Abstract
Parturition is an inflammatory process mediated to a significant extent by macrophages. Progesterone maintains uterine quiescence in pregnancy, and a proposed functional withdrawal of progesterone classically regulated by nuclear progesterone receptors (nPRs) leads to labor. Progesterone can impact the functions of macrophages despite the reported lack of expression of nPRs in these immune cells. Therefore, in this study we investigated the effects of the activation of the putative membrane-associated progesterone receptor on the function of macrophages (a key cell for parturition) and discuss the implications of these findings for pregnancy and parturition. In murine macrophage cells (RAW264.7), activation of mPRs by progesterone modified to be active only extracellularly by conjugation to BSA (P4BSA, 1.0×10−7 mol/l) caused a pro-inflammatory shift in mRNA expression profile, with significant up-regulation of the expression of cyclooxygenase 2 (Ptgs2), Il1B, and Tnf and down-regulation of membrane progesterone receptor alpha (Paqr7) and oxytocin receptor (Oxtr). Pretreatment with PD98059, a MEK 1/2 inhibitor, significantly reduced P4BSA-induced Il1B, Tnf and Ptgs2 mRNA. Inhibition of protein kinase A (PKA) by H89 blocked P4BSA-induced Il1B and Tnf mRNA levels. P4BSA induced rapid phosphorylation of MEK1/2 and cAMP responsive element binding protein (CREB, a downstream target of PKA). This phosphorylation was inhibited by pretreatment with PD98059 and H89, respectively, revealing that MEK1/2 and PKA are two of the components involved in mPR signaling. Taken together, these data demonstrate that changes in membrane progesterone receptor alpha expression and signaling in macrophages are associated with the inflammatory responses; and that these changes might contribute to the functional withdrawal of progesterone related to labor.
Keywords: Membrane progesterone receptor, progesterone, macrophages, inflammatory response
Introduction
Parturition is an inflammatory process observed at term and preterm. Evidence from human and animal studies has demonstrated that leukocytes infiltrate myometrium, cervix and decidua during and prior to the process of labor, playing a critical role in parturition (Mackler et al., 1999; Thomson et al., 1999; Hamilton et al., 2012; Care et al., 2013; Shynlova et al., 2013). In mice, uterine macrophage numbers increase during the period of early pregnancy and then decline near term (Mackler et al., 1999). In contrast, macrophage numbers in the cervix increase at term and their numbers peak on the day before delivery. Therefore, it has been suggested that macrophage trafficking between uterus and cervix, and associated cytokine production, contribute to the termination of pregnancy (Mackler et al., 1999). Decidual macrophage infiltration has also been shown in both term labor and idiopathic preterm labor in humans and rats prior to parturition, indicating an initiating role of inflammatory events in labor (Hamilton et al., 2012). Taken together, these studies highlight the importance of inflammatory cell infiltration into reproductive tissues as a physiological mechanism regulating pregnancy maintenance and parturition.
17-hydroxyprogesterone caproate injections have been shown to prevent preterm delivery in high-risk women (Meis et al., 2003). Progesterone (P4) maintains pregnancy by promoting uterine quiescence until parturition is initiated by certain forms of withdrawal of this “progesterone block” (Csapo, 1956). In humans, maternal levels of circulating P4 do not change during spontaneous labor or in the weeks preceding labor (Pieber et al., 2001). Therefore, alternative mechanisms of functional P4 withdrawal have been proposed (Zakar & Hertelendy, 2007; Mesiano et al., 2011). P4 exerts its actions through the classical intracellular nuclear progesterone receptors (nPRs) (Mulac-Jericevic et al., 2000; Conneely et al., 2003; Merlino et al., 2007), leading to the translocation of hormone-receptor complexes into the nucleus, where they bind to hormone-responsive elements of DNA to regulate gene transcription (Webster et al., 2002). However, some of the effects of P4 are not related to its transcriptional activity (Gellersen et al., 2009). In 2003, putative mPR receptors (mPRα, β and γ) were cloned, shedding new light on P4 receptor research (Zhu et al., 2003).
P4 elicits a variety of functional effects on immune cell types, including dendritic cells (DCs), monocytes, lymphocytes, and macrophages. P4 shifts the proinflammatory activity of DCs toward a more tolerogenic state (Kammerer et al., 2000; Liang et al., 2006) and promotes a T helper 2 (Th2)-biased profile that is a prerequisite for fetal survival and the maintenance of pregnancy (Piccinni et al., 1995; Raghupathy, 1997; Szekeres-Bartho et al., 2009; Sykes et al., 2012). Functional P4 withdrawal may contribute to the switch from a Th2 to a Th1 dominant phenotype via the actions of progesterone-induced blocking factor in lymphocytes toward the end of pregnancy (Szekeres-Bartho & Chaouat, 1990; Szekeres-Bartho et al., 1990; Druckmann & Druckmann, 2005; Raghupathy et al., 2009). Interestingly, classical nPRs are undetectable or expressed at very low levels in these immune cells. For instance, several studies have demonstrated the absence of nPRs in peripheral blood leukocytes, T lymphocytes, immortalized T cells (Jurkat cells), the murine RAW 264.7 macrophage cell line, as well as in mouse bone marrow derived macrophages (Mansour et al., 1994; Mulac-Jericevic et al., 2000; Merlino et al., 2007; Dosiou et al., 2008; Ndiaye et al., 2012). Since then, several studies have provided evidence for activation of mPRs in reproductive tissues and immune cells, and suggest that these mPRs act as G-protein coupled receptors (GPCRs) in fish oocytes (Zhu et al., 2003), human myocytes (Karteris et al., 2006), human T lymphocytes and Jurkat T cells (Dosiou et al., 2008) (Ndiaye et al., 2012). Because P4 elicits a variety of functional effects on immune cell types, even in those lacking nPRs (Dressing et al., 2011), the functions of P4-mediated mPR activation and signaling are of great interest. Since mPRs are putative GPCRs (Dosiou & Giudice, 2005; Karteris et al., 2006; Thomas et al., 2007; Dosiou et al., 2008; Dressing et al., 2011) and activation of GPCRs leads to downstream activation of cAMP-dependent protein kinase A (PKA), mitogen-activated protein kinase kinase (MEK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways, we investigated the potential involvement of mPR, PKA, MEK1/2, and PI3K/Akt in murine macrophage responses to P4.
Materials and methods
Reagents and antibodies
Progesterone 3-(O-carboxymethyl)oxime:BSA–fluorescein isothiocyanate conjugate (P4-BSA) (a cell-impermeable form of progesterone) (Gaetjens & Pertschuk, 1980), lipopolysaccride (LPS, Cat# L2262, a MEK 1/2 activation positive control ), Forskolin (Cat#F3917, a PKA activation positive control), and dihydrochloride hydrate (H89) (Cat#B1427, a PKA inhibitor) were from Sigma Chemical Co (St. Louis, MO). PD98059 (Cat# 9900, a MEK1/2 inhibitor) and LY294002 (Cat#99901, a PI3K inhibitor) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal anti-IL1B (Cat#ab9722) was from Abcam (Cambridge, MA). Antibodies to GAPDH (Cat#5174), CREB (cAMP responsive element binding protein, Cat#9197), phospho-CREB (Cat#9198), MEK (Cat#9126), phospho-MEK (Cat#9154), p38 (Cat#9212) and phospho-p38 (Cat#9211) were from Cell Signaling Technology. Antibodies to ERK (Cat#sc-135900), phospho-ERK (Cat#sc-7383), and HRP-conjugated anti-Rabbit (Cat#sc-2030) and anti-mouse (Cat#sc-2031) secondary antibodies were from Santa Cruz Biotechnology (Dallas, Texas).
Cell culture
Mouse RAW 267.4 macrophage cells from American Type Culture Collection (ATCC, Rockville, MD) were maintained from passage 5 to 25 in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% FBS, 1% L-glutamine, 1% penicillin and streptomycin in a humidified incubator with 5% CO2 at 37°C to 80–90% confluence. For treatment, RAW 264.7 cells were cultured in duplicate in 12-well plates (for RNA and signal transduction experiments) or 100 mm Petri-dishes for protein extraction for 24 hours before being subjected to serum-free medium with 1% L-glutamine and 1% penicillin and streptomycin overnight. Cells were then pre-treated with either a PKA inhibitor H89 (3.0×10−5 mol/l), a MEK1/2 inhibitor PD98059 (2.0×10−5 mol/l) or a PI3K/Akt inhibitor LY294002 (1.0×10−5 mol/l) for one hour in fresh serum-free medium followed by incubation with control (medium, or medium plus vehicle) or P4BSA (1×10−7 mol/l) for two mins, 15 mins or 30 mins for signal transduction experiments and four hours for RNA and protein isolation.
RT-PCR
Total RNA was extracted using TRIzol® Reagent (Life Technologies). RNA concentrations were measured using NANODROP 2000 (Thermo Scientific, Wilmington, DE) and 500 ng of RNA from each sample was used to generate cDNA using qScript™ cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). Messenger RNA (mRNA) expression was determined by semiquantitative real-time polymerase chain reaction (RT-PCR) using an ABI StepOnePlus RT-PCR instrument (Applied Biosystems, Carlsbad, CA). Reactions were performed in duplicate in 10 µl volumes using 1 µl diluted cDNA (5×) and 9 µL mixture of TaqMan Universal Master Mix Reagents (Roche, Branchburg, NJ) and TaqMan Gene Expression Assay for mPRα (Paqr7 , Mm00510958_m1), TNF (Tnf, Mm00443260_g1), IL1B (Il1B, Mm00434228_m1), COX2 (Ptgs2, Mm00478374_m1), iNOS (Nos2, Mm00440502_m1), oxytocin receptor (Oxtr, Mm01182684_m1), cAMP responsive element binding protein 3 (Creb3, Mm00457268_m1), and steroid receptor coactivator-2 (SRC2) (Ncoa2, Mm00500749_m1) (Life Technologies). The cycling conditions were 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. The mRNA level of each gene of interest was normalized against GAPDH (Gapdh, Mm99999915_g1), as the levels of the reference gene (Gapdh) did not differ among treatment groups in our study (data not shown). Gene expression is presented as fold change relative to respective controls and is plotted on a logarithmic scale with base 2.
Western blot
To extract total cell lysates for detection of protein expression, cells at the indicated time points were washed with 1× ice-cold PBS twice in dishes and scraped into conical tubes and again washed with PBS. Cells were then solubilized in cold lysis buffer containing 0.02 mol/l HEPES, pH 7.4, 0.15 mol/l NaCl, 1.0×10−9 mol/l EDTA, 1% Nonident P-40 (IGEPAL-CA-630) supplemented with cOmplete ULTRA protease inhibitors (Roche Applied Science, Mannheim, Germany) on ice for 20 mins. Supernatants were collected after centrifugation at 12,000 × g for 10 mins at 4°C. Protein concentrations were determined using Pierce™ BCA™ Protein Assay kit (Thermo Scientific) and a total of 30 µg of protein lysate was subjected to electrophoresis. For signal transduction studies, cells were washed with 1× PBS twice and then immediately lysed by adding 200 µl of 2× SDS sample buffer with 10% 2-Mercaptoethanol and kept on ice for 10 min. The suspension was sonicated for 20 s to shear DNA and to reduce the sample viscosity. Samples were heated at 95–100°C for 5 mins. After being cooled on ice for 2 mins, the samples were centrifuged for 2 min at 12,000 × g before 10 µL of supernatant was loaded into each well of 4–12% precast SDS-PAGE gels (Life Technologies) and transferred to PVDF membranes using a semi-dry transfer system (Bio-Rad, Hercules, CA). The membranes were blocked with 5% non-fat milk (NFM, Bio-Rad) in Tris-buffered saline (TBS, 0.02 mol/l Tris–HCl, 0.137 mol/l NaCl, pH=7.5) with 0.1% Tween-20 (TBST) for an hour on a shaker at room temperature and then probed with appropriate primary antibodies in 5% NFM in TBST overnight at 4°C. The membranes were washed 4× each for 10 mins with TBST and then incubated with secondary antibodies for one hour at room temperature. The chemiluminescent signal was detected using ECL Plus from Amersham (GE Healthcare Life Science, Piscataway, NJ) and captured by a STORM phosphor imager (Molecular Dynamics, Piscataway, NJ). The density of each band was quantified with ImageJ (NIH) and normalized to GAPDH or the respective total protein and presented as fold change to control.
Statistical Analysis
Kruskal-Wallis ANOVA was used to test overall heterogeneity and difference among groups. If significant differences were identified, post-hoc tests were performed by multiple comparisons of means, allowing for non-normality in the data. Adjusted p-values were computed using a bootstrap re-sampling method with step-down tests. Statistical analysis was performed on the SAS 9.3 (Cary, NC) platform, and p values <0.05 were considered statistically significant.
Results
RAW 264.7 cells express mPRα but do not express nuclear progesterone receptors
To verify reports that RAW 264.7 cells lack classical nuclear progesterone receptors (PRs), protein expression of PR-A and PR-B, the two isoforms of PR, was evaluated by western blot. As shown in Figure 1, total cell extracts were used to detect protein levels of PRs in several cell lines. While MCF-7 (a human breast adenocarcinoma cell line) and T47D (a human ductal breast epithelial tumor cell line) cells are known to highly express nPRs (Horwitz et al., 1982; Cho et al., 1994), MDA MB 231 (a human breast adenocarcinoma cell line) cells are known not to express nPRs (Dressing et al., 2011). We detected PR-A and PR-B protein in both MCF-7 and T47D cells, but neither was present in MDA MB 231 or RAW 264.7 cells, confirming the previous reports that RAW 264.7 cells lack nuclear PRs.
Figure 1.
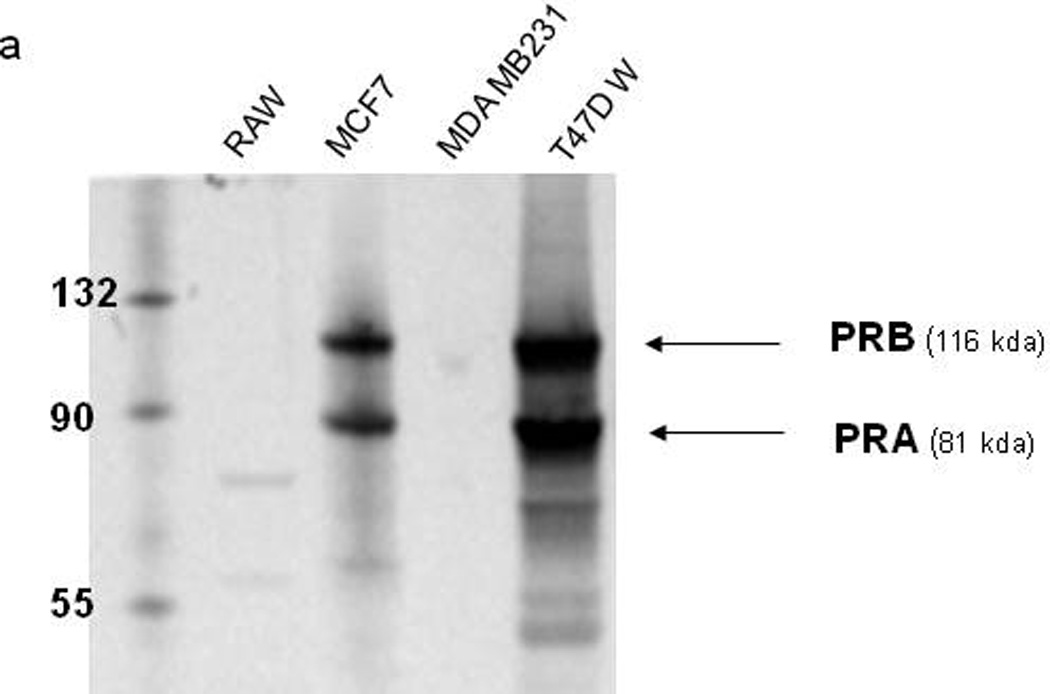
Expression of nuclear progesterone receptors (nPRs) in RAW264.7 cells, MCF-7 cells, MDA-MB-231 cells, and T47D wild type cells. Whole cell lysates were subjected to western blotting analysis to detect the expression of PR-A and PR-B.
Although we were able to detect expression of Paqr7 mRNA in RAW 264.7 cells (see data below), attempts to detect mPRα protein by western blot were not fully confirmed, probably due to the non-specificity of commercially available antibodies (See Supplemental Data Figure 1). Other groups, using a custom-made mPRα antibody generated by Dr. Peter Thomas at University of Texas (Thomas, 2008), have demonstrated that RAW 264.7 cells express mPRα protein (Dressing et al., 2011).
P4BSA-induced mPR activation induces MEK-dependent increases in Il1B and Ptgs2 mRNA expression
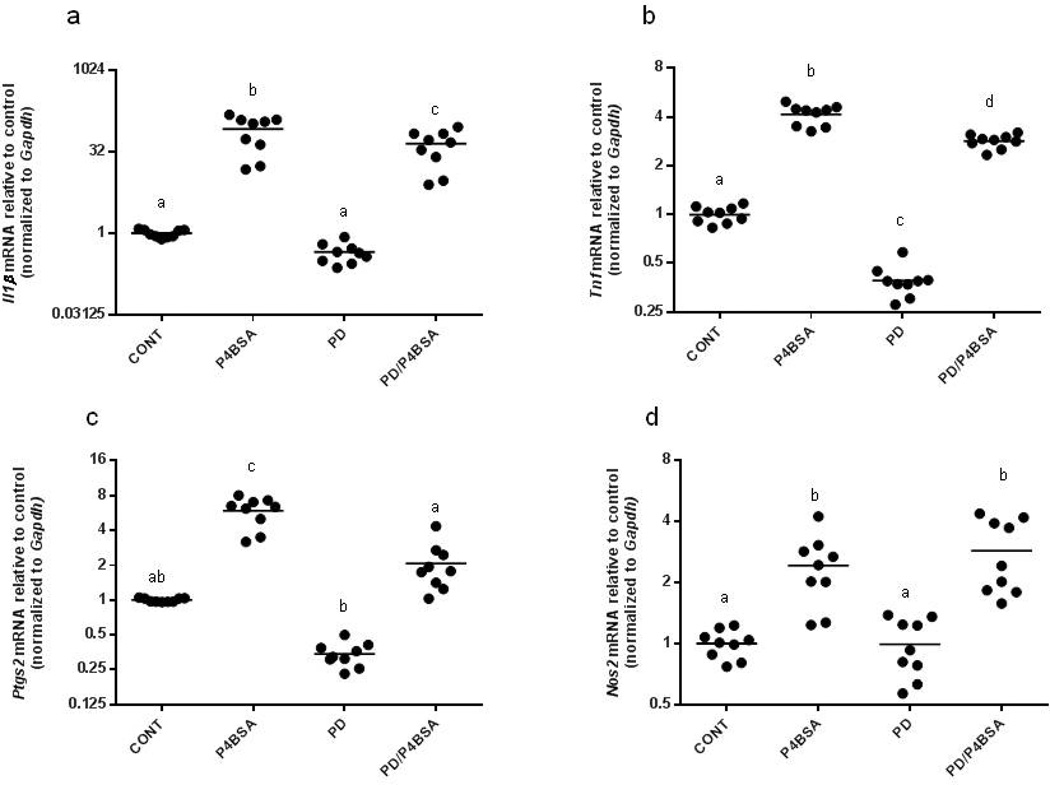
To study the functions of membrane-bound P4 receptors specifically, we used BSA-conjugated progesterone (P4BSA), which is cell-impermeable. Cellular responses to P4BSA were assessed, along with whether these effects are dependent on MEK1/2, signaling components of GPCR pathways. RAW 264.7 murine macrophages were pretreated without or with 2.0×10−5 mol/l PD98059 (a specific inhibitor of MEK1/2) for one hour and then incubated with P4BSA (1.0×10−7 mol/l) for four hours. We found that stimulation of mPRs with P4BSA resulted in significant increases of Il1B, Tnf, Ptgs2 and Nos2 mRNA transcripts (expressed as fold change of untreated controls; Fig. 2 a–d), indicating a pro-inflammatory role for P4 in RAW 264.7 macrophage cells. PD98059 alone did not change the expression of Il1B, Ptgs2, and Nos2 mRNA expression (Fig. 2a, c, and d), but significantly reduced the basal expression of Tnf (Fig. 2b). PD98059 pre-incubation followed by P4BSA stimulation significantly reduced P4BSA-induced Il1B, Tnf, and Ptgs2 mRNA expression (Fig. 2a, b, and c), but not Nos2 mRNA expression (Fig. 2d). These data suggest that MEK1/2 activity contributes to P4BSA-induced expression of Il-1B and Ptgs2 and is partially responsible for P4BSA-induced Tnf expression.
Figure 2.
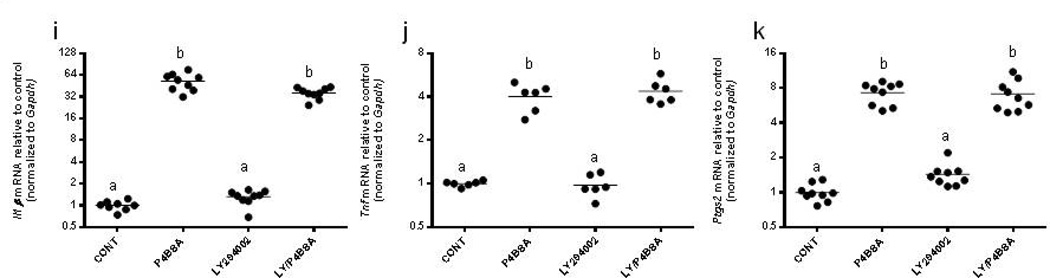
Effects of MEK1/2 inhibition, PKA inhibition or PI3K/Akt inhibition on P4BSA-induced increases in Il1B, Tnf, Ptgs2 and Nos2 gene transcripts. RAW264.7 cells were pretreated in the absence or presence of the MEK1/2 inhibitor PD98059 (2.0×10−5 mol/l) (a–d), the PKA inhibitor H89 (3.0×10−5 mol/l) (e–h) or the PI3K/Akt inhibitor LY294002 (1.0×10−5 mol/l) (i–k) for one hour and then stimulated with or without 1.0×10−7 mol/l P4-BSA for four hours. Il1B, Tnf, Ptgs2 and Nos2 mRNA expression was evaluated by RT-PCR and normalized to Gapdh mRNA levels. The experiment was repeated six to nine times and the results were expressed as fold changes of untreated controls. Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above indicate significant difference at p<0.05.
P4BSA-induced up-regulation of Il1B and Tnf, but not Ptgs2 or Nos2, is PKA-dependent
Since PKA is one of the main kinases in the cAMP-related signal transduction pathway upon GPCR activation, we evaluated the potential involvement of PKA in P4BSA-induced mPR activation. RAW 264.7 cells were pretreated without or with 3.0×10−5 mol/l H89 (a PKA inhibitor) for one hour and then stimulated with P4BSA (1.0×10−7 mol/l) for four hours. Inhibition of the activity of PKA by H89 completely eliminated the P4BSA-induced increase in Il1B mRNA expression (Fig. 2e). P4BSA-induced Tnf mRNA was significantly reduced, but not completely blocked, by H89 treatment (Fig. 2f). In contrast, inhibition of PKA had no effect on P4BSA-induced Ptgs2 or Nos2 expression (Fig. 2 g–h), although it increased baseline expression of these mRNAs. These results show that PKA is an important regulator of mPR-mediated Il1B and Tnf and baseline Ptgs2 and Nos2 expression.
P4BSA-induced upregulation of Il1B, Tnf, and Ptgs2 mRNA is not dependent on PI3k/Akt signaling
RAW 264.7 cells were pretreated without or with 1.0×10−5 mol/l LY294002, a specific inhibitor of PI3k/Akt, for one hour followed by stimulation with P4BSA (1.0×10−7 mol/l) for four hours. Pharmacological blockade of the PI3k/Akt pathway did not normalize P4BSA-induced Il1B, Tnf, and Ptgs2 mRNA expression (Fig. 2 i–k), indicating that PI3K/Akt signaling does not mediate the mPR-induced up-regulation of these pro-inflammatory mediators.
P4BSA-induced IL1B protein expression is dependent on PKA and MEK1/2
To further characterize the involvement of PKA and MEK1/2 in the P4BSA-induced Il1B up-regulation seen in RAW264.7 cells, IL1B protein expression was measured by western blot analysis using whole-cell extracts after treatments. P4BSA administration at 1.0×10−7 mol/l for four hours caused a significant increase of IL1β protein expression (Fig. 3 a–c); Fig. 3a is a representative western blot, and Figs. 3b and 3c contain the relevant densitometric analyses (n=4). Pretreatment with either 3.0×10−5 mol/l H89 or 2.0×10−5 mol/l PD98059 for one hour prior to P4BSA treatment significantly diminished P4BSA-induced IL1B protein expression, confirming the involvement of PKA and MEK1/2 in this pathway.
Figure 3.
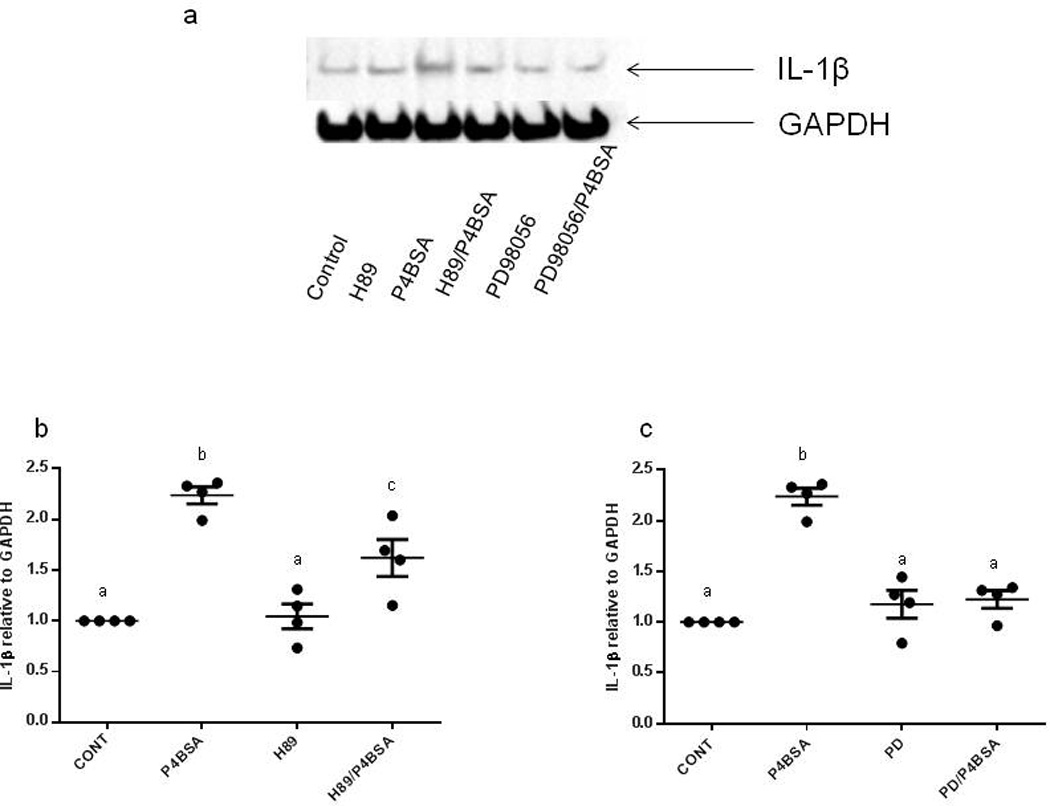
Effect of PKA or MEK1/2 inhibition on P4BSA-induced increases in IL1B protein expression. RAW264.7 cells were pretreated in the absence or presence of PKA inhibitor H89 (3.0×10−5 mol/l) or MEK1/2 inhibitor PD98059 (2.0×10−5 mol/l) for one hour and then stimulated with or without 1.0×10−7 mol/l P4-BSA for four hours. IL-1B expression was examined by western blotting using an anti-IL1B antibody (1:1000 dilution) and normalized to GAPDH levels. The experiment was repeated four times A representative blot is shown (a). The chemiluminescent signals were quantified using ImageJ (NIH) and the results were expressed as fold changes of untreated controls (b, c). Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above indicate significant difference at p<0.05.
Potential downstream targets of MEK1/2 involved in mPR signaling
Since MEK1/2 are activated by serine phosphorylation and, in turn, phosphorylate downstream kinases to initiate the signal cascade (Wortzel & Seger, 2011), we examined the effects of MEK1/2 inhibition on P4BSA-induced phosphorylation of MEK1/2, ERK1/2 and p38. Fig. 4a, Fig. 5a and Fig. 6a are representative blots demonstrating that 1.0×10−7 mol/l P4BSA stimulation for two minutes significantly increases phosphorylation of MEK1/2 at Ser 217/221, but not ERK1/2 at Tyr 202/204 or p38 at Tyr 180/182. Two minutes of 10 ng/mL LPS stimulation induced phosphorylation of MEK, but not ERK1/2 or p38 phosphorylation. P4BSA stimulation for 15 minutes did not increase the phosphorylation levels of either MEK1/2 at Ser 217/221 (Fig. 4b), ERK1/2 (Fig. 5b) at Tyr 202/204 or p38 at Tyr 180/182 (Fig. 6b). The observations after 15 minutes treatment were similar to those after 30 minutes (data not shown). At all three time points P4BSA treatment also did not alter MEK1/2, ERK1/2 or p38 total protein levels. These experiments were repeated five to ten times and the optical densitometry analyses are summarized in Fig. 4 (c–d), Fig. 5 (c–d), and Fig. 6 (c–d), Pretreatment with the MEK1/2 inhibitor 2.0×10−5 mol/l PD98059 significantly suppressed basal phosphorylation of MEK (Fig. 4 a–d) and ERK1/2 (Fig. 5 a–d), but it had no effect on p38 (Fig. 6 a–d) phosphorylation in the absence of a stimulus. The increase of phosphorylation of MEK1/2 at Ser 217/221 by P4BSA treatment for two minutes was prevented by pretreatment of PD98059 (Fig. 4a and 4b). These data together with results displayed in (Figs. 2 and 4) demonstrated that P4BSA-induced phosphorylation of MEK1/2 is involved in P4BSA-induced inflammatory responses.
Figure 4.
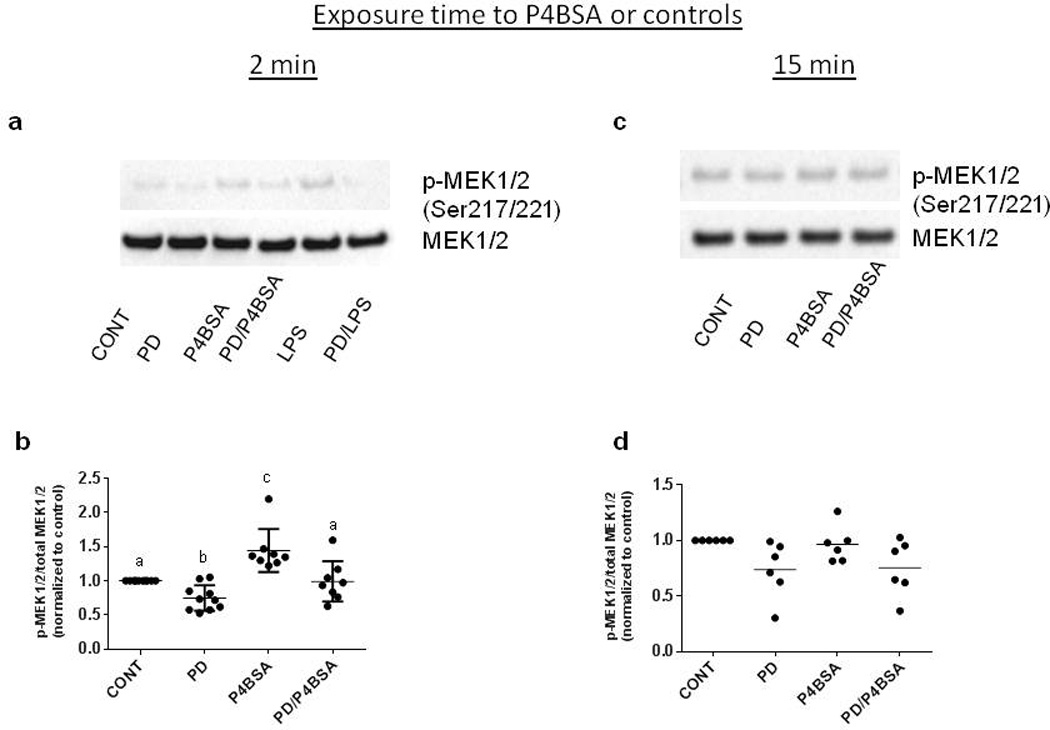
Effects of PD98059 on P4BSA-stimulated activation of MEK1/2 in macrophages. RAW264.7 cells were pretreated in the absence or presence of the MEK1/2 inhibitor PD98059 (2.0×10−5 mol/l) for one hour and then stimulated with or without 1.0×10−7 mol/l P4BSA for two (a and b) or 15 minutes (c and d). Phosphorylation levels of MEK1/2 were examined by western blotting and normalized to total MEK1/2. Representative blots are shown in (a and c). Experiments were repeated six times. The chemiluminescent signals were quantified using ImageJ (NIH) and the results are expressed as fold change of untreated controls (b and d). Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above indicate significant difference at p<0.05.
Figure 5.
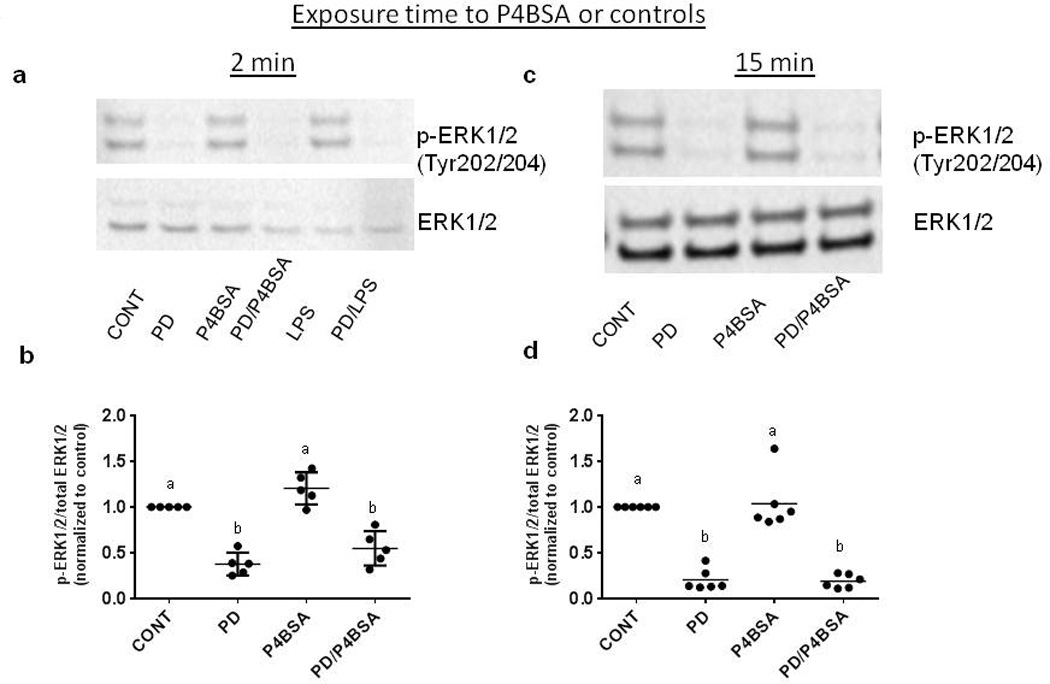
Effects of PD98059 on ERK1/2 phosphorylation in P4BSA-stimulated macrophages. The treatment was similar to what has been described in Fig. 6. Phosphorylation levels of ERK1/2 were examined by western blotting and normalized to total ERK1/2. Representative blots are shown in (a and c). Experiments were repeated six times. The chemiluminescent signals were quantified using ImageJ (NIH) and the results are expressed as fold changes of untreated controls (b and d). Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above indicate significant difference at p<0.05.
Figure 6.

Effects of PD98059 on p38 phosphorylation in P4BSA-stimulated macrophages. The treatment was similar to what has been described in Fig. 6. Phosphorylation levels of p38 were examined by western blotting and normalized to total p38. Representative blots are shown in (a and c). Experiments were repeated six times. The chemiluminescent signals were quantified using ImageJ (NIH) and the results are expressed as fold changes of untreated controls (b and d). Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above indicate significant difference at p<0.05.
Potential targets of PKA in the mPR-activated pathway
Since PKA catalytic subunit alpha (Cα) phosphorylates cAMP-response element binding protein (CREB) on Ser 133 (Gonzalez & Montminy, 1989) and CREB is an essential component of the cAMP signaling pathway in the regulation of Il1B and Ptgs2 transcription (Chandra et al., 1995; Ghosh et al., 2007), we tested whether inhibition of PKA affects CREB phosphorylation in macrophages stimulated with P4BSA. RAW 264.7 cells were pretreated with 3.0×10−5 mol/l H89 for one hour prior to treatment with 1.0×10−7 mol/l P4BSA for two, 15 or 30 mins. Cell lysates were collected and subjected to western blotting to determine the phosphorylation levels of CREB at Serine 133 using total CREB as a loading control. P4BSA significantly increased phosphorylation of CREB at S133 after two minutes of stimulation (Fig. 7a and 7b), but not after 15 minutes stimulation (Fig. 7c and 7d). Inhibition of PKA by H89 did not affect phosphorylation of CREB at any time point. The rapid and transient P4BSA-induced CREB phosphorylation was blocked by pretreatment with H89 (Fig. 7a and 7b). Observations after 15 mins treatment were similar to those after 30 mins (data not shown). These data suggest that the signaling of PKA through CREB might be responsible for the effects of P4BSA on the gene expression of the targets studied.
Figure 7.

Effect of PKA inhibition on CREB phosphorylation in P4BSA-activated macrophages. RAW264.7 cells were pretreated in the absence or presence of PKA inhibitor H89 (3.0×10−5 mol/l) for one hour and then stimulated with or without 1.0×10−7 mol/l P4BSA for two or 15 minutes. Phosphorylation levels of CREB were examined by western blotting using anti-phospho-CREB and normalized to total CREB levels. Representative blots are shown in (a and c). These experiments were repeated six times. The chemiluminescent signals were quantified using ImageJ (NIH) and the results were expressed as fold changes of untreated controls (b and d). The results are expressed as fold changes of untreated controls. Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above would indicate significant difference at p<0.05.
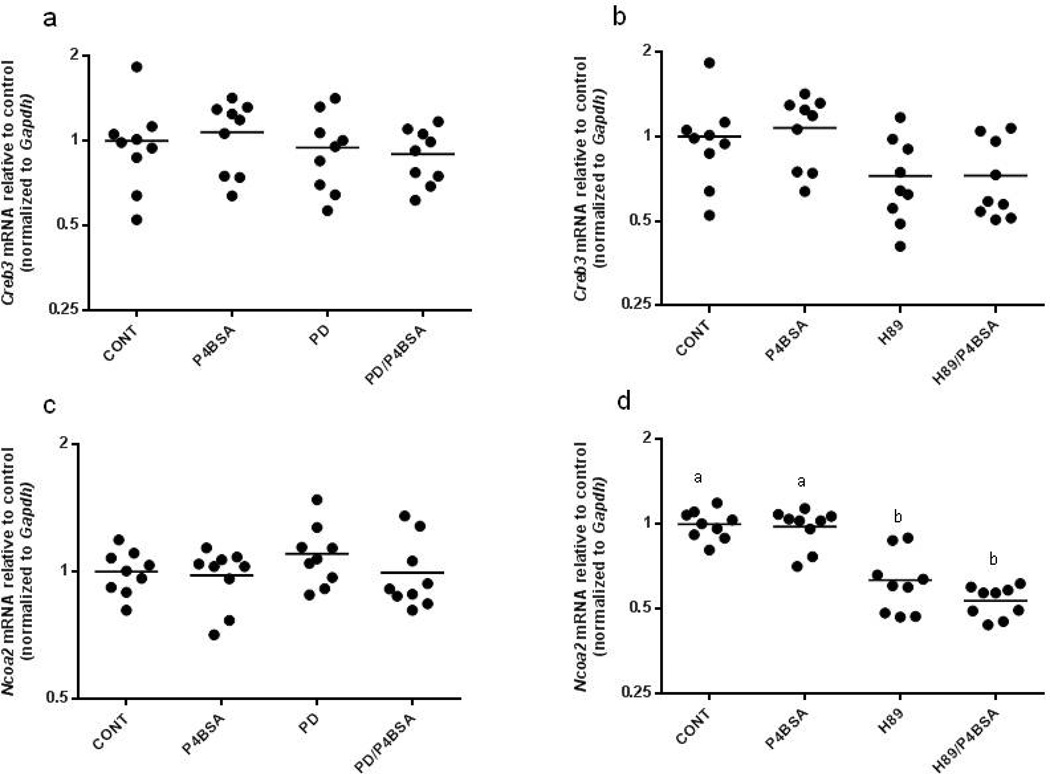
P4BSA did not affect the mRNA levels of the transcription factors Creb3 and Ncoa2
As studies have demonstrated the involvement of CREB in the regulation of Il1B and Ptgs2 transcription (Chandra et al., 1995; Ghosh et al., 2007) and down-regulation of SRC2 (Ncoa2) has been implicated in the effects of mPRs activation in human myometrial cells (Karteris et al., 2006), we determined the effects of mPR activation via P4BSA treatment on Creb3 and Ncoa2 expression in RAW264.7 cells. As shown in Fig. 8 (a and c), pretreatment with 2.0×10−5 mol/l PD98059 did not affect the basal levels of Creb3 and Ncoa2 mRNA expression. Subsequent 1.0×10−7 mol/l P4BSA stimulation also did not affect the transcriptional levels of both genes. Pretreatment of 3.0×10−5 mol/l H89 did not change the expression levels of Creb3 (Fig. 8b), but did significantly decrease the mRNA level of Ncoa2 (Fig. 8d). There was no additional down-regulation of Ncoa2 transcription when PKA inhibition was followed by P4BSA stimulation (Fig. 8d). These data demonstrate that H89 regulates basal mRNA level of Ncoa2.
Figure 8.
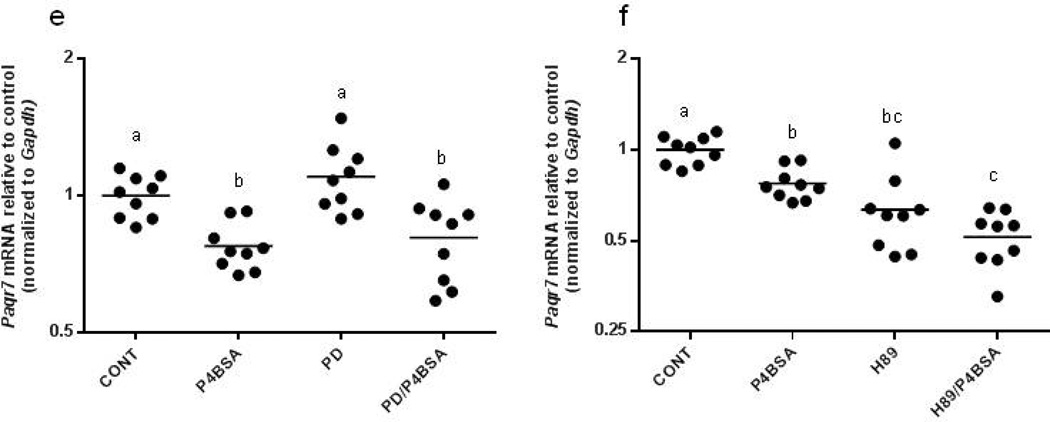
Effects of MEK1/2 inhibition or PKA inhibition on Creb3, Ncoa2, Paqr7 and Oxtr gene expression in P4BSA-treated macrophages. RAW264.7 cells were pretreated in the absence or presence of 2.0×10−5 mol/l MEK1/2 inhibitor PD98059 (a, c, e, and g) or 3.0×10−5 mol/l PKA inhibitor H89 (b, d, f and h) for one hour and then stimulated with or without 1.0×10−7 mol/l P4BSA for four hours. Gene transcripts were evaluated by RT-PCR and normalized to Gapdh mRNA levels. The experiment was repeated nine times and the results were expressed as fold changes of untreated controls. Kruskal-Wallis ANOVA with post-hoc test was performed and groups with different letters above indicate significant difference at p<0.05.
P4BSA down-regulates mPRa (Paqr7) and oxytocin receptor (Oxtr) mRNA expression in RAW264.7 cells
Since ligand-induced down-regulation of receptor mRNA levels is a mechanism to diminish receptor signal (Hadcock & Malbon, 1988) and oxytocin receptor is reportedly down-regulated by P4 through miR-200 (Renthal et al., 2010), we explored the mechanisms through which progesterone might regulate cellular responses in murine macrophages. Paqr7 and Oxtr expression were evaluated by real-time PCR after the administration of the treatments described above. We observed that P4BSA treatment significantly reduced the mRNA expression levels of Paqr7 (Fig. 8 e–f) and Oxtr (Fig. 8g–h). Interestingly, MEK1/2 inhibition (Fig. 8e) did not affect either baseline Paqr7 or P4BSA-induced down-regulation of Paqr7, while PKA blockade (Fig. 8f) resulted in significant down-regulation of Paqr7 expression at baseline. Pretreatment with H89 did not affect P4BSA’s ability to down-regulate Paqr7. In comparison, inhibition of either MEK or PKA alone reduced baseline mRNA levels of Oxtr (Fig. 8g and 8h). Oxtr down-regulation by P4BSA stimulation was not affected by pretreatment with the MEK inhibitor or PKA inhibitor. These data suggest P4BSA induced down-regulation of both Paqr7 and Oxtr expression. PKA regulates steady-state Paqr7 expression whereas both PKA and MEK regulate steady-state Oxtr expression in murine macrophages.
Discussion
During pregnancy, decidual macrophages exhibit an immunosuppressive phenotype that is required to maintain immunological homeostasis and support immune tolerance of the fetus. As the placenta develops, macrophages are recruited around spiral arteries to support vascular remodeling by producing pro-angiogenic factors. At the end of the pregnancy, classically activated macrophages engage in the cervical remodeling process toward the onset of labor (Lee et al., 2012). Therefore, macrophages, among other immune cells, are pivotal for the maintenance of pregnancy and initiation of labor. P4 has both immunosuppressive and immunostimulatory effects on macrophages (Miller & Hunt, 1996), demonstrating the plasticity and versatility of these cells, depending on the biological environment. Despite the reported various effects of progesterone on macrophages, most studies have failed to detect the expression of the so-called classical nPRs in macrophages (Miller & Hunt, 1996; Dressing et al., 2011). On the other hand, recent advances in this area have revealed the existence of mPRs in macrophages (Dressing et al., 2011).This suggests that they might be responsible for progesterone’s action in these immune cells.
In this study, we demonstrate that activation of a putative family of mPRs by a cell-impermeable form of P4 resulted in a pro-inflammatory profile in murine macrophages. This cell-impermeable form of P4 induced robust increases in the mRNA expression of pro-inflammatory markers such as Il1B, Tnf, Ptgs2 and Nos2. Both PKA and MEK1/2 are involved in regulating the mRNA and protein expression of Il1B. MEK1/2 also regulates Tnf and Ptgs2 transcription. P4BSA-stimulated Tnf mRNA production is also mediated by PKA. Furthermore, P4 regulates mRNA expression of one of its own receptors, Paqr7, and of Oxtr, thus providing potential mechanisms by which P4 affects cellular events in macrophages.
The roles of membrane progesterone receptors in pregnancy and labor are diverse and complex. These roles are almost certainly tissue-, cell type-, and gestational age-specific. Pro-inflammatory actions of progesterone have been shown in other cell types (i.e. other than the macrophages we studied). For example, exposure to progesterone activates a wide array of genes involved in several biological processes, including cell adhesion, cell survival and inflammation in the mammary gland (Santos et al., 2009). Feng et al. (Feng et al., 2014) demonstrated diminished progesterone receptor membrane component-1(PGRMC1) at the rupture site among preterm prelabor rupture of membranes (PPROM) subjects compared to preterm non-labor or term-non-labor subjects. They deduced from the data that PGRMC1 functions to maintain fetal membrane integrity based on the previous studies that nuclear progesterone receptor expression is negative in the chorion and amnion. Our study was in agreement with the previous studies (Mackler et al., 1999; Thomson et al., 1999; Shynlova et al., 2013) which demonstrate infiltration of macrophages to decidua and cervix and the inflammatory responses associated with the infiltration might be the keys for the initiation of labor. The uterus also undergoes a switch from quiescence to contraction near term due to a proposed functional withdrawal of progesterone (Tan et al., 2012). In this model, classical anti-inflammatory nuclear progesterone receptor (PR)-B is down-regulated and PR-A (pro-inflammatory) is increased at the onset of labor. The net results of this altered expression of PRA/PRB in uterus are thought to be increased expression of pro-inflammatory factors and contraction-associated proteins to promote labor. Therefore our results in macrophages stimulated by P4BSA are in agreement with the overall concept that labor is an inflammatory event in which macrophages play an active role. Furthermore, we provide evidence that not the classical nuclear PRs, but mPRs are responsible for the action of progesterone on macrophages.
Actions for mPRs activated specifically by P4BSA have been described in recent years. Blackmore et al. demonstrated that P4 covalently linked to BSA is capable of eliciting Ca2+ influx in human sperm. The conclusion drawn from the study is that there is a receptor that is most likely present in the plasma membrane of the spermatozoa and that the P4-binding site resides in the extracellular portion of the receptor (Blackmore et al., 1991). In a cell model system of human parafollicular cells, P4-BSA increases the release of calcitonin through activation of adenylyl cyclase and PKA (Lu & Tsai, 2007). Flock et al. (Flock et al., 2013) reported that activation of mPRs by P4BSA is responsible for the increased secretion of glucagon-like peptide-1 in enteroendocrine cells in vitro and improvement in glucose tolerance in vivo. Our study, using a murine macrophage cell line expressing at least one of the mPRs, mPRα, but not expressing classical nuclear progesterone receptors, is consistent with the above-mentioned studies and suggests that the site of progesterone’s action in macrophages is the plasma membrane.
Several lines of evidence suggest that mPRs have functional characteristics of GPCRs in fish oocytes (Zhu et al., 2003), MDA-MB-231 (human breast adenocarcinoma) cells transfected with mPRα (Zhu et al., 2003; Kelder et al., 2010), human myocytes (Sulke et al., 1985), human T lymphocytes and Jurkat T cells (Dosiou et al., 2008) (Ndiaye et al., 2012). However, others question the subcellular localization and signal coupling of this family of receptors (Fernandes et al., 2008; Smith et al., 2008). To date, the seven-transmembrane-domain topology, a hallmark of the GPCR, has never been conclusively demonstrated in mPRs, but is deduced from the amino acid sequence of mPRs. Furthermore, there is a large deviation from canonical G-protein-induced signaling pathways downstream of activation of mPRs. Although early reports indicated that P4 can promote ERK1/2 phosphorylation in fish oocytes and MDA-MB-231 cells transfected with mPRα (Zhu et al., 2003), in human myometrial cells both progesterone and BSA-conjugated progesterone did not increase the phosphorylation levels of ERK1/2 (Karteris et al., 2006). P4BSA was also reported to induce phosphorylation of p38 MAPK in human myometrial cells (Karteris et al., 2006), but when Krietsch et al. (Krietsch et al., 2006) stably expressed human, sea trout, and Fugu mPRα in HEK293 and MDA-MB-231 cells, progesterone administration did not decrease cAMP levels or increase phosphorylation levels of ERK and p38. In the present study, P4BSA induces a rapid and transient phosphorylation of MEK1/2 and CREB, but not ERK and p38, demonstrating that MEK1/2 and CREB/PKA are two of the signaling components involved in the pathways triggered by activation of mPRs in macrophages.
Down-regulation of GPCRs through ligand binding (a form of negative feedback) is one of the distinctive characteristics of this large family of receptors (Hadcock & Malbon, 1988). The diminished steady-state level of GPCR expression following prolonged treatment with an agonist is the combined result of increased degradation and decreased synthesis as a consequence of a decrease in the level of its mRNA (Drake et al., 2006) . Our data suggest that reduced expression of Paqr7 at four hours may be one of the adaptive mechanisms that function to desensitize macrophages to the inflammatory environment, representing a potential negative feedback mechanism for immune cells to withstand further inflammatory insults. Further study using prolonged stimulation with P4-BSA is warranted to test whether the down-regulation of Paqr7 parallels diminishing pro-inflammatory responses in these macrophages and whether this down-regulation is in concert with a global functional withdrawal of progesterone in myometrium prior to labor.
Oxytocin induces uterine contraction in labor (Fuchs et al., 1984), and increased Oxtr mRNA expression in the uterus occurs prior to delivery in many species (Murata et al., 2000). Progesterone blocks both preterm labor and the associated increase in myometrial Oxtr expression in ovariectomized rats (Ou et al., 1998), suggesting a role for P4 in regulating Oxtr mRNA expression. In fact, Renthal et al. demonstrated that P4 through binding to its nPRs directly up-regulated zinc finger E-box binding homebox protein 1 and suppression of miR-200b/429, which in turn resulted in the down-regulation of contraction-associated genes such as connexin 43 and Oxtr in immortalized human myocytes (Renthal et al., 2010). Other studies also revealed that P4 suppressed Oxtr mRNA expression in endometrial epithelial cells (Kombe et al., 2003) and bovine lymphocytes (Ndiaye et al., 2008). RAW 264.7 macrophages express OXTR (Szeto et al., 2008) and in our study we demonstrated that extracellular P4 down-regulates the expression of Oxtr in these murine macrophages. Further studies are needed to study the physiological significance of hormonal regulation of Oxtr expression in macrophages within the context of reproductive systems.
In summary, a non cell-permeable form of P4 elicits pro-inflammatory responses and down-regulates gene transcripts of Paqr7 and Oxtr in macrophages, most likely through binding to mPRs in a process dependent on PKA and MEK1/2. Changes in mPR expression or activation by progesterone may represent a novel pathway that contributes to the regulation of inflammatory responses in macrophages and overall regulation of parturition by progesterone.
Supplementary Material
Acknowledgments
This study is funded by NIH R01HD056118 March of Dimes #21-FY10-202 and Satter Foundation.
Footnotes
Disclosure: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Blackmore PF, Neulen J, Lattanzio F, Beebe SJ. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. The Journal of biological chemistry. 1991;266:18655–18659. [PubMed] [Google Scholar]
- Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. The Journal of clinical investigation. 2013;123:3472–3487. doi: 10.1172/JCI60561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra G, Cogswell JP, Miller LR, Godlevski MM, Stinnett SW, Noel SL, Kadwell SH, Kost TA, Gray JG. Cyclic AMP signaling pathways are important in IL-1 beta transcriptional regulation. J Immunol. 1995;155:4535–4543. [PubMed] [Google Scholar]
- Cho H, Aronica SM, Katzenellenbogen BS. Regulation of progesterone receptor gene expression in MCF-7 breast cancer cells: a comparison of the effects of cyclic adenosine 3’,5’-monophosphate, estradiol, insulin-like growth factor-I, and serum factors. Endocrinology. 1994;134:658–664. doi: 10.1210/endo.134.2.7507831. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–778. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- Csapo A. Progesterone block. The American journal of anatomy. 1956;98:273–291. doi: 10.1002/aja.1000980206. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Giudice LC. Natural killer cells in pregnancy and recurrent pregnancy loss: endocrine and immunologic perspectives. Endocrine reviews. 2005;26:44–62. doi: 10.1210/er.2003-0021. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Giudice LC. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. The Journal of endocrinology. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circulation research. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76:11–17. doi: 10.1016/j.steroids.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. The Journal of steroid biochemistry and molecular biology. 2005;97:389–396. doi: 10.1016/j.jsbmb.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Feng L, Antczak BC, Lan L, Grotegut CA, Thompson JL, Allen TK, Murtha AP. Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM) Placenta. 2014;35:331–333. doi: 10.1016/j.placenta.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Fernandes MS, Brosens JJ, Gellersen B. Honey, we need to talk about the membrane progestin receptors. Steroids. 2008;73:942–952. doi: 10.1016/j.steroids.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Flock GB, Cao X, Maziarz M, Drucker DJ. Activation of enteroendocrine membrane progesterone receptors promotes incretin secretion and improves glucose tolerance in mice. Diabetes. 2013;62:283–290. doi: 10.2337/db12-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. American journal of obstetrics and gynecology. 1984;150:734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- Gaetjens E, Pertschuk LP. Synthesis of fluorescein labelled steroid hormone-albumin conjugates for the fluorescent histochemical detection of hormone receptors. Journal of steroid biochemistry. 1980;13:1001–1003. doi: 10.1016/0022-4731(80)90177-6. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Human reproduction update. 2009;15:119–138. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Garcia GE, Crosby K, Inoue H, Thompson IM, Troyer DA, Kumar AP. Regulation of Cox-2 by cyclic AMP response element binding protein in prostate cancer: potential role for nexrutine. Neoplasia. 2007;9:893–899. doi: 10.1593/neo.07502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Hadcock JR, Malbon CC. Down-regulation of beta-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5021–5025. doi: 10.1073/pnas.85.14.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, Lye SJ, Jones RL. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biology of reproduction. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Mockus MB, Lessey BA. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell. 1982;28:633–642. doi: 10.1016/0092-8674(82)90218-5. [DOI] [PubMed] [Google Scholar]
- Kammerer U, Schoppet M, McLellan AD, Kapp M, Huppertz HI, Kampgen E, Dietl J. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. The American journal of pathology. 2000;157:159–169. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to membrane progesterone receptor alpha (mPRalpha) and to nuclear progesterone receptor: correlation with physicochemical properties assessed by comparative molecular field analysis and identification of mPRalpha-specific agonists. Steroids. 2010;75:314–322. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombe A, Sirois J, Goff AK. Prolonged progesterone treatment of endometrial epithelial cells modifies the effect of estradiol on their sensitivity to oxytocin. Steroids. 2003;68:651–658. doi: 10.1016/s0039-128x(03)00094-1. [DOI] [PubMed] [Google Scholar]
- Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam EW, Huhtaniemi I, Brosens JJ, Gellersen B. Human homologs of the putative G protein-coupled membrane progestin receptors (mPRalpha, beta, and gamma) localize to the endoplasmic reticulum and are not activated by progesterone. Mol Endocrinol. 2006;20:3146–3164. doi: 10.1210/me.2006-0129. [DOI] [PubMed] [Google Scholar]
- Lee Y, Sooranna SR, Terzidou V, Christian M, Brosens J, Huhtinen K, Poutanen M, Barton G, Johnson MR, Bennett PR. Interactions between inflammatory signals and the progesterone receptor in regulating gene expression in pregnant human uterine myocytes. Journal of cellular and molecular medicine. 2012;16:2487–2503. doi: 10.1111/j.1582-4934.2012.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Sun L, Wang Q, Hou Y. Progesterone regulates mouse dendritic cells differentiation and maturation. International immunopharmacology. 2006;6:830–838. doi: 10.1016/j.intimp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Lu CC, Tsai SC. The cyclic AMP-dependent protein kinase A pathway is involved in progesterone effects on calcitonin secretion from TT cells. Life sciences. 2007;81:1411–1420. doi: 10.1016/j.lfs.2007.08.046. [DOI] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biology of reproduction. 1999;61:879–883. doi: 10.1095/biolreprod61.4.879. [DOI] [PubMed] [Google Scholar]
- Mansour I, Reznikoff-Etievant MF, Netter A. No evidence for the expression of the progesterone receptor on peripheral blood lymphocytes during pregnancy. Hum Reprod. 1994;9:1546–1549. doi: 10.1093/oxfordjournals.humrep.a138746. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O’Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. The New England journal of medicine. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. The Journal of clinical endocrinology and metabolism. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci. 2011;18:6–19. doi: 10.1177/1933719110382922. [DOI] [PubMed] [Google Scholar]
- Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life sciences. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- Murata T, Murata E, Liu CX, Narita K, Honda K, Higuchi T. Oxytocin receptor gene expression in rat uterus: regulation by ovarian steroids. The Journal of endocrinology. 2000;166:45–52. doi: 10.1677/joe.0.1660045. [DOI] [PubMed] [Google Scholar]
- Ndiaye K, Poole DH, Pate JL. Expression and regulation of functional oxytocin receptors in bovine T lymphocytes. Biology of reproduction. 2008;78:786–793. doi: 10.1095/biolreprod.107.065938. [DOI] [PubMed] [Google Scholar]
- Ndiaye K, Poole DH, Walusimbi S, Cannon MJ, Toyokawa K, Maalouf SW, Dong J, Thomas P, Pate JL. Progesterone effects on lymphocytes may be mediated by membrane progesterone receptors. Journal of reproductive immunology. 2012;95:15–26. doi: 10.1016/j.jri.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Ou CW, Chen ZQ, Qi S, Lye SJ. Increased expression of the rat myometrial oxytocin receptor messenger ribonucleic acid during labor requires both mechanical and hormonal signals. Biology of reproduction. 1998;59:1055–1061. doi: 10.1095/biolreprod59.5.1055. [DOI] [PubMed] [Google Scholar]
- Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Molecular human reproduction. 2001;7:875–879. doi: 10.1093/molehr/7.9.875. [DOI] [PubMed] [Google Scholar]
- Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunology today. 1997;18:478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- Raghupathy R, Al-Mutawa E, Al-Azemi M, Makhseed M, Azizieh F, Szekeres-Bartho J. Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. Journal of reproductive immunology. 2009;80:91–99. doi: 10.1016/j.jri.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SJ, Aupperlee MD, Xie J, Durairaj S, Miksicek R, Conrad SE, Leipprandt JR, Tan YS, Schwartz RC, Haslam SZ. Progesterone receptor A-regulated gene expression in mammary organoid cultures. The Journal of steroid biochemistry and molecular biology. 2009;115:161–172. doi: 10.1016/j.jsbmb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Lye SJ. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. Journal of cellular and molecular medicine. 2013;17:90–102. doi: 10.1111/j.1582-4934.2012.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Kupchak BR, Garitaonandia I, Hoang LK, Maina AS, Regalla LM, Lyons TJ. Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–1173. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulke AN, Jones DB, Wood PJ. Hormonal modulation of human natural killer cell activity in vitro. Journal of reproductive immunology. 1985;7:105–110. doi: 10.1016/0165-0378(85)90064-6. [DOI] [PubMed] [Google Scholar]
- Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators of inflammation. 2012;2012:967629. doi: 10.1155/2012/967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Chaouat G. Lymphocyte-derived progesterone-induced blocking factor corrects resorption in a murine abortion system. Am J Reprod Immunol. 1990;23:26–28. doi: 10.1111/j.1600-0897.1990.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. Journal of reproductive immunology. 2009;83:60–64. doi: 10.1016/j.jri.2009.06.262. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Szekeres G, Debre P, Autran B, Chaouat G. Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cellular immunology. 1990;125:273–283. doi: 10.1016/0008-8749(90)90083-4. [DOI] [PubMed] [Google Scholar]
- Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. American journal of physiology. Endocrinology and metabolism. 2008;295:E1495–E1501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. The Journal of clinical endocrinology and metabolism. 2012;97:E719–E730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Frontiers in neuroendocrinology. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annual review of immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes & cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. American journal of obstetrics and gynecology. 2007;196:289–296. doi: 10.1016/j.ajog.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.