Abstract
Background
Hematopoietic progenitor cells (HPCs) are mobilized into the peripheral blood (PB) and then sequestered in injured tissue after trauma. Nonselective β-adrenergic blockade (BB) has been shown to cause a decrease in mobilization of HPCs to the periphery and to injured tissue. Given the vast physiologic effects of nonselective BB, the aim of this study is to delineate the role of selective BB in HPC growth and mobilization.
Methods
Rats underwent daily intraperitoneal injections of propranolol (Prop), atenolol (B1), butoxamine (B2), or SR59230A (B3) for 3 days to induce BB. All groups then underwent lung contusion (LC). HPC presence was assessed by GEMM, BFU-E, and CFU-E colony growth both in injured lung and bone marrow (BM). Flow cytometry, using c-kit and CD71, was used to determine mobilization into PB.
Results
LC alone decreased BM HPC growth in all erythroid cell types and increased their number in injured lung (all *p < 0.05). β-Blockade with Prop, B2, and B3 blockades restored BM HPC growth to control levels and decreased HPCs recovered in the injured lung. Similarly, Prop, B2, and B3 blockade prevented HPC mobilization to PB. B1 blockade with atenolol had no impact on HPC growth and mobilization following LC.
Conclusions
Nonselective BB reduced suppression of HPC growth in BM after injury and prevented the mobilization and subsequent sequestration of HPCs in injured tissue. Our data have shown that this effect is mediated through the B2 and B3 receptors. Therefore, after trauma, treatment with selective B2 or B3 blocker may attenuate the BM suppression associated with tissue injury.
Keywords: Bone marrow, Trauma, Mobilization, β-Blockers
Hematopoietic suppression after shock and trauma has been observed both in animal models and humans.1–3 Prolonged bone marrow (BM) dysfunction after severe injury is part of the multiple organ failure seen after trauma, and it is manifest clinically as a persistent anemia despite normal or even elevated erythropoietin levels.4 In addition, the persistent anemia and multiple blood transfusions are believed to be major contributors of morbidity and mortality.5,6 The etiology of BM suppression after injury is multifactorial, and it is associated with impaired growth of hematopoietic progenitor cells (HPCs) and stromal cells within the BM, along with a mobilization of HPCs into the peripheral circulation.7 Shah et al.8 have shown that HPCs mobilize to peripheral blood (PB), sequester in injured tissue, and are likely involved in tissue repair.
The trafficking of HPCs to and from the BM is a complex and highly regulated process that is not fully understood. The sympathetic nervous system, through its BM innervations, has been proposed as a crucial regulator of HPC mobilization.9,10 Granulocyte-colony stimulating factor (G-CSF) promotes HPC mobilization by suppressing the expression of adhesion molecules between the HPCs and the BM matrix.9 Katayama et al.9 found that HPC mobilization following G-CSF injection was significantly reduced following chemical sympathectomy with 6-hydroxydopamine. Despite the evidence supporting the role of the sympathetic nervous system in HPC trafficking, the exact mechanism involved and its role after trauma have not been shown.
Major trauma induces a significant and sustained release of catecholamines, and the activation of β-adrenergic receptors by catecholamines has been shown to induce hypermetabolism, tachycardia, and increased myocardial oxygen demand.11–13 β-Adrenergic blocades (BBs) have beneficial cardiac, metabolic, and immunomodulatory effects.14–16 In addition, BB use has been associated with improved outcomes in both burn and trauma patients.14,17 One potential mechanism, which may account for the improved outcomes with BB in times of stress involves an inhibition of immunomodulation caused by catecholamines.18
Previous work in our laboratory has shown that norepinephrine impacts BM progenitor cell growth and mobilization in a dose-dependent manner and that nonselective BB with propranolol reduced HPC mobilization into the periphery and HPC growth in injured tissue.19,20 However, given the extensive physiologic and immunomodulatory effects of nonselective BB, the aim of this study is to delineate the role of specific β-adrenergic receptors in HPC growth and mobilization.
MATERIALS AND METHODS
Experimental Design
To study the effects of selective β-blockade on HPC growth and mobilization, rats were pretreated with once daily intraperitoneal injections of either nonselective or selective BB for 3 days. Intraperitoneal injection was preferred because of ease of injection and reduced stress associated with sedation and placement of a catheter for long-term intravenous access. Propranolol (Prop) at 10 mg/kg (Sigma-Aldrich, St. Louis, MO) was given as a nonselective BB. For selective BB, atenolol (B1), butoxamine (B2), or SR59230A (B3) were given at 5 mg/kg (Sigma-Aldrich, St. Louis, MO). Study groups consisted of an unmanipulated control (UC), a lung contusion (LC) alone, and after 3 days of pretreatment with a designated BB, each of the experimental groups underwent unilateral LC.
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA), weighing 300 g to 400 g, were housed under barrier-sustained conditions and kept at 25°C with 12-hour light/dark cycles. The rats had free access to water and chow (Teklan 22/5 Rodent Diet W-8640, Harlan Teklad, Madison, WI). All rats were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. The New Jersey Medical School Animal Care and Use Committee approved all animal protocols.
LC
Rats were weighed and anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg). Unilateral LC was performed by using a blast wave of a percussive nail gun (Craftsman 968514 Stapler, Sears Brands, Chicago, IL) applied to a 12-mm small metal plate placed on the right axilla of the rat. This model has been shown to produce a clinically relevant LC as demonstrated by Badami et al.7 Animals were allowed to recover from LC and were killed at 3 hours. The right contused lung and bilateral femurs were harvested for progenitor cell clonogenic assays.
Physiologic Monitoring
Before LC, rats from each of the experimental groups underwent physiologic monitoring using the CODA noninvasive tail-cuff acquisition system (Kent Scientific Corporation, Torrington, CT). Six parameters were measured (systolic pressure, diastolic pressure, mean arterial pressure, heart rate (HR), tail blood volume, and tail blood flow) to determine the effectiveness of BB.
HPC Cultures
BM was obtained by flushing each femur with 5 mL of cold Iscove’s Modified Dulbecco’s Medium (IMDM; Sigma-Aldrich, St. Louis, MO) to ensure cell separation. The right contused lung was mechanically shredded using two 19-gauge needles in 5 mL of cold IMDM. Both lung tissue and femurs were centrifuged at 1500g for 15 minutes and suspended in IMDM containing 10% fetal calf serum (Hyclone Laboratories, Logan, UT). Lung or BM mononuclear cells (2 × 106) were plated in duplicate in IMDM media containing 30% fetal calf serum, 2% bovine serum albumin, 1% methylcellulose, rat growth factor, penicillin/streptomycin (GIBCO, Grand Island, NY), 2 × 10−4 mol/L 2-mercaptoethanol, and glutamine (Cellgro; Mediatech, Herndon, VA). BFU-E and CFU-E cultures were supplemented with 3.6 U/mL rhEpo and 3 U/mL rhIL-3 (Genetics Institute, Cambridge, MA), and CFU-GEMM cultures were supplemented with 2.4 U/mL rhGM-CSF (Genetics Institute, Cambridge, MA). Cultures were incubated at 37°C in 5% CO2. CFU-E colonies were counted at day 7, BFU-E colonies on day 14, and CFU-GEMM colonies on day 18 by an observer who was blinded to the origin of the samples.
Flow Cytometry
Rat PB was collected in heparinized syringes by performing cardiac puncture. Two hundred microliters of the heparinized blood was transferred to 5-mL polypropylene culture tubes (VWR, West Chester, PA) along with 2 mL of prediluted BD Phosflow Lysis/Fix Buffer (BD Pharmingen, San Diego, CA). This solution was incubated in a 37°C water bath for 10 minutes and then centrifuged at 300g for 10 minutes. The supernatant was discarded, and the cells were washed with phosphate buffered saline by centrifugation at 300g for 10 minutes. The cells were resuspended in 100 μL of BD Pharmingen Stain Buffer (FBS; BD Pharmingen, San Diego, CA) and incubated with the primary antibody (4 μL c-Kit) for 30 minutes at 37°C. The primary antibody was c-Kit (C-19) rabbit polyclonal immunoglobulin G (Santa Cruz Biotechnology, Santa Cruz, CA) and fluorochromes included mouse anti-rabbit immunoglobulin G-phycoerythrin (Santa Cruz Biotechnology, Santa Cruz, CA) and fluoroscein isothiocyanate mouse anti-rat CD71 (OX-26; BD Pharmingen, San Diego, CA). The cells were again washed and resuspended in the stain buffer, treated with the secondary antibodies (2 μL phycoerythrin and 10 μL fluoroscein isothiocyanate), and allowed to incubate for 20 minutes in the dark. After a final wash, the cells were transferred to BD Falcon 5-mL polystyrene culture tubes (BD Biosciences, Bedford, MA) in 500 mL of stain buffer and analyzed on a BD FACSCalibur using Summit Mo Flo software (Cytomation, Fort Collins, CO).
Statistical Analysis
All data are expressed as mean ± SD. Statistical analyses were performed using Student’s t test or analysis of variance and the Tukey-Kramer multiple comparisons test where appropriate. A p value of <0.05 was considered significant.
RESULTS
Effectiveness of BB
Tail-cuff parameters of animals pretreated with BB were recorded, and systolic blood pressure (SBP) and HR were measured and compared with UC. Prop treatment produced a significant decrease in both HR and SBP when compared with UC (396 ± 30* and 118 ± 4* vs. 440 ± 32 and 140 ± 8; N = 3–4 rats per group; *p < 0.05). Selective B1 blockade produced a decrease in the HR with little impact on the SBP (411 ± 7 and 138 ± 9, respectively). Selective B2 blockade decreased both HR and SBP, 391 ± 22 and 122 ± 6, similar to what was seen with Prop. However, selective B3 blockade had no effect on either HR or SBP (433 ± 24 and 139 ± 7).
BM Hematopoietic Colony Formation
As demonstrated previously (Sal), LC resulted in approximately a 40% to 50% a statistically significant suppression of CFU-GEMM, BFU-E, and CFU-E growth in BM 3 hours after injury when compared with UC (Fig. 1, A–C). The addition of nonspecific BB with propranolol protected BM HPC growth. Similarly, pretreatment with a B2 or B3 blocker before LC resulted in a restoration of HPC growth to that of control values throughout all lineages (Fig. 1, A–C). In contrast, animals pretreated with a B1 blocker had ~30% to 50% suppression of CFU-GEMM, BFU-E, and CFU-E growth, which was similar to LC alone.
Figure 1.
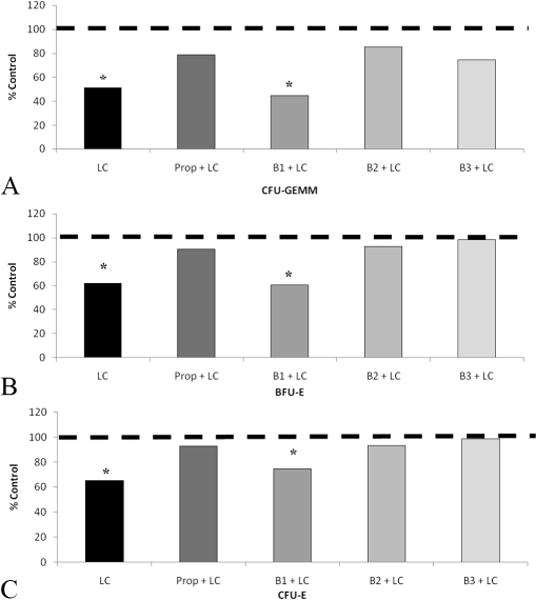
A–C, BM suppression at 3 hours after LC is restored by pretreatment with Prop, B2, and B3 blockades. Similar patterns occur in all HPC types. *p < 0.05 when compared with UC. UC is represented by dashed line. N = 5–8 per group.
HPC Growth in Injured Tissue
There is an eight-tenfold increase in HPC growth in injured tissue after LC (Fig. 2, A–C). This statistically significant increase in the homing of HPCs to injured tissue was consistent for CFU-GEMM, BFU-E, and CFU-E cell lines. Rats pretreated with Prop, B2, or B3 blocker before LC resulted in a lack of growth and sequestration of HPCs in injured tissue (Fig. 2, A–C). However, selective BB with B1 blocker, atenolol, resulted in an increase in HPC growth in injured tissue, similar to LC alone.
Figure 2.
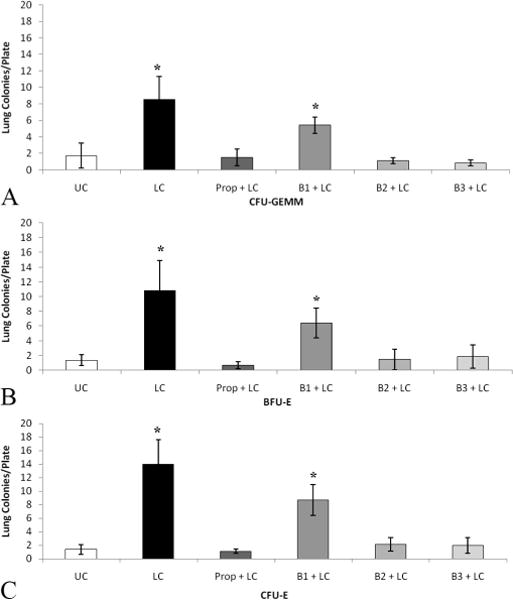
A–C, HPCs sequester in the contused lung at 3 hours, which is prevented by Prop, B2, and B3 blockades. Similar patterns occur in all HPC types. *p < 0.05 when compared with UC. N = 7–11 per group.
HPC Mobilization
To determine whether selective BB prevented the mobilization of HPCs from the BM into the peripheral circulation, we examined the PB using flow cytometry. Cells were labeled with c-kit and CD71, which are markers of early HPCs. In rats subject to LC alone, there was a threefold increase in the mobilization of HPCs into the periphery when compared with the UC (Fig. 3). Pretreatment with Prop, B2, or B3 blocker before LC prevented HPC mobilization into the PB after lung injury (Fig. 3). However, B1 blockade with atenolol, similar to LC resulted in increased HPC mobilization into PB.
Figure 3.
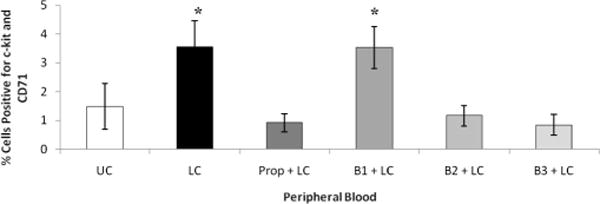
After LC, there is an increase in HPC mobilization into PB. This is prevented by Prop, B2, and B3 blockades. *p < 0.05 when compared with UC. N = 5–9 per group.
DISCUSSION
It has been established that there is normal continuous low-grade flux of HPCs into and out of the BM.21 This mobilization of HPCs into the peripheral circulation has been demonstrated to be centrally mediated and can be induced by various substances, including G-CSF, commonly used for BM transplantation.21–23 Both inflammation and infection have been shown to induce HPC mobilization from the BM.24 HPC mobilization is a multifactorial process that involves both endogenous serine proteases that exist within the BM niches and stimulatory effects that are processed through the sympathetic nervous system.25 Catecholamines not only play a role in HPC mobilization but also affect HPC progenitor cell growth within BM.10,19 Spiegel et al.10 demonstrated that adrenergic stimulation increased early multipotential CD34+ progenitor growth in BM. In contrast, our study demonstrates that after LC, BM erythroid HPC growth is inhibited. These differences may be explained by our previous work19,26 that demonstrates that norepinephrine impacts BM HPC growth in a dose-dependent manner, being inhibitory at severe stress levels. It seems that both the degree and duration of catecholamine stimulation impacts BM HPC growth. Katayama et al.9 demonstrated that there is a direct link with the sympathetic nervous system and HPC egress from BM under normal conditions. Similarly, this study shows that after lung injury, there is increased HPC mobilization into PB. Upregulation of elastase, matrix metalloproteinases, and serine proteases secondary to stress have been shown to lead to HPC mobilization.27
Traumatic injury has been shown to cause a dramatic increase in sympathetic stimulation leading to elevation of urinary norepinephrine and epinephrine levels for up to a week after severe injury.28 At severe stress levels (10−3 mol/L), both epinephrine and norepinephrine completely inhibited BM erythroid progenitor growth in vitro.26 Because severely injured patients are at risk of developing multiple organ failure, of which BM failure is one component, the prolonged persistent hypercatecholamine state is one potential mechanism of BM suppression. In addition to those findings, our previous work has shown that norepinephrine stimulation leads to an increase in mobilization of HPCs into the peripheral circulation as well as an increase in sequestration of HPCs in injured tissue.8,19 Both lung injury alone and hemorrhagic shock have been shown to cause a prolonged and exaggerated mobilization of HPCs into the peripheral circulation as well as injured tissue.4,8 This exaggerated HPC mobilization has been associated with a profound suppression of HPC growth within the BM.4,7,8 This marked suppression of BM function is measured by a decrease in BM HPC growth that lasts for up to 14 days in severely injured trauma patients.4 The data presented here show that pretreatment with propranolol or selective B2 or B3 blocker prevents the early profound suppression of HPC growth within the BM.
The administration of β-adrenergic antagonists leads to the pharmacologic blockade of β-adrenergic receptors, which are categorized into three subtypes, B1, B2, and B3. To ensure adequacy of BB, dosing was given to produce physiologic effect. All BB for this study appeared successful as demonstrated by the observed effects on HR and blood pressure, specific to their receptor interaction. All β-adrenoreceptors are members of the G protein coupled receptors. G proteins activate adenylate cyclase and increase intracellular cyclic adenosine monophosphate. B1 receptors are found mainly in cardiac, kidney, and adipose tissue. B2 receptors are located in the vascular smooth muscle, heart, lung gastrointestinal tract, liver, pancreas, and skeletal muscle. Our selective B2 blocker decreased both HR and blood pressure as B2 receptors can be found not only in vascular smooth muscle but also cardiac tissue. B3 receptors have been demonstrated on adipocytes, and the most potent and selective B3 antagonists belong to a class of aryloxypropanolaminotetralin, of which our B3 antagonist, SR59230A, is the most potent.29 In this study, selective B2 and B3 blockers may be acting either to directly decrease the catecholamine surge, which has been shown to affect BM HPC growth, or by blocking these specific β-adrenergic receptors and preventing HPC mobilization. Each mechanism is a potential cause of posttraumatic BM suppression, and the loss of BM cells may be an important contributor to the development of early organ dysfunction.8 The avoidance of prolonged suppression of BM erythropoietic lineages after trauma may reduce the prolonged anemia seen and therefore avoid the use of blood transfusion.
Blockade of the β-adrenergic receptors have cardiac, vascular, and immunologic effects.30 Although the beneficial cardiovascular effects of BB are clear, their immunomodulating properties and subsequent clinical consequence remain unclear. Administration of norepinephrine and epinephrine to healthy volunteers induced alterations in circulating immune cells via activation of B2 receptors.31 Using B1 selective blocker, there was no specific alteration in cellular immune function.30 Similarly, we demonstrated that with B1 selective blockade, there was no alteration in BM HPC growth and mobilization after LC. Therefore, because B1 receptors are predominantly in cardiac tissue, this may explain their lack of immunomodulatory effect. In a burn injury and sepsis model, Muthu et al.32 provided evidence for the role β-adrenergic receptors in the expansion and differentiation of myeloid committed stem cells and further examined the role of the B2 receptor subtype within the BM microenvironment. Looking at circadian fluctuations of HPC trafficking under steady–state conditions has shown that norepinephrine directly activates B3 adrenergic receptors leading to rhythmic fluctuations of HPCs.21 These findings imply the involvement of specific β-adrenergic receptors at different stages of HPC growth and differentiation.
Our current work uses our LC model, which has been proven to simulate the nonlethal blunt chest trauma that leads to HPC mobilization and sequestration in injured tissue.7 Previously, our lab has demonstrated that given before injury, nonselective BB reduced the suppression of HPC growth in BM after injury and prevented the mobilization and subsequent homing of HPCs to injured tissue.20 This study demonstrates that the selective blockade of either B2 or B3 adrenergic receptors provided a statistically significant decrease in HPC mobilization into the peripheral circulation as well as decreased growth of HPCs in injured lung tissue. Thus, providing evidence that the egress of HPCs from the BM after injury is mediated specifically by B2 and B3 receptors. In addition, we have also demonstrated that the B1 receptor seems to have no role in this process.
BM cells have been shown to be critical for wound healing.8 The findings presented in this study demonstrate that Prop, B2, and B3 blockades prevent the mobilization of HPCs after injury, and previous work with Prop pretreatment has shown that despite a decrease in HPC mobilization, 7 days after LC, the lung tissue has healed.20 Therefore, the mobilization of HPCs after injury appears to represent a range of physiologic response that is based on the degree and timing of trauma and stress that ultimately leads to either normal wound healing or organ dysfunction. Normal wound healing requires the delivery of BM cells to injured tissue in a time and dose-dependent fashion. Norepinephrine has been shown to produce dose-dependent effects of BM HPC growth and HPC mobilization in rats.19 In addition, once cells mobilize to injured tissues, the inflammatory milieu is integral to successful wound healing. Stromal derived factor-1, interleukin-1, tumor necrosis factor-α, and transforming growth factor-β (TGF-β) all play a role in mesenchymal stem cell wound healing. There is an alteration in the stromal derived factor-1 gradient after injury that may play a role in HPC mobilization.33 TGF-β is a potent inhibitor of HPC differentiation and is associated with the development of scarring and fibrosis.34 TGF-β has also been shown to be increased in the BM of trauma patients, and thus may play a role in BM suppression and wound healing.35 From these data, it appears that mobilization of BM cells after injury is necessary for wound healing, however, ongoing stress and daily catecholamine surges drive a sustained mobilization leading to a distant organ dysfunction and abnormal wound healing that may potentially be abrogated by selective B2 or B3 blockade.
In summary, the results of this study demonstrate that pretreatment with propranolol or selective B2 or B3 blockade before LC inhibits BM suppression and prevents HPC mobilization and sequestration in injured tissue. In addition, the mobilization of HPCs after injury is mediated by the B2 and B3 adrenergic receptors. Therefore, the use of B2 or B3 blocker after severe trauma may minimize BM suppression and allow for more coordinated wound healing.
Acknowledgments
Supported by the National Institutes of Health grant K08 NIH GM078304 and the Clowes American College of Surgeons/American Association for the Surgery of Trauma Award.
Footnotes
Presented at the 68th Annual Meeting of the American Association for the Surgery of Trauma, October 1–3, 2009, Pittsburgh, Pennsylvania.
References
- 1.Livingston DH, Gentile PS, Malangoni MA. Bone marrow failure after hemorrhagic shock. Circ Shock. 1990;30:255–263. [PubMed] [Google Scholar]
- 2.Mohr AM, Upperman JS, Taneja R, Wang MT, Rameshwar P, Livingston DH. Differential effects of acute hypoxia and endotoxin on the secretion and expression of bone marrow interleukin-1 and interleukin-6. Shock. 1997;7:324–331. doi: 10.1097/00024382-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Fontes B, Moore FA, Moore EE, et al. Gut ischemia induces bone marrow failure and increases risk of infection. J Surg Res. 1994;57:505–509. doi: 10.1006/jsre.1994.1176. [DOI] [PubMed] [Google Scholar]
- 4.Livingston DH, Anjaria D, Wu J, et al. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandromme MJ, McGwin G, Jr, Marques MB, Kerby JD, Rue LW, III, Weinberg JA. Transfusion and pneumonia in the trauma intensive care unit: an examination of the temporal relationship. J Trauma. 2009;67:97–101. doi: 10.1097/TA.0b013e3181a5a8f9. [DOI] [PubMed] [Google Scholar]
- 6.Claridge JA, Sawyer RG, Schulman AM, McLemore EC, Young JS. Blood transfusions correlate with infections in trauma patients in a dose-dependent manner. Am Surg. 2002;68:566–572. [PubMed] [Google Scholar]
- 7.Badami CD, Livingston DH, Sifri ZC, et al. Hematopoietic progenitor cells mobilize to the site of injury after trauma and hemorrhagic shock in rats. J Trauma. 2007;63:596–602. doi: 10.1097/TA.0b013e318142d231. [DOI] [PubMed] [Google Scholar]
- 8.Shah S, Ulm J, Sifri ZC, Mohr AM, Livingston DH. Mobilization of bone marrow cells to the site of injury is necessary for wound healing. J Trauma. 2009;67:315–322. doi: 10.1097/TA.0b013e3181a5c9c7. [DOI] [PubMed] [Google Scholar]
- 9.Katayama Y, Battista M, Kao W, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel A, Shivtiel S, Kalinkovich A, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 11.Bryan RM., Jr Cerebral blood flow and energy metabolism during stress. Am J Physiol. 1990;259:H269–H280. doi: 10.1152/ajpheart.1990.259.2.H269. [DOI] [PubMed] [Google Scholar]
- 12.Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–492. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman TM, Horton JW. Characterization of cardiac beta-adrenergic receptors in the guinea pig heart: application to study of beta-adrenergic receptors in shock models. J Surg Res. 1993;55:516–523. doi: 10.1006/jsre.1993.1177. [DOI] [PubMed] [Google Scholar]
- 14.Auerback AD, Goldman L. Beta-blockers and reduction in cardiac events in non-cardiac surgery: clinical applications. JAMA. 2002;287:1445–1447. doi: 10.1001/jama.287.11.1445. [DOI] [PubMed] [Google Scholar]
- 15.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 16.Friese RS, Barber R, McBride D, Bender J, Gentilello LM. Could beta blockade improve outcome after injury by modulating the inflammatory profiles? J Trauma. 2008;64:1061–1068. doi: 10.1097/TA.0b013e3181684cf0. [DOI] [PubMed] [Google Scholar]
- 17.Arbabi S, Campion EM, Hemmila MR, et al. Beta-blocker use is associated with improved outcomes in adult trauma patients. J Trauma. 2007;62:56–62. doi: 10.1097/TA.0b013e31802d972b. [DOI] [PubMed] [Google Scholar]
- 18.Oberbeck R, van Griensven M, Nickel E, Tschernig T, Wittwer T, Pape HC. Influence of beta-adrenoceptor antagonists on hemorrhage-induced cellular immune suppression. Shock. 2002;18:331–335. doi: 10.1097/00024382-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ulm JP, Mohr AM, Sifri ZC, et al. The role of norepinephrine in progenitor cell mobilization to sites of injury. Shock. 2008;29S:11. [Google Scholar]
- 20.Beiermeister K, Brett K, Patel P, et al. The effect of beta blockade on progenitor cell growth and mobilization. Shock. 2009;31S:7. [Google Scholar]
- 21.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 22.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiologic migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 23.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 24.Papayannopoulou T. Current mechanistic scenarios is hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- 25.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem/progenitor cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59:884–890. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 27.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor 1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca RB, Mohr AM, Wang L, et al. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect (Larchmt) 2004;5:385–393. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 29.Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective beta 3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol. 1996;49:7–14. [PubMed] [Google Scholar]
- 30.Oberbeck R, Kobbe P. Beta-adrenergic antagonists: indications and potential immunomodulatory side effects in the critically ill. Curr Med Chem. 2009;16:1082–1090. doi: 10.2174/092986709787581770. [DOI] [PubMed] [Google Scholar]
- 31.Schedlowski M, Hosch W, Oberbeck R, et al. Catecholamines modulate human NK cell circulation and function via spleen-independent beta 2-adrenergic mechanisms. J Immunol. 1996;156:93–99. [PubMed] [Google Scholar]
- 32.Muthu K, Iyer S, He LK, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keck BM, Beiermeister K, Sifri ZC, et al. Role of SDF-1in homing of progenitor cells to sites of injury. Shock. 2009;31S:86. [Google Scholar]
- 34.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu JC, Livingston DH, Hauser CJ, Deitch EA, Rameshwar P. Trauma inhibits erythroid burst-forming unit and granulocyte-monocyte colony-forming unit growth through the production of TGF-beta1 by bone marrow stroma. Ann Surg. 2001;234:224–232. doi: 10.1097/00000658-200108000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]


