Abstract
Objective
The diagnosis of primary aldosteronism (PA) among the older-aged population has posed a crucial challenge. Among patients over 50 years old, this trial assessed comparability of the performance of two PA diagnostic tests: losartan and captoril suppression tests.
Methods
A post-hoc subgroup analysis from a prospective cohort was conducted by TAIPAI (Taiwan Primary Aldosteornism Investigation) group between July 2003 and July 2006. Of the 160 patients in the cohort, 60 patients over 50 years received captopril and losartan tests to confirm PA.
Results
Among the 60 patients over 50 years old, 31 patients had PA confirmed by standardized protocol. The area under the receiver-operating characteristic (ROC) curve of the post-captopril aldosterone was significantly less than that of the post-losartan plasma aldosterone concentration (0.87 vs. 0.94, p = 0.02). Using ARR>35 with PAC>10 ng/dL, the specificity was 82.76% vs. 93.1% and the sensitivity was 77.42% vs. 87.10% for the captopril and losartan tests, respectively. The equivalence between the two tests were confirmed by exact McNemar test (p= 1.0).
Conclusion
The losartan test showed comparable accuracy to confirm PA. Verification of this “elderly-friendly” confirmatory test will be the first step to prepare the specific diagnostic model of PA for older-aged population.
Keywords: Primary aldosteronism, aldosterone-renin ratio, plasma aldosterone concentration, plasma renin activity, hypertension, captopril, losartan
Introduction
As populations are aging worldwide, the global burden of hypertension has dramatically increased as the elderly persons have the highest prevalence of this disease.1-3. The unique characteristic of hypertension in the near-elderly and elderly is that secondary causes are more prevalent and different compared to the younger population.4, 5 Primary aldosteronism (PA) has long been considered a rare cause of hypertension in near-elderly and elderly.6 An increasing body of literature has demonstrated a greater prevalence of PA, about 10% in patients with increasing hypertension severity, presenting a growing concern.7 A long-standing of aldosterone excess has been proofed to induce arterial stiffness and endothelial injury8. PA is also related to kidney damage9, metabolic syndrome10, and left ventricular hypertrophy.11. Therefore, an accurate and safe diagnostic test for these populations is an unaddressed clinical and epidemiological concern.
As the capacity to adjust excess fluid level is gradually declining in near-elderly and elderly, the oral sodium loading, saline infusion and fludrocortisone suppression tests may not be the first choice especially if patients also have been diagnosed with heart failure, advanced liver disease, and renal insufficiency. Consequently, the captopril challenge test may be the only suitable diagnostic test. Compared to the captopril test, the losartan test has demonstrated potentially better diagnostic accuracy in general population while losartan also provides a better safety profile than captopril regarding the development of angioedema, a potentially life-threatening event.12-14 In this post-hoc analysis, we aimed to verify the performance of the losartan test for PA in patients older than 50 years old. As PA becomes increasingly prevalent in this specific age range, the safety issues and applicability of diagnostic tests would become a critical concern in this population.
Materials and Methods
Patients
From July 2003 to January 2006, 64 patients older than 50 years old were enrolled into the Taiwan Primary Aldosteronism Investigation (TAIPAI) database after follow-up screening at or referral to the National Taiwan University Hospital and its affiliated hospitals. Since this was a subgroup analysis from our previous published prospective cohort study investigating the diagnostic accuracy of losartan test,12 we carefully examined the study power according to the subgroup sample size. Based on the study sample size of 30 in each group with a 0.70 proportion of positive ratings, and 0.60 kappa, we have 90% power for a 2-sided test.15 The initial evaluation included: 1) an age at onset of hypertension or hypokalemia of < 35 years; 2) difficult-to-control hypertension after therapy initiation; 3) the clinical occurrence of a hypertensive crisis; 4) the presence of hypokalemia or metabolic alkalosis, or a random aldosterone-renin ratio (ARR) > 35 ngdL−1 per ngmL−1h−1; and 5) evidence of adrenal incidentaloma with hypertension or hypokalemia. Difficult-to-control hypertension was defined as patients taking more than three antihypertensive medications while their blood pressure still did not reach their therapeutic target16. Hypertensive crises are characterized by severely elevated blood pressure (BP), usually higher than 180/110 mmHg, along with progressive or impending target organ damage17. The ethnic composition of our cohort reflected a typical Taiwanese population18.
All antihypertensive medications were discontinued for at least 21 days before the study. Diltiazem and/or doxazosin were administered for control of marked high blood pressure when required.19 Medications that might interfere with the renin-aldosterone axis such as steroids, sex hormones, licorice, or non-steroidal anti-inflammatory drugs were also withheld for at least 6 weeks. All patients consumed a low-salt diet with 6 g of NaCL daily and were supplied with potassium during the testing period if hypokalemia was identified. The database was constructed for quality assurance in one medical center (National Taiwan University Hospital, Taipei, Taiwan) and its three branch hospitals in different cities (National Taiwan University Hospital Yun-Lin branch, Yun-Lin, southern Taiwan; En Chu Kong Hospital, Taipei County; and, Tao-Yuan General Hospital, Tao-Yuan, middle Taiwan). All patients with intention to confirm and requiring a suppression test or adrenal venous sampling (AVS) were recruited and the data were prospectively collected. This post-hoc subgroup analysis from the previous prospective cohort study was approved by the Institutional Review Board of National Taiwan University Hospital(NTUH No. 9461700402).
Confirmation of primary aldosteronism
In this subcohort, a diagnosis of pheochromocytoma was made in one patient, renal artery stenosis was diagnosed in one patient, and polycystic kidney disease was diagnosed in one patient. Only one patient who did not give his informed consent was excluded. The remaining 60 patients received both captopril and losartan tests (Figure 1). The two tests were performed on 2 consecutive days. As previously reported, the time-to-peak plasma concentration of losartan was proportional to the dose and was ~1.5 h with the 50 mg dose.20 With oral captopril, the time-to-peak was >0.5 h.21 In our previous head-to-head comparative study,12 we have set the sampling period at least one half-life of plasma renin activity (PRA) or plasma aldosterone concentration (PAC) because the biological half-life of aldosterone is ~30 min22 and the half-life of PRA is ~15 min.23. The patients were asked to sit for at least 10 minutes for the baseline blood samples at 9 am, and allowed to ambulate moderately until the second sampling. The second blood samples were obtained either 1 hour after the administration of 50 mg of captopril, or 2 hour after the administration of 50 mg of losartan12.
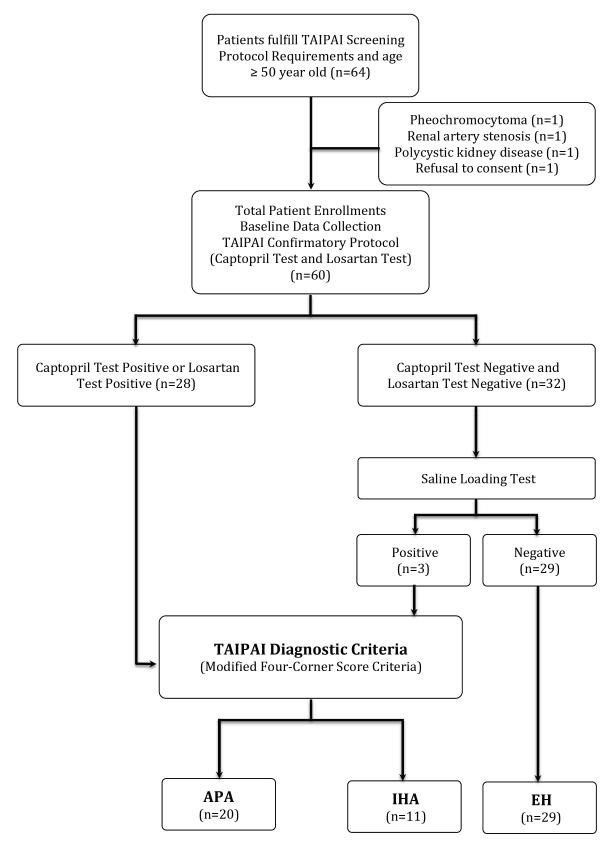
Figure 1.
Study flow diagram. The diagnosis of primary aldosteronism is based on Taiwan Primary Aldosteronism Investigation Group (TAIPAI) protocols.24
Aldosterone-renin ratio >35 after administration of captopril or losartan indicated a positive result. A plasma aldosterone concentration >10 ng/dl after saline infusion is positive for the test (see text). APA, aldosterone-producing adenoma; EH, essential hypertension; IHA, idiopathic hyperaldosteronism.
An ARR >35 with a PAC >10 ng/dL (>277 pmol/l) after the administration of captopril or losartan was defined as a positive test for PA. We constructed an ARR >35 (ngdL−1 per ngmL−1h−1) because this value had the best sensitivity and specificity to differentiate PA from EH in the TAIPAI database. Patients with both negative captopril and losartan suppression tests underwent a saline infusion test on a separate day to evaluate the autonomous secretion of aldosterone. After at least 1 hour in the supine position, two liters of 0.9% NaCl solution were administrated intravenously from 8:00 to 12:00 am, and blood samples for PRA and PAC were drawn before and at the end of the saline infusion. Patients in whom the PAC >10 ng/dl after saline infusion were diagnosed with PA in TAIPAI database.24
Differential Imaging studies
A computerized tomography (CT) of the adrenal glands with a non-ionic iodinated contrast agent was done on all enrolled patients, with at least 3-mm contiguous slices in a normal surrounding. Although there were no strict measurements of normal adrenal size, CT imaging was considered abnormal when any volume thicker than 10 mm3 was detected. Those with inconclusive CT findings underwent dexamethasone suppression adrenocortical scintigraphy with CT (NP-59, I-131-6-beta-iodomethyl-19-norcholesterol & NP59-SPECT/CT).25 Bilateral adrenal venous sampling (AVS) was required if image studies were ambiguous. Successful venous cannulation was defined as the ratio of the cortisol level of the adrenal vein to that of the inferior vena cava > 3. Lateralization of aldosterone secretion was defined by a greater than four-fold difference in the aldosterone/cortisol ratio between the two adrenal glands.24
Histopathological studies
All of the surgically removed adenomas were re-evaluated by a histopathologist in the TAIPAI study group who was blinded to the clinical data. A histological diagnosis of aldosterone-producing adenomas (APA) was based on well-defined, encapsulated tumors predominantly consisting of foamy clear cells.26 Adenoma appeared as nodules of clear cells in sheets or nests that were sharply demarcated by a pseudo-capsule and compressed the non-neoplastic, uninvolved adrenal gland.27 Adenomas were differentiated from nodular adrenal hyperplasia by their solitary and well-circumscribed nature.27, 28 Adrenal glands from the idiopathic hyperaldosteronism (IHA) patients were marked by diffuse hyperplasia of cells resembling those of normal zona glomerulosa with or without macro- or micro-nodules.29
Measure of aldosterone and renin
The concentration of aldosterone was measured by radioimmunoassay (RIA) using commercial kits (Aldosterone MAIA Kit, Biochem ImmunoSystems, Bologna, Italy) as previously described.30, 31 The detection limit was 10.0 pg mL−1 with a 90% confidence interval, with the normal range of 70-350 pg mL−1 in an upright position. PRA was measured as the generation of angiotensin I in vitro using a commercially available RIA kit (Incstar Corporation, Stillwater, Minnesota, US). Its normal range was 2.63 ± 1.32 ng mL−1h−1 with patient in an upright position. In our three centers over a period of 13 years, the same aldosterone and renin assays were used.
Diagnostic criteria
Identification of APA in hypertensive patients required all of the following “modified 4 corners score” criteria:12, 24, 32 1) evidence of autonomous excess aldosterone production based on an ARR > 35 ngdL−1 per ngmL−1h−1 and a PAC > 10 ng dL−1after any confirmatory test; (2) lateralization of aldosterone secretion at adrenal vein sampling or during dexamethasone suppression NP-59 SPECT/CT;25 (3) evidence of adenoma on a CT; and 4) pathologically proven adenoma after an adrenalectomy, and cure of hypertension without antihypertensive agents or improved hypertension, potassium, PAC, and PRA as described.32
IHA was established based on the following criteria:33 1) evidence of autonomous excess aldosterone production based on an ARR > 35 ngdL−1 per ngmL−1h−1 and a PAC > 10 ng dL−1 after any confirmatory test; 2) non-lateralization of aldosterone secretion at adrenal vein sampling, or after undergoing dexamethasone suppression adreno-cortical scintigraphy;25 3) evidence of bilateral diffuse enlargement on a CT; and 4) evidence of diffuse cell hyperplasia in the pathology studies. In patients with negative captopril and losartan tests, the pre-specified ARR < 35 ngdL−1 per ngmL−1h−1 and PAC < 25 ng dL−1, and negative salt-loading results were considered to be diagnostic of essential hypertension (EH).
Statistical analysis
The primary objective of the study was to compare the diagnostic accuracy of the losartan test vs. the captopril test for PA in patients older than 50 years old. The data were provided as the mean values ± standard deviation (s.d.). As the data of PAC, PRA and calculated ARR were not normally distributed, median level with interquartile range were provided34.
Statistical analyses were performed using STATA version 12.0 statistical software (StataCorp LP, College Station, Texas). A normal distribution was attained by appropriate transformations of skewed variables such as PAC and ARR. Comparisons of variables between PA and EH were based on t-test statistics. The κ-test was used to evaluate the agreement of defining PA between captopril and losartan suppression tests. The results were expressed as a kappa coefficients and were classified according to the scale of Landis and Koch.35 The exact McNemar test was used to check the equality among captopril and losartan tests and the reference standard—the “modified 4 corners score” criteria. The sensitivity, specificity, accuracy, positive percent agreement and negative percent agreement for both losartan and captopril tests were calculated and compared by using receiver operating characteristic (ROC) curve. In addition, the age- and potassium-adjusted probabilities of having PA according to the results of both suppression tests were also computed. The P-value equating significance was <0.05.
Results
Demography of study population
Among 60 hypertensive patients who had underwent the confirmatory TAIPAI protocol24 (31 women and 29 men; mean age, 60.9±7.5 years), 28 patients had positive captopril or losartan tests (Figure 1). Also, three patients with negative captopril and losartan tests were diagnosed based on a positive saline-loading test and abnormal imaging findings. Finally, 31 patients (16 women and 15 men; mean age, 57.9±5.3 years) had the diagnosis of PA, 20 patients were diagnosed with APA, and 11 patients were diagnosed with IHA. All of the EH patients had been affirmed by the negative saline infusion test. AVS was performed in total 12 patients with positive confirmatory tests (4 patients for initial negative CT imaging; 2 patients with bilateral adenoma; 1 for bilateral diffusely nodular adrenal gland on CT imaging; 5 patients with incompatible NP59-SPECT/CT and CT findings).
The demographics of PA patients and EH patients are shown in Table 1. EH patients tended to be older. There were no statistically significant differences in sex, BMI, systolic and diastolic blood pressures between the PA and EH groups. The basal levels of serum potassium (SK), PRA, PAC, and ARR were statistically significantly different between the PA and EH patients (all P < 0.05).
Table 1.
Main Demographic and biochemical characteristics of the study patients
| PA (n=31) | EH (n=29) | p value | |
|---|---|---|---|
| Age, y | 57.9 (5.3) | 64.1 (8.1) | 0.001 |
| Sex, male(%) | 15 (48.4) | 14 (48.3) | 0.99 |
| SBP, mmHg | 156.1 (22.5) | 163.7 (17.6) | 0.16 |
| DBP, mmHg | 93.5 (13.1) | 89.6 (13.9) | 0.29 |
| BMI, kg/m2 | 25.6 (2.8) | 26.2 (3.7) | 0.55 |
| Creatinine, mg/dL | 1.1 (0.3) | 1.2 (0.4) | 0.28 |
| Potassium, mmol/L | 3.5 (0.8) | 4.3 (0.6) | < 0.001 |
| Captopril test | |||
| PAC (ng/dL) | 25.3 (16.4-46.8) | 14.1 (9.8-18.4) | < 0.001 |
| PRA (ng/ml/h) | 0.2 (0.06-0.5) | 3.2 (0.7-6.3) | < 0.001 |
| ARR | 225.6 (52.1-950) | 4.9 (1.9-10.9) | < 0.003 |
| Losartan test | |||
| PAC (ng/dL) | 39.8 (20.6-57.5) | 12.2 (9.8-18.2) | < 0.001 |
| PRA (ng/ml/h) | 0.3 (0.1-0.5) | 2.8 (1.0-4.7) | 0.006 |
| ARR | 144.6 (41.1-545) | 7.2 (3.3-12.0) | 0.001 |
Data as the mean values ± standard deviation (SD) except for PAC, PRA, and ARR which were presented as median with interquartile range.
Abbreviations: APA, aldosterone-producing adenoma; ARR, aldosterone-renin ratio (ng/dl per ng/ml/h); BMI, body mass index; DBP, diastolic blood pressure; EH, essential hypertension; IHA, idiopathic hyperaldosteronism; MBP, mean blood pressure; NS, not significant; PAC, plasma aldosterone concentration; PRA, plasma renin activity; SBP, systolic blood pressure.
Changes of PAC and ARR after captopril and losartan suppression tests
In all enrolled patients, the pretest PACs of the captopril and losartan tests were not statistically significantly different (34.5 ± 5.5 ng/dl vs. 29.8 ± 2.9 ng/dl, P = 0.34). However, the postcaptopril PAC was lower than the post-losartan PAC in PA patients (34.7 ± 4.9 ng/dl vs. 53.5 ± 8.2 ng/dl, P = 0.03). In EH patients, there was no difference in the postcaptopril and postlosartan PAC (16.1 ± 2.0 ng/dl vs. 15.1 ± 2.0 ng/dl, P = 0.72). There was a statistically significant correlation between the postcaptopril PAC and postlosartan PAC (r = 0.43, P = 0.0006) in all patients.
The performance of captopril and losartan tests on patients older than 50 years old with PA and EH
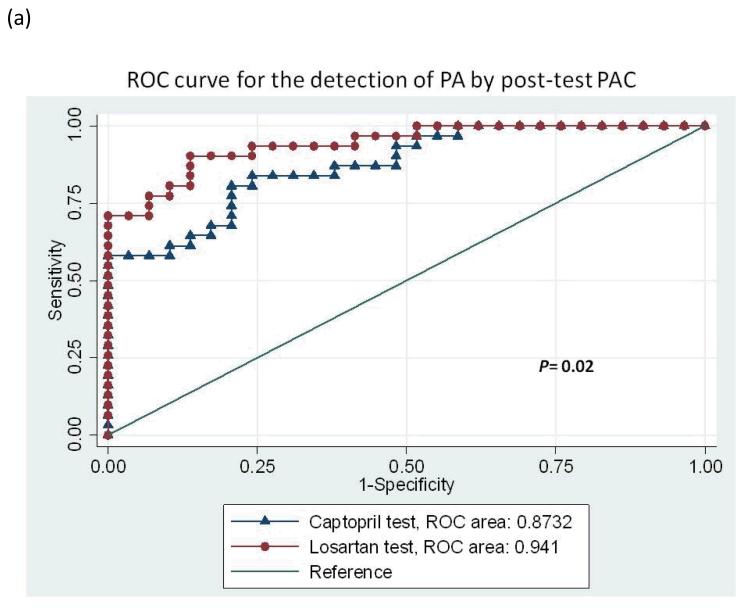
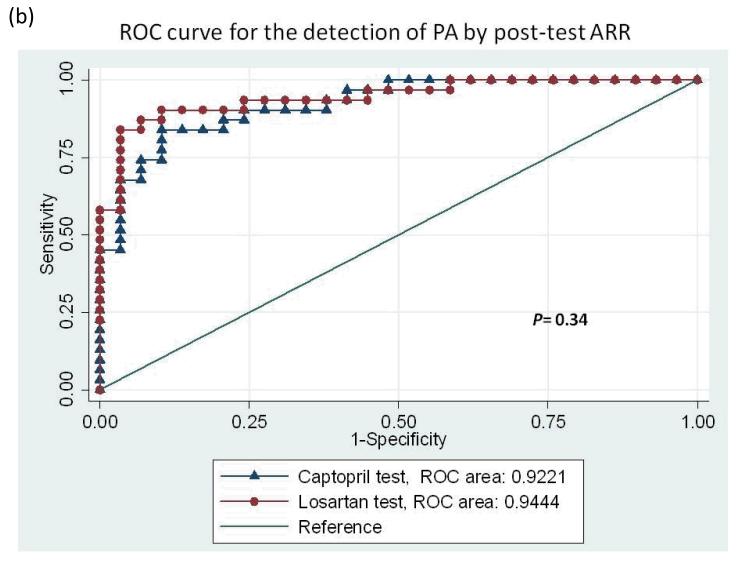
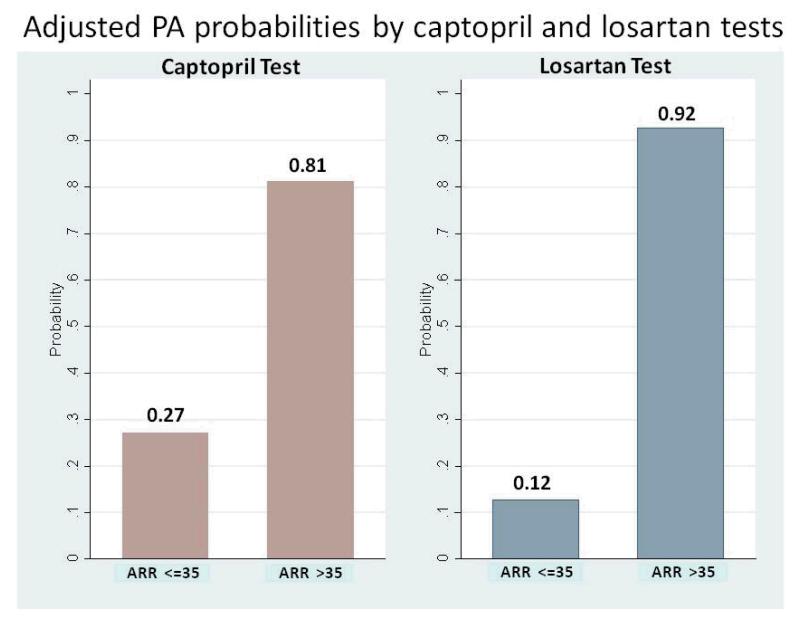
With the diagnostic criteria for PA based on an ARR >35 after captopril or losartan, the area under the ROC curve analyzed with the post-test ARR revealed no difference between the captopril and losartan tests after adjusted by age and serum potassium level (0.92 vs. 0.94, P = 0.34; Figure 2a). When the ROC curve was analyzed with the post-test PAC to differentiate PA from EH, the area under the curve of post-captopril PAC was inferior to that of post-losartan PAC adjusted by age and serum potassium (0.87 vs. 0.94, P = 0.02; Figure 2b). Using an ARR >35 with a PAC >10 ng/dl, the specificity was 82.76% vs. 93.1% and the sensitivity was 77.42% vs. 87.10% for the captopril test vs. the losartan test respectively. An accuracy of 80.0% for the diagnosis of PA from EH was achieved with the captopril test, with moderate agreement by the κ-test (k = 0.60, p<0.01) while the accuracy for losartan test was 90.0% and k =0.8 (p<0.01). The exact McNemar test comparing captopril and losartan tests revealed equivalence (p=1.00). The positive percent agreement was 82.8% and negative percent agreement was 83.9% between captopril and losartan tests (Table 2). The probability of having PA using post-test ARR cut-off 35 was 81% vs. 92% for the captopril test vs. the losartan test respectively (Figure 3).
Figure 2.
Receiver-operating characteristic (ROC) curves for the detection of all primary aldosteronism by (a) post-test PAC, and (b) post-test ARR. PAC, plasma aldosterone concentration; ARR, aldosterone-renin ratio.
Table 2.
Comparison of diagnostic performances between losartan and captopril tests.
| Captopril test | Losartan test | |
|---|---|---|
| Sensitivity | 77.42% | 87.10% |
| (58.90%-90.41%) | (70.17%-96.37%) | |
| Specificity | 82.76% | 93.10% |
| (64.23%-94.15%) | (77.23%-99.15%) | |
| Positive predictive value | 82.76% | 93.1% |
| (64.23%-94.15%) | (77.2%-99.2%) | |
| Negative predictive value | 77.42% | 87.1 |
| (58.9%-90.4%) | (70.2%-96.4%) | |
| Accuracy | 80.0% | 90.0% |
| Kappa coefficient comparing to | 0.60 | 0.80 |
| reference standard (s.e.m.) | (0.40-0.80) | (0.65-0.95) |
| Kappamax | 0.93 | 0.93 |
| Exact McNemar test comparing to reference standard |
p=0.77 | p=0.69 |
| Positive percent agreement | 0.8 | 0.9 |
| comparing to reference standard | (0.69-0.91) | (0.82-0.98) |
| Negative percent agreement | 0.8 | 0.9 |
| comparing to reference standard | (0.69-0.91) | (0.82-0.98) |
| Kappa coefficient between the two tests (s.e.m.) |
0.67 (0.48-0.86) | |
| Exact McNemar test between the two tests |
p=1.00 | |
Figure 3.
The adjusted probability of having PA by using post-captopril and post-losartan ARR at the cut-off value of 35 ng/dl per ng/ml/h. ARR, aldosterone-renin ratio.
Discussion
This study verified the diagnostic value of losartan challenge test in near-elderly and elderly patients, a subpopulation of major interest in current global healthcare.36, 37 The losartan test was initially thought to be less diagnostically powerful but has been recently validated as a comparable test as captopril test in the general population by our previously published head-to-head study.12 The current nested case-cohort investigated the diagnostic accuracy of the losartan test in population over 50 years old. Since the pharmacodynamics/pharmacokinetic profiles are different among older-aged patients for ACEI, analyzing this subgroup provides additional clinical insight to previously published work. 12, 38, 39 In addition, our study has demonstrated a good agreement between captopril and losartan tests with kappa value of 0.67 (p<0.01).
In this study, we found that the frequency of APA is higher than IHA in patients over 50 years in contrast to current concept of PA regarding the prevalence of subtype.40 This “reversing” phenomenon reflects the multiple and intertwined problems of approaching PA in the near-elderly and elderly. First, the prevalence of PA in elder persons has rarely been the focus of contemporary literature, perhaps the most significant grey area in the management of hypertension on older-aged patients. Second, the presence of clinical comorbidities poses noteworthy limitations to current PA diagnostic system such as adherence and tolerance to multi-step diagnostic tests. Finally, the cut-off value in many morbid subgroups remains undetermined e.g. patients with end stage renal disease (ESRD) and congestive heart failure (CHF). All above-mentioned perspectives may lower the awareness of primary clinicians toward PA in daily practice with a blunt and insensitive approach.
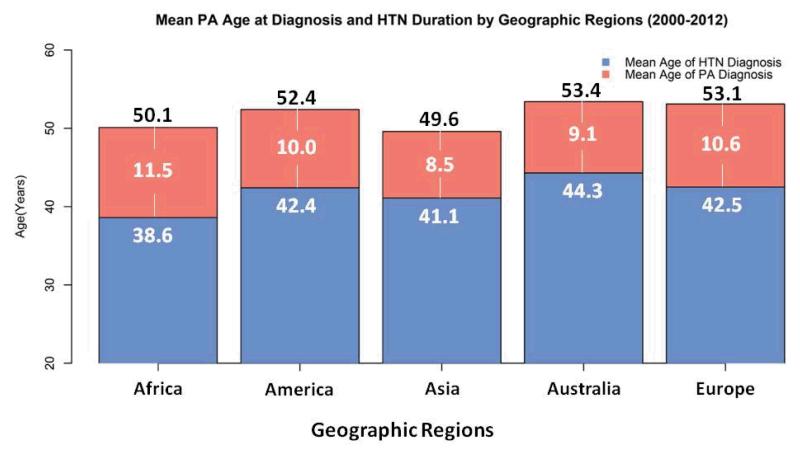
Although previously thought to be an extremely rare condition, PA now has been considered as one of the most common causes of secondary hypertension.41, 42 Also, a family history of early hypertension (<40 years40), indicating an increased pretest probability, has long been considered a triggering factor of the PA screening test.40, 43 However, the prevalence of PA may be stable across all age spectrum.4 Meanwhile, the average age at which PA was diagnosed in recent published studies from five continents is 52.1 years old, nearly the U.S. Census Bureau’s definition of “older” population (Table 3).44 The average hypertension duration is 10.1 years which echoes the tendency of delayed diagnosis and the wastage of medical resources under current clinical practices (Table 3). Furthermore, the hypothetical PA onset age is approximately 41.8 years old (Table 3 & Figure 4). By updating the epidemiological profile, public awareness of PA in cases over 50 years old has the potential to increase.
Table 3.
Recent studies (study population more than 10 patients) of primary aldosteronism (PA) provide both mean age at PA’s diagnosis and the patients’ mean duration of hypertension including primary care and referral settings published between January 1, 2000 and July 4, 2012 from two electronic databases, MEDLINE and EMBASE.
The key words “primary aldosteronism” or “hyperaldosteronism” were used and two reviewers (C.C. Kuo and V.C. Wu) independently screened the titles, abstracts, and contents to identify potentially eligible studies and to avoid the duplication by collate authors’ name and affiliation to minimize the possibility of extracting repeat data from the same study group.
| Continents AuthorReference, Year |
Number of PA patients |
Mean Age of PA at diagnosis, years |
Mean Duration of HTN, years |
Country |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | |||
| Africa | ||||
|
| ||||
| Rayner54, 2000 | 69 | 50.1(12.6) | 11.5(9.8) | South Africa |
|
| ||||
| America | ||||
|
| ||||
| Williams55, 2006 | 11 | 49.5 (6.3) | 9.6 (6.6) | United States |
| Umpierrez56, 2007 | 14 | 57(6) | 15(7) | United States |
| Murashima57, 2009 | 56 | 50.6 (10.0) | 13.0 (9.4) (n=45) |
United States |
| Stehr58, 2010 | 30 | 54.6(10.4) | 3.2(1.8) | Chile |
| Continental Summary | 111 | 52.4 (9.6) | 10.0 (8.5) (n=100) |
-- |
|
| ||||
| Asia | ||||
|
| ||||
| Loh31, 2000 | 16 | 50.6(11.3) | 8.6 (6.9) | Singapore |
| Horita59, 2001, | 25 | 45.4 (11.2) | 5.1 (4.6) | Japan |
| Benchetrit60, 2002 | 20 | 56(8.9) | 12(8.9) | Israel |
| Fukudome61, 2002 | 46 | 44(6.8) | 7(5.4) | Japan |
| Takakuwa62, 2002 | 13 | 38.9(6.2) | 5.4 (3.8) | Japan |
| Matsumura63, 2006 | 25 | 47.2(11) | 7.6(6.0) | Japan |
| Satoh64, 2007 | 87 | 52.4(12.1) | 12.9(19.6) | Japan |
| Kim65, 2010 | 27 | 45(4) | 5.6(7.9) | Korea |
| Mukherjee66, 2010 | 13 | 60.2 (7.9) | 16.2(10.8) | Singapore |
| Kuo9, 2011 | 346 | 48.6 (11.8) | 7.2(7.2) | Taiwan |
| Nakajima67, 2011 | 76 | 53.9 (10.7) | 11 (8.4) | Japan |
| Continental Summary | 694 | 49.6 (11.6) | 8.5 (10.0) | -- |
|
| ||||
| Australia | ||||
|
| ||||
| Sukor68, 2009 | 40 | 52.2(9.5) | 10.6(10.1) | Australia |
| Pimenta69, 2011 | 21 | 55.8(7.7) | 6.1(5.5) | Australia |
| Continental Summary | 61 | 53.4 (9.0) | 9.1 (9.0) | -- |
|
| ||||
| Europe | ||||
|
| ||||
| Rossi70, 2002 | 66 | 54.6(10.8) | 5(4.9) | Italy |
| Enberg71,2004 | 27 | 49.3 (13.3) | 7.5 (6.0) | Sweden |
| Juutilainen72, 2005 | 38 | 55(12.3) | 11(6.2) | Finland |
| Lumachi73, 20 05 | 98 | 47.7 (10.1) | 4.4(1.7) | Italy |
| Ribstein74, 2005 | 25 | 49(10) | 9(5) | France |
| Fallo10, 2006 | 85 | 55(9) | 12.3(9.3) | Italy |
| Sechi75, 2006 | 50 | 53(12) | 10(6) | Italy |
| Zacharieva76, 2006 | 64 | 46.2 (11.7) | 9.7 (12.2) | Bulgaria |
| Fogari77, 2007 | 177 | 48(7) | 9.3 (3.6) | Italy |
| Giacchetti78, 2007 | 61 | 51(10.5)) | 8.8(6.3) | Italy |
| Bernini79, 2008 | 23 | 54.0(12.0) | 7.5(6.7) | Italy |
| Mourad80, 2008 | 48 | 52(11) | 11.4(1.1) | France |
| Matrozova81, 2009 | 460 | 51.9 (5.5) | 12.3 (5.6) (n=438) |
France |
| Reincke82, 2009 | 408 | 60(10) | 12(9) | Germany |
| Somloova83, 2010 | 100 | 50.0 (8.5) | 10.9 (8.4) | Czech |
| Continental Summary | 1730 | 53.1 (10.0) | 10.6 (7.3) (n=1708) |
-- |
|
| ||||
| Overall Five Continents | ||||
|
| ||||
| Overall Summary | 2665 | 52.1 (10.6) | 10.1 (8.3) (n=2632) |
|
Hypothetical mean age of PA onset = the average of the five continents’ (mean age of PA at diagnosis - mean duration of hypertension) = 41.8 years
PA, primary aldosteronism; HTN, hypertension
Figure 4.
The mean PA age at diagnosis and hypertension duration summarized from recent PA studies between January 2000 and July 2012 in five continents. The number in each red bar indicates the mean duration of hypertension.
The call for adequate diagnostic testing of PA in near-elderly and elderly is still largely unaddressed as are the clinical practice guidelines for other more common diseases.45 Current three-step diagnostic system of PA greatly compromises the adherence among the elderly. Furthermore, multiple comorbid conditions often limit the selection of diagnostic tests. In patients with hypertensive pulmonary edema, congestive heart failure or kidney diseases that are vulnerable to fluid-overload, salt-loading and fludrocortisones tests are obviously inappropriate. Furthermore, to avoid possible angioedema from captopril in such a susceptible population,14 losartan test turns out to be a feasible choice. From the standpoints of safety and test adherence, conducting a large prospective study to verify losartan test in an ethnically diverse population would be fundamental to formulate a specific diagnostic model for patients over 50 years old.
In this post-hoc analysis restricting the sample to patients over 50 years old, the proportion of APA is significantly higher than IHA. Although this prevalence profile might be affected by the selection criteria in our TAIPAI screening protocol,24 the curability of APA offers a cost-benefit opportunity to avoid unnecessary long-term anti-hypertensive medication. The accurate diagnosis of IHA also facilitates the use of effective target therapies such as spironolactone and eplerenon.46 And timely diagnosis of PA in elder population may considerably lower the risk of long-term complications ranging from cardiovascular diseases47, 48, kidney damage9, to metabolic syndrome49 related to aldosterone excess and consequently interrupt the vicious cycles of PA and coexisting organ damages.
The strength of our study is its prospective head-to-head design and that all patients underwent both captopril and losartan suppression tests. Moreover, the rigorous protocol based three-step investigation minimizes the possibilities of misclassification and ascertainment bias.24, 40
There are some limitations to the study. First, as a study investigating a confirmatory test, larger numbers would offer a more accurate unbiased estimation on the sensitivity and specificity of the losartan test.50 The relatively small sample size may also mask the true difference due to insufficient statistical power. Yet the results seem promising since even with limited sample size the losartan test is comparable to captopril test as a useful confirmatory tool in hypertensive patients over 50 years. Second, a selection bias may be inherent in the selection criteria. Regarding the proportion of subtypes of PA, the TAIPAI protocol employed hypokalemia as a selection criteria which may lead to selective inclusion of more severe form of PA, APA.51 Third, spectrum bias is an inevitable issue for studies conducted in tertiary medical centers, which may overestimate the performance of the diagnostic tests. This bias may be even more prominent for confirmatory tests due to the study population would have been highly selected after the screening protocol. However due to the head-to-head design, it is fair to compare both captopril and losartan tests and verify the “rule in” and “rule out” performances of the losartan test.52
Also, we are aware that confirmatory tests based on perturbation of the renin-angiotensin system will lead to exclude patients with Angiotensin II responsive APA53. Therefore, our finding may not be generalized to non-Angiotensin II autonomous APA. Finally, the target population restricted to Asian population in our study; therefore, the statistical inference toward other race/ethnicities needs to be further validated.
In summary, we found that the losartan test is comparable to captopril test in patients older than 50 years old. With comparable diagnostic accuracy, the losartan test has better safety profile compared to the captopril test which is an advantage to the case management for patients over 50 years old. We believe the verification of this “elderly-friendly” confirmatory test will be the first step to prepare the specific diagnostic model of PA for near-elderly and elderly in contrast to current one-size-fits-all practice.
Funding Acknowledgements
This research was supported in part by National Heart, Lung, and Blood Institute training grant T32 HL7024-38, Ta-Tung Kidney Foundation, and the Taiwan National Science Council (grant NSC 96-2314-B-002-164, grant NSC 96-2314-B-002-033-MY3, and grant NSC 97-2314-B-002-155-MY2).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005 Jan 15-21;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005 Jul 27;294(4):466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 3.Wolf-Maier K, Cooper RS, Banegas JR, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003 May 14;289(18):2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GH, Jr., Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994 May;12(5):609–615. doi: 10.1097/00004872-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Dluhy RG. Uncommon forms of secondary hypertension in older patients. Am J Hypertens. 1998 Mar;11(3 Pt 2):52S–56S. doi: 10.1016/s0895-7061(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 6.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011 May 17;57(20):2037–2114. doi: 10.1016/j.jacc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Mosso L, Carvajal C, Gonzalez A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003 Aug;42(2):161–165. doi: 10.1161/01.HYP.0000079505.25750.11. [DOI] [PubMed] [Google Scholar]
- 8.Wu VC, Lo SC, Chen YL, et al. Endothelial progenitor cells in primary aldosteronism: a biomarker of severity for aldosterone vasculopathy and prognosis. J Clin Endocrinol Metab. 2011 Oct;96(10):3175–3183. doi: 10.1210/jc.2011-1135. [DOI] [PubMed] [Google Scholar]
- 9.Kuo CC, Wu VC, Tsai CW, Wu KD. Relative kidney hyperfiltration in primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2011 Jun;12(2):113–122. doi: 10.1177/1470320310391331. [DOI] [PubMed] [Google Scholar]
- 10.Fallo F, Veglio F, Bertello C, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006 Feb;91(2):454–459. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 11.Lin YH, Wang SM, Wu VC, et al. The association of serum potassium level with left ventricular mass in patients with primary aldosteronism. Eur J Clin Invest. 2011 Jul;41(7):743–750. doi: 10.1111/j.1365-2362.2010.02462.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu VC, Chang HW, Liu KL, et al. Primary Aldosteronism: Diagnostic Accuracy of the Losartan and Captopril Tests. Am J Hypertens. 2009 May 14;22(8):821–827. doi: 10.1038/ajh.2009.89. [DOI] [PubMed] [Google Scholar]
- 13.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008 Jan 1;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Borges M, Gonzalez-Aveledo LA. Angiotensin-converting enzyme inhibitors and angioedema. Allergy Asthma Immunol Res. 2010 Jul;2(3):195–198. doi: 10.4168/aair.2010.2.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005 Mar;85(3):257–268. [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Vidt DG. Hypertensive crises: emergencies and urgencies. J Clin Hypertens (Greenwich) 2004 Sep;6(9):520–525. doi: 10.1111/j.1524-6175.2004.03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su TC, Hwang LC, You SL, Chen CJ. Ethnic variation in hypertension prevalence of women in Taiwan. J Hum Hypertens. 2009 Mar;23(3):160–167. doi: 10.1038/jhh.2008.120. [DOI] [PubMed] [Google Scholar]
- 19.Wu KD, Liao TS, Chen YM, et al. Preoperative diagnosis and localization of aldosterone-producing adenoma by adrenal venous sampling after administration of metoclopramide. J Formos Med Assoc. 2001 Sep;100(9):598–603. [PubMed] [Google Scholar]
- 20.Munafo A, Christen Y, Nussberger J, et al. Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist. Clin Pharmacol Ther. 1992 May;51(5):513–521. doi: 10.1038/clpt.1992.56. [DOI] [PubMed] [Google Scholar]
- 21.Capoten, Captopril . Product Information. Bristol-Myers Squibb; Princeton, NJ: 1996. [Google Scholar]
- 22.Tait JF, Tait SA, Little B, Laumas KR. The disappearance of 7-H-3-d-aldosterone in the plasma of normal subjects. J Clin Invest. 1961 Jan;40:72–80. doi: 10.1172/JCI104239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skrabal F. Half-life of plasma renin activity in normal subjects and in malignant hypertension. Klin Wochenschr. 1974 Dec 15;52(24):1173–1174. doi: 10.1007/BF01466736. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CC, Wu VC, Huang KH, et al. Verification and evaluation of aldosteronism demographics in the Taiwan Primary Aldosteronism Investigation Group (TAIPAI Group) J Renin Angiotensin Aldosterone Syst. 2011 Sep;12(3):348–357. doi: 10.1177/1470320310391329. [DOI] [PubMed] [Google Scholar]
- 25.Yen RF, Wu VC, Liu KL, et al. 131 I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med. 2009 Oct;50(10):1631–1637. doi: 10.2967/jnumed.109.064873. [DOI] [PubMed] [Google Scholar]
- 26.Aiba M, Suzuki H, Kageyama K, et al. Spironolactone bodies in aldosteronomas and in the attached adrenals. Enzyme histochemical study of 19 cases of primary aldosteronism and a case of aldosteronism due to bilateral diffuse hyperplasia of the zona glomerulosa. Am J Pathol. 1981 Jun;103(3):404–410. [PMC free article] [PubMed] [Google Scholar]
- 27.Omura M, Sasano H, Fujiwara T, Yamaguchi K, Nishikawa T. Unique cases of unilateral hyperaldosteronemia due to multiple adrenocortical micronodules, which can only be detected by selective adrenal venous sampling. Metabolism. 2002 Mar;51(3):350–355. doi: 10.1053/meta.2002.30498. [DOI] [PubMed] [Google Scholar]
- 28.Novitsky YW, Kercher KW, Rosen MJ, Cobb WS, Jyothinagaram S, Heniford BT. Clinical outcomes of laparoscopic adrenalectomy for lateralizing nodular hyperplasia. Surgery. 2005 Dec;138(6):1009–1016. doi: 10.1016/j.surg.2005.09.027. discussion 1016-1007. [DOI] [PubMed] [Google Scholar]
- 29.Wu VC, Chueh SC, Chang HW, et al. Association of kidney function with residual hypertension after treatment of aldosterone-producing adenoma. Am J Kidney Dis. 2009 Oct;54(4):665–673. doi: 10.1053/j.ajkd.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Chang HW, Chu TS, Huang HY, et al. Down-regulation of D2 dopamine receptor and increased protein kinase Cmu phosphorylation in aldosterone-producing adenoma play roles in aldosterone overproduction. J Clin Endocrinol Metab. 2007 May;92(5):1863–1870. doi: 10.1210/jc.2006-2338. [DOI] [PubMed] [Google Scholar]
- 31.Loh KC, Koay ES, Khaw MC, Emmanuel SC, Young WF., Jr. Prevalence of primary aldosteronism among Asian hypertensive patients in Singapore. J Clin Endocrinol Metab. 2000 Aug;85(8):2854–2859. doi: 10.1210/jcem.85.8.6752. [DOI] [PubMed] [Google Scholar]
- 32.Rossi GP, Belfiore A, Bernini G, et al. Comparison of the captopril and the saline infusion test for excluding aldosterone-producing adenoma. Hypertension. 2007 Aug;50(2):424–431. doi: 10.1161/HYPERTENSIONAHA.107.091827. [DOI] [PubMed] [Google Scholar]
- 33.Wu VC, Chueh SC, Chang HW, et al. Bilateral aldosterone-producing adenomas: differentiation from bilateral adrenal hyperplasia. Qjm. 2008 Jan;101(1):13–22. doi: 10.1093/qjmed/hcm101. [DOI] [PubMed] [Google Scholar]
- 34.Weinberger MH, Fineberg NS. The diagnosis of primary aldosteronism and separation of two major subtypes. Arch Intern Med. 1993 Sep 27;153(18):2125–2129. [PubMed] [Google Scholar]
- 35.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968 Oct;70(4):213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 36.Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004 Jun 29;109(25):3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00. [DOI] [PubMed] [Google Scholar]
- 37.Renehan AG, Howell A. Preventing cancer, cardiovascular disease, and diabetes. Lancet. 2005 Apr 23-29;365(9469):1449–1451. doi: 10.1016/S0140-6736(05)66399-4. [DOI] [PubMed] [Google Scholar]
- 38.Chobanian AV. The use of angiotensin converting enzyme inhibitors in elderly patients with hypertension. J Am Geriatr Soc. 1987 Mar;35(3):269–270. doi: 10.1111/j.1532-5415.1987.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 39.Reid JL. Angiotensin converting enzyme inhibitors in the elderly. Br Med J (Clin Res Ed) 1987 Oct 17;295(6604):943–944. doi: 10.1136/bmj.295.6604.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008 Sep;93(9):3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 41.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011 May 31;123(21):2434–2506. doi: 10.1161/CIR.0b013e31821daaf6. [DOI] [PubMed] [Google Scholar]
- 42.Mattsson C, Young WF., Jr. Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006 Apr;2(4):198–208. doi: 10.1038/ncpneph0151. quiz, 191 p following 230. [DOI] [PubMed] [Google Scholar]
- 43.Rossi GP. A comprehensive review of the clinical aspects of primary aldosteronism. Nat Rev Endocrinol. 2011;7(8):485–495. doi: 10.1038/nrendo.2011.76. [DOI] [PubMed] [Google Scholar]
- 44.U.S.Census.Bureau . In: Age Data of the United States. U.S. Census Bureau PD, editor. 2011. [Google Scholar]
- 45.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005 Aug 10;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 46.Karagiannis A, Tziomalos K, Papageorgiou A, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother. 2008 Mar;9(4):509–515. doi: 10.1517/14656566.9.4.509. [DOI] [PubMed] [Google Scholar]
- 47.Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008 Jan 14;168(1):80–85. doi: 10.1001/archinternmed.2007.33. [DOI] [PubMed] [Google Scholar]
- 48.Lin YH, Wu XM, Lee HH, et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism. J Hypertens. 2012 Aug;30(8):1606–1613. doi: 10.1097/HJH.0b013e3283550f93. [DOI] [PubMed] [Google Scholar]
- 49.Fallo F, Pilon C, Urbanet R. Primary Aldosteronism and Metabolic Syndrome. Horm Metab Res. 2011 Nov 24; doi: 10.1055/s-0031-1295412. [DOI] [PubMed] [Google Scholar]
- 50.Dendukuri N, Joseph L. Bayesian approaches to modeling the conditional dependence between multiple diagnostic tests. Biometrics. 2001 Mar;57(1):158–167. doi: 10.1111/j.0006-341x.2001.00158.x. [DOI] [PubMed] [Google Scholar]
- 51.Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med. 1994 Dec 1;121(11):877–885. doi: 10.7326/0003-4819-121-11-199412010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978 Oct 26;299(17):926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 53.Gordon RD, Hamlet SM, Tunny TJ, Klemm SA. Aldosterone-producing adenomas responsive to angiotensin pose problems in diagnosis. Clin Exp Pharmacol Physiol. 1987 Mar;14(3):175–179. doi: 10.1111/j.1440-1681.1987.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 54.Rayner BL, Opie LH, Davidson JS. The aldosterone/renin ratio as a screening test for primary aldosteronism. S Afr Med J. 2000 Apr;90(4):394–400. [PubMed] [Google Scholar]
- 55.Williams JS, Williams GH, Raji A, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006 Feb;20(2):129–136. doi: 10.1038/sj.jhh.1001948. [DOI] [PubMed] [Google Scholar]
- 56.Umpierrez GE, Cantey P, Smiley D, et al. Primary aldosteronism in diabetic subjects with resistant hypertension. Diabetes Care. 2007 Jul;30(7):1699–1703. doi: 10.2337/dc07-0031. [DOI] [PubMed] [Google Scholar]
- 57.Murashima M, Trerotola SO, Fraker DL, Han D, Townsend RR, Cohen DL. Adrenal venous sampling for primary aldosteronism and clinical outcomes after unilateral adrenalectomy: a single-center experience. J Clin Hypertens (Greenwich) 2009 Jun;11(6):316–323. doi: 10.1111/j.1751-7176.2009.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stehr CB, Mellado R, Ocaranza MP, et al. Increased levels of oxidative stress, subclinical inflammation, and myocardial fibrosis markers in primary aldosteronism patients. J Hypertens. 2010 Oct;28(10):2120–2126. doi: 10.1097/HJH.0b013e32833d0177. [DOI] [PubMed] [Google Scholar]
- 59.Horita Y, Inenaga T, Nakahama H, et al. Cause of residual hypertension after adrenalectomy in patients with primary aldosteronism. Am J Kidney Dis. 2001 May;37(5):884–889. doi: 10.1016/s0272-6386(05)80002-2. [DOI] [PubMed] [Google Scholar]
- 60.Benchetrit S, Bernheim J, Podjarny E. Normokalemic hyperaldosteronism in patients with resistant hypertension. Isr Med Assoc J. 2002 Jan;4(1):17–20. [PubMed] [Google Scholar]
- 61.Fukudome Y, Fujii K, Arima H, et al. Discriminating factors for recurrent hypertension in patients with primary aldosteronism after adrenalectomy. Hypertens Res. 2002 Jan;25(1):11–18. doi: 10.1291/hypres.25.11. [DOI] [PubMed] [Google Scholar]
- 62.Takakuwa H, Shimizu K, Izumiya Y, et al. Dietary sodium restriction restores nocturnal reduction of blood pressure in patients with primary aldosteronism. Hypertens Res. 2002 Sep;25(5):737–742. doi: 10.1291/hypres.25.737. [DOI] [PubMed] [Google Scholar]
- 63.Matsumura K, Fujii K, Oniki H, Oka M, Iida M. Role of aldosterone in left ventricular hypertrophy in hypertension. Am J Hypertens. 2006 Jan;19(1):13–18. doi: 10.1016/j.amjhyper.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 64.Satoh F, Abe T, Tanemoto M, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007 Nov;30(11):1083–1095. doi: 10.1291/hypres.30.1083. [DOI] [PubMed] [Google Scholar]
- 65.Kim RM, Lee J, Soh EY. Predictors of resolution of hypertension after adrenalectomy in patients with aldosterone-producing adenoma. J Korean Med Sci. 2010 Jul;25(7):1041–1044. doi: 10.3346/jkms.2010.25.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherjee JJ, Khoo CM, Thai AC, Chionh SB, Pin L, Lee KO. Type 2 diabetic patients with resistant hypertension should be screened for primary aldosteronism. Diab Vasc Dis Res. 2010 Jan;7(1):6–13. doi: 10.1177/1479164109350556. [DOI] [PubMed] [Google Scholar]
- 67.Nakajima Y, Yamada M, Taguchi R, et al. Cardiovascular complications of patients with aldosteronism associated with autonomous cortisol secretion. J Clin Endocrinol Metab. 2011 Aug;96(8):2512–2518. doi: 10.1210/jc.2010-2743. [DOI] [PubMed] [Google Scholar]
- 68.Sukor N, Gordon RD, Ku YK, Jones M, Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab. 2009 Jul;94(7):2437–2445. doi: 10.1210/jc.2008-2803. [DOI] [PubMed] [Google Scholar]
- 69.Pimenta E, Gordon RD, Ahmed AH, et al. Cardiac dimensions are largely determined by dietary salt in patients with primary aldosteronism: results of a case-control study. J Clin Endocrinol Metab. 2011 Sep;96(9):2813–2820. doi: 10.1210/jc.2011-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002 Oct;15(10 Pt 1):896–902. doi: 10.1016/s0895-7061(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 71.Enberg U, Volpe C, Hoog A, et al. Postoperative differentiation between unilateral adrenal adenoma and bilateral adrenal hyperplasia in primary aldosteronism by mRNA expression of the gene CYP11B2. Eur J Endocrinol. 2004 Jul;151(1):73–85. doi: 10.1530/eje.0.1510073. [DOI] [PubMed] [Google Scholar]
- 72.Juutilainen AM, Voutilainen ET, Mykkanen L, Niskanen L. Plasma aldosterone to renin ratio predicts treatment response in primary aldosteronism: is volume loading needed? Blood Press. 2005;14(4):245–250. doi: 10.1080/08037050510034329. [DOI] [PubMed] [Google Scholar]
- 73.Lumachi F, Ermani M, Basso SM, Armanini D, Iacobone M, Favia G. Long-term results of adrenalectomy in patients with aldosterone-producing adenomas: multivariate analysis of factors affecting unresolved hypertension and review of the literature. Am Surg. 2005 Oct;71(10):864–869. [PubMed] [Google Scholar]
- 74.Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol. 2005 May;16(5):1320–1325. doi: 10.1681/ASN.2004100878. [DOI] [PubMed] [Google Scholar]
- 75.Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006 Jun 14;295(22):2638–2645. doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- 76.Zacharieva S, Orbetzova M, Elenkova A, et al. Diurnal blood pressure pattern in patients with primary aldosteronism. J Endocrinol Invest. 2006 Jan;29(1):26–31. doi: 10.1007/BF03349173. [DOI] [PubMed] [Google Scholar]
- 77.Fogari R, Preti P, Zoppi A, Rinaldi A, Fogari E, Mugellini A. Prevalence of primary aldosteronism among unselected hypertensive patients: a prospective study based on the use of an aldosterone/renin ratio above 25 as a screening test. Hypertens Res. 2007 Feb;30(2):111–117. doi: 10.1291/hypres.30.111. [DOI] [PubMed] [Google Scholar]
- 78.Giacchetti G, Ronconi V, Turchi F, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007 Jan;25(1):177–186. doi: 10.1097/HJH.0b013e3280108e6f. [DOI] [PubMed] [Google Scholar]
- 79.Bernini G, Galetta F, Franzoni F, et al. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008 Dec;26(12):2399–2405. doi: 10.1097/HJH.0b013e32831286fd. [DOI] [PubMed] [Google Scholar]
- 80.Mourad JJ, Girerd X, Milliez P, Lopez-Sublet M, Lejeune S, Safar ME. Urinary aldosterone-to-active-renin ratio: a useful tool for predicting resolution of hypertension after adrenalectomy in patients with aldosterone-producing adenomas. Am J Hypertens. 2008 Jul;21(7):742–747. doi: 10.1038/ajh.2008.175. [DOI] [PubMed] [Google Scholar]
- 81.Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2009 Apr;53(4):605–610. doi: 10.1161/HYPERTENSIONAHA.108.122002. [DOI] [PubMed] [Google Scholar]
- 82.Reincke M, Rump LC, Quinkler M, et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009 Mar;94(3):869–875. doi: 10.1210/jc.2008-1851. [DOI] [PubMed] [Google Scholar]
- 83.Somloova Z, Widimsky J, Jr., Rosa J, et al. The prevalence of metabolic syndrome and its components in two main types of primary aldosteronism. J Hum Hypertens. 2010 Oct;24(10):625–630. doi: 10.1038/jhh.2010.65. [DOI] [PubMed] [Google Scholar]