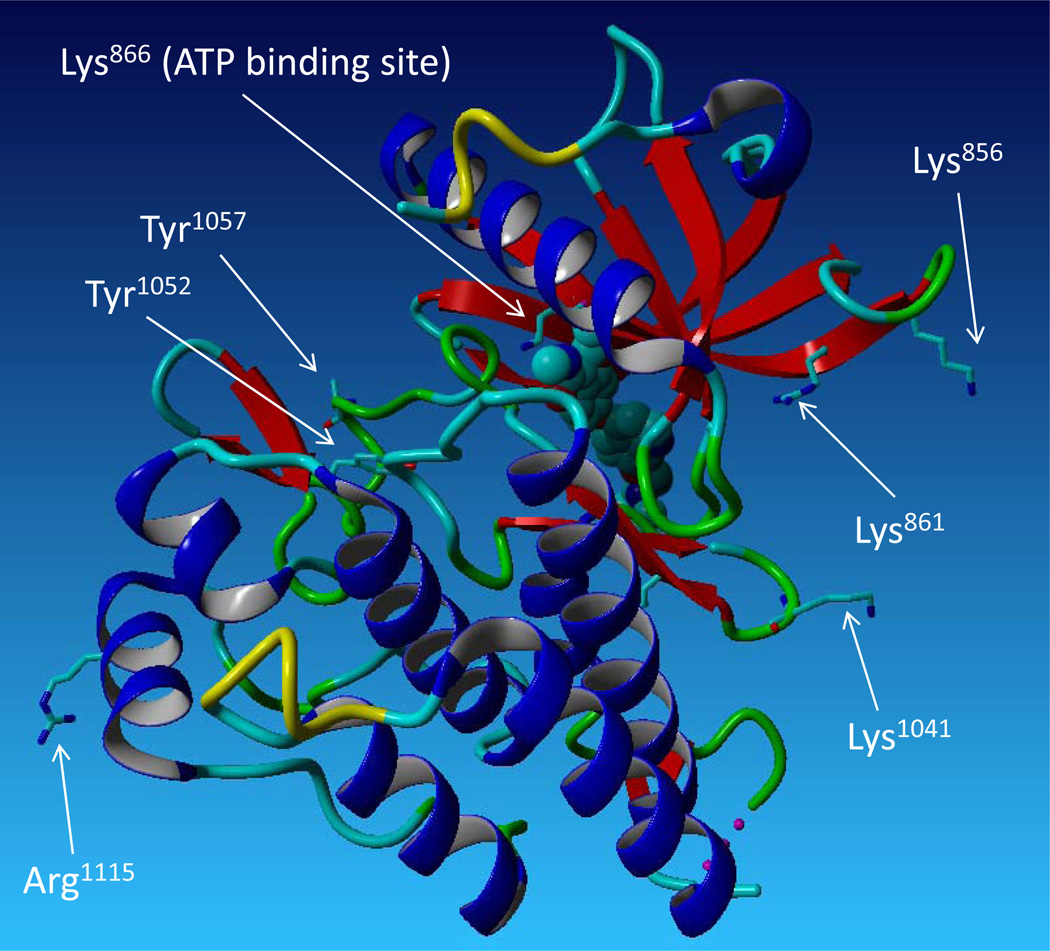
Activation of RTKs is tightly regulated by phosphorylation of one or two key Tyr residues located in the activation loop of the kinase domain [9]. Recent evidence suggests that methylation also modulates the activation of RTKs. Methylation of Lys831 in the kinase domain of VEGFR-1 by SMYD3 was reported to increase its kinase activation [53]. Interestingly, VEGFR-1 has low kinase activity and plays a dichotomous role in angiogenesis. In certain circumstances, it stimulates and, in other cases, it inhibits angiogenesis [54, 55]. However, it is not known whether the methylation status of VEGFR-1 is responsible for its paradoxical activities in different cellular environments. Our recent study demonstrated that VEGFR-2 also undergoes both Arg and Lys methylation. Mass spectrometry analysis identified five methylated residues including, Arg817, Lys856, Lys861, Lys1041, and Arg1115. Interestingly, Lys856 and Lys861 are located in the C-loop of the kinase domain, upstream of the ATP binding site at Lys866, but their methylation does not appear to be involved in the kinase activation of VEGFR-2 [56]. Lys1041 is located in the unstructured and flexible linker region between the two β sheets of the kinase domain (Figure 6), and its methylation is necessary for ligand-mediated tyrosine phosphorylation and kinase activation of VEGFR-2[56]. However, several key questions regarding methylation of VEGFR-2 remain to be answered. For example: what methyltransferases are involved in the methylation of VEGFR-2? Since multiple Lys and Arg residues have been documented as methylation sites on VEGFR-2, it is likely that multiple PRMTs and KMTs are involved. Furthermore, because methylation of VEGFR-2 is not regulated by ligand stimulation [56], it remains unclear what regulates methylation of VEGFR-2. Thus, it seems likely that mechanisms other than ligand stimulation might exist to govern methylation of VEGFR-2. Finally, it will be important to dissect the role of methylation at specific residues on VEGFR-2. Among the five residues shown to be subject to methylation on VEGFR-2, methylation of Lys1041 is linked to kinase activation; the role of the remaining methylated residues remains unknown.
Figure 6.
The crystal structure of VEGFR-2 and the location of the methylation sites including Lys1041, Lys866, Lys856 and Arg1115 are shown. Lys861, an ATP binding site, and the location of Tyr1052 and Tyr1057 (tyrosine autophosphorylation sites) are indicated.
Role of Protein acetylation in protein kinases signaling
Acetylation can occur on the N-termini of proteins and on the side chain of lysine residues of various proteins, including histones and non-histone proteins. N-terminal acetylation is an irreversible modification that occurs co-translationally and is a widespread modification on eukaryotic proteins [57]. Although early studies identified lysine acetylation as a common PTM on histones and transcription factors [58], emerging data now suggest that lysine acetylation is also a major PTM in non-histone proteins and plays significant role in signal transduction, as discussed below. The addition of an acetyl group on the side chain of lysines is expected to reduce positive charges and change electrostatic properties of the protein.
Lysine acetyltransferases (KATs), which are historically known as histone acetyltransferases (HATs) are responsible for catalysis of lysine acetylation. Lysine acetylation is a reversible PTM, which is accomplished by the activity of lysine deacetylases (KDACs)/histone deacetylases (HDACs). A number of lines of evidence indicate that lysine acetylation regulates the kinase activity of protein kinases. A study by Sabo et al., 2008 indicates that lysine acetylation of Cyclin-Dependent Kinase 9 (CDK9) in the kinase domain by GCN5 and P/CAF KATs inhibits the kinase activity of CDK9 [59]. Similarly, acetylation of Salt-inducible kinase 2 (SIK2), an AMP-activated protein kinase family kinase, has been reported to inhibit its kinase activity, whereas deacetylation increased SIK2 kinase activity [60]. In other cases, lysine acetylation appears to increase the kinase activity of protein kinases. For example, a recent study suggests that VEGFR-2 undergoes ligand-dependent lysine acetylation, which is mediated by p300/CBP, GCN5 and P/CAF KATs. MALDI-TOF/TOF mass spectrometry analysis showed that five lysine residues including, Lys929, Lys937, Lys939, Lys947, and Lys1053 on mouse VEGFR-2 are subject to acetylation. Curiously, Lys1053 is located in the activation loop of the kinase domain, which may positively regulate kinase activity of VEGFR-2[61]. Similarly, lysine acetylation of pyruvate kinase M2 isoform (PKM2) is reported to increase its Kinase activity [62].
Acetylation has been proposed a regulatory modification that rivals protein phosphorylation [63]. Accumulating data indicate that acetylation has dynamic effects on protein kinases, however the precise mechanisms and effects of acetylation on protein kinases remain unclear. It is increasingly evident that acetylation plays a significant role in signal transduction and cellular events independent of transcription [57]. Given that lysines are being targeted for various modifications, including ubiquitination and methylation, it is highly likely that acetylation could also influence ubiquitination and methylation of kinases and subsequently their degradation, substrate binding and biological activities.
Conclusions and Perspectives
The study of VEGF ligands and receptors has been area of intense research in the past two decades. One major clinical interest has been to develop angiogenesis-based therapies to treat diseases involving excessive angiogenesis, such as cancer and neovascular ocular diseases, or insufficient angiogenesis, such as ischemic heart diseases [2]. Major progress toward anti-angiogenesis therapy has been achieved in the treatment of cancer and AMD. Anti-angiogenesis therapy has benefited many patients with cancer; however, insufficient efficacy, intrinsic refractoriness, and development of resistance have significantly limited the number of patients who experience the therapeutic benefits of these therapies [3]. A deeper understanding of the molecular mechanisms by which cell signaling molecules and PTMs regulate angiogenic and tumorigenic signaling events should generate a new perspective in disease diagnosis and treatment. Equally important is the growing perception that development of an understanding how the disruption of PTMs in pathological conditions such as cancer contributes to tumor progression could significantly improve our understanding of disease processes. Ultimately, understanding how inappropriate PTMs contribute to tumor progression, angiogenesis and resistance to cancer treatments could lead to the identification of novel cancer therapies and possible biomarkers.
Acknowledgments
The authors sincerely apologize to all those colleagues whose important work in the field was not cited in this paper owing to space limitations.
Funding: This work was supported in part through a grant from the NIH/National Eye Institute (R01EY017955 to N.R.) and Massachusetts Lions Foundation; and through grant P41 RR010888/GM104603 from NIH/NIGMS and NIH/National Heart, Lung, and Blood Institute contract HHSN268201000031C (to C.E.C.).
Footnotes
Conflict of Interest: The authors have declared no conflict of interest.
References
- 1.Folkman J. Angiogenesis. Annual review of medicine. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. The Journal of clinical investigation. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung AS, Kowanetz M, Wu X, Zhuang G, Ngu H, Finkle D, et al. Differential drug class-specific metastatic effects following treatment with a panel of angiogenesis inhibitors. The Journal of pathology. 2012;227:404–416. doi: 10.1002/path.4052. [DOI] [PubMed] [Google Scholar]
- 8.Singh M, Ferrara N. Modeling and predicting clinical efficacy for drugs targeting the tumor milieu. Nature biotechnology. 2012;30:648–657. doi: 10.1038/nbt.2286. [DOI] [PubMed] [Google Scholar]
- 9.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. Journal of cell science. 2006;119:615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 11.Tao Q, Backer MV, Backer JM, Terman BI. Kinase insert domain receptor (KDR) extracellular immunoglobulin-like domains 4–7 contain structural features that block receptor dimerization and vascular endothelial growth factor-induced signaling. The Journal of biological chemistry. 2001;276:21916–21923. doi: 10.1074/jbc.M100763200. [DOI] [PubMed] [Google Scholar]
- 12.Nacev BA, Grassi P, Dell A, Haslam SM, Liu JO. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. The Journal of biological chemistry. 2011;286:44045–44056. doi: 10.1074/jbc.M111.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan S, Meyer RD, Lugo R, Rahimi N. Identification of PDCL3 as a novel chaperone protein involved in the generation of functional VEGF receptor 2. The Journal of biological chemistry. 2013;288:23171–23181. doi: 10.1074/jbc.M113.473173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 16.Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PloS one. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. The EMBO journal. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AJ, Meyer RD, Band H, Rahimi N. The carboxyl terminus of VEGFR-2 is required for PKC-mediated down-regulation. Molecular biology of the cell. 2005;16:2106–2118. doi: 10.1091/mbc.E04-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer RD, Singh AJ, Rahimi N. The carboxyl terminus controls ligand-dependent activation of VEGFR-2 and its signaling. The Journal of biological chemistry. 2004;279:735–742. doi: 10.1074/jbc.M305575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AJ, Meyer RD, Navruzbekov G, Shelke R, Duan L, Band H, et al. A critical role for the E3-ligase activity of c-Cbl in VEGFR-2-mediated PLCgamma1 activation and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5413–5418. doi: 10.1073/pnas.0700809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Z, Li X, Massena S, Kutschera S, Padhan N, Gualandi L, et al. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. The Journal of experimental medicine. 2012;209:1363–1377. doi: 10.1084/jem.20111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramani J, Ghosh M, Rahman MM, Caromile LA, Gerber C, Rezaul K, et al. Tyrosine phosphorylation of CD13 regulates inflammatory cell-cell adhesion and monocyte trafficking. J Immunol. 2013;191:3905–3912. doi: 10.4049/jimmunol.1301348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dayanir V, Meyer RD, Lashkari K, Rahimi N. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. The Journal of biological chemistry. 2001;276:17686–17692. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- 25.Holmqvist K, Cross MJ, Rolny C, Hagerkvist R, Rahimi N, Matsumoto T, et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. The Journal of biological chemistry. 2004;279:22267–22275. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- 26.Meyer RD, Latz C, Rahimi N. Recruitment and activation of phospholipase Cgamma1 by vascular endothelial growth factor receptor-2 are required for tubulogenesis and differentiation of endothelial cells. The Journal of biological chemistry. 2003;278:16347–16355. doi: 10.1074/jbc.M300259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nature reviews Molecular cell biology. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Frontiers in bioscience : a journal and virtual library. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer RD, Srinivasan S, Singh AJ, Mahoney JE, Gharahassanlou KR, Rahimi N. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and down-regulation. Molecular and cellular biology. 2011;31:2010–2025. doi: 10.1128/MCB.01006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaik S, Nucera C, Inuzuka H, Gao D, Garnaas M, Frechette G, et al. SCF(beta-TRCP) suppresses angiogenesis and thyroid cancer cell migration by promoting ubiquitination and destruction of VEGF receptor 2. The Journal of experimental medicine. 2012;209:1289–1307. doi: 10.1084/jem.20112446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. The Journal of biological chemistry. 2005;280:20995–21003. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
- 32.Issbrucker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, et al. p38 MAP kinase--a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:262–264. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- 33.Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K, Sessa WC. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. The Journal of biological chemistry. 2001;276:30359–30365. doi: 10.1074/jbc.M009698200. [DOI] [PubMed] [Google Scholar]
- 34.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Molecular cell. 2005;18:263–272. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Berger SL. The emerging field of dynamic lysine methylation of non-histone proteins. Current opinion in genetics & development. 2008;18:152–158. doi: 10.1016/j.gde.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Paik WK, Paik DC, Kim S. Historical review: the field of protein methylation. Trends in biochemical sciences. 2007;32:146–152. doi: 10.1016/j.tibs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Katz JE, Dlakic M, Clarke S. Automated identification of putative methyltransferases from genomic open reading frames. Molecular & cellular proteomics : MCP. 2003;2:525–540. doi: 10.1074/mcp.M300037-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Bedford MT. Arginine methylation at a glance. Journal of cell science. 2007;120:4243–4246. doi: 10.1242/jcs.019885. [DOI] [PubMed] [Google Scholar]
- 39.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nature reviews Molecular cell biology. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 40.Dillon SC, Zhang X, Trievel RC, Cheng X. The SET-domain protein superfamily: protein lysine methyltransferases. Genome biology. 2005;6:227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrossian TC, Clarke SG. Uncovering the human methyltransferasome. Molecular & cellular proteomics : MCP. 2011;10:M110 000976. doi: 10.1074/mcp.M110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajul-Arifin K, Teasdale R, Ravasi T, Hume DA, Mattick JS, Group RG, et al. Identification and analysis of chromodomain-containing proteins encoded in the mouse transcriptome. Genome research. 2003;13:1416–1429. doi: 10.1101/gr.1015703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bienz M. The PHD finger, a nuclear protein-interaction domain. Trends in biochemical sciences. 2006;31:35–40. doi: 10.1016/j.tibs.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell research. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li D, Roberts R. WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cellular and molecular life sciences : CMLS. 2001;58:2085–2097. doi: 10.1007/PL00000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bedford MT, Frankel A, Yaffe MB, Clarke S, Leder P, Richard S. Arginine methylation inhibits the binding of proline-rich ligands to Src homology 3, but not WW, domains. The Journal of biological chemistry. 2000;275:16030–16036. doi: 10.1074/jbc.M909368199. [DOI] [PubMed] [Google Scholar]
- 48.Zhu W, Mustelin T, David M. Arginine methylation of STAT1 regulates its dephosphorylation by T cell protein tyrosine phosphatase. The Journal of biological chemistry. 2002;277:35787–35790. doi: 10.1074/jbc.C200346200. [DOI] [PubMed] [Google Scholar]
- 49.Jansson M, Durant ST, Cho EC, Sheahan S, Edelmann M, Kessler B, et al. Arginine methylation regulates the p53 response. Nature cell biology. 2008;10:1431–1439. doi: 10.1038/ncb1802. [DOI] [PubMed] [Google Scholar]
- 50.Yamagata K, Daitoku H, Takahashi Y, Namiki K, Hisatake K, Kako K, et al. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Molecular cell. 2008;32:221–231. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi H, Chang SS, Hsu JL, Hung MC. Signaling cross-talk in the resistance to HER family receptor targeted therapy. Oncogene. 2014;33:1073–1081. doi: 10.1038/onc.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu JM, Chen CT, Chou CK, Kuo HP, Li LY, Lin CY, et al. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nature cell biology. 2011;13:174–181. doi: 10.1038/ncb2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunizaki M, Hamamoto R, Silva FP, Yamaguchi K, Nagayasu T, Shibuya M, et al. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by SMYD3. Cancer research. 2007;67:10759–10765. doi: 10.1158/0008-5472.CAN-07-1132. [DOI] [PubMed] [Google Scholar]
- 54.Meyer RD, Mohammadi M, Rahimi N. A single amino acid substitution in the activation loop defines the decoy characteristic of VEGFR-1/FLT-1. The Journal of biological chemistry. 2006;281:867–875. doi: 10.1074/jbc.M506454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahimi N, Dayanir V, Lashkari K. Receptor chimeras indicate that the vascular endothelial growth factor receptor-1 (VEGFR-1) modulates mitogenic activity of VEGFR-2 in endothelial cells. The Journal of biological chemistry. 2000;275:16986–16992. doi: 10.1074/jbc.M000528200. [DOI] [PubMed] [Google Scholar]
- 56.Hartsough EJ, Meyer RD, Chitalia V, Jiang Y, Marquez VE, Zhdanova IV, et al. Lysine Methylation Promotes VEGFR-2 Activation and Angiogenesis. Science signaling. 2013;6:ra104. doi: 10.1126/scisignal.2004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Molecular cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 59.Sabo A, Lusic M, Cereseto A, Giacca M. Acetylation of conserved lysines in the catalytic core of cyclin-dependent kinase 9 inhibits kinase activity and regulates transcription. Molecular and cellular biology. 2008;28:2201–2212. doi: 10.1128/MCB.01557-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang FC, Tan BC, Chen WH, Lin YH, Huang JY, Chang HY, et al. Reversible acetylation regulates salt-inducible kinase (SIK2) and its function in autophagy. The Journal of biological chemistry. 2013;288:6227–6237. doi: 10.1074/jbc.M112.431239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zecchin A, Pattarini L, Gutierrez MI, Mano M, Mai A, Valente S, et al. Reversible acetylation regulates vascular endothelial growth factor receptor-2 activity. Journal of molecular cell biology. 2014;6:116–127. doi: 10.1093/jmcb/mju010. [DOI] [PubMed] [Google Scholar]
- 62.Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki N, et al. Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Molecular cell. 2013;52:340–352. doi: 10.1016/j.molcel.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? The EMBO journal. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]