Abstract
Traumatic injury is a significant cause of morbidity and mortality worldwide. Microcirculatory activation and injury from hemorrhage contributes to organ injury. Many adaptive responses occur within the microcirculatory beds to limit injury including up regulation of heme oxygenase (HO) enzymes, the rate limiting enzymes in the breakdown of heme to carbon monoxide (CO), iron, and biliverdin. Here we tested the hypothesis that CO abrogates trauma induced injury and inflammation protecting the microcirculatory beds.
Methods.
C57Bl/6 mice underwent sham operation or hemorrhagic shock to a mean arterial pressure of 25mmHg for 120 minutes. Mice were resuscitated with Lactated Ringer’s at 2X the volume of maximal shed blood. Mice were randomized to receive CO-releasing molecule (CO-RM) or inactive CO-RM at resuscitation. A cohort of mice was pretreated with tin protoporphyrin-IX (SnPP) to inhibit endogenous CO generation by heme oxygenases (HO). Primary mouse liver sinusoidal endothelial cells were cultured for in vitro experiments.
Results.
CO-RM protected against hemorrhagic shock/resuscitation (HS/R) organ injury and systemic inflammation and reduced hepatic sinusoidal endothelial injury. Inhibition of HO activity with SnPP exacerbated liver hepatic sinusoidal injury. HS/R in vivo or cytokine stimulation in vitro resulted in increased endothelial expression of adhesion molecules that was associated with decreased leukocyte adhesion in vivo and in vitro.
Conclusions.
HS/R is associated with endothelial injury. HO enzymes and CO are involved in part in diminishing this injury and may prove useful as a therapeutic adjunct that can be harnessed to protect against endothelial activation and damage.
Introduction
Traumatic injury is a leading cause of death in our society and accounts for significant morbidity and mortality worldwide. Bleeding and the development of hemorrhagic shock as a component of traumatic injury contribute significantly to the mortality from trauma(1). Additionally, it is thought that hemorrhagic shock as a consequence of traumatic injury is the area in which interventions could have the greatest impact to decrease mortality.
The influence of traumatic shock extends well beyond the direct site of injury. The release of cellular products from injured tissues into the systemic circulation, combined with decreased tissue perfusion and the consequences of hemorrhage, predisposes to widespread tissue injury and inflammation(2). The influences are pronounced on the vascular endothelium and microcirculation(3-5) As a result of these processes, the endothelium is activated and inflammatory signaling is initiated. One manifestation includes the increased expression of adhesion molecules and integrins, which promote the adhesion of platelets and leukocytes(3, 6)and it is this combination that leads to further endothelial injury, inflammation, and coagulopathy.
Cell signaling in response to trauma and hemorrhage drives the injury response. Additionally, a number of adaptive cell signaling pathways are integral to limit the extent of injury and inflammation, including heme oxygenase signaling(7-11). Heme oxygenase enzymes are the rate limiting enzymes in the breakdown of heme to carbon monoxide, biliverdin and free iron. Heme oxygenase-1 is the inducible isoform that is up regulated by many different stressors and in nearly all instances functions to limit inflammation. Heme oxygenase-2 is constitutively expressed in many cell types, including the vascular endothelium. Over-expression of heme oxygenase-1 or administration of exogenous carbon monoxide is protective against organ injury in multiple models, including hemorrhagic and septic shock(7, 12). Additionally, HO expression and CO delivery are vasoprotective and limit endothelial injury and death(13-16). Others and we have demonstrated that CO increases reactive oxygen species resulting in activation of redox-sensitive transcription factors or stress signaling, which in turn increases expression of antioxidant enzymes and other adaptive responses to stress(17, 18). The cellular mechanisms of protection by CO in the liver in response hemorrhagic shock continue to be investigated. The purpose of these experiments were to test the hypothesis that CO-releasing molecules serve to limit vascular injury following hemorrhagic shock and that exogenously delivered carbon monoxide can prevent endothelial cell activation, injury, and inflammation.
Materials and Methods
Hemorrhagic shock model
The University of Pittsburgh Institution Animal Care and Use Committee approved animal protocols. The experiments were performed in adherence to the National Institutes of Health Guidelines on the Use of Laboratory Animals. Hemorrhagic shock was performed as described previously (12). Briefly, C57BL/6 mice weighing 23 to 27 g were anesthetized with pentobarbital (70 mg/kg i.p.). The right and the left femoral arteries were cannulated. The left arterial catheter was connected to a monitor to follow MAP and heart rate. Blood was withdrawn for more than 10 min via the right femoral artery to achieve a MAP of 25 mmHg. Blood was withdrawn and returned to the animal as needed to maintain a MAP of 25 mmHg for a total of 120 minutes. Sham animals were cannulated but were not subjected to hemorrhage. At the end of the shock period, mice were resuscitated with Ringer’s lactate solution using a total of two times the volume of maximum shed blood. Sham mice were subject to the same surgical procedures but did not undergo hemorrhage. Blood was not returned as part of the resuscitation protocol. Sham and shock mice were randomized to either no further treatment, CO-Releasing Molecule (CO-RM)-421 (Alfama, 5 mg/kg), or inactive CO-RM-421. CO-RM or inactive CO-RM was delivered intravenously in a volume of 100 microliters diluted in lactated Ringers at the time of resuscitation. Four hours after the onset of resuscitation, the mice were euthanized, and serum and organs were collected. Survival studies were carried out for 24 hours from resuscitation.
Serum cytokine and ALT measurements
MAGPIX Multiplex assay kit (Millipore) was used according to the manufacturer’s instructions to measure the Levels of the cytokines IL-6, TNF-α, G-CSF and IL-1α. Alanine aminotransferase (ALT) was determined using an iSTAT Analyzer (Abbott, Princeton, NJ). Soluble ICAM-1 (CD54) was measured using enzyme-linked immunoabsorbant assay (ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Immunohistochemistry/immunocytochemistry
Primary mouse sinusoidal endothelial cells were seeded onto collagen cross-linked coverslips. At the end of specified treatments, cells were fixed in 2% paraformaldehyde in phosphate buffered saline (PBS) for 1 hour, rinsed 3 times in PBS, permeabilized with 0.1% Triton X-100 in PBS for 20 minutes. Liver tissue that was fixed in 2% paraformaldehyde for 2 hours, dehydrated in 30% sucrose for 12 hours, and then frozen were sectioned (7 μm) onto gelatin-coated slides. Cells or tissues were blocked in 5% non-immune goat serum in PBS for 30 minutes at room temperature. Primary antibody to ICAM-1 (Abcam; 1:100) in PBS was added to cells/sections for 1 hour at room temperature. Samples were washed 5 times with PBS followed by incubation with Cy3 (1:1000, Jackson ImmunoResearch Laboratories) secondary antibodies diluted in PBS. Samples were washed three times with PBS, followed by a single wash with PBS prior to 30-second incubation with Hoeschst nuclear stain. Nuclear stain was removed and samples were washed with PBS prior to coverslipped using Gelvatol (23 g poly[vinyl alcohol 2000], 50 ml glycerol, 0.1% sodium azide to 100 ml PBS). Positively stained cells in 6 random fields were imaged on a Fluoview 1000 confocal scanning microscope (Olympus, Melville,NY). Imaging conditions were maintained at identical settings within each antibody-labeling experiment with original gating performed using the negative control.
Scanning Electron Microscopy
For electron microscopy, livers were perfused through the portal vein with PBS and fixed with 2.5% glutaraldehyde. Liver tissue was the cut into small blocks (3mm3) and washed thoroughly in 3 changes of 0.1 M PBS for 15 minutes each. These tissues were then treated in 1% OsO4 in 0.1 M PBS for 60 minutes and washed thoroughly in 3 changes 0.1 M PBS for 15 minutes each. Finally it was dehydrated in graded series of alcohol (30%, 50%, 70%, 90% and 100% in PBS) for 15 minutes each. After slices were mounted on aluminum stubs they were sputter coated with gold/palladium (Cressington Auto 108, Cressington, UK). Imaging was accomplished using a JEM-6360 SEM (JEOL, Peabody, MA) at 10-15 kV(19).
Intravital microscopy
Briefly, the left lobe of the liver was gently exteriorized through a subcostal abdominal incision and positioned over a glass optical port in a specially designed microscope stage. Sterile gauze was placed slightly below and to either side of the exposed lobe to facilatate drainage of irrigating fluid. The liver was covered by Saran wrap (Dow Chemical Co., Midland, MI) to stabilize its position and limit movements induced by respiration. Suffusion of a modified Krebs solution serves to keep the liver warm and provide an aqueous interface between the liver and the microscope objective. The stage was positioned so the liver could be observed by either brightfield or fluorescence microscopy using a Leitz ELR microscope.
Three post-sinusoidal venules (20-25 μm) in each mouse were identified for the quantitation of leukocyte-endothelial adherence. The number of rolling leukocytes (those traveling at a slower rate than the red blood cell column) that passed an arbitrary reference point in the venule during 1 min was counted. The number of firmly adherent leukocytes (those adhering to the vessel wall for a minimum of 30 seconds) were counted and standardized per endothelial surface area.
Endothelial permeability
Hemorrhagic shock was performed as described above. 100 μl of Evans blue dye (10g/L) was injected 30 min prior to sacrifice. At sacrifice, the mouse was perfused through the femoral artery with 10 ml of PBS at a rate of 0.55 ml/min using a syringe infusion pump. The liver, kidney, and lungs were harvested, blotted dry and weighed and then washed in 5ml of PBS. The organs were then placed in 5 ml of formamide at 37 degrees Celsius for 48 hours and subsequently centrifuged at 3000 r.p.m. for 10 min and the supernatant was harvested. Using Multi-mode microplate reader (SynergyMX), the optical density was measured at a wavelength of 630 nm. The concentration was determined using the standard curve (0-64 μg/ml). Readings were corrected to the mass of the tissue sample for each experiment.
Cell culture and treatment
Mouse liver sinusoidal endothelial cells were purchased from Cell Biologics and cultured in Mouse Endothelial Cell Medium (Cell biologics). Cells were used between passages 1 to 2. Cells were stimulated for 24 hours with a mixture of interferon-gamma (100 u/ml) TNF-α (500u/ml) and IL-1β. (100u/ml). They were treated with or without CORM-A1 (Gift from Carlos Romao, ITQB, Oeiras, Portugal) at a concentration of 20 μM. Flow cytometry was used to confirm purity by confirming CD31 (ebioscience, PE-Cy7) and CD144 (BD, FITC) positive staining.
Reverse Transcriptase-Polymerase Chain Reaction
Cells grown on 6 cm plate were scraped after adding 200 μl of PBS. Cell pellets were collected after centrifugation at 10,000 rpm for 10 min. RNA was prepared by utilizing a silica-gel based membrane method using the RNeasy Midi Kit (Qiagen) according to the manufacturer’s instructions. An on-column DNase digestion using RNase-free DNase (Qiagen) was performed to rid the samples of genomic DNA. One microgram of RNA was used to generate cDNA using oligo dT primers (Qiagen) and Omniscript (Qiagen) reverse transcriptase. PCR reaction mixtures were prepared using SYBR Green PCR master mix (PE Applied Biosystems, Foster City, CA). SYBR Green two-step real-time RT-PCR for ICAM-1, VCAM-1, p-selectin, e-selectin and β-actin was performed as described. All samples were run in duplicate. The level of gene expression for each sample was normalized to [beta]-actin mRNA expression using the comparative threshold cycle (CT) method.
Endothelial-Leukocyte Adhesion assay
Endothelial leukocyte adhesion assay was performed using Vybrant™ Cell Adhesion Assay Kit (V-13181; Life Technologies, Grand Island, NY) as described by the manufacturer. Briefly, hepatic sinusoidal endothelial cells were seeded into a 96 well plate for two days. They were stimulated for 6 hours with a mixture of interferon-gamma (100 u/ml) TNF-α (500u/ml) and IL-1.β (100u/ml). Cells were treated with or without CORM-A1 at a concentration of 20 μM. Isolated mouse polymorphonuclear leukocytes were isolated as described previously(20) and were labeled with calcein AM. They were co-cultured with the stimulated endothelial cells for 4 hours at 37°C. Prior to co-culture, media was changed removing the cytokine mixture and CORM. Non-adherent cells were washed and the fluorescence was measured using a fluorescein filter set (calcein has an absorbance maximum of 494 nm and an emission maximum of 517 nm).
Statistics
The data was then analyzed in Sigma plot and utilizing ANOVA analysis analyzed for significance. Data is presented as mean ± standard error of the mean.
Results
Carbon monoxide releasing molecules decrease organ injury and limit systemic inflammation following hemorrhagic shock and resuscitation
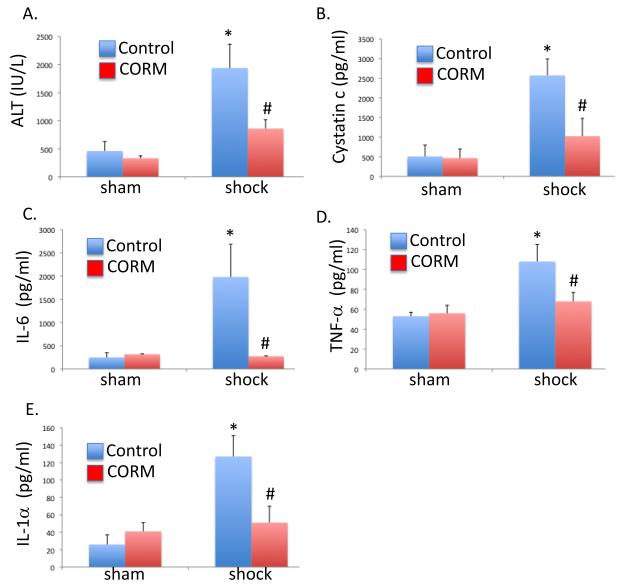
Survival at 24 hours was determined following hemorrhagic shock and resuscitation (HS/R) with CO-RM or iCO-RM. Survival was 58% with iCO-RM and was 92% with CO-RM (n=12 per group). Liver and kidney injury 4 hours after HS/R or sham was determined using serum measurements of serum ALT and cystatin A. HS/R resulted in liver and kidney injury, however this was attenuated in mice receiving CO-RM (P<0.05; Figure 1A,B). Inflammatory cytokines elaborated after an insult have numerous effects including the activation of endothelial cells, increased expression of adhesion molecules, and leukocyte recruitment(21). Serum levels of IL-6, TNF-α and IL-1α were determined 4 hours after resuscitation (Figure 1C-E). In the HS/R group, the levels of IL-6, G-CSF, TNF-α and IL-1α were increased compared to the sham group (P<0.05). Treatment with CORM significantly limited HS/R-induced cytokine production (P<0.05).
Figure 1.
Carbon monoxide-releasing molecule protected against hemorrhagic shock and resuscitation-induced organ injury and inflammation. A, B. HS/R resulted in liver and kidney injury as determined by serum ALT and cystatin C, respectively (*P<0.05 compared to sham, control mice). CORM treatment limited organ injury (#P<0.05 compared to shock, control mice). C-E. HS/R increased serum IL-6, TNF-α, and IL-1α levels (*P<0.05 compared to sham, control mice). Mice treated with CORM protected against HS/R-induced elaboration of cytokines (#P<0.05 compared to shock, control mice).
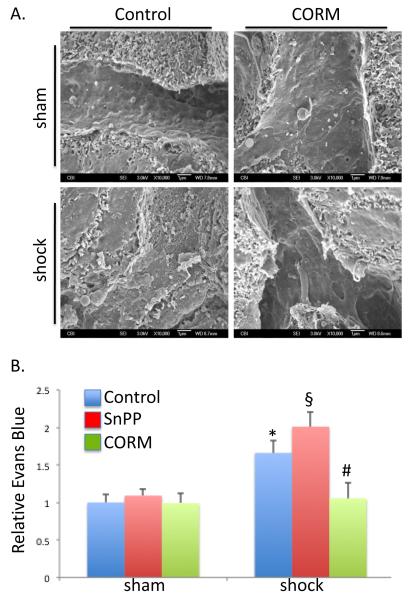
CO preserves hepatic sinusoidal structure and decreases Evans blue leakage from hepatic microcirculation in HS/R
Hemorrhagic shock and resuscitation leads to microcirculatory derangement, but the extent of injury and the mechanisms responsible for this injury are poorly understood. The influence of heme oxygenase enzymes and therefore endogenous CO, as well as supplemental CO-RM was determined. Hepatic sinusoidal integrity was assessed using scanning electron microscopy. HS/R resulted in loss of normal endothelium fenestrations, rounding of cells, and increases in the number of adherent leukocytes. Intravenous CO-RM limited these changes and maintained normal structure of the hepatic sinusoids by preserving the endothelial integrity and sinusoidal fenestrations (Figure 2A). In order to determine if these structural changes led to gross changes in endothelial function and increased vascular permeability, Evans blue dye was injected and relative tissue levels in the liver were determined. Evans blue is a dye that binds to albumin, a protein that usually does not extravasate into the interstitial space by virtue of its high molecular weight and electronegative charge(4). An increase in Evans blue in the interstitial space indirectly implies an increase in vascular permeability to albumin and therefore increased permeability. HSR resulted in an increase in the level of Evans blue dye extravasated in the liver compared to sham control mice (Figure 2B; 1.66±0.17 fold higher; P<0.05). Pretreatment with tin protoporphyrin-IX (SnPP), a selective inhibitor of heme oxygenase enzyme activity exacerbated the extravasation of dye (2.01±0.19 fold higher than sham; P<0.05 compared to vehicle treated sham or HS/R). CO-RM minimized the endothelial leakage as demonstrated by a significant drop in Evans blue accumulation compared with non-CORM treated HS/R animals (1.05±0.2 fold increase over sham control; P<0.05). Thus, CO preserves the structural and functional integrity of hepatic microcirculation.
Figure 2.
Carbon monoxide-releasing molecule limited HS/R-induced hepatic microvascular injury. A. HS/R resulted in hepatic sinusoidal endothelial ultrastructural damage as demonstrated by scanning electron microscopy. Loss of normal cellular structure and hepatic sinusoidal endothelial cell fenestrations are visualized. CORM protected against these changes. B. HS/R-damaged the hepatic microvasculature as determined by Evan’s blue leak into liver tissue (*P<0.05 compared to sham, control mice). Inhibition of heme oxygenase activity with tin protoporphyrin (SnPP-IX) exacerbated microvascular injury (§P<0.05 compared to shock, control mice), whereas CORM limited this injury (#P<0.05 compared to shock, control mice).
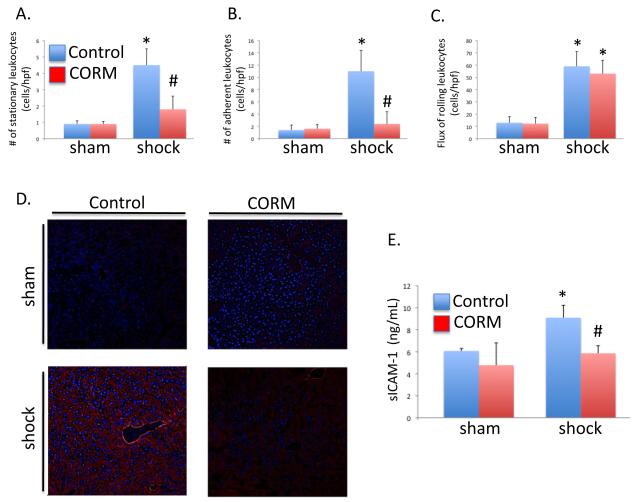
CO decreases HS/R induced recruitment of leukocyte into the liver and decreases HS/R-induced expression of ICAM-1 in hepatic sinusoidal endothelium
Leukocyte recruitment into most tissues, including the liver, is a multistep process that requires the interaction of endothelial adhesion molecules and leukocyte glycoprotein ligands such as integrins(22). The initial step in this process involves tethering and rolling of leukocytes mediated primarily by selectins expressed on the endothelium(23). This is followed by leukocyte adhesion, which is facilitated by the interaction of leukocyte integrins and endothelial intercellular adhesion molecules such as ICAM-1 and VCAM-1(23). Finally, leukocytes extravasate into the tissue following a chemokine gradient. Leukocyte adhesion and fixation were determined using intravital microscopy. HS/R increased the number of adherent or stationary leukocytes compared to the sham mice (0.9±0.2 and 1.4±0.8 in sham vs. 4.5±1 and 11±3.4 cells/high powered field in HS/R, respectively (Figures 3A,B) while CO-RM significantly decreased the number of stationary and adherent cells in response to HS/R (1.8±0.8. and 2.4±2.0 cells/high powered field, respectively; P<0.05 compared to HS/R).
Figure 3.
Hemorrhagic Shock/Resuscitation-induced adhesion molecule expression and leukocyte adhesion are minimized by CO-RM. A, B. HS/R increased the number of adherent and stationary leukocytes compared to sham, control mice (*P<0.05), whereas CO-RM treatment prevented these changes (#P<0.05 compared to shock, control mice). C. CO-RM treatment limited HS/R-induced hepatic ICAM protein levels (red) as determined by immunohistochemistry. D. Furthermore, CO-RM treatment limited HS/R-induced increased levels of soluble ICAM in the serum (*P<0.05 compared to sham, control; #P<0.05 compared to shock, control).
To assess the effect of CO on the expression of endothelial adhesion molecules such as ICAM-1, we performed immunofluorescent staining of hepatic tissue sections and measured sICAM-1 in the serum. The levels of ICAM-1 was increased in the hepatic sinusoids in HS/R animals compared to sham controls and were reduced following the treatment with CO-RM (Figure 3C). sICAM-1 is a dimeric protein generated by proteolytic cleavage of membrane bound ICAM-1 by MMP-9 and elastase(24). Shocked animals had a 1.50 fold increase in sICAM-1 compared to controls, and CO-RM treatment prevented this increase in the serum (Figure 3D).
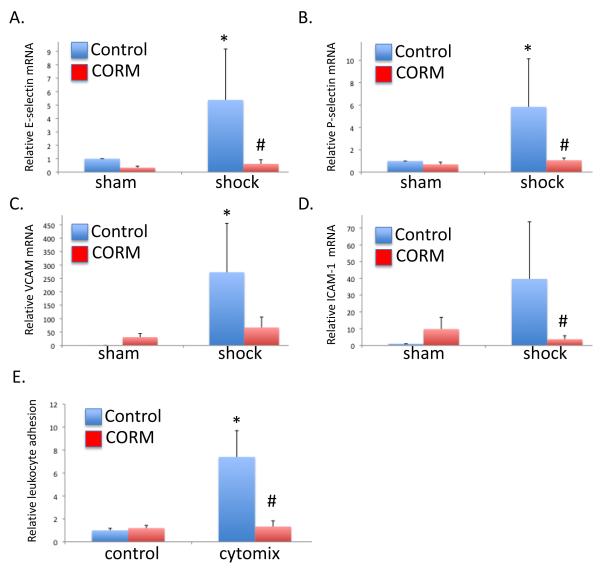
CO reduces cytokine-induced expression of adhesion molecules in cultured sinusoidal endothelial cells and reduces in vitro leukocyte-endothelial adhesion
Hepatic sinusoidal endothelial cells were cultured and stimulated with a mixture of cytokines including interferon-gamma, TNF-α and IL-1β and with CO-RM or iCO-RM (the CO-RM molecule without CO) at a concentration of 20 μM. The cells were 96.5% positive for CD 31, 72.2% positive for CD 144 and 85.0 % double positive, validating the purity and identity of the cultured cells (25)}. Using RT-PCR, mRNA levels of P-selectin, E-selectin ICAM-1 and VCAM-1 were measured. Cytokine stimulation increased RNA levels of all these adhesion molecules in iCO-RM treated but not in CO-RM treated cells (Figures 4A-D).
Figure 4.
Carbon monoxide treatment limited cytokine mixture [cytomix- a combination of TNF-α (500 U/ml), IL-β (100 U/ml), and IFN-γ (1,00 U/ml)] induced increased in liver sinusoidal endothelial cell adhesion molecule expression and leukocyte adhesion. A-D. Cytomix increased expression of E-selectin, P-selectin, VCAM, and ICAM-1 in cultured primary liver sinusoidal endothelial cells (*P<0.05 compared to non-cytokine treated, vehicle controls), and CO-RM limited these increases (#P<0.05 compared to cytokine treated, vehicle). E. Cytomix-induced neutrophil adhesion (7.4±2.3 fold increase compared to non-cytomix, vehicle controls; *P<0.05) was minimized by CO-RM treatment (1.33±0.5 fold increase compared to non-cytomix, vehicle controls; #P<0.05 compared to cytomix, vehicle treated cells).
To analyze the effect of CO on the endothelial leukocyte interaction, we performed an adhesion assay as described in the method section. While cytomix stimulation lead to an increase in PMN adhesion, CO-RM abrogated this effect (Figure 4E). Together, these data suggest that CO-RM reduced the level of leukocyte adhesion via reduction in expression of endothelial adhesion molecules.
Discussion
The current study demonstrated that the use of CO-RM as an adjunct in the treatment of hemorrhagic shock and resuscitation has salutary effects. CO-RM decreased the levels of pro inflammatory cytokines that are induced by hemorrhage and resuscitation. In addition, CO-RM prevented hepatic and renal injury as demonstrated by decreased levels of ALT and cystatin C. These data further showed that CO-RM protects the hepatic micro vascular circulation structurally and functionally. CO-RM also reduced the number of stationary and adherent leukocytes in the post-sinusoidal venules as demonstrated by intravital microscopy and decreased neutrophil adhesion to sinusoidal endothelial cells in vitro. Furthermore, there was decreased expression of adhesion molecules both in vivo and in vitro. These findings suggest that CO-RM protects against hemorrhagic shock and resuscitation-induced hepatic injury, in part by maintaining microvascular and endothelial integrity.
Hemorrhagic shock and resuscitation is known to cause a general ischemia and reperfusion injury. This is accompanied by an inflammatory response, an increase in endothelial adhesion molecules expression and recruitment of leukocytes into different organs(3, 5). Leukocytes, mainly neutrophils, release reactive oxygen intermediates that increase oxidative stress to endothelial cells, and contribute to endothelial dysfunction(26). Furthermore neutrophils increase microvascular permeability and induce endothelial dysfunction by secreting chemokines such as CXCL1, 2, 3, and 8, and by the release of heparin-binding protein(27). Data in this manuscript shows that CO-RM decreases the expression of endothelial adhesion molecules, which play a major role in leukocyte extravasation into the liver(22). This may explain in part how CO-RM preserves hepatic sinusoidal endothelial integrity. It is likely that the insult of hemorrhagic shock and resuscitation concurrently activates the endothelium and circulating inflammatory cells, and that the injury to the microvasculature is both secondary to direct influences in the endothelium as well as from activated leukocytes and platelets. We did not test the effects of CO-RM on activated leukocytes, but numerous reports describe the effects of HO-1 and CO on leukocyte activation(28-30). The influence of CO-RM is also likely to be secondary to both direct effects on the endothelium as well as these circulating cells. Of note, primary hepatic sinusoidal cells may alter their phenotype with passage when culturing in vitro. This may change the expression of certain gene products from their normal basal state and can alter the results.
The data in this manuscript evaluating the adhesion of neutrophils in the post-sinusoidal venules can not be assumed to correspond to changes that occur within the sinusoids. Adhesion molecule expression may not be necessary for neutrophil accumulation within hepatic sinusoids, but have been shown to play a role in extravasation and injury. Moreover, most hepatic neutrophil extravasation occurs in the sinusoids.
It was previously shown that the preservation of the hepatic microcirculatory structural and functional integrity in HS/R is critical to prevent hepatic injury(31). There is a direct correlation between sinusoidal dysfunction and liver failure in HS/R(31). An additional consequence of preventing endothelial cell activation and microvascular injury is the maintenance of microcirculatory blood flow. Blood flow was not examined in these investigations, but it has been reported that CO regulates hepatic portal blood flow(8, 32-34). Pannen et al described the role of endogenous CO in preventing hepatic microcirculatory failure following hemorrhagic shock(8). When HO-1 activity was inhibited using SnPP-IX, blood flow through the hepatic sinusoids was reduced, a finding that was more prominent in the shock-treated animals versus controls. Reduced flow was also accompanied by an increase in sinusoidal resistance. In addition, previous investigations from our group have demonstrated that CO can prevent the development of hepatic hypoxia in the setting of hemorrhage(12). We speculate that the maintenance of hepatic blood flow and subsequently oxygen delivery to the liver plays a role in preventing hepatic injury and hypoxia. In addition, other effects of CO potentially explain the paradoxical limitation of hepatic hypoxia in HS/R. Others and we have shown that the HO-CO system may mediate beneficial effects by reducing cellular metabolic activity so as to maintain adequate cellular oxygen tension(7). CO as well as HO-1 induction decreases cellular respiration yet maintains cellular ATP levels even under hypoxic conditions (7, 12).
In conclusion, our data show that the use of CO-RM as an adjunct in the treatment of hemorrhagic shock is potently hepatoprotective. It inhibits the inflammatory cascade initiated by hemorrhagic shock and resuscitation, protects end organ damage notably hepatic injury elicited in part by tissue hypoxia, and it preserves hepatic microcirculation integrity. Further investigations are needed to delineate the mechanism behind the salutary effects of CO-RM in HS/R in liver injury.
Supplementary Material
Acknowledgments
Funding: This work is supported by National Institutes of Health grants R01 GM082830 (BSZ), Veterans Affairs Merit Award 1I01BX000566 (BSZ), and Department of Defense DM102439 (BSZ), 5R01GM088666 (LEO) and CIMIT Center for Integration of Medicine and Innovative Technology (LEO).
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Mollen KP, Levy RM, Prince JM, Hoffman RA, Scott MJ, Kaczorowski DJ, Vallabhaneni R, et al. Systemic inflammation and end organ damage following trauma involves functional TLR4 signaling in both bone marrow-derived cells and parenchymal cells. J Leukoc Biol. 2008;83:80–88. doi: 10.1189/jlb.0407201. [DOI] [PubMed] [Google Scholar]
- 3.Scalia R, Armstead VE, Minchenko AG, Lefer AM. Essential role of P-selectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J Exp Med. 1999;189:931–938. doi: 10.1084/jem.189.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schumacher J, Binkowski K, Dendorfer A, Klotz KF. Organ-specific extravasation of albumin-bound Evans blue during nonresuscitated hemorrhagic shock in rats. Shock. 2003;20:565–568. doi: 10.1097/01.shk.0000093540.78705.71. [DOI] [PubMed] [Google Scholar]
- 5.van Meurs M, Wulfert FM, Knol AJ, De Haes A, Houwertjes M, Aarts LP, Molema G. Early organ-specific endothelial activation during hemorrhagic shock and resuscitation. Shock. 2008;29:291–299. doi: 10.1097/SHK.0b013e318145a7c1. [DOI] [PubMed] [Google Scholar]
- 6.Akgur FM, Zibari GB, McDonald JC, Granger DN, Brown MF. Kinetics of P-selectin expression in regional vascular beds after resuscitation of hemorrhagic shock: a clue to the mechanism of multiple system organ failure. Shock. 2000;13:140–144. doi: 10.1097/00024382-200013020-00008. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhaneni R, Kaczorowski DJ, Yaakovian MD, Rao J, Zuckerbraun BS. Heme oxygenase 1 protects against hepatic hypoxia and injury from hemorrhage via regulation of cellular respiration. Shock. 2010;33:274–281. doi: 10.1097/SHK.0b013e3181b0f566. [DOI] [PubMed] [Google Scholar]
- 8.Pannen BH, Kohler N, Hole B, Bauer M, Clemens MG, Geiger KK. Protective role of endogenous carbon monoxide in hepatic microcirculatory dysfunction after hemorrhagic shock in rats. J Clin Invest. 1998;102:1220–1228. doi: 10.1172/JCI3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rensing H, Bauer I, Peters I, Wein T, Silomon M, Jaeschke H, Bauer M. Role of reactive oxygen species for hepatocellular injury and heme oxygenase-1 gene expression after hemorrhage and resuscitation. Shock. 1999;12:300–308. doi: 10.1097/00024382-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Rensing H, Jaeschke H, Bauer I, Patau C, Datene V, Pannen BH, Bauer M. Differential activation pattern of redox-sensitive transcription factors and stress-inducible dilator systems heme oxygenase-1 and inducible nitric oxide synthase in hemorrhagic and endotoxic shock. Crit Care Med. 2001;29:1962–1971. doi: 10.1097/00003246-200110000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Kubulus D, Rensing H, Paxian M, Thierbach JT, Meisel T, Redl H, Bauer M, et al. Influence of heme-based solutions on stress protein expression and organ failure after hemorrhagic shock. Crit Care Med. 2005;33:629–637. doi: 10.1097/01.ccm.0000156295.48075.49. [DOI] [PubMed] [Google Scholar]
- 12.Zuckerbraun BS, McCloskey CA, Gallo D, Liu F, Ifedigbo E, Otterbein LE, Billiar TR. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock. 2005;23:527–532. [PubMed] [Google Scholar]
- 13.Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 15.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui TY, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 16.Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, Bach FH. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal. 2002;4:321–329. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- 17.Choi YK, Por ED, Kwon YG, Kim YM. Regulation of ROS production and vascular function by carbon monoxide. Oxid Med Cell Longev. 2012;2012;9:794237. doi: 10.1155/2012/794237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, Billiar TR, Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 19.Lagoa CE, Vodovotz Y, Stolz DB, Lhuillier F, McCloskey C, Gallo D, Yang R, et al. The role of hepatic type 1 plasminogen activator inhibitor (PAI-1) during murine hemorrhagic shock. Hepatology. 2005;42:390–399. doi: 10.1002/hep.20797. [DOI] [PubMed] [Google Scholar]
- 20.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133:895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386:302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowalewska PM, Burrows LL, Fox-Robichaud AE. Intravital microscopy of the murine urinary bladder microcirculation. Microcirculation. 2011;18:613–622. doi: 10.1111/j.1549-8719.2011.00123.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee WY, Kubes P. Leukocyte adhesion in the liver: distinct adhesion paradigm from other organs. J Hepatol. 2008;48:504–512. doi: 10.1016/j.jhep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21:5213–5223. doi: 10.1038/sj.onc.1205684. [DOI] [PubMed] [Google Scholar]
- 25.Katz DL, Evans MA, Chan W, Nawaz H, Comerford BP, Hoxley ML, Njike VY, et al. Oats, antioxidants and endothelial function in overweight, dyslipidemic adults. J Am Coll Nutr. 2004;23:397–403. doi: 10.1080/07315724.2004.10719384. [DOI] [PubMed] [Google Scholar]
- 26.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 27.DiStasi MR, Ley K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30:547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarady JK, Otterbein SL, Liu F, Otterbein LE, Choi AM. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am J Respir Cell Mol Biol. 2002;27:739–745. doi: 10.1165/rcmb.4816. [DOI] [PubMed] [Google Scholar]
- 29.Urquhart P, Rosignoli G, Cooper D, Motterlini R, Perretti M. Carbon monoxide-releasing molecules modulate leukocyte-endothelial interactions under flow. J Pharmacol Exp Ther. 2007;321:656–662. doi: 10.1124/jpet.106.117218. [DOI] [PubMed] [Google Scholar]
- 30.Nakahira K, Kim HP, Geng XH, Nakao A, Wang X, Murase N, Drain PF, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer I, Bauer M, Pannen BH, Leinwand MJ, Zhang JX, Clemens MG. Chronic ethanol consumption exacerbates liver injury following hemorrhagic shock: role of sinusoidal perfusion failure. Shock. 1995;4:324–331. doi: 10.1097/00024382-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Bauer M, Pannen BH, Bauer I, Herzog C, Wanner GA, Hanselmann R, Zhang JX, et al. Evidence for a functional link between stress response and vascular control in hepatic portal circulation. Am J Physiol. 1996;271:G929–935. doi: 10.1152/ajpgi.1996.271.5.G929. [DOI] [PubMed] [Google Scholar]
- 33.Kubulus D, Mathes A, Pradarutti S, Raddatz A, Heiser J, Pavlidis D, Wolf B, et al. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock. 2008;29:583–590. doi: 10.1097/SHK.0b013e318157e526. [DOI] [PubMed] [Google Scholar]
- 34.Suematsu M, Suzuki H, Delano FA, Schmid-Schonbein GW. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation. 2002;9:259–276. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.