Abstract
Background/Objectives
What drives overconsumption of food is poorly understood. Alterations in brain structure and function could contribute to increased food seeking. Recently brain orbitofrontal cortex volume has been implicated in dysregulated eating but little is know how brain structure relates to function.
Subjects/Methods
We examined obese (n=18, age=28.7.4±8.3 years) and healthy control women (n=24, age=27.4±6.3 years) using a multimodal brain imaging approach. We applied magnetic resonance and diffusion tensor imaging to study brain gray and white matter volume as well as white matter integrity, and tested whether orbitofrontal cortex volume predicts brain reward circuitry activation in a taste reinforcement-learning paradigm that has been associated with dopamine function.
Results
Obese individuals displayed lower gray and associated white matter volumes (p<.05 family wise error (FWE)-small volume corrected) compared to controls in the orbitofrontal cortex, striatum, and insula. White matter integrity was reduced in obese individuals in fiber tracts including the external capsule, corona radiata, sagittal stratum, and the uncinate, inferior fronto-occipital, and inferior longitudinal fasciculi. Gray matter volume of the gyrus rectus at the medial edge of the orbitofrontal cortex predicted functional taste reward-learning response in frontal cortex, insula, basal ganglia, amygdala, hypothalamus and anterior cingulate cortex in control but not obese individuals.
Conclusions
This study indicates a strong association between medial orbitofrontal cortex volume and taste reinforcement-learning activation in the brain in control but not in obese women. Lower brain volumes in the orbitofrontal cortex and other brain regions associated with taste reward function as well as lower integrity of connecting pathways in obesity may support a more widespread disruption of reward pathways. The medial orbitofrontal cortex is an important structure in the termination of food intake and disturbances in this and related structures could contribute to overconsumption of food in obesity.
Keywords: Obesity, Gray Matter, White Matter, Reward, DTI, VBM, Orbitofrontal, SPSRQ
Introduction
With more than a third of the US population obese1 (defined as body mass index, BMI, of ≥30 kg/m2), understanding the neurobiology of disturbed eating behaviors is of significant importance. What, when and how much we eat is influenced, at least in part, by brain circuits involving taste perception and afferents to motivational and hedonic pathways,2 which likely play an important role in obesity (OB).3 In recent years, it has further been suggested that individuals prone to OB and aberrant eating behaviors could get ‘addicted’ to food, as the same neural pathways that reinforce natural appetitive behaviors are also activated in response to addictive drugs.4, 5
The networks processing taste and taste-reward are complex. After taste stimulation in the mouth, neurons project via brainstem and thalamus to the primary taste cortex, comprised of the insula, and from there to the ventral striatum, amygdala, hypothalamus, and orbitofrontal cortex (OFC).6 Within that circuitry, the brain neurotransmitter dopamine is associated with the motivational aspect of approaching foods and provides a learning signal in response to cues that predict taste and other rewards.7
Food restriction and weight loss are associated with heightened dopamine-related brain reward response in rodents,8 while overconsumption of food leads to addiction-like dopamine D2 receptor down-regulation in the striatum.9 In line with those findings are human functional imaging studies indicating a reduction in brain response to food receipt in OFC and striatum in OB.10 In addition, our group11 recently found OB displayed diminished brain response during a dopamine-related taste reward learning task in ventral striatum and insula. In that task subjects learn to predict taste stimuli in response to conditioned visual cues but when a taste stimulus is received or omitted unexpectedly then this “prediction error” is associated with activation or depression of dopamine neuronal activity.12 In contrast, underweight individuals showed increased brain response in this task,11 further supporting the idea that over- and underweight states may be associated with opposite brain alterations. Other brain research in OB, summarized in a recent meta-analysis of functional magnetic resonance imaging studies that used visual food stimuli provided evidence that OB is associated with increased brain response in brain regions that evaluate potentially rewarding stimuli such as the prefrontal cortex, but decreased activation in areas involved in cognitive control and interoceptive awareness including the insula and dorsolateral prefrontal cortex.13
Altered brain gray (GM) and white matter (WM) structure might be directly related to altered brain function and behavior,14 however previous research in OB has been inconsistent, finding both increases and decreases in brain volume being associated with higher BMI and OB in frontal, temporal, and limbic brain regions.15, 16
Most recently, we found in individuals with the eating disorders anorexia (AN) and bulimia (BN) nervosa increased gray matter volume in the gyrus rectus across age groups and different illness states.17, 18 The gyrus rectus is the medial part of the OFC and has been associated with value attribution to food stimuli, taste pleasantness, and food avoidance.19 Thus, the gyrus rectus could also be a key component in the pathophysiology of OB. We had previously hypothesized that increased gyrus rectus volume could contribute to early satiation and chronic or episodic food restriction in AN or BN.17 In contrast, in OB, gyrus rectus volumes might be smaller compared to controls, and thus making it more difficult for individuals with OB to terminate food intake.
In addition to measurement of GM and WM volume, magnetic resonance imaging allows to study integrity of brain WM tracts by mapping water diffusivity in WM structures.20 Diffusivity can be measured as fractional anisotropy (FA) along WM axons related to axon myelination and density, as well as the apparent diffusion coefficient (ADC), assessing water diffusivity at the voxel level. Higher FA is related to better myelination, whereas higher ADC indicates dispersed water diffusion reflecting cell damage.20 Few studies have employed this method in OB, showing higher BMI associated with lower WM integrity in fornix and corpus callosum,21 or decreased WM integrity in corticospinal tracts, mammillary bodies and corpus callosum.22
In summary, studies suggest both structural and functional GM and WM alterations in OB, but the studies have been inhomogeneous. What is especially missing in our understanding of brain function in OB is an integrated concept of how brain structure could mechanistically be involved in brain function that drives food intake.
In the present study we explored reward circuitry by measuring brain GM and WM volume together with WM integrity in OB compared to healthy control women, and examined directly potential interactions between brain volume and reward prediction response from a dopamine associated reward learning paradigm that we reported before.11 Testing such an interaction has not been reported previously. Our primary hypothesis was that OB would display reduced GM and associated WM volumes in the gyrus rectus. Additionally, we hypothesized that gyrus rectus volume might be directly related to normal brain taste reward learning response, as it computes reward values, responds to the amount of food eaten and controls how much we eat,23, 24 but that this relationship would be disturbed in OB.
Subjects and Methods
Participants
Forty-two right-handed healthy women similar in age participated, 18 obese (OB) and 24 normal weight controls. All imaging procedures occurred during the first 10 days of the follicular phase of the menstrual cycle.
Screening and Study Inclusion
Participants were recruited through local advertisements in the Denver/Metro area. After complete description of study procedures, written informed consent was obtained. The Colorado Multiple Institutional Review Board approved all research procedures. Controls had a lifetime history of healthy body weight (between 90% and 110% of ideal body weight since menarche), no eating or weight concerns, and were free from any lifetime major medical or psychiatric illness. OB had a BMI ≥ 30. Individuals taking any medication other than oral contraceptives were excluded.
Study participants completed: 1. Eating Disorder Inventory-3 (EDI-3) for Drive for Thinness, Bulimia, and Body Dissatisfaction.25 2. Temperament and Character Inventory (TCI) for Novelty Seeking, Reward Dependence and Harm Avoidance.26 3. Spielberger State and Trait Anxiety Inventory (STAI).27 4. Beck Depression Inventory (BDI).28 5. Revised Sensitivity to Punishment and Reward Questionnaire (SPSRQ).29
Imaging Procedures
MRI Acquisition for Brain GM and WM Volumes
Structural brain images were acquired on a GE Signa 3T scanner, using an axial three-dimensional (3D) T1-weighted magnetization-prepared rapid acquisition gradient echo (IR-prepped fast spoiled gradient recall, FSPGR, field of view 22 cm, flip angle 10°, slice thickness 1.2 mm, scan matrix 256×256, TR/TE/TI= 10/3/450 ms, ASSET, 1 NEX, voxel size 0.89 mm3).
MRI Acquisition for Functional Imaging
Brain images were acquired on a GE Signa 3T scanner for blood oxygen dependent (BOLD) T2* weighted echo-planar imaging (EPI), voxel size 3.4 × 3.4 × 2.6mm, TR 2100ms, TE 30ms, angle 70°, 30 slices, interleaved acquisition, 2.6mm slice thickness with 1.4mm gap.
MRI Acquisition for Diffusion Tensor Imaging (DTI)
Diffusion-weighted images (DWI) for DTI mapping included 25 DWI diffusion directions and one T2-weighted (b=0) baseline image. Each image included 45 slices acquired in axial anterior-posterior commissure orientation (128×128 matrix, TR/TE=16000/82.6 ms, field of view = 26 cm, b-value=1000, ASSET, slice thickness/gap = 2.6/0 mm).
Functional Imaging Taste Reward Paradigm
Subjects completed a fMRI taste reward conditioning task described previously,11 learning the association between conditioned visual (CS) and unconditioned taste stimuli (US) and unexpected violation of those learned associations. This violation elicits the so-called prediction error, which has been associated with activation or depression of brain dopamine reward circuits.
Each participant’s individual prediction error signal was modeled based on trial sequence. The predicted value (V̂) at any time (t) within a trial is calculated as a linear product of weights (wi) and the presence of the conditioned visual stimuli (CS) at time t, coded in a stimulus representation vector xi(t) where each stimulus xi is represented separately at each moment in time:
The predicted stimulus value at each time point t in the trial is updated by comparing the predicted value at time t+1 to that actually observed at time t, leading to the prediction error δ(t):
where r(t) is the reward at time t. The parameter γ is a discount factor, which determines the extent to which rewards arriving sooner are more important than rewards that arrive later during the task, with γ=0.99. The weights wi relate to how likely a particular unconditioned taste stimuli (US) follows the associated CS and are updated on each trial according to the correlation between prediction error and the stimulus representation:
where α is a learning rate. Among various learning rates (0.2, 0.5, 0.7) a slow α=0.7 was the best fit for study groups. The initial reward values were 1 for Sucrose and 0 for No solution. The trial-to-trial prediction error was regressed with brain activation across all trials within each subject. For more detailed methods see Frank et al 2012. 11
GM and WM Analysis using Voxel Based Morphometry (VBM)
Pre-processing of T1-weighted images was performed using SPM8 VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/download/) in Matlab R2009b, 7.9.0 (MathWorks, Natick, MA, USA). Images were normalized to MNI space using high-dimensional diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL),30 using a custom template to normalize images based off individual brain images rather than a common template. Non-linear modulation was used, which produces template aligned tissue class images, but multiplies voxel values by non-linear components only, correcting volumes for individual brain sizes directly to the data instead of to the statistical model, thus reducing distortions.31, 32 Images were smoothed to an 8-mm full-width at half maximum Gaussian kernel.
DTI Image Analysis
DTI datasets were processed using NordicICE 2.3.12 MRI toolbox (http://www.nordicneurolab.com) for 3-dimensional axonal fiber tracking using “Fiber Assignment by Continuous Tacking” (FACT).33 Fibers are tracked continuously based on water diffusion. Where the tract leaves the voxel and enters the next, the direction is changed to that of the neighboring voxel. Exhaustive search tracking and a principal eigenvector angle stopping threshold of 41° was used, minimum fiber length 5 mm with only fractional anisotropy values greater than 0.2.33, 34
Whole brain FA and ADC maps were normalized to the average age-specific T1 template, smoothed with a 6-mm FWHM filter and masked with a WM mask and compared across groups using SPM8.
To delineate the WM pathways associated with clusters of significant FA groups difference, we applied probabilistic tractography using the Oxford Centre for Functional MRI of the Brain, (FMRIB) Diffusion Toolbox 4.1.3 (http://www.fmrib.ox.ac.uk/fsl) for preprocessing and PROBTRACKX toolbox for tractography35 using default parameters of 5000 samples, 0.2 curvature threshold, and loopcheck options applied. From each individual’s path distribution estimation, a mean image was created using fslmaths toolbox.
Statistical Analyses
GM and WM Volume Differences
A general linear model (GLM) whole-brain analysis was used (SPM8), a factorial design modeled with diagnosis as 2-level factor (controls and OB) and depression scores and total intracranial volume (TIV) as covariates. Initially, a voxel-wise F-test was performed, p<0.001 uncorrected, extent threshold>50 voxels. Results were corrected using SPM8 anatomical automatic labeling (AAL) atlas derived a priori defined anatomical regions involved in taste and reward processing (OFC, amygdala, ACC, insula, operculum, caudate, putamen, nucleus accumbens, pallidum, hippocampus, thalamus, hypothalamus, and midbrain), family-wise error (FWE) corrected at p<0.05, and regional volumes that reached significance were extracted.17, 18
GM Volume and Reward Correlation Analysis
Based on the areas of significant group difference in GM volume, we extracted GM volumes for those anatomical regions (amygdala, caudate, OFC, hippocampus, and ACC), and conducted a within-group (controls and OB separately) whole brain regression analysis between those GM volumes and taste-reward task brain response. Depression and TIV were used as covariates. A voxel-wise F-test was performed, and contrast maps were created with an initial threshold set at a p<0.005 uncorrected and 50 voxel threshold. Results were corrected using a priori defined anatomical regions (same as used for volumetric analyses), FWE-corrected at p<0.05, and regional activation that reached significance was reported.
DTI Analysis
A GLM was used for group comparison similar to volumetric analyses. A voxel-wise F-test was performed; threshold set at a p<0.05 FWE-corrected and extent threshold >5 voxels. We used this threshold to be conservative as there are no anatomical regions whole brain activation that can be corrected to. For the resulting clusters, mean FA and ADC values were extracted using the SPM marsbar toolbox. The WM bundles identified as significantly different across groups were then identified by visual inspection using the ‘MRI Atlas of Human White Matter’36 and ‘Dissecting the White Matter Tracts: Interactive Diffusion Tensor Imaging Teaching Atlas’ by Hutchins et al., an online atlas (http://www.asnr2.org/neurographics).
Demographic, behavioral and extracted brain data were analyzed using SPSS (IBM-SPSS, Chicago, IL) and independent-samples t-test. Linear regression analyses to test behavior–brain relationships were applied for age, BMI, and reward and punishment sensitivity. Significant correlations were corrected for the false discovery rate using the method proposed by Benjamini & Hochberg (1995).37
Results
Demographic and Behavioral Data (Table 1)
Table 1.
Demographic variables for healthy control women and obese women.
| Control Women, n=24 | Obese Women, n=18 | t | p | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| Age (Years) | 27.42 | 6.28 | 28.67 | 8.30 | −0.56 | 0.581 |
| Body Mass Index (kg/m2) | 21.64 | 1.26 | 34.78 | 4.44 | −12.18 | <0.001 |
| Harm Avoidance | 9.58 | 3.99 | 12.61 | 4.91 | −2.20 | 0.033 |
| Novelty Seeking | 17.92 | 5.16 | 20.94 | 5.29 | −1.86 | 0.070 |
| Reward Dependence | 16.96 | 3.71 | 16.67 | 3.60 | 0.26 | 0.800 |
| Depression (BDI) | 1.13 | 0.95 | 4.67 | 4.78 | −3.10 | 0.006 |
| Drive for thinness (EDI-3) | 2.63 | 3.41 | 11.67 | 7.35 | −4.84 | <0.001 |
| Body Dissatisfaction (EDI-3) | 4.38 | 4.25 | 26.17 | 9.04 | −9.47 | <0.001 |
| Bulimia (EDI-3) | 0.79 | 1.22 | 10.22 | 11.28 | −3.53 | 0.003 |
| Punishment Sensitivity | 4.04 | 1.85 | 6.78 | 4.29 | −2.53 | 0.019 |
| Reward Sensitivity | 4.42 | 2.84 | 6.22 | 4.49 | −1.59 | 0.119 |
| State Anxiety | 32.67 | 11.79 | 36.78 | 13.84 | −1.04 | 0.305 |
| Trait Anxiety | 33.92 | 11.35 | 39.44 | 11.16 | −1.57 | 0.124 |
OB and healthy controls were similar in age. OB showed the expected higher BMI, as well as higher scores on Depression (but within normal limits), Drive for Thinness, Bulimia, Body Dissatisfaction, Punishment Sensitivity, and Harm Avoidance. State and Trait anxiety as well as Novelty Seeking and Reward Sensitivity were similar in both groups.
GM and WM Volume Results (Table 2, Fig. 1)
Table 2.
Whole brain and regional gray and white matter brain volumes across groups. Values for regional brain volumes are fractions of respective tissue type per volume.
| Whole Brain Volumes | Control Women, n =24 | Obese Women, n=18 | t | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Gray Matter Volume (cm3) | 629.65 | 62.12 | 627.98 | 51.13 | 0.09 | 0.926 |
| White Matter Matter Volume (cm3) | 494.00 | 44.34 | 492.01 | 42.41 | 0.15 | 0.884 |
| Cerebral Spinal Fluid Volume (cm3) | 226.81 | 30.63 | 254.14 | 28.76 | −2.94 | 0.005 |
| Total Intracranial Volume (cm3) | 1350.47 | 105.28 | 1374.13 | 100.49 | −0.73 | 0.467 |
|
| ||||||
| Anatomical Region | Cluster Size | p FWE-Corr | T/Z at peak | MNI Coordinates | ||
| GM Volume Ratio | x | y | z | |||
|
| ||||||
| L Amygdala, BA 38 | 154 | 0.001 | 4.36/4.09 | −26 | 5 | −29 |
| R Caudate | 59 | 0.011 | 4.23/3.79 | 8 | 18 | −9 |
| R Superior Orbitofrontal Cortex, BA 11 | 38 | 0.008 | 4.39/3.91 | 9 | 56 | −24 |
| R Medial Orbitofrontal Cortex, Gyrus Rectus | 4 | 0.017 | 3.98/3.61 | 17 | 23 | −12 |
| R Medial Orbitofrontal Cortex, Gyrus Rectus, BA 11 | 12 | 0.032 | 3.71/3.40 | 6 | 56 | −23 |
| R Anterior Cingulum, BA 32 | 2 | 0.033 | 3.91/3.55 | 6 | 20 | −8 |
| R Hippocampus | 80 | 0.021 | 3.97/3.60 | 35 | −13 | −14 |
| L Hippocampus | 232 | 0.004 | 4.62/4.08 | −36 | −9 | −18 |
| WM Volume Ratio | ||||||
|
| ||||||
| L Amygdala | 63 | 0.003 | 4.26/3.82 | −30 | −4 | −29 |
| R Caudate | 192 | 0.001 | 5.16/4.45 | 15 | 8 | 21 |
| L Caudate | 354 | 0.001 | 5.18/4.47 | −15 | 8 | 19 |
| R Medial Orbitofrontal Cortex | 36 | 0.015 | 3.92/3.56 | 8 | 30 | −14 |
| L Medial Orbitofrontal Cortex | 98 | 0.001 | 4.93/4.29 | −12 | 50 | −14 |
| L Middle Orbitofrontal Cortex | 3 | 0.021 | 3.93/3.57 | −15 | 50 | −18 |
| L Superior Orbitofrontal Cortex | 185 | 0.003 | 4.82/4.22 | −14 | 48 | −15 |
| R Insula | 135 | 0.005 | 4.78/4.19 | 30 | 27 | 12 |
| R Insula, BA 13 | 22 | 0.045 | 3.87/3.52 | 38 | −13 | 22 |
| L Insula, BA 13 | 22 | 0.029 | 4.04/3.65 | −36 | −25 | 22 |
| L Insula | 7 | 0.049 | 3.81/3.48 | −44 | −3 | −9 |
| R Medial Orbitofrontal Cortex, Gyrus Rectus | 29 | 0.030 | 3.71/3.40 | 8 | 29 | −15 |
| L Medial Orbitofrontal Cortex, Gyrus Rectus | 97 | 0.001 | 4.93/4.30 | −12 | 48 | −15 |
| R Anterior Cingulum | 272 | <0.001 | 6.00/4.98 | 9 | 29 | 22 |
| R Anterior Cingulum | 1 | 0.002 | 4.92/3.57 | 11 | 50 | 10 |
| R Anterior Cingulum | 24 | 0.011 | 4.35/3.89 | 11 | 47 | 4 |
| R Anterior Cingulum | 4 | 0.043 | 3.78/3.45 | 9 | 48 | 13 |
| R Anterior Cingulum | 53 | 0.048 | 3.73/3.42 | 11 | 32 | −11 |
| L Anterior Cingulum | 437 | 0.002 | 5.01/4.35 | −2 | 24 | 21 |
| L Anterior Cingulum | 70 | 0.002 | 5.03/4.36 | −15 | 48 | 7 |
| R Hippocampus | 139 | 0.014 | 4.11/3.71 | 35 | −15 | −23 |
| L Hippocampus | 65 | <0.001 | 5.46/4.64 | −30 | −12 | −24 |
| R Rolandic Operculum | 11 | 0.042 | 3.66/3.36 | 38 | −13 | 21 |
| R Rolandic Operculum | 21 | 0.006 | 4.46/3.96 | 44 | −28 | 22 |
| R Rolandic Operculum | 11 | 0.019 | 3.66/3.36 | 45 | −24 | 22 |
| L Rolandic Operculum | 179 | 0.019 | 4.08/3.68 | −39 | −24 | 22 |
Control Women > Obese Women; Whole-brain volumes represented as raw values. Regional brain volume contrasts based on group comparison corrected for total intracranial volume and depression. FWE-corr = family-wise error corrected; L = left; MNI = Montreal Neurological Institute; R = right.
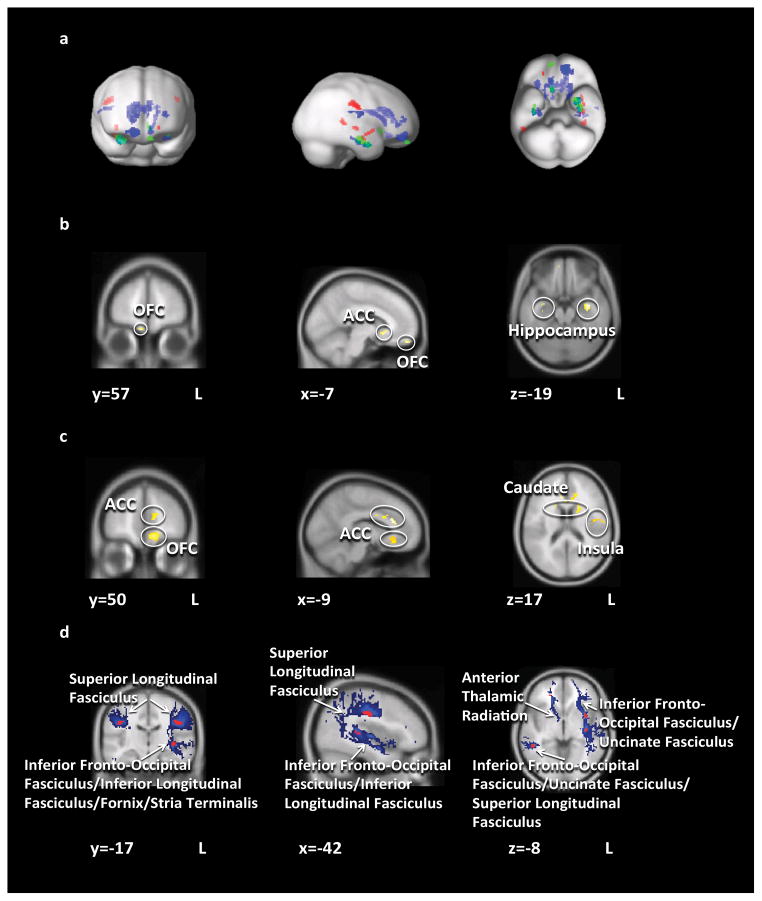
Figure 1.
Structural brain results. a) Areas of significant gray matter volume (green), white matter volume (blue), and white matter integrity (red) differences b) Gray matter volume Control Women > Obese Women; c) White matter volume Control Women > Obese Women; d) Areas of significant FA difference (red, Control Women > Obese Women) overlayed on mean group probabilistic tractography path distribution estimations (blue). Abbreviations: OFC = orbitofrontal cortex; ACC = anterior cingulate cortex.
Total GM and WM brain volume was similar between groups. OB displayed less total cerebral spinal fluid volume than controls.
Localized GM and associated WM volumes (expressed as ratio of GM or WM per voxel) were reduced in OB compared to controls in the amygdala, caudate, ACC, hippocampus, OFC, and insular WM. Neither GM nor WM volumes were greater in OB compared to controls in any brain region.
GM Volume and Taste Reward Task Correlation Results (Table 3, Fig.2)
Table 3.
Regions of significantly negative correlation between right and left gyrus rectus gray matter volume and taste reward activation in control and obese women.
| Control Women | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Anatomical Region | Peak Level | MNI Coordinates | ||||
| kE | p FWE-Corr | T/Z at peak | x | y | z | |
|
Right Gyrus Rectus
|
||||||
| L Amygdala | 26 | 0.036 | 3.74/3.22 | −28 | 0 | −24 |
| L Caudate | 238 | 0.023 | 4.68/3.80 | −18 | 24 | 8 |
| L Anterior Cingulum | 59 | 0.023 | 4.81/3.88 | −4 | 12 | 26 |
| R Medial OFC | 31 | 0.023 | 4.47/3.68 | 2 | 38 | −10 |
| L Medial OFC | 17 | 0.024 | 4.54/3.72 | 0 | 38 | −10 |
| L Hippocampus | 28 | 0.030 | 4.52/3.71 | −22 | −34 | 0 |
| R Hypothalamus | 3 | 0.007 | 3.86/3.29 | 8 | −6 | −8 |
| L Insula | 346 | 0.013 | 5.28/4.13 | −42 | 2 | −12 |
| L Insula | 26 | 0.017 | 5.16/4.07 | −36 | −22 | 22 |
| L Putamen | 91 | 0.037 | 4.42/3.65 | −26 | 10 | 12 |
| L Rolandic Operculum | 52 | 0.002 | 6.25/4.60 | −40 | −20 | 22 |
|
| ||||||
|
Left Gyrus Rectus
|
||||||
| L Amygdala | 23 | 0.043 | 3.64/3.15 | −28 | −2 | −26 |
| L Hippocampus | 60 | 0.012 | 5.03/4.00 | −22 | −34 | 0 |
| L Insula | 116 | 0.030 | 4.83/3.89 | −40 | 2 | −14 |
| L Rolandic Operculum | 53 | 0.017 | 4.97/3.96 | −40 | −20 | 22 |
|
| ||||||
| Obese Women | ||||||
|
| ||||||
|
Right Gyrus Rectus
|
||||||
| R Rolandic Operculum | 17 | 0.003 | 7.48/4.48 | 50 | −22 | 20 |
| L Rolandic Operculum | 155 | 0.039 | 5.71/3.90 | −48 | −10 | 14 |
|
| ||||||
|
Left Gyrus Rectus
|
||||||
| L Rolandic Operculum | 164 | 0.030 | 5.91/3.97 | −56 | −20 | 16 |
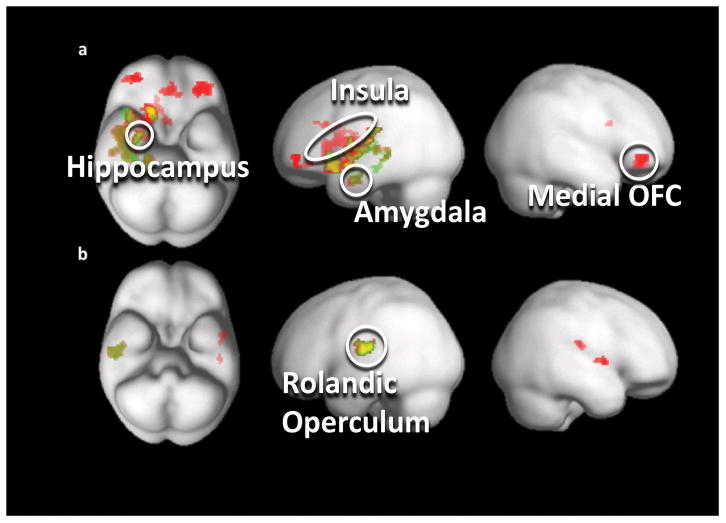
Figure 2.
Gray matter volume and reward correlation analysis results. Significantly negative correlation between gyrus rectus right (red) and gyrus rectus left (green) gray matter volume and reward response in a) control women and b) obese women. Abbreviations: OFC = orbitofrontal cortex.
There was a significantly negative correlation between left and right gyrus rectus GM volume and reward response in controls in the ACC, amygdala, striatum, hypothalamus, hippocampus, rolandic operculum, and insula. There was a significantly negative correlation between gyrus rectus GM volume and rolandic operculum functional brain reward response in OB.
DTI Results (Table 4, Fig. 1)
Table 4.
Regions of significant fractional anisotropy and apparent diffusion coefficient differences, Control Women > Obese Women.
| Anatomical Region/Pathway | Cluster Size | p FWE-Corr | T/Z at peak | MNI Coordinates | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
|
FA
|
||||||
| R Anterior Corona Radiata - ATR/UF/IFOF | 6 | 0.026 | 5.74/4.82 | 20 | 42 | −6 |
| R Superior Corona Radiata -SLF/IFOF/UF | 30 | 0.003 | 6.57/5.31 | 42 | −20 | 34 |
| R Sagittal Stratum - IFOF/ILF | 11 | 0.007 | 6.21/5.11 | 50 | −44 | −4 |
| L External Capsule - UF/IFOF/SLF | 18 | 0.006 | 6.29/5.15 | −36 | 4 | −8 |
| L External Capsule - IFOF | 15 | 0.013 | 6.01/4.99 | −38 | −16 | −2 |
| L Sagittal Stratum - IFO/ILF | 19 | 0.01 | 6.11/5.05 | −40 | −34 | 6 |
| L Sagittal Stratum - IFOF/ILF/FX/ST | 7 | 0.025 | 5.76/4.84 | −40 | −6 | −16 |
| L Superior Corona Radiata - SLF | 104 | <0.001 | 8.21/6.16 | −46 | −16 | 30 |
|
| ||||||
|
ADC
|
||||||
| R Sagittal Stratum - IFOF/ILF | 5 | 0.015 | 5.74/4.82 | 32 | −52 | −8 |
| L Superior Corona Radiata - STR/CPT/CST | 5 | 0.002 | 6.48/5.26 | −18 | −16 | 66 |
UF = uncinate fasciculus; IFOF = inferior fronto-occipital fasciculus; ATR = anterior thalamic radiation; SLF = superior longitudinal fasciculus; ILF = inferior longitudinal fasciculus; FX = fornix; ST = stria terminalis; STR = superior thalamic radiation; CPT = corticopontine tract; CST = corticospinal tract.
DTI FA was reduced in OB compared to controls in bilateral anterior corona radiata, superior corona radiata, sagittal stratum, and external capsule. DTI FA was not greater in OB compared to controls in any brain region. ADC was greater in OB compared to controls in the right sagittal stratum and left superior corona radiata. The fiber paths that connect those brain regions, as determined by probabilistic tractography, included the inferior fronto-occipital fasciculus, superior longitudinal fasciculus, anterior thalamic radiation, and uncinate fasciculus.
Demographic and Behavioral Correlation Results
Controls
WM volume was significantly positively correlated with age in the left insula (r=.542, p<.043; x=−44, y=−3, z=−9) and right gyrus rectus (r=.573, p<.017; x=8, y=29, z=−15).
FA was significantly negatively correlated with age in the right sagittal stratum (r=−.521, p<.018; x=50, y=−44, z=−4) and with BMI in the right superior corona radiata (r=−.473, p<.039; x=42, y=−20, z=34).
OB
GM volume was significantly negatively correlated with age in the right gyrus rectus (r=−.678, p<.012; x=17, y=23, z=−12 and r=−.615, p<.040; x=6, y=56, z=−23).
FA was significantly negatively correlated with age in the right anterior corona radiata (r=−.644, p<.008; x=20 y=42 z=−6).
Discussion
The present study indicates that OB is associated with decreases in GM and associated WM volumes in OFC, insula, amygdala, striatum, hippocampus, and ACC as well as decreased WM integrity in the corona radiata, sagittal stratum, and external capsule. Gyrus rectus GM volume predicted negatively brain activation during a reward learning paradigm in taste and reward-related regions including the insula, striatum, amygdala, hypothalamus and hippocampus in controls, but not OB, perhaps suggesting a potential mechanism that could alter reward function and eating regulation in OB.
As expected, OB showed higher BMI, and scored higher on body dissatisfaction, bulimic symptoms and drive for thinness. OB displayed greater SPSRQ29 sensitivity to punishment than controls, similar to adults with AN and BN,38, 39 suggesting increased sensitivity to negative reinforcement in OB.
GM and Associated WM Volume
OB displayed lower regional GM and associated WM volume than controls in OFC, insula, caudate, amygdala, hippocampus and ACC, a network of regions that contributes to taste and reward processing,3 as well as motivation, emotion processing and food intake control.24 More specifically, the OFC regulates when to stop eating a particular food, the ACC is important in anticipating food reward,19, 40 and the insula as the primary taste cortex processes taste quality, 11 but gets also activated in response to visual food stimuli13 and has been associated with ‘craving’ or ‘wanting’ of rewards in drug addiction,41 food-cravings,19 and hunger.42 The caudate receives direct input from the insula and is thought to regulate the incentive properties of food,3 and in OB, activation in this region has been associated with food cravings and food reward anticipation.19, 43 Importantly, the striatum including the caudate contains dopaminergic terminals from the midbrain that are involved in the motivational aspect of food approach. The amygdala drive dopamine activation in the reward cycle,44 and greater activation in OB has been implicated in studies involving visual food cues.19 Altogether it is possible that reduced volumes in this circuitry may contribute to altered internal feedback mechanisms in response to food, leading to an inability to stop food intake when physiologically enough food would have been eaten.
GM Volume and Reward Correlation Analysis
Importantly and novel in this study, we conducted a regression analysis between GM volume and brain response during a taste reward conditioning task.11 This showed a negative structure-function relationship between gyrus rectus GM volume and brain reward activation in reward-related regions including the amygdala, caudate, putamen, insula, ACC, hippocampus, rolandic operculum and hypothalamus in controls, but not OB. In OB there was only a significantly negative association between gyrus rectus GM volume and taste reward response activation in the rolandic operculum.
The gyrus rectus is the medial part of the OFC and further defined by a caudal agranular and dysgranular layer (area 14) that transitions antero-superiorly into the granular layer (area 11).45 The OFC is an important component of hedonic and motivational aspects of reward23 and has been implicated in addiction.46 The OFC is connected to all sensory modalities6 and is integral in controlling reward- and punishment-related behavior.23 The gyrus rectus’ role in regulating sensory specific satiety has particular implication for obesity, as a dysfunction in this structure could contribute to overeating.47 Little is known though whether connections of the OFC within the taste reward circuitry are in a positive or negative feedback fashion.
The current literature suggest that obese individuals have heightened brain response to visual food cues, but reduced brain satiety response.19 Our results in the control group suggests that the larger the OFC volume the smaller brain response during reward learning as expressed by predication error response, which has not been shown before. Thus the OFC may regulate motivational pathways for food approach. In OB however, smaller OFC volume together with a lack of relationship of OFC volume and functional taste reward response suggests that a feedback mechanism between OFC and taste reward pathways is disturbed, which could interfere with healthy control of eating. It is unclear however, whether smaller gyrus rectus volume is a premorbid vulnerability for OB or whether it is adaptive to excessive food intake, for instance in response to inflammatory processes that have been associated with OB and could impact brain structure.48
The correlation between gyrus rectus volume and the rolandic operculum was preserved in OB. The rolandic operculum covers the superior posterior insula and has been implicated in somatosensory processing of taste stimuli.49–51 One possible explanation for the preserved structure-function relationship between gyrus rectus GM volume and rolandic operculum in the obese group is that somatosensory processing of taste and somatic sensations involved in food intake remain intact, while the circuitry for integration of the rewarding aspects of taste may be disrupted.3
WM Integrity
OB displayed reduced WM integrity (lower FA) in the external capsule, which lies between the putamen medially and claustrum laterally. The external capsule connects ventral and medial prefrontal cortices with limbic regions,52 contains fibers from both the uncinate fasciculus and inferior fronto-occipital fasciculus, and connects the amygdala and hippocampus with prefrontal and OFC regions. Thus those pathways connect GM regions that showed reduced volume in OB in this study, and locally altered fiber connections between these regions may further suggest disruption of a larger taste reward circuitry.
Other WM tracts with reduced integrity were the sagittal stratum that conveys fibers from the parietal, occipital, cingulate, and temporal regions to the thalamus and contains the inferior longitudinal fasciculus, a long association system connecting visual pathways in the occipito-temporal cortices.52 The corona radiata, a collection of fiber bundles that extend from the internal capsule to cerebral cortex,52 basal ganglia and spinal cord, has been associated with central taste disorders.53 In summary, OB displayed localized disrupted WM integrity in many fiber tracts that connect frontal and limbic regions of the brain. While speculative, it is possible that reduced integrity of WM tracts between OFC and limbic brain regions involved in reward processing may contribute to the lack of OFC volume-taste reward learning signal relationship in OB. Surprisingly, OB did not display decreases in fornix integrity as has been previously reported in OB21 and other eating problems.18, 54, 55 Differing results may be explained by differences in inclusion of age, depression, and gender, but this will require study in a larger sample.
A limitation is the potentially confounding effect from normalization of brain images to a template. In order to minimize such effects, our methods included a template that was based off the current study population, and a correction for individual brain sizes that was directly applied to the data instead of to the statistical model, thus reducing distortions.31, 32 The present study is also limited to individuals who are already obese and so we cannot discern whether these differences are pre-morbid or are a result of the OB. Additionally, we examined brain structure and function only in female individuals and thus we cannot generalize the findings to males. Future studies are needed to address these concerns.
In summary, this multimodal imaging study is the first in OB research to study and integrate brain GM and associated WM volume, white matter connectivity, and functional response from a reward learning task that has been associated with dopaminergic pathways. Importantly we find in OB a pattern of GM volume reduction across the taste reward system. Gyrus rectus GM volume predicted reward learning response in controls, but not OB, suggesting a disrupted pathway between the OFC and its associated connections in OB. This finding is further supported by the reduction in WM integrity in OB in fiber tracts that connect frontal with limbic and subcortical brain regions, which could further contribute to disturbed reward circuitry feedback. Whether the alterations found exist premorbidly or whether these are alterations in response to specific eating patterns need further study.
Acknowledgments
The authors would like to thank all the individuals who participated in this study.
This work was supported by NIMH grant K23 MH080135-01A2, NIMH grant R01 MH096777, and by the Davis Foundation Award of the Klarman Family Foundation Grants Program in Eating Disorders (all GKWF).
Footnotes
Conflicts of Interests Statement
The authors declare no conflicts of interest.
All authors contributed significantly to this manuscript.
References
- 1.Odgen C, Carroll M. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1976–1980 Through 2007–2008. Hyattsville, MD: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 2.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 3.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2013;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73(9):804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156(4):865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30(39):13105–13109. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37(9):2031–2046. doi: 10.1038/npp.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 13.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8(4):e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao X, Xu D, Bansal R, Dong Z, Liu J, Wang Z, et al. Multimodal magnetic resonance imaging: The coordinated use of multiple, mutually informative probes to understand brain structure and function. Human Brain Mapping. 2013;34(2):253–271. doi: 10.1002/hbm.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: a voxel-based morphometric study. Neuroimage. 2006;31(4):1419–1425. doi: 10.1016/j.neuroimage.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 16.Brooks SJ, Benedict C, Burgos J, Kempton MJ, Kullberg J, Nordenskjold R, et al. Late-life obesity is associated with smaller global and regional gray matter volumes: a voxel-based morphometric study. Int J Obes (Lond) 2013;37(2):230–236. doi: 10.1038/ijo.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170(10):1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank GK, Shott ME, Hagman JO, Yang TT. Localized brain volume and white matter integrity alterations in adolescent anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2013;52(10):1066–1075. e1065. doi: 10.1016/j.jaac.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13(1):43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nature Reviews Neuroscience. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 21.Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, et al. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity. 2011;19(3):500–504. doi: 10.1038/oby.2010.312. [DOI] [PubMed] [Google Scholar]
- 22.Karlsson HK, Tuulari JJ, Hirvonen J, Lepomaki V, Parkkola R, Hiltunen J, et al. Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel-based morphometric study. Obesity. 2013;21(12):2530–2537. doi: 10.1002/oby.20386. [DOI] [PubMed] [Google Scholar]
- 23.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 24.Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol Hung. 2008;95(2):131–164. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- 25.Garner D. Eating Disorder Inventory™-3 (EDI™-3) Psychological Assessment Resources, Inc; Lutz, FL: 2004. [Google Scholar]
- 26.Cloninger C, Przybeck T, Svarkic D, Wetzel R. The temperament and character inventory (TCI): A guide to its development and use. Center for Psychobiology of Personality, Washington University; St. Louis, MO: 1994. [Google Scholar]
- 27.Spielberger CD. Manual for the State-Trate Anxiety Inventory. Consulting Psychologists Press, Inc; Palo Alto, CA: 1983. [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Torrubia R, Avila C, Molto J, Caseras X. The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences. 2001;31:837–862. [Google Scholar]
- 30.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 32.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Olivi A, Hertig SJ, van Zijl P, Mori S. Automated fiber tracking of human brain white matter using diffusion tensor imaging. Neuroimage. 2008;42(2):771–777. doi: 10.1016/j.neuroimage.2008.04.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mori S, Wakana S, van Zijl P, Nagae-Poetscher L. MRI Atlas of Human White Matter. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 38.Jappe LM, Frank GK, Shott ME, Rollin MD, Pryor T, Hagman JO, et al. Heightened sensitivity to reward and punishment in anorexia nervosa. Int J Eat Disord. 2011;44(4):317–324. doi: 10.1002/eat.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frank GK, Reynolds JR, Shott ME, O’Reilly RC. Altered temporal difference learning in bulimia nervosa. Biol Psychiatry. 2011;70(8):728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23(4):1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32(1):56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 44.Pauli WM, Hazy TE, O’Reilly RC. Expectancy, ambiguity, and behavioral flexibility: separable and complementary roles of the orbital frontal cortex and amygdala in processing reward expectancies. J Cogn Neurosci. 2012;24(2):351–366. doi: 10.1162/jocn_a_00155. [DOI] [PubMed] [Google Scholar]
- 45.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nature Neuroscience. 2012;15(1):13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10(3):334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 47.Kringelbach ML, O’Doherty J, Rolls E, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 48.Shefer G, Marcus Y, Stern N. Is obesity a brain disease? Neurosci Biobehav Rev. 2013;37(10 Pt 2):2489–2503. doi: 10.1016/j.neubiorev.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Ruch TC, Patton HD. The relation of the deep opercular cortex to taste. Fed Proc. 1946;5(1 Pt 2):89. [PubMed] [Google Scholar]
- 50.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerf-Ducastel B, Van De Moortele PF, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses. 2001;26(4):371–383. doi: 10.1093/chemse/26.4.371. [DOI] [PubMed] [Google Scholar]
- 52.Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Onoda K, Ikeda M, Sekine H, Ogawa H. Clinical study of central taste disorders and discussion of the central gustatory pathway. J Neurol. 2012;259(2):261–266. doi: 10.1007/s00415-011-6165-z. [DOI] [PubMed] [Google Scholar]
- 54.Mettler LN, Shott ME, Pryor T, Yang TT, Frank GK. White matter integrity is reduced in bulimia nervosa. Int J Eat Disord. 2013;46(3):264–273. doi: 10.1002/eat.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazlouski D, Rollin MD, Tregellas J, Shott ME, Jappe LM, Hagman JO, et al. Altered fimbria-fornix white matter integrity in anorexia nervosa predicts harm avoidance. Psychiatry Res. 2011;192(2):109–116. doi: 10.1016/j.pscychresns.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]