Abstract
The leukemogenic CALM-AF10 fusion protein is found in patients with immature acute myeloid and T-lymphoid malignancies. CALM-AF10 leukemias display abnormal H3K79 methylation and increased HOXA cluster gene transcription. Elevated expression of HOXA genes is critical for leukemia maintenance and progression; however, the precise mechanism by which CALM-AF10 alters HOXA gene expression is unclear. We previously determined that CALM contains a CRM1-dependent nuclear export signal (NES), which is both necessary and sufficient for CALM-AF10-mediated leukemogenesis. Here, we find that interaction of CALM-AF10 with the nuclear export receptor CRM1 is necessary for activating HOXA gene expression. We show that CRM1 localizes to HOXA loci where it recruits CALM-AF10, leading to transcriptional and epigenetic activation of HOXA genes. Genetic and pharmacological inhibition of the CALM-CRM1 interaction prevents CALM-AF10 enrichment at HOXA chromatin, resulting in immediate loss of transcription. These results provide a comprehensive mechanism by which the CALM-AF10 translocation activates the critical HOXA cluster genes. Furthermore, this report identifies a novel function of CRM1: the ability to bind chromatin and recruit the NES-containing CALM-AF10 transcription factor.
Keywords: leukemia, CRM1, Leptomycin B, Hoxa9
Introduction
Controlled regulation of homeobox HOX cluster genes is critical for normal proliferation and differentiation in embryogenesis(1). During hematopoiesis, HOXA genes are transcribed at high levels in hematopoietic stem cells and immature progenitor cells, and their expression is downregulated upon differentiation and maturation (2, 3). HOXA9 and HOXA10 are overexpressed in the majority of human acute myeloid leukemias (AMLs) (4, 5). In mouse leukemia models, ectopic expression of HOXA9 or HOXA10 confers a growth advantage to immature myeloid cells and is sufficient to cause leukemia in vivo (6, 7). Therefore, deregulation of the HOXA pathway is a common and driving mechanism of leukemic transformation.
Excessive levels of HOXA gene expression are seen in leukemias harboring CALM-AF10 rearrangements, which are found in pediatric and adult patients with immature acute myeloid and T-lymphoid malignancies (8–10). CALM-AF10 leukemia cells display a global reduction in H3K79 methylation, while HOXA cluster genes are locally hypermethylated, corresponding with their elevated expression (11–13). AF10 contains an essential octapeptide/leucine zipper (OM-LZ) domain that interacts with DOT1L, the sole mammalian H3K79 methyltransferase (14). DOT1L is thought to be aberrantly targeted to chromatin by CALM-AF10, leading to global changes in H3K79 methylation and gene expression (12). DOT1L inhibitors have recently been developed and shown to suppress HOXA gene expression and reduce viability of CALM-AF10 leukemia cells (15, 16). Therefore, aberrant recruitment of DOT1L by CALM-AF10 is critical for HOXA expression and leukemia cell survival, yet the mechanism by which this occurs is unclear.
We previously determined that CALM contains a nuclear export signal (NES) that is essential for CALM-AF10-mediated leukemogenesis (13). NES motifs interact with the nuclear export receptor CRM1, which in turn drives translocation of NES-containing proteins from the nucleus to the cytoplasm through the nuclear pore complex (17). Indeed, CALM-AF10 undergoes nucleocytoplasmic shuttling, resulting in predominant cytoplasmic localization (13, 18). Nuclear export of CALM-AF10 causes cytoplasmic mislocalization of DOT1L, which likely contributes to global H3K79 hypomethylation that is seen in CALM-AF10 leukemia cells (13). However, we also observed that the CALM NES is necessary for CALM-AF10 to upregulate HOXA gene expression. In these studies, we sought to establish how the CALM NES motif affects the ability of CALM-AF10 to activate HOXA gene expression, specifically via interaction with the nuclear export receptor, CRM1.
We demonstrate that acute treatment with the CRM1 inhibitor Leptomycin B (LMB) results in loss of HOXA transcript levels in CALM-AF10 cells. This occurs prior to epigenetic changes, and the effects of LMB treatment on HOXA transcript levels are kinetically similar to those of Actinomycin D, suggesting that CALM-AF10 transcriptionally regulates HOXA genes in a CRM1-dependent manner. We also show that CALM-AF10 binds HOXA loci, while mutation of the NES motif or treatment with LMB prevents this interaction. Furthermore, we find that CRM1 localizes to HOXA loci in both CALM-AF10 and wild-type cells. These data support a model by which CRM1 directly recruits CALM-AF10 to the HOXA cluster, resulting in increased HOXA gene expression and ultimately cellular transformation.
Methods
Cell culture
Retroviral packaging Plat-E(19) and HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin (Life Technologies, Carlsbad, CA, USA). MEF lines were immortalized with SV40 T/t antigen, as described previously (20). MEFs were maintained in the same basic medium supplemented with non-essential amino acids and glutamine. Murine leukemia cell lines were generated from mice with CALM-AF10- or Hoxa9/Meis1-derived acute leukemias, as described previously (13). Leukemic bone marrow cells were grown in RPMI 1640 medium supplemented with 5 ng/ml recombinant murine IL-3 (PeproTech, Rocky Hill, NJ, USA), 10% FBS, glutamine, penicillin and streptomycin. Human U937 (ATCC, Manassas, VA, USA) and P31/Fujioka (21) cells were grown in the same medium without IL-3.
Transfection/Infection of cell lines
Retroviral plasmid constructs were derived as described previously. Plat-E cells were transfected by the calcium phosphate method. MEFs were infected by co-culture with filtered Plat-E supernatant in the presence of Polybrene (2 µg/ml). Transfection/infection efficiencies were verified by GFP percentage by flow cytometry (Accuri C6, Ann Arbor, MI, USA).
Real Time qRT-PCR
Total RNA was isolated from MEFs or leukemic cells using the RNeasy Mini kit (Qiagen, Valencia, CA, USA). RNA was reverse transcribed using the iScript kit (Bio-Rad, Hercules, CA, USA). Quantitative PCR amplification was performed using either the iQ Sybr Mix (Bio-Rad) with the iQ5 Optical System (Bio-Rad) or iTaq Universal SYBR Green Supermix (Bio-Rad) with the ViiA 7 Real Time PCR System (Life Technologies). In MEFs, Hoxa gene expression levels were normalized to the levels of Gapdh and then to empty vector by the comparative threshold (CT) method. For pharmacological inhibitor studies, equal amounts of RNA were used for reverse transcription reactions, and gene expression is shown relative to time zero. Primers used for qRT-PCR are listed in Supplementary Table 1.
Chromatin Immunoprecipitation (ChIP)
Di-methylated H3K79 ChIP assays were performed as described previously (13). For ChIP with anti-Flag and anti-CRM1 antibodies, formaldehyde-fixed cells were lysed with a mild lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mm EDTA, 1% Triton-X) and sonicated to an average fragment size of 1.5 kb. Immunoprecipitation was performed with anti-FLAG M2 Affinity Gel (Sigma-Aldrich, St. Louis, MO, USA) or anti-CRM1 (Santa Cruz, Dallas, TX or Bethyl, Montgomery, TX) antibodies incubated at 4oC for 3–5 hr or overnight, respectively. Salmon sperm-conjugated protein G sepharose beads (35 µL, Millipore, Billerica, MA, USA) were added to anti-CRM1 ChIPs and rocked at 4°C for an additional 3 hr. Following RNAse A and proteinase K treatment, input and ChIP DNA were purified with a PCR purification kit (Qiagen) and amplified by real time RT-PCR. Amplification values were normalized to input. Primer sequences used to amplify genomic DNA are listed in Supplementary Table 2.
Co-Immunoprecipitation and Western blotting
For co-immunoprecipitation experiments, MEFs stably expressing empty vector, Flag-tagged CALM-AF10 or Flag-tagged CALM(mutNES)-AF10 were fixed, lysed, and sonicated as for ChIP experiments. Anti-Flag M2 affinity gel was used to IP Flag-tagged constructs. After washing with lysis buffer, Laemmli sample buffer was added to the gel and samples were boiled for 15 min. Western blots were performed according to standard protocols. Primary antibodies included: CRM1 (Santa Cruz), CALM (Sigma), Histone H3 (Cell Signaling, Danvers, MA, USA), and di-methylated H3K79 (Cell Signaling). Fluorescently conjugated secondary antibodies were incubated for 1 hr at RT, and blots were developed using the Odyssey Infra-red imaging system (Li-Cor Biosciences, Lincoln, NE, USA).
Luciferase Reporter Assays
HEK293 cells were transiently co-transfected with pTAL-Hoxa9-LUC (a kind gift from Jay Hess (22)) and MSCV-IRES-eGFP CALM-AF10, CALM(mutNES)-AF10 or a size-matched anti-sense CALM-AF10 constructs by the calcium phosphate method. Luciferase assays were performed using the Luciferase Assay Kit (Promega, Madison, WI, USA) according to manufacturer’s instructions and measured using a luminometer (Promega). To correct for transfection differences, luciferase values are normalized to percent GFP-positive cells and are shown relative to the size-matched anti-sense control.
Pharmacological Inhibitor Studies
Leptomycin B (LMB) was obtained from Sigma Aldrich, and Actinomycin D (Act D) was obtained from Lundbeck Inc (Deerfiled, IL, USA). MEFs were treated with 0.7 nM LMB for 2.5 hr before cells were either collected for RNA isolation or fixed for ChIP. Murine and human leukemia cells were treated with 0.7 nM LMB or 5 µg/mL Act D for 0, 60, 150, or 300 min, and RNA was isolated. The DOT1L inhibitor, SGC0946 was obtained from the Structural Genomics Consortium (SGC) and used at 1 µM for up to 4 days.
Statistical Analysis
Data are presented as mean plus or minus SEM (n = 3 or more). Statistical analysis was performed by Student’s t-test when two groups were compared. Statistical analysis was performed by one-way ANOVA followed by Dunnett’s post-test when three or more groups of data were compared. Statistical analyses of gene expression with LMB or ActD were performed by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test. Values were considered statistically significant when P values were less than 0.05 (unless specified otherwise in the Figure Legends). All analyses were carried out using PRISM software (GraphPad Software, Inc.).
Results
Interaction of CALM-AF10 with CRM1 is necessary to upregulate Hoxa gene expression
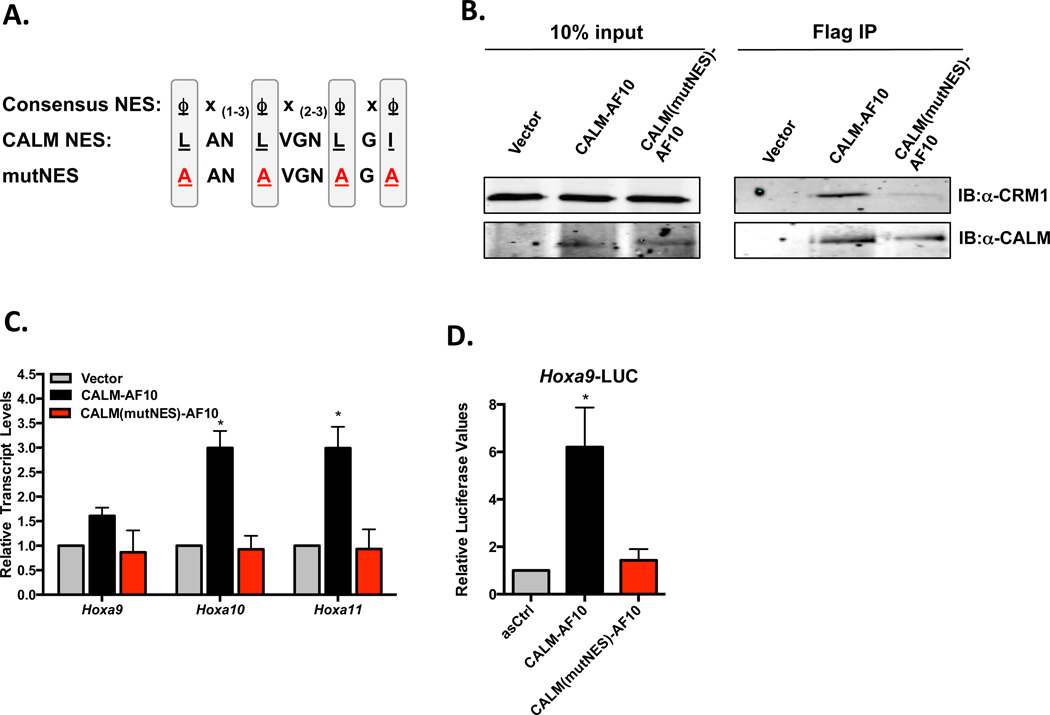
CALM-AF10 interacts with the nuclear export receptor CRM1 via a NES within aa 544–553 of CALM (Figure 1A). Point mutation of hydrophobic residues within the NES abolishes interaction with CRM1 (Figure 1B) and also abrogates the ability of CALM-AF10 to activate Hoxa expression in murine embryonic fibroblasts (MEFs) (previously reported (13) and shown in Figure 1C). Furthermore, while CALM-AF10 transcriptionally activates the Hoxa9 promoter in a luciferase reporter assay (Hoxa9-LUC), CALM(mutNES)-AF10 does not alter luciferase levels (Figure 1D). Therefore, the CALM-derived NES motif is essential for CALM-AF10-mediated upregulation of Hoxa gene expression.
Figure 1. CALM-AF10 interacts with CRM1 via the CALM-derived NES, which is necessary for Hoxa upregulation in MEFs.
(A) Alignment of the NES within CALM (aa 544–553) and the consensus sequence of CRM1-dependent NES where ϕ represents any hydrophobic residue and x represents any amino acid. The hydrophobic aa of the CALM NES were point mutated to alanines (A) to create the CALM(mutNES)-AF10 mutant. (B) Co-immunoprecipitation experiments were performed to assess the interaction of CALM-AF10 and CRM1. MEFs stably infected with empty Vector, Flag-CALM-AF10 or Flag-CALM(mutNES)-AF10 were treated as described for ChIP experiments using Anti-Flag affinity gel. Co-IP complexes were visualized by SDS-PAGE using antibodies against CRM1 or CALM (recognizes CALM-AF10 fusions). (C) Hoxa cluster transcript levels were measured by qRT-PCR in MEFs expressing empty vector or CALM(mutNES)-AF10. Results are normalized to GAPDH and then to vector controls by the ΔΔCt method. Results are shown as mean ± SEM from at least 3 experiments. (D) Luciferase assays were performed using the Hoxa9-LUC reporter construct and either anti-sense CALM-AF10 (asCtrl), CALM-AF10, or CALM(mutNES)-AF10. Results are shown relative to asCtrl and as mean ± SEM from 4 separate experiments. Statistical analysis was performed by one-way ANOVA; *P<0.05.
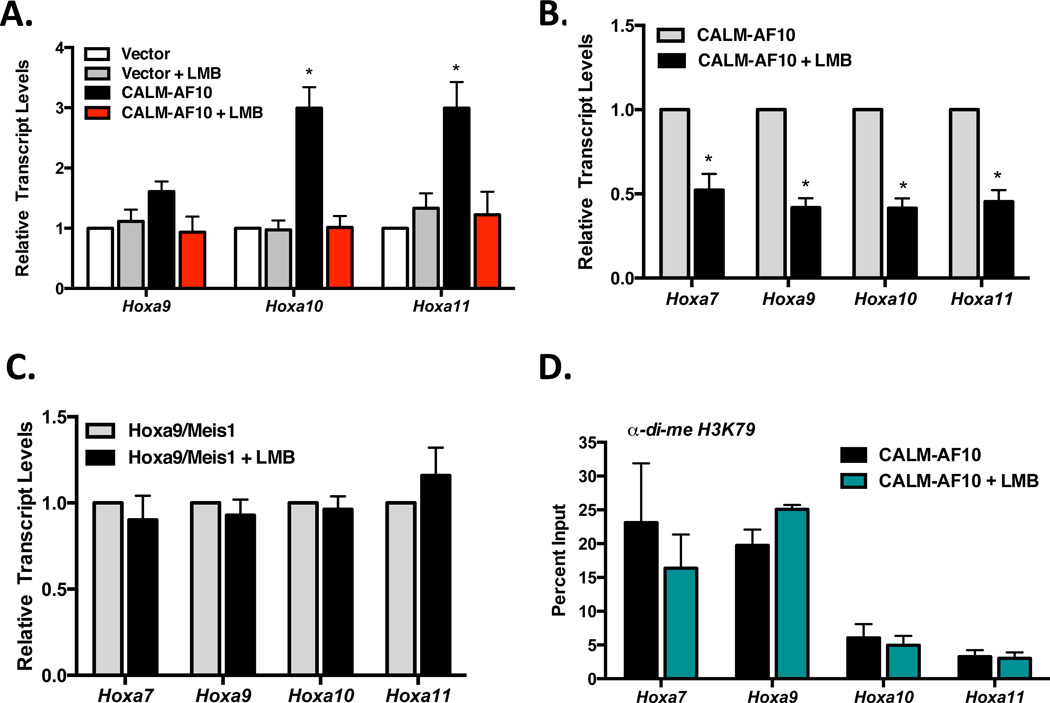
To determine whether interaction with CRM1 is specifically necessary for elevated Hoxa expression, we treated CALM-AF10 or vector expressing cells with Leptomycin B (LMB). LMB is a well-characterized CRM1 inhibitor that covalently modifies the CRM1 NES binding domain, thereby preventing interaction of CRM1 with cargo molecules, such as CALM-AF10 (23). As shown in Figure 2A, short treatment with LMB (2.5 h, 0.7 nM) abolished CALM-AF10-induced expression of Hoxa9, Hoxa10 and Hoxa11 in MEFs.
Figure 2. Inhibition of CRM1 with Leptomycin B reduces Hoxa cluster expression in CALM-AF10 cells.
(A) MEFs stably expressing empty vector or CALM-AF10 were treated with 0.7 nM LMB for 2.5 h, and Hoxa transcript levels were measured by real time qRT-PCR. Results are normalized to GAPDH and then to untreated empty vector MEFs by the ΔΔCt method. Statistical analysis was performed by one-way ANOVA for each Hoxa gene followed by Dunnett’s multiple comparison test; *P<.01. (B) Murine CALM-AF10 or (C) Hoxa9/Meis1 leukemia cells were grown in the absence or presence of LMB (0.7 nM, 2.5 h). Hoxa transcript levels were measured by qRT-PCR. Results are normalized to GAPDH and then to untreated cells by the ΔΔCt method. Statistical analysis was performed by Student’s t-test; *P<0.05. (D) ChIP analysis of di-methylated H3K79 in the promoter regions of Hoxa cluster genes in murine CALM-AF10 leukemia cells. Results were generated by qRT-PCR and are shown as a percent of input. For all panels, results are shown as mean ± SEM compiled from at least 3 separate experiments.
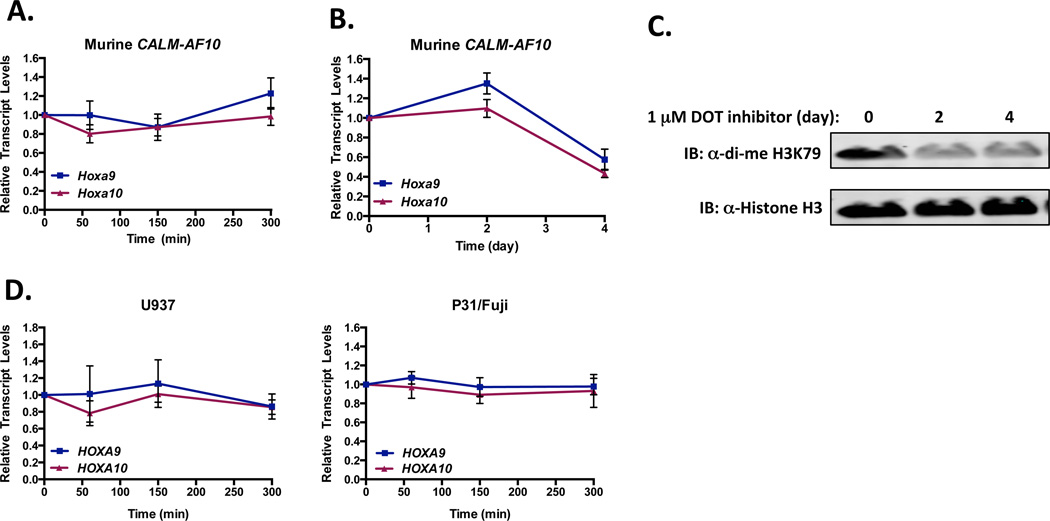
To extend the observations made in MEFs, murine CALM-AF10 leukemia cells were treated with LMB, and Hoxa transcript levels were measured. As shown in Figure 2B, 2.5 h LMB treatment resulted in at least a 50% reduction of Hoxa7–11 transcript levels in CALM-AF10 cells. To verify that these effects are specific to CALM-AF10 leukemia cells, we tested leukemia cells derived by co-transduction of Hoxa9 and Meis1 (Hoxa9/Meis1), which do not aberrantly express endogenous Hoxa genes. In contrast, Hoxa transcript levels were unchanged in Hoxa9/Meis1 leukemic cells after 2.5 h LMB treatment (Figure 2C). Together, these results suggest that the interaction of CALM-AF10 with CRM1 is necessary for upregulation of Hoxa cluster gene expression.
Upregulation of Hoxa genes in CALM-AF10 leukemias is thought to be a consequence of perturbed H3K79 methylation across Hoxa loci (11, 15). Because 2.5 h LMB treatment resulted in reduced Hoxa transcript levels, we assessed its effect on levels of di-methylated H3K79 in murine CALM-AF10 leukemic cells. As shown in Figure 2D, H3K79 methylation at Hoxa loci is unchanged following 2.5 h LMB treatment. Therefore, we conclude that H3K79 hypermethylation by itself is not sufficient to maintain Hoxa gene expression. This suggests that in addition to enabling DOT1L-mediated histone methylation, CALM-AF10 regulates Hoxa transcript levels through another mechanism.
Treatment with the CRM1 inhibitor Leptomycin B hinders transcription of Hoxa genes in CALM-AF10 leukemia cells
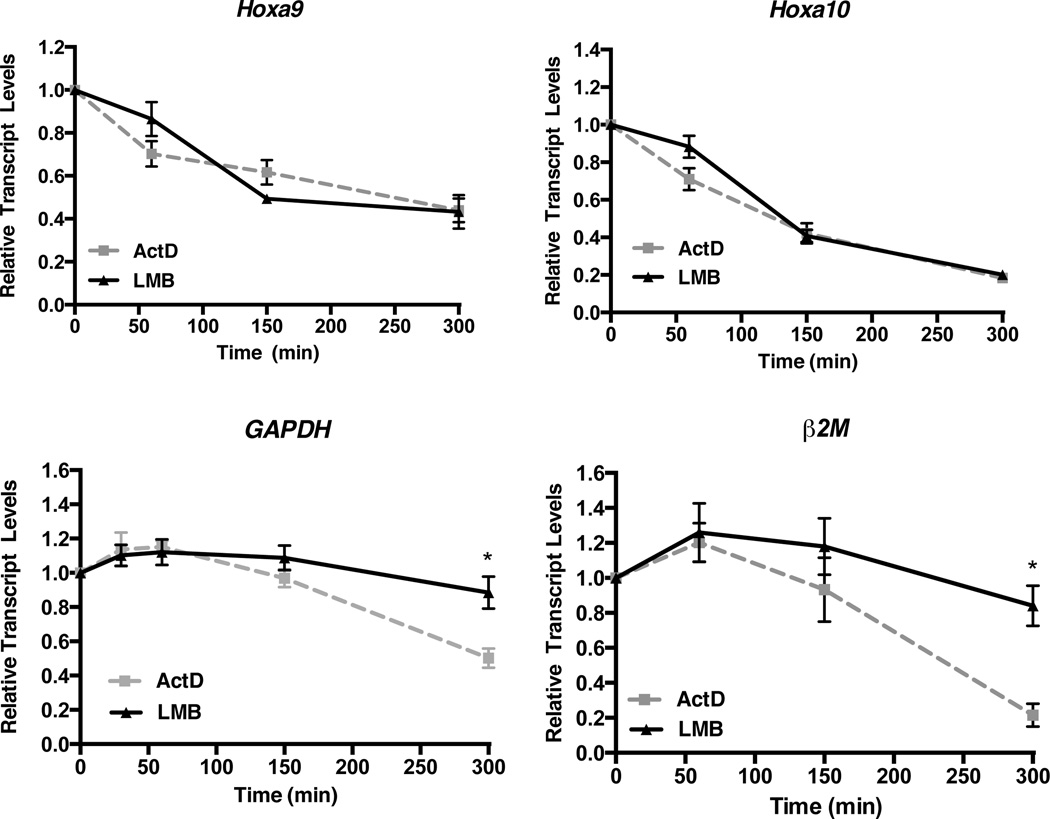
To explore whether the CRM1/CALM-AF10 interaction directly affects transcription of Hoxa genes, we compared the activity of LMB to that of Actinomycin D (ActD) in murine CALM-AF10 leukemia cells. Because ActD prevents RNA Polymerase-mediated transcriptional elongation, Hoxa transcript levels in ActD treated cells reflect mRNA stability rather than active transcription (24). As shown in Figure 3, incubation with ActD (5 µg/ml) results in loss of Hoxa9, Hoxa10, Gapdh, and β2M transcript levels over the course of 300 min (5 hr). Likewise, treatment with LMB (0.7 nM) results in reduced Hoxa9 and Hoxa10 transcript levels with kinetics similar to ActD (Figure 3). Importantly, Gapdh and β2M transcript levels are not decreased in LMB treated cells, demonstrating that LMB does not block all active transcription in CALM-AF10 leukemia cells. These findings suggest that blocking the CRM1/CALM-AF10 interaction with LMB specifically inhibits aberrant Hoxa9 and Hoxa10 transcription that occurs in these leukemias.
Figure 3. Leptomycin B and Actinomycin D inhibit Hoxa expression with similar kinetics in murine CALM-AF10 leukemia cells.
Murine CALM-AF10 leukemia cell lines were treated with 5 µg/ml Actinomycin D (ActD) or 0.7 nM LMB for 60, 150, or 300 min, and real time qRT-PCR was performed. Hoxa9, Hoxa10, Gapdh or β2M transcript levels were normalized to time zero (untreated). Results are shown as mean ± SEM from at least 3 experiments. Statistical analysis was performed by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test; *P<0.05.
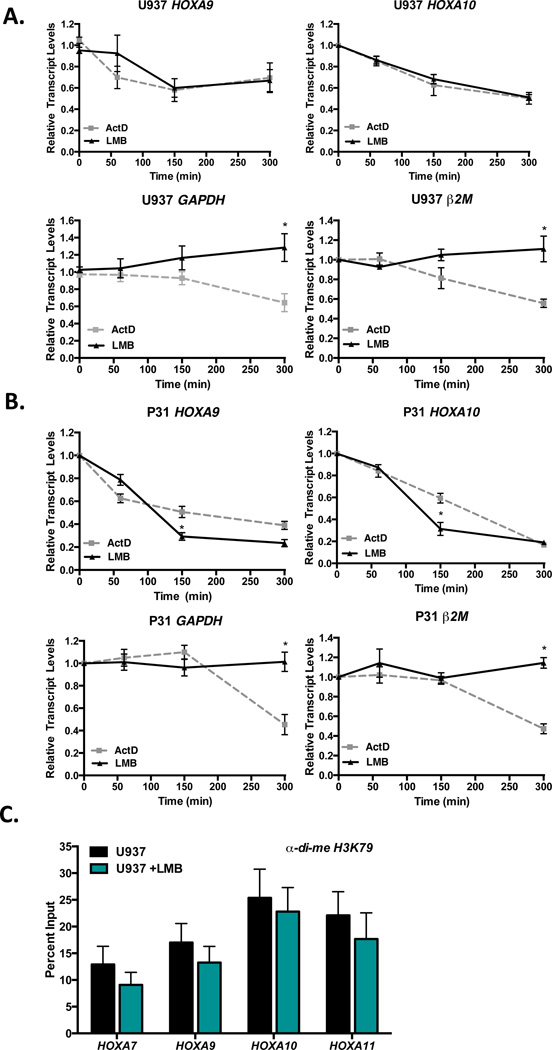
To extend our observations of the effects of LMB on Hoxa gene expression to human disease, we treated two human CALM-AF10 cell lines, U937 and P31/Fujioka (P31) with ActD or LMB. Both U937 and P31 cells are derived from patients with hematopoietic disorders driven by CALM-AF10 translocations (13, 21, 25). Cells were incubated with ActD (5 µg/ml) or LMB (0.7 nM) for up to 300 min, and transcript levels were measured by qRT-PCR. In both U937 (Figure 4A) and P31 cells (Figure 4B), HOXA9 and HOXA10 levels are reduced in the presence of LMB with kinetics similar to ActD. In contrast, GAPDH and β2M are reduced in the presence of ActD, but their levels remain unchanged upon exposure to LMB. These results are in agreement with our previous studies that showed that both U937 and P31 cell viabilities are reduced following exposure to LMB treatment (13). Next, we measured the levels of di-methylated H3K79 in U937 cells treated with LMB. As shown in Figure 4C, H3K79 methylation at HOXA loci is unchanged following 2.5 h LMB treatment, similar to that observed in murine CALM-AF10 leukemic cells (Figure 2D). We conclude that LMB-mediated CRM1 inhibition represses HOXA gene transcription independently of altered H3K79 methylation in both murine and human CALM-AF10 leukemia cells.
Figure 4. Leptomycin B and Actinomycin D inhibit HOXA expression with similar kinetics in human CALM-AF10 leukemia cell lines.
Human CALM-AF10-positive leukemia cell lines U937 (A) and P31/Fujioka (P31; B) were treated with 5 µg/ml ActD or 0.7 nM LMB for 60, 150, or 300 min, and real time qRT-PCR was performed. HOXA9, HOXA10, GAPDH or β2M transcript levels were normalized to time zero (untreated). Results are shown as mean ± SEM from at least 3 experiments. Statistical analysis was performed by two-way ANOVA followed by Bonferroni’s Multiple Comparison Test; *P<0.05 (C) ChIP analysis of di-methylated H3K79 in the promoter regions of HOXA cluster genes in human CALM-AF10 U937 cells. Results were generated by qRT-PCR and are shown as a percent of input. Results are shown as mean ± SEM from 3 experiments.
The rapid reduction of Hoxa transcripts induced by LMB in CALM-AF10 cells contrasts with the maintenance of H3K79 methylation at Hoxa loci (Figures 2D and 4C). Epigenetic deregulation of H3K79 methylation via aberrant recruitment and/or activity of DOT1L is thought to regulate overexpression of Hoxa genes in CALM-AF10 leukemias. Hence, pharmacological inhibitors of DOT1L have recently been developed for clinical use and have been shown to reduce Hoxa gene expression in these cells (15, 16). To test whether pharmacological inhibition of DOT1L reduces Hoxa gene expression with kinetics similar to ActD and LMB, we treated murine CALM-AF10 leukemia cells with the DOT1L inhibitor SGC0946 (1 µM) (26). Unlike LMB and ActD, SGC0946 does not alter Hoxa9 or Hoxa10 transcript levels over the course of 5 hr of treatment (Figure 5A). Similar to published studies, we found that Dot1L inhibition does lead to decreased Hoxa9 and Hoxa10 transcript levels after 4 days of treatment (Figure 5B) (15, 16). Likewise, cellular di-methylated H3K79 levels are reduced after 2 days of treatment with SGC0946 (Figure 5C). Next, we verified that HOXA9 or HOXA10 transcript levels are not altered in human CALM-AF10 leukemic cells treated with the DOT1L inhibitor SGC0946 over the course of 5 hr (Figure 5D). Congruent with recent findings (16), we also observed that the U937 cells are insensitive to long term DOT1L inhibition, as both cell growth and di-me H3K79 levels did not change following 6 days of treatment with SGC0946 (data not shown). From these results, we conclude that ActD and LMB treatment inhibit Hoxa9 and Hoxa10 transcript levels with similar kinetics in both human and murine CALM-AF10 cells. In contrast, murine CALM-AF10 cells require a more prolonged exposure to a DOT1L inhibitor, while human U937 cells show minimal response to DOT1L inhibition.
Figure 5. Inhibition of DOT1L with SGC0946 lowers Hoxa transcript levels with slower kinetics than LMB in murine CALM-AF10 cells.
(A-B) Murine CALM-AF10 cells were treated with either DMSO or 1 µM SGC0946, a specific DOT1L inhibitor, for 60, 150, or 300 min (A) or 2–4 days (B), and Hoxa9 and Hoxa10 transcript levels were measured by real time qRT-PCR. Hoxa9 and Hoxa10 transcript levels are normalized to GAPDH and then to respective DMSO-treated controls by the ΔΔCt method. Results are shown as mean ± SEM from at least 3 experiments. (C) Representative western blot of di-methylated H3K79 and Histone H3 levels in CALM-AF10 leukemic cells treated with DMSO (4 days) or SGC0946 (1 mM; 2–4 days). (D) Human CALM-AF10 leukemic cells (U937, left; P31/Fuji, Right) were treated with either DMSO or 1 µM SGC0946 for 60, 150, or 300 min. HOXA9 and HOXA10 transcript levels were measures by real time qRT-PCR, normalized to GAPDH and to respective DMSO-treated controls by the ΔΔCt method. Results are shown as mean ± SEM from 3 experiments.
CALM-AF10 is targeted to Hoxa loci in a CRM1-dependent manner
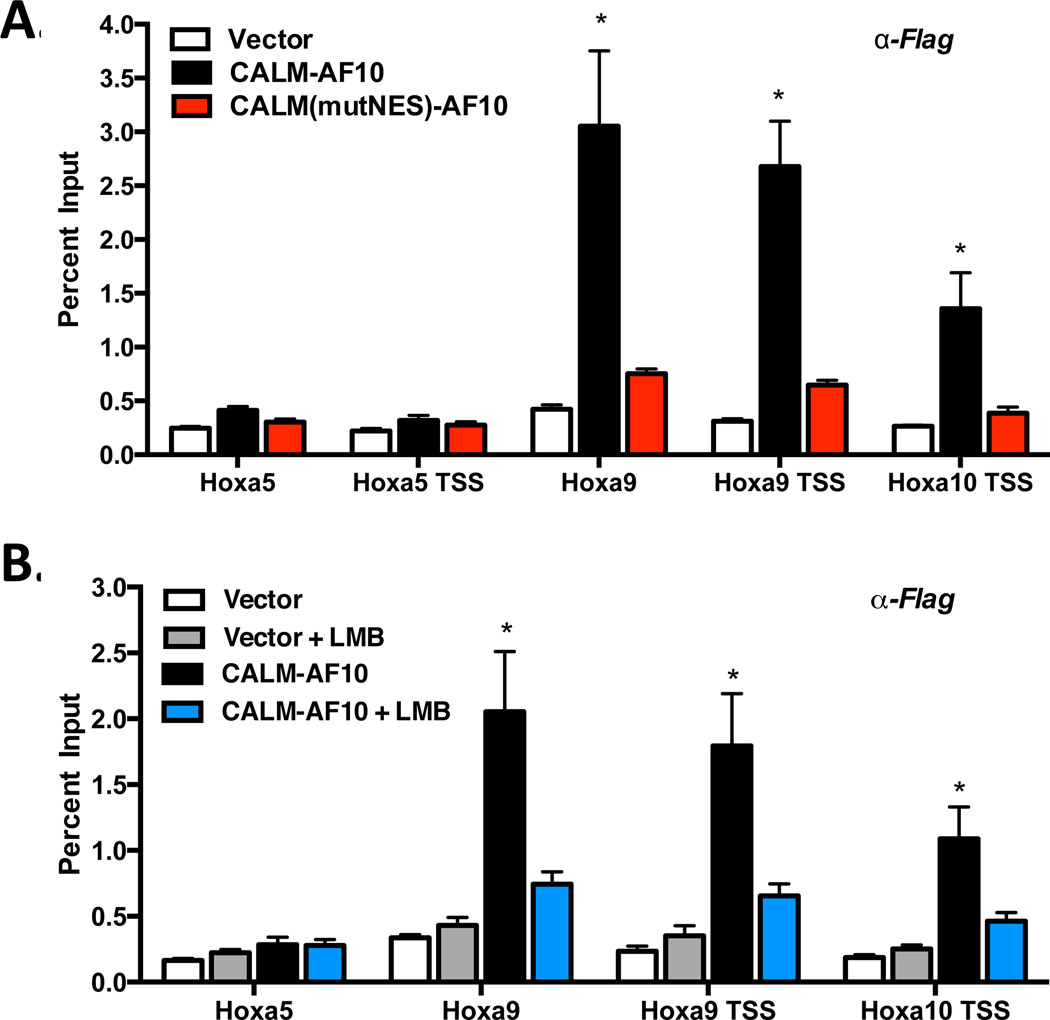
Our results thus far support a model in which CALM-AF10 must interact with CRM1 (via the NES) to upregulate Hoxa gene transcription. However, it remains unclear whether these effects at the Hoxa locus are directly or indirectly mediated by CALM-AF10. To assess whether the effects of CALM-AF10 on Hoxa gene transcription are direct, we performed chromatin immunoprecipitation (ChIP) using anti-Flag antibodies in MEFs stably expressing empty vector, Flag-tagged CALM-AF10, or Flag-tagged CALM(mutNES)-AF10. As shown in Figure 6A, CALM-AF10 is enriched at the transcriptional start site (TSS) of Hoxa9 and Hoxa10 and within the coding region of Hoxa9. However, we did not find enrichment of CALM-AF10 at the TSS or within the first exon of Hoxa5, in contrast to the report by Okada and colleagues (11). To determine whether the CALM-AF10 NES motif is required to interact with chromatin, we studied the point mutant CALM(mutNES)-AF10 that does not bind CRM1 and is unable to activate Hoxa genes or transform murine bone marrow (13). Interestingly, while CALM(mutNES)-AF10 is an entirely nuclear protein (13), we did not observe enrichment of CALM(mutNES)-AF10 at Hoxa9 or Hoxa10 chromatin loci (Figure 6A). Therefore, point mutation of the hydrophobic residues within the NES abrogates the ability of CALM-AF10 to bind chromatin and subsequently activate transcription of the Hoxa genes.
Figure 6. CALM-AF10 presence at Hoxa loci is dependent on CRM1 interaction.
(A) ChIP was performed using anti-Flag antibodies in MEFs expressing empty Vector, Flag-CALM-AF10, or Flag-CALM(mutNES)-AF10. (B) Vector or CALM-AF10 expressing MEFs were treated with 0.7 nM LMB for 2.5 h, and ChIP using anti-Flag antibodies was performed. Hoxa amplification was measured by real time qPCR and shown as a percent of input. Results represent mean ± SEM from at least 3 experiments. Statistical analysis was performed by one-way ANOVA for each Hoxa gene, followed by Dunnett’s Multiple Comparison Test; *P<0.05.
To further establish whether interaction with CRM1 is required for CALM-AF10 localization at Hoxa chromatin, we treated CALM-AF10-expressing cells with LMB and performed ChIP using anti-Flag antibodies. Strikingly, we observed a significant loss of CALM-AF10 enrichment at Hoxa9 and Hoxa10 chromatin following only 2.5 hr treatment with LMB (Figure 6B), mirroring the phenotype of CALM(mutNES)-AF10 (Figure 6A). These results are also congruent with the kinetics of Hoxa transcript reduction caused by LMB (Figure 2A). Therefore, we conclude that the localization of CALM-AF10 at Hoxa loci requires interaction between the CALM-derived NES and CRM1. Both genetic and pharmacologic inhibition of this interaction not only prevents CALM-AF10 from localizing to Hoxa chromatin but also blocks CALM-AF10-mediated activation of Hoxa gene transcription.
CRM1 is enriched at Hoxa loci in the presence and absence of CALM-AF10
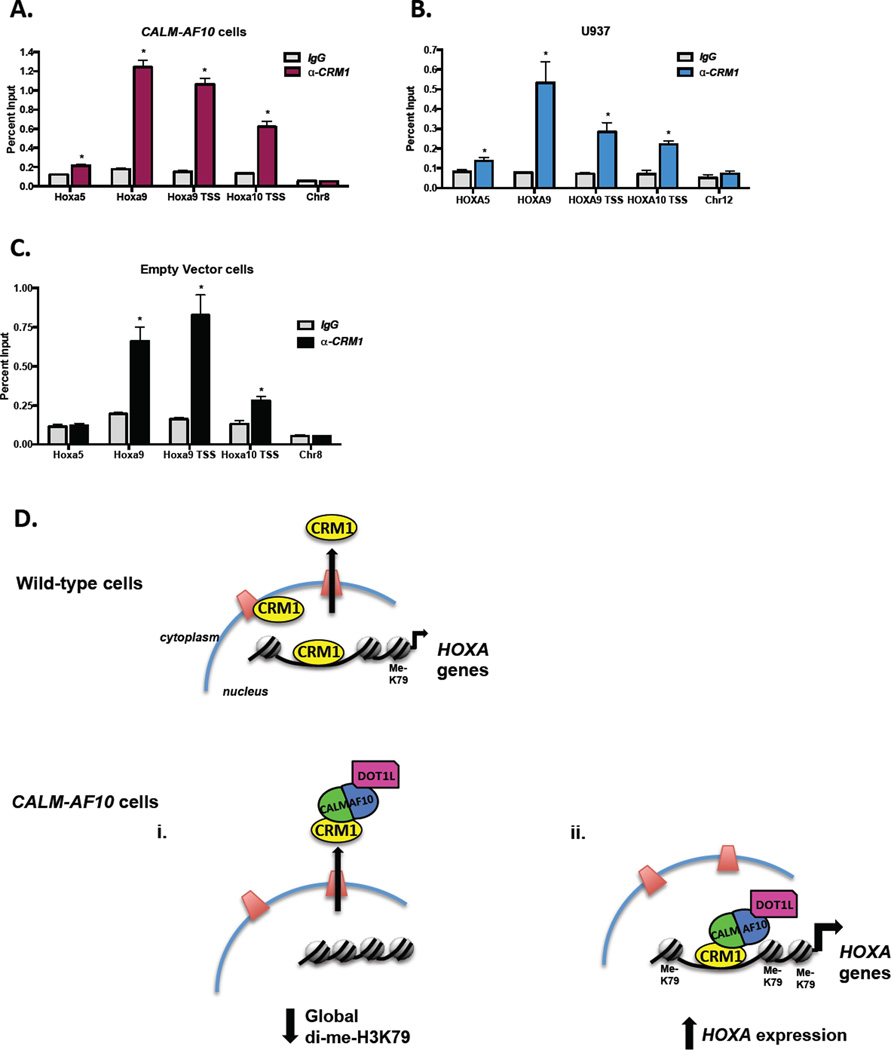
Our observation that CALM-AF10 interacts with Hoxa chromatin in a CRM1-dependent manner led us to hypothesize that CRM1 itself may bind Hoxa loci thus allowing for recruitment of CALM-AF10. However, as the major mammalian nuclear export receptor, CRM1 is solely thought to affect gene regulation through its translocation of nuclear factors to the cytoplasm. It was therefore surprising to find significant enrichment of CRM1 at Hoxa chromatin in MEFs expressing CALM-AF10 (Figure 7A). The pattern of CRM1 localization across the Hoxa cluster is similar to that seen with CALM-AF10 with highest enrichment at the TSS and within the first exon of Hoxa9 (Figure 6A–B). Importantly, we did not observe CRM1 enrichment at a region of heterochromatin (Chr8, Figure 7A), suggesting that CRM1 localizes to specific loci. These results can be recapitulated using a different anti-CRM1 antibody (Figure S1A).
Figure 7. CRM1 interacts with Hoxa chromatin in both the presence and absence of CALM-AF10.
(A) ChIP analyses were performed using anti-CRM1 antibodies in MEFs expressing CALM-AF10. Hoxa amplification was measured by real time qPCR and shown as a percent of input. A region of heterochromatin (Chr8) was used as a negative control. (B) ChIP analyses were performed using anti-CRM1 antibodies in CALM-AF10-positive U937 cells. HOXA amplification was measured by real time qPCR and shown as a percent of input. A region of heterochromatin (Chr12) was used as a negative control. (C) CRM1 ChIP experiments were performed using anti-CRM1 antibodies in MEFs stably infected with an empty vector. Results represent mean ± SEM from at least 3 experiments. Statistical analysis was performed by Student’s t-test; *P<0.05. (D) Schematic model demonstrating the molecular mechanisms of CALM-AF10-mediated leukemogenesis. In wild-type (non-CALM-AF10) cells, in addition to mediating protein nuclear export, CRM1 also localizes to HOXA loci. CRM1 mediates the leukemogenic effects of CALM-AF10 through two major mechanisms. (i) CRM1-mediated nuclear export of CALM-AF10 results in cytoplasmic mislocalization of DOT1L and subsequent global H3K79 hypomethylation, potentially resulting in genomic instability. (ii) Within the nucleus, CRM1 recruits CALM-AF10 to HOXA loci, resulting in transcriptional and epigenetic activation of the leukemogenic HOXA genes.
The observation that CRM1 binds Hoxa chromatin in CALM-AF10 expressing MEFs led us to question whether CRM1 is also present at HOXA loci in leukemia cells. We performed ChIP using anti-CRM1 antibodies in the human CALM-AF10 leukemia cell line, U937. Similar to experiments performed in MEFs, CRM1 is enriched at HOXA9 and HOXA10 gene regions, but we did not detect enrichment at a heterochromatin region (Chr12, Figure 7B). The observations that CRM1 binds HOXA chromatin and that HOXA transcript levels are sensitive to LMB in human CALM-AF10 leukemic cells (Figure 4) support a mechanism by which CRM1 mediates the effects of CALM-AF10 on HOXA transcription during leukemogenesis.
Finally, we sought to determine whether the localization of CRM1 at Hoxa loci is dependent on CALM-AF10. We performed ChIP using an anti-CRM1 antibody in MEFs expressing an empty retroviral vector. As shown in Figures 7C and S1B, we observed similar enrichment of CRM1 at Hoxa chromatin in the absence of CALM-AF10. The presence of CRM1 at Hoxa loci in wild-type MEFs suggests a model whereby CRM1 recruits CALM-AF10 to DNA. In other words, the CALM-AF10 fusion protein is able to hijack this normal function of CRM1, allowing for CALM-AF10 localization at the Hoxa gene cluster and resulting in H3K79 hypermethylation, increased transcription and ultimately cellular transformation.
Discussion
In previous work, we showed that CALM contains a CRM1-dependent NES that is essential for CALM-AF10-mediated transformation (13). Interaction with CRM1 mediates nuclear export of CALM-AF10 and leads to cytoplasmic mislocalization of the AF10 binding partner, DOT1L (Figure 7D) (13). Mislocalization of DOT1L to the cytoplasm is associated with global H3K79 hypomethylation, which in turn may contribute to genomic instability (12). In the current studies, we continue to explore the molecular mechanisms by which the CALM-AF10 translocation drives leukemogenesis.
We have demonstrated that in addition to mediating nuclear export of CALM-AF10, the interaction of CRM1 with CALM-AF10 directly regulates HOXA cluster gene expression. We found that short treatment (2.5 h) with the CRM1 inhibitor, LMB, results in loss of Hoxa transcript levels in both MEFs and leukemia cells expressing CALM-AF10. These effects are specific to CALM-AF10 cells, as Hoxa levels are unchanged following LMB treatment in vector-expressing MEFs and in Hoxa9/Meis1-driven leukemia cells (Figures 2A and 2C). From these results, we conclude that Hoxa gene expression is particularly sensitive to CRM1 inhibition in CALM-AF10 cells.
Prior to these studies, CALM-AF10-mediated upregulation of Hoxa gene expression was solely thought to be a consequence of DOT1L-mediated H3K79 hypermethylation at this locus. However, we observed that although Hoxa transcript levels were reduced in the presence of LMB, di-methylated H3K79 levels remained unchanged (Figures 2D and 4C). These results suggest that exposure to LMB impairs Hoxa gene expression via a mechanism that is distinct from DOT1L-mediated methylation. Indeed, while a reduction in Hoxa9 and Hoxa10 transcript levels requires a prolonged treatment with the DOT1L inhibitor (4 days in murine leukemic cells), HOXA levels are reduced after only 2.5 hours in either murine or human CALM-AF10 cells. The effects of LMB on HOXA9 and HOXA10 transcript levels are similar to those seen with inhibition of RNA Polymerase in both mouse and human CALM-AF10 leukemia cells. These results suggest that in addition to facilitating H3K79 methylation at HOXA loci, CALM-AF10 may also drive transcription of HOXA9 and HOXA10.
To further explore how CRM1 affects Hoxa regulation by CALM-AF10, we studied the direct binding of CALM-AF10 at Hoxa loci. We found that interaction of CALM-AF10 with Hoxa loci was inhibited by both mutation of the NES motif and treatment with LMB. Therefore, enrichment of CALM-AF10 at Hoxa loci requires interaction between the CALM-derived NES and CRM1. Likewise, we found that CRM1 also localizes to HOXA loci in MEFs and human leukemia cells expressing CALM-AF10. The binding pattern of CRM1 across HOXA9 and HOXA10 is similar to that observed for CALM-AF10. Finally, we found that CRM1 localizes to Hoxa chromatin in MEFs that do not express CALM-AF10.
Taken together, these data support a mechanistic model of CALM-AF10-mediated leukemogenesis that is critically dependent on the CALM-CRM1 interaction (Figure 7D). In non-CALM-AF10 cells, CRM1 not only mediates nuclear export, but also localizes to Hoxa loci for reasons that at this time remain unclear. Both of these roles for CRM1 are exploited by interaction with the aberrant CALM-AF10 fusion protein, which drives leukemic transformation. Specifically, as discussed earlier, CRM1 mediates nuclear export of CALM-AF10 resulting in mislocalization of DOT1L and global loss of di-methylated H3K79 levels (Figure 7Di). Global H3K79 hypomethylation may alter structure and organization of the genome and has been shown to contribute to genome instability (12), each of which could contribute to the leukemic state. In addition to mediating nuclear export, the localization of CRM1 to HOXA loci results in recruitment of CALM-AF10, which in turn increases both H3K79 methylation and transcription of HOXA genes (Figure 7Dii). Our data demonstrate the during acute LMB treatment, H3K79 hypermethylation is not sufficient to maintain HOXA gene expression. However, maintenance of H3K79 methylation has previously been shown to be necessary for HOXA expression and leukemogenesis (11, 15). Taken together, these findings implicate CALM-AF10 in control of both the immediate transcription and maintained epigenetic state of the HOXA genes via interaction with both CRM1 and DOT1L. The HOXA genes are key effectors of leukemogenesis, and their overexpression ultimately contributes to cellular transformation.
While elucidating the mechanisms of CALM-AF10-driven leukemias, we discovered a novel role for CRM1: its ability to bind HOXA gene loci and to modulate their epigenetic and transcriptional status through recruitment of CALM-AF10. While this is the first demonstration of CRM1 at chromatin in mammalian cells, the CRM1 ortholog, Xpo1 was found to be associated with transcriptionally active genes in a genome-wide ChIP screen in S. cerevisiae (27). In addition, other members of the nuclear transport machinery, such as nucleoporins, have been shown to bind DNA and transcriptionally regulate gene expression (28, 29). In the future, it will be important to assess the physiologic role of CRM1 at chromatin and to determine whether it interacts with other components of the nuclear pore and plays a direct role in gene regulation. Because CALM-AF10 is recruited to HOXA chromatin via interaction with CRM1, we predict that under normal circumstances, CRM1 may recruit NES-containing factors to specific genomic loci. CRM1 is known to interact with many transcription factors that contain NES motifs (such as p53(30), STAT1(31), MAP2K1(32) and IκB(33)) and mediate their nuclear/cytoplasmic localization. Therefore, it is tempting to speculate that in addition to mediating nuclear export, CRM1 may directly interact with transcription factors at DNA to regulate gene expression. In support of this notion, mutation of the NES motif or treatment with LMB has been shown to inhibit the ability of transcription factors Oct-6 and Sox10 to activate their downstream targets (34, 35). Likewise, acute (6 h) treatment with the CRM1 inhibitor KPT-276 results in decreased cell cycle gene expression in myeloma cells, similar to our observations of HOXA transcript levels in leukemia cells (36).
The critical role of CRM1 in CALM-AF10-mediated leukemogenesis, may extend to other leukemic fusion proteins. This might be particularly relevant in the case of leukemogenic fusions involving the nucleoporins NUP98 or NUP214, which physiologically interact with CRM1 and are also associated with HOXA gene overexpression (37–39). Intriguingly, it has recently been proposed that CRM1 may directly cooperate with NUP98/NUP214 in binding chromatin and regulating transcription during leukemogenesis (40). In the future, it will be interesting to assess a more general role for CRM1 in regulating transcription in leukemias that overexpress HOXA genes.
The use of CRM1 inhibitors for the treatment of hematologic malignancies was first attempted more than 20 years ago. Although initial clinical trials with Leptomycin B in humans were associated with significant toxicity (41), several novel CRM1 inhibitors have recently been developed and are being tested in preclinical trials for patients with hematological malignancies, such as AML (42–44). Based on our observations in CALM-AF10 leukemias, we hypothesize that inhibition of CRM1 may affect tumor cell viability by directly blocking the transcription of effector genes such as HOXA9 and HOXA10, in addition to their well-recognized ability to prevent nuclear export of NES containing proteins.
In summary, we have identified a novel molecular mechanism by which the CALM NES enables CALM-AF10-mediated activation of HOXA gene expression via interaction with the nuclear export receptor, CRM1. Genetic and pharmacologic inhibition of CRM1 prevents CALM-AF10 enrichment at HOXA loci, resulting in loss of HOXA gene transcription. CRM1 is first present at HOXA chromatin where it recruits CALM-AF10, highlighting a novel role for CRM1 in gene regulation through direct loci interaction, rather than nuclear export. We conclude that CRM1 is a central mediator of and promising therapeutic target in CALM-AF10-mediated leukemogenesis.
Supplementary Material
Acknowledgements
We appreciate the helpful critiques, comments and suggestions of Chi Dang and Rob Wechsler-Reya. We thank Jay Hess for providing the Hoxa9-LUC construct. This work was supported by NCI R01 CA 109281 (DSW), a V Foundation/Applebee’s Grant (DSW), a St. Baldrick’s Research Grant (DSW) and a Hyundai Hope Grant (CPL).
Footnotes
Conflict of Interest Disclosure
The authors declare no competing financial interests.
Author Contributions
A.E.C. and J.M.H. designed and performed experiments, analyzed data and wrote the paper. D.S.W. and C.P.L. designed experiments and wrote the paper.
Supplementary information is available at Leukemia’s website.
References
- 1.Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 2.Sauvageau G, Lansdorp PM, Eaves CJ, Hogge DE, Dragowska WH, Reid DS, et al. Differential expression of homeobox genes in functionally distinct CD34+ subpopulations of human bone marrow cells. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12223–12227. doi: 10.1073/pnas.91.25.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pineault N, Helgason CD, Lawrence HJ, Humphries RK. Differential expression of Hox, Meis1, and Pbx1 genes in primitive cells throughout murine hematopoietic ontogeny. Experimental hematology. 2002 Jan;30(1):49–57. doi: 10.1016/s0301-472x(01)00757-3. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence HJ, Rozenfeld S, Cruz C, Matsukuma K, Kwong A, Komuves L, et al. Frequent co-expression of the HOXA9 and MEIS1 homeobox genes in human myeloid leukemias. Leukemia. 1999 Dec;13(12):1993–1999. doi: 10.1038/sj.leu.2401578. [DOI] [PubMed] [Google Scholar]
- 5.Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999 Oct 15;286(5439):531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 6.Thorsteinsdottir U, Sauvageau G, Hough MR, Dragowska W, Lansdorp PM, Lawrence HJ, et al. Overexpression of HOXA10 in murine hematopoietic cells perturbs both myeloid and lymphoid differentiation and leads to acute myeloid leukemia. Molecular and cellular biology. 1997 Jan;17(1):495–505. doi: 10.1128/mcb.17.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroon E, Krosl J, Thorsteinsdottir U, Baban S, Buchberg AM, Sauvageau G. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 1998 Jul 1;17(13):3714–3725. doi: 10.1093/emboj/17.13.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohlander SK, Muschinsky V, Schrader K, Siebert R, Schlegelberger B, Harder L, et al. Molecular analysis of the CALM/AF10 fusion: identical rearrangements in acute myeloid leukemia, acute lymphoblastic leukemia and malignant lymphoma patients. Leukemia. 2000 Jan;14(1):93–99. doi: 10.1038/sj.leu.2401614. Epub 2000/01/19. eng. [DOI] [PubMed] [Google Scholar]
- 9.Dik WA, Brahim W, Braun C, Asnafi V, Dastugue N, Bernard OA, et al. CALM-AF10+ T-ALL expression profiles are characterized by overexpression of HOXA and BMI1 oncogenes. Leukemia. 2005 Nov;19(11):1948–1957. doi: 10.1038/sj.leu.2403891. Epub 2005/08/19. eng. [DOI] [PubMed] [Google Scholar]
- 10.Caudell D, Zhang Z, Chung YJ, Aplan PD. Expression of a CALM-AF10 fusion gene leads to Hoxa cluster overexpression and acute leukemia in transgenic mice. Cancer Res. 2007 Sep 1;67(17):8022–8031. doi: 10.1158/0008-5472.CAN-06-3749. Epub 2007/09/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada Y, Jiang Q, Lemieux M, Jeannotte L, Su L, Zhang Y. Leukaemic transformation by CALM-AF10 involves upregulation of Hoxa5 by hDOT1L. Nat Cell Biol. 2006 Sep;8(9):1017–1024. doi: 10.1038/ncb1464. Epub 2006/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin YH, Kakadia PM, Chen Y, Li YQ, Deshpande AJ, Buske C, et al. Global reduction of the epigenetic H3K79 methylation mark and increased chromosomal instability in CALM-AF10-positive leukemias. Blood. 2009 Jul 16;114(3):651–658. doi: 10.1182/blood-2009-03-209395. Epub 2009/05/16. eng. [DOI] [PubMed] [Google Scholar]
- 13.Conway AE, Scotland PB, Lavau CP, Wechsler DS. A CALM-derived nuclear export signal is essential for CALM-AF10-mediated leukemogenesis. Blood. 2013 2013 Mar 13;121(23):4758–4768. doi: 10.1182/blood-2012-06-435792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada Y, Feng Q, Lin Y, Jiang Q, Li Y, Coffield VM, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005 Apr 22;121(2):167–178. doi: 10.1016/j.cell.2005.02.020. Epub 2005/04/27. eng. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Deshpande AJ, Banka D, Bernt KM, Dias S, Buske C, et al. Abrogation of MLL-AF10 and CALM-AF10-mediated transformation through genetic inactivation or pharmacological inhibition of the H3K79 methyltransferase Dot1l. Leukemia. 2012 Nov 9;27(4):813–822. doi: 10.1038/leu.2012.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011 Jul 12;20(1):53–65. doi: 10.1016/j.ccr.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997 Sep 19;90(6):1051–1060. doi: 10.1016/s0092-8674(00)80371-2. Epub 1997/10/10. eng. [DOI] [PubMed] [Google Scholar]
- 18.Archangelo LF, Glasner J, Krause A, Bohlander SK. The novel CALM interactor CATS influences the subcellular localization of the leukemogenic fusion protein CALM/AF10. Oncogene. 2006 Jul 6;25(29):4099–4109. doi: 10.1038/sj.onc.1209438. [DOI] [PubMed] [Google Scholar]
- 19.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000 Jun;7(12):1063–1066. doi: 10.1038/sj.gt.3301206. Epub 2000/06/29. eng. [DOI] [PubMed] [Google Scholar]
- 20.Scotland PB, Heath JL, Conway AE, Porter NB, Armstrong MB, Walker JA, et al. The PICALM protein plays a key role in iron homeostasis and cell proliferation. PloS one. 2012;7(8):e44252. doi: 10.1371/journal.pone.0044252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narita M, Shimizu K, Hayashi Y, Taki T, Taniwaki M, Hosoda F, et al. Consistent detection of CALM-AF10 chimaeric transcripts in haematological malignancies with t(10;11)(p13;q14) and identification of novel transcripts. Br J Haematol. 1999 Jun;105(4):928–937. doi: 10.1046/j.1365-2141.1999.01433.x. Epub 1999/11/11. eng. [DOI] [PubMed] [Google Scholar]
- 22.Muntean AG, Tan J, Sitwala K, Huang Y, Bronstein J, Connelly JA, et al. The PAF complex synergizes with MLL fusion proteins at HOX loci to promote leukemogenesis. Cancer Cell. 2010 Jun 15;17(6):609–621. doi: 10.1016/j.ccr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobell HM. Actinomycin and DNA transcription. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5328–5331. doi: 10.1073/pnas.82.16.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyling MH, Martinez-Climent JA, Zheng M, Mao J, Rowley JD, Bohlander SK. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4804–4809. doi: 10.1073/pnas.93.10.4804. Epub 1996/05/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu W, Chory EJ, Wernimont AK, Tempel W, Scopton A, Federation A, et al. Catalytic site remodelling of the DOT1L methyltransferase by selective inhibitors. Nature communications. 2012;3:1288. doi: 10.1038/ncomms2304. [DOI] [PubMed] [Google Scholar]
- 27.Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA. Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell. 2004 May 14;117(4):427–439. doi: 10.1016/s0092-8674(04)00448-9. [DOI] [PubMed] [Google Scholar]
- 28.Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010 Feb 5;140(3):360–371. doi: 10.1016/j.cell.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Capelson M, Liang Y, Schulte R, Mair W, Wagner U, Hetzer MW. Chromatin-bound nuclear pore components regulate gene expression in higher eukaryotes. Cell. 2010 Feb 5;140(3):372–383. doi: 10.1016/j.cell.2009.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999 Mar 15;18(6):1660–1672. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBride KM, McDonald C, Reich NC. Nuclear export signal located within theDNA-binding domain of the STAT1transcription factor. EMBO J. 2000 Nov 15;19(22):6196–6206. doi: 10.1093/emboj/19.22.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997 Nov 20;390(6657):308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 33.Johnson C, Van Antwerp D, Hope TJ. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IkappaBalpha. EMBO J. 1999 Dec 1;18(23):6682–6693. doi: 10.1093/emboj/18.23.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baranek C, Sock E, Wegner M. The POU protein Oct-6 is a nucleocytoplasmic shuttling protein. Nucleic acids research. 2005;33(19):6277–6286. doi: 10.1093/nar/gki947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehberg S, Lischka P, Glaser G, Stamminger T, Wegner M, Rosorius O. Sox10 is an active nucleocytoplasmic shuttle protein, and shuttling is crucial for Sox10-mediated transactivation. Molecular and cellular biology. 2002 Aug;22(16):5826–5834. doi: 10.1128/MCB.22.16.5826-5834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt J, Braggio E, Kortuem KM, Egan JB, Zhu YX, Xin CS, et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia. 2013 Dec;27(12):2357–2365. doi: 10.1038/leu.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda A, Sarma NJ, Abdul-Nabi AM, Yaseen NR. Inhibition of CRM1-mediated nuclear export of transcription factors by leukemogenic NUP98 fusion proteins. The Journal of biological chemistry. 2010 May 21;285(21):16248–16257. doi: 10.1074/jbc.M109.048785. Epub 2010/03/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Vlierberghe P, van Grotel M, Tchinda J, Lee C, Beverloo HB, van der Spek PJ, et al. The recurrent SET-NUP214 fusion as a new HOXA activation mechanism in pediatric T-cell acute lymphoblastic leukemia. Blood. 2008 May 1;111(9):4668–4680. doi: 10.1182/blood-2007-09-111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghannam G, Takeda A, Camarata T, Moore MA, Viale A, Yaseen NR. The oncogene Nup98-HOXA9 induces gene transcription in myeloid cells. The Journal of biological chemistry. 2004 Jan 9;279(2):866–875. doi: 10.1074/jbc.M307280200. [DOI] [PubMed] [Google Scholar]
- 40.Takeda A, Yaseen NR. Nucleoporins and nucleocytoplasmic transport in hematologic malignancies. Seminars in cancer biology. 2014 Mar 18; doi: 10.1016/j.semcancer.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. British journal of cancer. 1996 Aug;74(4):648–649. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Etchin J, Sanda T, Mansour MR, Kentsis A, Montero J, Le BT, et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br J Haematol. 2013 Apr;161(1):117–127. doi: 10.1111/bjh.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etchin J, Sun Q, Kentsis A, Farmer A, Zhang ZC, Sanda T, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia. 2013 Jan;27(1):66–74. doi: 10.1038/leu.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranganathan P, Yu X, Na C, Santhanam R, Shacham S, Kauffman M, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood. 2012 Aug 30;120(9):1765–1773. doi: 10.1182/blood-2012-04-423160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.