Abstract
In remote ischemic conditioning (RIC) brief, reversible episodes of ischemia with reperfusion in one vascular bed, tissue or organ confer a global protective phenotype and render remote tissues and organs resistant to ischemia/reperfusion injury. The peripheral stimulus can be chemical, mechanical or electrical and involves activation of peripheral sensory nerves. The signal transfer to the heart or other organs is through neuronal and humoral communications. Protection can be transferred, even across species, with plasma-derived dialysate and involves nitric oxide, stromal derived factor-1α, microRNA-144, but also other, not yet identified factors. Intracardiac signal transduction involves: adenosine, bradykinin, cytokines, and chemokines, which activate specific receptors; intracellular kinases; and mitochondrial function. RIC by repeated brief inflation/deflation of a blood pressure cuff protects against endothelial dysfunction and myocardial injury in percutaneous coronary interventions, coronary artery bypass grafting and reperfused acute myocardial infarction. RIC is safe and effective, noninvasive, easily feasible and inexpensive.
Keywords: acute myocardial infarction, coronary artery bypass grafting, myocardial ischemia, reperfusion
Historical background and concept of RIC
Remote ischemic conditioning (RIC) is the intriguing phenomenon whereby brief, reversible episodes of ischemia and reperfusion applied in one vascular bed, tissue, or organ confers global protection, rendering remote tissues and organs resistant to ischemia/reperfusion injury. Its discovery 2 decades ago in heart (1) was not serendipitous, but evolved from a mathematical model developed by Whittaker and Przyklenk, in which brief episodes of preconditioning ischemia in one coronary bed were predicted to trigger activation, release, or transport of one or more unknown “protective factors” throughout the myocardium (2–4). To test this hypothesis, anesthetized dogs underwent 4 episodes of 5 min ischemia applied in the left circumflex coronary territory, followed by a 1-h sustained ischemic insult in the left anterior descending coronary artery bed. As anticipated, compared with controls subjected to left anterior descending occlusion alone, animals that received brief antecedent episodes of circumflex occlusion before sustained left anterior descending occlusion displayed a robust reduction of infarct size, (1).
Evolution of the paradigm
Although this first report of “intracardiac” RIC was provocative and met with considerable skepticism (4), the concept also engendered curiosity and raised the question: can the RIC paradigm be extrapolated to other remote triggers?
Spatial evolution: from intracardiac to interorgan RIC
During the past two decades, multiple variations on the theme of RIC have been investigated, encompassing both in vitro and in vivo models. Cardioprotection by collection and transfer of perfusate among isolated buffer-perfused hearts is a notable example (5–8). Specifically, coronary effluent released from donor rabbit hearts throughout a standard, conventional preconditioning stimulus (3 cycles of 5 min global ischemia with 10 min reperfusion) or a time-matched control period was collected, reoxygenated, warmed, and used as the perfusate for 2 cohorts of naïve, acceptor hearts. All 4 groups of hearts then underwent 40 min of sustained global ischemia. Infarct sizes were significantly smaller in both donor hearts subjected to brief preconditioning ischemia, and naïve acceptor hearts that received the effluent from preconditioned donors, versus donor and acceptor controls. There was no difference in the magnitude of the infarct-sparing effect seen in donor-preconditioned and acceptor-preconditioned groups, implying that the efficacy of cardioprotection triggered by RIC was comparable to that achieved by conventional ischemic preconditioning (5). This general strategy, involving transfer of effluent or perfusate has been refined to include collection of serum following brief preconditioning ischemia applied in vivo and its administration to either isolated hearts or cultured cells subjected to a sustained ischemic or hypoxic insult (9–11). This strategy also provided evidence of cross-species protection by RIC, including treatment of isolated buffer-perfused rabbit hearts with human serum (9,11).
It could be argued that intracardiac RIC or cardioprotection achieved by transfer of perfusate between hearts are laboratory curiosities providing mechanistic insight, but of limited translational relevance. Accordingly, the observation of interorgan RIC was a pivotal preclinical advance (12). Initial evidence revealed that brief episodes of ischemia/reperfusion in kidney and mesentery rendered the heart resistant to infarction (12–15). Moreover, a number of studies documented RIC-induced attenuation of ischemia/reperfusion injury in brain, lungs, liver, kidney, intestine, skin, and other tissues (reviewed in (16)). However, the first reported seminal extension of interorgan RIC in a clinically-relevant, large animal (swine) model (17) demonstrating that brief episodes of peripheral limb ischemia, achieved by simple inflation/deflation of a standard blood pressure cuff on one or more limbs, was sufficient to evoke a profound reduction in myocardial infarct size accelerated subsequent implementation of phase II trials aimed at establishing efficacy in patients (17).
Conceptual evolution: from ischemic to non-ischemic triggers
In the aforementioned studies, intercardiac and interorgan RIC were (by definition) initiated by a brief ischemic stimulus. However, accumulating evidence from a spectrum of in vivo and in vitro models (some involving perfusate transfer among models) suggests that transient ischemia or interruption of blood flow is not a requisite trigger for remote protection. Multiple alternative triggers capable of recapitulating the infarct-sparing effect of RIC have been proposed, including peripheral nociception (initiated by skin incisions made on the abdomen and termed “remote preconditioning of trauma”), direct peripheral nerve stimulation, and noninvasive transcutaneous nerve stimulation and electroacupuncture (18–23). Perhaps the most attractive, for its potential as a clinical cardioprotective strategy, is nontraumatic peripheral nociception instigated by chemical stimulation of sensory C-fibers in the skin (18,21): A >70% reduction in infarct size was reported in mice treated with 0.1% capsaicin cream, applied topically to a 2 cm2 area of skin along the abdominal midline 15 min before the onset of coronary artery occlusion, compared with untreated controls (18). In spite of its inherent appeal, this concept has not yet been translated to clinical investigation.
Temporal variants: remote preconditioning, preconditioning and postconditioning
In all studies discussed thus far, the remote conditioning stimulus was administered prophylactically in the ~30 to 40 min period before the onset of sustained myocardial ischemia. However, pretreatment is not a requirement for RIC-induced cardioprotection: reduction of infarct size has also been described with concurrent application of the remote ischemic stimulus during sustained coronary occlusion (remote ischemic perconditioning) or at the time of reperfusion (remote ischemic postconditioning) (24,25).
The first documentation of infarct size reduction with remote perconditioning utilized brief renal ischemia/reperfusion as the trigger, applied during the final minutes of coronary artery occlusion (26). This approach provided proof-of-principle, but has obvious practical limitations as therapy. However, evidence from the swine model demonstrated a significant infarct-sparing effect of four 5 min cycles of intermittent limb ischemia administered during a 40 min period of left anterior descending coronary occlusion (27), providing the rationale for the landmark clinical trial in which limb ischemia was applied during transport of patients with suspected acute myocardial infarction (AMI) to the hospital (28). Cardioprotection with remote ischemic postconditioning was first demonstrated in swine (29), and subsequently corroborated in other models, including rabbit and rat (8,30). In each case, the protective stimulus was initiated immediately upon relief of the sustained myocardial ischemia.
The preclinical consensus
A general consensus regarding RIC has emerged: with rare exceptions (31), there is consistent evidence among diverse models and species that brief ischemia/reperfusion applied in a remote tissue or organ confers cytoprotection against ischemia/reperfusion injury. When the heart is the target organ, the gold standard of RIC-induced protection is reduction of myocardial infarct size. However, remote ischemic preconditioning (RIPC) protects the myocardium, but also other parenchymal organs (16) and, notably, the vasculature. Endothelial dysfunction from ischemia/reperfusion can serve as a surrogate for studies on cardioprotection by RIC in healthy humans, but it is unclear whether extrapolation from preservation of peripheral vasomotion to cardioprotection is also true mechanistically (32).
Among the multiple variants of RIC, is any option superior for evoking cardioprotection? Interorgan (rather than intracardiac) conditioning, achieved via intermittent limb ischemia or, potentially, via nontraumatic peripheral nociception, is among the more appealing and practical strategies. Studies where peripheral limb ischemia is the RIC stimulus have mostly employed 3 or 4 episodes of 5 min arm and/or leg ischemia interspersed with 5 min reperfusion periods. However, these are empiric choices, the optimal algorithm has not been identified, and it has been postulated that “hyperconditioning” (i.e., an as-yet undefined, excessive number of conditioning episodes) may be deleterious (33,34). With regard to timing, outcomes of the limited number of head-to-head comparisons revealed no apparent difference in efficacy of RIPC, remote preconditioning, and postconditioning (35,36). The paradigms of remote ischemic perconditioning and postconditioning may be particularly relevant, as they expand the potential scope for clinical translation of RIC.
Signal transduction of RIC
Neuronal signal transfer from the remote organ to the heart
Signal transduction to the heart from the remote organ where the RIC protocol initiates protection appears to involve the somatosensory system, the spinal cord, and the autonomous nervous system. The stimulus can originate not only from local ischemia/reperfusion injury in an organ other than the heart (e.g., mesentery (12,14,37) or limb (35,38–42)), but also from local surgical trauma (18–20,41), local activation of sensory fibers by capsaicin (18,21,35), bradykinin (14,20), or adenosine (39), and local electrical nerve stimulation (21,23). Accordingly, local anesthesia with lidocaine (18) or a sensory nerve blocker (21) and transection of the peripheral nerve (21,35,39,40) abrogated protection by RIPC, although femoral nerve transection did not abrogate protection by limb RIPC in mice in one study (42).
The local release mechanism in response to a nociceptive stimulus involves protein kinase Cγ in rats (20) and is inhibited by a nitric oxide donor (39). Whereas the causal involvement of peripheral nociceptive sensory nerves is unequivocal, the nature and transfer to the heart of the released transmitter molecule through neuronal or humoral pathways remains ambiguous. A blood-derived dialysate was able to transfer protection to a recipient bioassay heart after local peripheral adenosine or capsaicin administration, peripheral nerve stimulation, or RIPC (21,39), supporting the notion of humoral transfer of a neuronally-released signal molecule. This is also suggested by studies in humans, where the dialysate from diabetics after RIC provided protection only in the absence of diabetic neuropathy (11).
Abrogation of protection by RIPC with spinal cord transection at T7-T10 (18,40) or intrathecal spinal opioid receptor blockade with naloxone (41) and infarct size reduction by spinal cord stimulation by C8-T2 (43) favor a spinal reflex response. The efferent pathway appears to involve the autonomous nervous system. The ganglionic blocker, hexamethonium, abrogated protection by local bradykinin administration or RIPC in most (12,14,18), but not all (38) studies. Another ganglionic blocker, trimetaphan, also abrogated RIPC’s protection from ischemia/reperfusion-induced endothelial dysfunction in humans (44). Cardiac sympathetic nerves are involved in attenuation of the observed infarct size reduction upon spinal cord stimulation, and this effect is attenuated by the α1-blocker, prazosin, and the β-blocker, timolol (43). Another β-blocker, propranolol, also abrogated protection by peripheral surgical trauma (18). Vagotomy (35,40) or atropine (40,45) abrogated the protection by limb preconditioning (35,40).
In conclusion, local injury during remote organ preconditioning activates nociceptive fibers, which release an unidentified molecule into the blood and/or signal through the spinal cord to activate both cardiac vagal and sympathetic efferents to release cardioprotective substances. Most of the previously discussed data originate from rodent models of RIC or from studies with transfer of dialysate to rodent hearts. However, neuronal involvement in protection by RIPC in humans undergoing coronary artery bypass grafting (CABG) or aortic valve surgery is suggested by its abrogation with propofol, but not isoflurane anesthesia (46–48).
Humoral signal transfer from the remote organ to the heart
In early studies of local preconditioning, coronary effluent from a preconditioned heart induced cardioprotection in a naïve acceptor heart (5). The presence of a circulating cardioprotective factor after RIPC was first demonstrated in a porcine transplant model (49), where RIPC of the limb in an acceptor pig provided potent cardioprotection to the subsequently transplanted and denervated donor heart. Subsequent studies confirmed the presence of a circulating element and further characterized the nature of the factor(s). In an isolated rabbit heart model (9), plasma from remotely preconditioned animals was cardioprotective when perfused into an isolated naïve heart. The plasma dialysate using a 15 kDa membrane was similarly cardioprotective. When processed over a C18 column, the small hydrophobic molecule eluate provided potent cardioprotection, along with a protective kinase signature. Importantly, when the dialysate was given to isolated fresh cardiomyocytes (excluding neuronal influence), the resistance of cardiomyocytes to simulated ischemia/reperfusion injury mimicked that of a local preconditioning stimulus. Subsequent animal studies using such Langendorff bioassays confirmed that RIC induced by femoral nerve stimulation, transcutaneous peripheral nerve stimulation, capsaicin, and even electroacupuncture appear to work, at least in part, via release of cardioprotective factors into the blood (21–23,39).
Langendorff bioassays have also been used to test for the presence of circulating cardioprotective factors in human RIC. Depending on whether peripheral neuropathy was present, dialyzed plasma from diabetic patients subjected to RIC had differential responses, confirming interaction between the neural and humoral components of remote conditioning, (11). While plasma from diabetic patients without neuropathy was highly cardioprotective in naïve acceptor rabbit hearts, patients with peripheral neuropathy failed to provide cardioprotective plasma. Most recently, RIPC had no effect on exercise performance in heart failure patients (50). However, in the isolated mouse heart bioassay, plasma from heart failure patients was cardioprotective at baseline, but provided no additional cardioprotection after RIPC. When the results were stratified for the degree of baseline cardioprotection, those with low baseline cardioprotective activity showed significant improvement in their exercise function after clinical RIPC, suggesting that some patients lacking a pre-existing cardioprotective milieu may benefit from RIPC. Several recent studies identified putative contributors to the humoral response. A recent proteomic study identified multiple potential cardioprotective targets released into the blood after a limb RIC protocol (51). Several specific circulating molecules also were studied in detail: the shear-stress-related release of nitric oxide, secondary to reactive hyperemia induced by transient limb ischemia, is expected to increase plasma nitrite in the blood, which is known to be cardioprotective (52). Conversely, RIPC was inactive in genetically-modified animals deficient in endothelial nitric oxide synthase (42). Pretreatment with the nitrite scavenger sulfanilamide abrogated the cardioprotective effect of plasma obtained after limb RIC in human volunteers, when used to perfuse naïve mouse hearts in a Langendorff bioassay. The clinical effect of nitrite is less certain. The NIAMI (Nitrites in Acute Myocardial Infarction) investigators (53) studied 229 patients with acute ST-segment elevation myocardial infarction (STEMI) randomized to receive an infusion of sodium nitrite or placebo. Nitrite failed to modify either myocardial infarction size or any of the secondary endpoints (e.g., troponin, creatine kinase, left ventricular function), suggesting that the clinical effect of RIC is beyond that of nitrite alone. Stromal-derived factor-1α is a small chemokine that fulfills the criteria for a putative circulating effector (9), and is cardioprotective via its interaction with its chemokine receptor 4 (54). Circulating plasma levels of stromal-derived factor-1α increased in rats subjected to RIPC by limb ischemia/reperfusion, and the cardioprotection of RIPC was partially abrogated by pretreatment of the animals with a specific inhibitor (55). The lack of complete abrogation in this model suggests involvement of other factors.
Finally, a microRNA was recently shown to play a role in the preconditioning effect of transient limb ischemia/reperfusion. MicroRNA-144 levels were increased in mouse myocardium after RIPC and markedly reduced after ischemia/reperfusion injury. In subsequent experiments, the effect of RIC was completely abrogated by the use of a specific antagomir to microRNA-144. Conversely, intravenous microRNA-144 was cardioprotective, both acutely and 3 days after administration. Importantly, microRNA-144 levels were increased in the plasma of mouse and humans subjected to limb RIC. Plasma carriage of microRNAs, to prevent digestion by circulating RNase, has been demonstrated within lipoprotein complexes in association with specific carrier proteins, such as argonaute, and in exosomes (56–59). Interestingly, the total number of exosomes in mouse plasma after RIC did not increase (60), although others observed increased numbers of exosomes following RIC in both rats and humans (61). However, the hairpin precursor of microRNA-144 in the exosome pellet increased 4-fold, and single-stranded microRNA-144 levels increased substantially in the plasma supernatant after RIC. Plasma microRNA-144 colocated with argonaute protein complexes, suggesting this may be the plasma carriage mechanism after release of microRNA-144 precursor from the exosome. While the exosome fraction was not tested for cardioprotective activity in that study, effluent from a preconditioned heart was able to protect a second heart unless microvesicles and exosomes were removed, demonstrating that protection depends upon their presence (62). In summary, more work is required to identify whether microRNA-144, other microRNAs, chemokines, and perhaps undiscovered circulating factors may act either alternately or in concert as the humoral signal transferring protection to the heart. Nitric oxide, stromal-derived factor-1α, and microRNA-144 are clearly humoral transfer signals, but do not fully explain the RIC phenomenon.
Signal transduction of RIC in the heart
The search for signaling molecules/mechanisms of RIC has largely focused on signals identified in local ischemic preconditioning and post-conditioning studies (63,64). Early studies using pharmacological antagonists identified the involvement of adenosine (13,26), bradykinin (14,19,65), opioids (37,66,67), epoxyeicosatrienoic acids (19), reactive oxygen species (66), and ATP-dependent potassium-channels (13,27), but could not dissect whether these molecules/mechanisms were involved in signal generation within the remote organ, transfer of the signal to the heart, cardioprotective signaling in the heart, or any combination of these steps. To attribute signaling to the heart, the signal must be demonstrated to localize in the myocardium or an antagonist must be given in the transfer fluid obtained after a RIC protocol in a donor organism, then administered to an isolated recipient and target heart. However, isolated bioassay hearts contain a number of different cellular compartments, in addition to cardiomyocytes including innervation, vasculature, interstitial cells, and matrix with resident leukocytes/immune cells. Also, most signaling molecules/mechanisms have thus far only been determined in rodent hearts, and translation to larger mammals or humans cannot be taken for granted. With these caveats in mind, there is solid evidence for a causal involvement of the ligands adenosine (10,68), bradykinin (18), interleukin 10 (in delayed RIPC) (69) and stromal-derived factor-1α (55) in the heart. Adenosine acts on its A1 receptor, which, in turn, interacts with δ and κ opioid receptors (68); bradykinin acts on its B2 receptor (18), and stromal-derived factor-1α acts on chemokine receptor 4 (55). Adenosine receptor activation results in improved mitochondrial function, evidenced by better respiration and reduced formation of reactive oxygen species (10). Bradykinin B2 receptor activation results in protein kinase Cε activation (18). The action of interleukin 10 results in increased phosphorylation of protein kinase B (Akt) and endothelial nitric oxide synthase (69). RIC consistently results in activation of the reperfusion injury salvage kinase (RISK) pathway, that is, increased phosphorylation of inositol-triphosphate kinase (70), Akt (8,69–72), extracellular-regulated kinase 1/2 (71) and glycogen synthase kinase 3β (73). RISK activation was also confirmed by abrogation of infarct size reduction with the respective pharmacological antagonists (8,70,71), and was not only seen in rodent, but also in pig hearts (70), in which RISK activation was previously not found important for protection by ischemic postconditioning (74). However, the study with RISK activation by remote ischemic preconditioning and perconditioning in pigs was confounded by the ambiguous finding that an adenosine antagonist abrogated RISK activation, rather than protection (70). RIC also consistently (18,38,43,65) results in activation of protein kinase C, a key molecule in cardioprotection (75) with a somewhat ambiguous role (76,77); in rodent hearts, protein kinase Cε is classically activated and shifted from the cytosolic to the particulate fraction (18,65). The role of hypoxia-inducible factor-1α (HIF-1α) in RIC is controversial; in one study, infarct size reduction by limb RIPC was abrogated in heterozygous knockout mice (72), but in another study, HIF-1α expression was increased by limb RIPC in wild-type mice, but was not a prerequisite for protection (73). HIF-1α protein expression is also increased in right atrial tissue of patients undergoing cardiac surgery under cardiopulmonary bypass with RIPC, but its causal involvement in the observed attenuation of troponin T release remains unclear (78). Late RIPC in rats increased heme oxygenase-1 protein expression and its inhibition by zinc protoporphyrin abrogated protection (79). As in local ischemic preconditioning (80), limb RIPC in rats not only reduced infarct size, but also preserved connexin 43 phosphorylation and localization at intercalated disks (81); the role of mitochondrial connexin 43 in RIC has not been addressed. An unbiased (mass spectrometry) proteomic search for phosphorylated proteins revealed that limb RIC increased expression of several phosphoproteins related to the sarcomeric Z-disc (82). A comprehensive immunoblotting approach for established cardioprotective proteins in right ventricular tissue of children undergoing repair of Fallot’s tetralogy revealed no differences in their phosphorylated forms without or with RIPC (83). In left ventricular biopsies from adult patients undergoing CABG, tyrosine-phosphorylated signal transducer and activator of transcription 5 was the only protein among more than 30 established cardioprotective proteins that was increased by RIPC (84). Autophagy appears to have no role in human RIPC (85). Mitochondria are clearly involved in cardioprotection by RIC. Human plasma from healthy volunteers undergoing a RIC protocol had increased nitrite concentration and increased the concentration of myocardial nitrite when transferred to an isolated mouse bioassay heart. Myocardial nitrite was converted to bioactive nitric oxide by myoglobin and reduced infarct size. In parallel mouse experiments, the same nitrite-nitric oxide pathway was activated by RIPC, induced S-nitrosation of mitochondrial proteins, and reduced complex I respiration and reactive oxygen species formation (42). In rabbits with limb RIPC, blockade of the mitochondrial aldehyde dehydrogenase-2 by cyanamide abrogated protection; in parallel experiments in humans with a functionally inactive enzyme polymorphism, endothelial protection by RIPC was eliminated, supporting that mitochondrial function is essential in RIC (86). Better preservation of mitochondrial respiration was also seen in right atrial tissue of patients undergoing CABG with RIPC, who also had lower incidence of postoperative atrial fibrillation (87). Apart from mitochondrial function, RIPC increases myocardial glycolytic flux in adult, but not in neonatal rabbit hearts, along with reduced infarct size in adult, but not in neonatal hearts (88). In isolated hearts from rats that underwent a RIPC protocol, myocardial microRNA-1, microRNA-2, heat shock protein 70, and programmed cell death protein expression were decreased (89). In right atrial tissue of patients undergoing CABG with RIPC, microRNA-388-3p expression was increased (87). The biological meaning of these changes in microRNA expression is unclear. Apparently, the intracardiac signal transduction of RIC largely resembles that of local ischemic preconditioning and postconditioning with significant involvement of nitric oxide, protein kinase C, the RISK pathway, and mitochondrial function. The data on myocardial signal transduction of RIC have not yet been integrated into a more complex and comprehensive scheme. Surprisingly, the role in RIC of the survival activating factor enhancement pathway, including signal transducer and activator of transcription 3, has not been addressed.
Clinical evidence for RIC
Effects of RIC on the heart: elective ischemia/reperfusion
Patients undergoing elective CABG and percutaneous coronary interventions (PCI) change as the demographics of the general population alter. Patients aged ≥75 years at the time of operation increased from 17% in 1999 to 29% in 2005 (90). Operated patients had more comorbidities, with increased rates of hypertension (from 43.7% in 1999 to 68.9% in 2007), obesity (from 13% to 17.5% in the same period) and worse functional and cardiac status (reduced ejection fraction, hemodynamic instability and shock) (90). Improvement in anesthetics, surgical and perioperative treatments allows surgeons to accept patients for operation, who, only a few years ago, would have been refused. Left ventricular ejection fraction <30% remains the most important determinant of outcome after isolated CABG (91). In elective CABG and PCI, adverse intermediate and long-term outcomes relate to periprocedural myocardial injury, including reduction of left ventricular ejection fraction; hence, the importance of cardioprotection beyond cardioplegia and off-pump surgery. Preconditioning by intermittent cross-clamping of the ascending aorta is invasive and has recently been comprehensively reviewed (64,92). The first (very small) clinical study evaluating the effect of RIPC on creatine kinase-myocardial band release in CABG patients was negative (93). Translation of RIPC’s protective potential to forearm endothelium-dependent vasomotion (17) initiated exploration of the cardioprotective potential of this approach using biomarkers as endpoint in cardiac surgery, such as pediatric cardiac surgery, CABG, and combined CABG and valvular surgery. Most studies, including one small pilot study of high-risk patients (93–102), demonstrated cardioprotective potential (46,84,87,103–116) (Table 1), with similar findings for elective PCI (117–125) (Table 2). Many studies only included a few patients. Type 2 error might explain the discrepant results and confounding factors, including age, comedication, anesthesia, comorbidity, and risk factors may also have influenced the efficacy of RIC (126). Concomitant therapy with beta-blockers (127,128) and statins (129) is cardioprotective, as is an anesthetic regimen using propofol or volatile anesthetics (46,48,128), and may interfere with the cardioprotective effect of RIC. The interference of propofol, which is cardioprotective per se, with further protection by RIC contrasts with the inherent cardioprotective effect of isoflurane, which does not interfere with RIPC (46,48), suggesting specific interaction of propofol with neuronal transfer of the protective RIPC signal. Although in experimental studies, diabetes mellitus attenuated the effect of local ischemic preconditioning (130), the degree of cardioprotection may depend on stimulus intensity (131) and diabetes duration (132), and the attenuation of protection by RIC seems minor in the clinical setting (133,134).
Table 1.
Clinical Studies of RIC in Cardiac Surgery
| Study | Number of patients (control/RI C) | Type of Surgery | RIC regimen | Endpoint | Outcome |
|---|---|---|---|---|---|
| Günaydin et al., 2000 (93) | 4/4 | CABG | Upper limb 2 cycles I/R (3/2 min) | CK (Sampled via coronary perfusion catheter 5 min after declamping) | No effect |
| Cheung et al., 2006 (103) | 20/17 | Pediatric cardiac surgery | Upper limb 4 cycles I/R (5/5 min) | TnT (AUC 24 h after surgery) Post-operative inotropic need Lung function | Reduced TnT; reduced inotropic score; reduced airway resistance |
| Hausenloy et al., 2007 (104) | 30/27 | CABG | Upper limb 3 cycles I/R (5/5 min) | TnT (AUC 72 h after surgery) | 43% reduction of TnT |
| Venugopal et al 2009 (105) | 22/23 | CABG (cold blood cardioplegia) | Upper limb 3 cycles I/R (5/5 min) | TnT (AUC 72 h after surgery) | 42% reduction of TnT |
| Hong et al., 2010 (106) | 65/65 | CABG (off-pump) | Upper limb 4 cycles I/R (5/5 min) | TnI (AUC 72 h after surgery) | 26% reduction of TnT, NS |
| Rahman et al., 2010 (96) | 82/80 | CABG | Upper limb 4 cycles I/R (5/5 min) | TnT (AUC 48 h after surgery) | No effect |
| Thielmann et al., 2010 (107) | 26/27 | CABG (crystalloid cardioplegic arrest) | Upper limb 3 cycles I/R (5/5 min) | TnI (AUC 72 h after surgery) | 45% reduction of TnI |
| Li (2010) (108) | 27/26 | Valve replacement | Lower limb 3 cycles I/R (4/4 min) | TnI (AUC 72 h after surgery) | 40% reduction of TnI |
| Zhou (2010) (109) | 30/30 | Pediatric cardiac surgery | Upper limb 2 cycles I/R (5/5 min) 24 h and 1 h prior to operation | CK-MB Inflammatory biomarkers (Plasma levels 2, 4, 12, and 24 h after surgery) Lung function | Reduced CK-MB and inflammator y biomarkers Improved postoperativ e lung function |
| Wagner et al., 2010 (110) | 34/32 | CABG (cold crystalloid cardioplegia) | Upper limb 3 cycles I/R (5/5 min) 18 h prior to operation | TnI (AUC 24 h after surgery) | Reduced TnI |
| Ali et al., 2010 (111) | 50/50 | CABG | Upper limb 3 cycles I/R (5/5 min) | CK-MB (Plasma levels 8, 16, 24, and 48 h after surgery) | Reduced CK-MB |
| Karuppasam y et al., 2011 (97) | 27/27 | CABG | Upper limb 3 cycles I/R (5/5 min) | TnI (AUC 48 h after surgery) | No effect |
| Wu et al., 2011 (112) | 25/25 | Mitral valve replacement | Upper limb 3 cycles I/R (5/5 min) and 3 cycles I/R (5/5 min) + 2 cycles I/R (10/10 min) | TnI (Plasma levels 4, 8, 12, 24, 48, and 72 h after surgery) | Reduced TnI with 3 cycles I/R (5/5 min) + 2 cycles I/R (10/10 min) but not 3 cycles I/R (5/5 min) |
| Kottenberg et al., 2012 (46) | 19/20 | CABG Propofol vs. isoflurane anesthesia | Upper limb 3 cycles I/R (5/5 min) | TnI (AUC 72 h after surgery) | Reduced TnI with isoflurane, but not propofol anesthesia |
| Young et al., 2012 (94) | 48/48 | Cardiac surgery (high- risk CABG and valve surgery) | Upper limb 3 cycles I/R (5/5 min) | TnT (Plasma levels 6 and 12 h surgery) | No effect |
| Heusch et al., 2012 (84) | 12/12 | CABG | Upper limb 3 cycles I/R (5/5 min) | TnI (AUC 72 h after operation) Phosphorylatio n of signal transducer and activator of transcription 5 (STAT5) | Reduced TnI STAT5 activation |
| Lee et al., 2012 (98) | 28/27 | Pulmonary hypertensive infants receiving ventricular septal defect repair | Lower limb 4 cycles I/R (5/5 min) | TnI AUC 24 h after surgery | No effect |
| Pavione et al., 2012 (99) | 10/12 | Pediatric cardiac surgery | Lower limb 4 cycles I/R (5/5 min) | TnI (Plasma levels 4, 12, 24, and 48 h after surgery) | No effect |
| Lucchinetti et al., 2012 (100) | 28/27 | CABG Opioids and propofol for induction and isoflurane for maintenance anesthesia | Lower limb 4 cycles I/R (5/5 min) | TnT (Plasma levels 24, 48, and 72 h after surgery | No effect |
| Xie et al., 2012 (113) | 35/38 | Valve surgery | Upper limb 3 cycles I/R (5/5 min) | TnI (Plasma levels 6, 12, 24, 48, and 72 h after surgery | Reduced TnI |
| Thielmann et al 2013 (114) | 167/162 | CABG (Cold crystalloid cardioplegia and cardiopulmonar y bypass) | Upper limb 3 cycles I/R (5/5 min) | TnI (AUC 72 h after surgery) | 27% reduction of TnI Reduced all- cause mortality |
| Ahmad et al., 2014 (101) | 32/35 | CABG On-pump | ? | CK-MB (Plasma levels 1, 12, 24, and 48 h after surgery) | No effect |
| Hong et al., 2014 (95) | 636/644 | Cardiac surgery (cardiac valve surgery; CABG; combined valve and CABG surgery; ascending aorta and aortic arch surgery; and congenital heart defect repair) | Upper limb 4 cycles I/R (5/5 min) - 2 cycles before and 2 cycles after cardiopulmonar y by-pass | Clinical outcome (composite of: death; myocardial infarction; arrhythmia; stroke; coma; renal damage; respiratory failure; gastrointestina l complications; and multiorgan failure) | No effect |
| Slagsvold et al., 2014 (87) | 30/30 | CABG | Upper limb 3 cycles I/R (5/5 min) | Mitochondrial oxidation | Improved mitochondri al respiration |
| McCrindle et al., 2014 (102) | 151/148 | Pediatric cardiac surgery | Lower limb 4 cycles I/R (5/5 min) | Duration of post-operative hospital stay | No effect |
| Holmberg et al., 2014 (116) | 23/23 | Cardiac surgery (CABG; valve surgery; ascending aorta; myxoma) | Upper limb 3 cycles I/R (5/5 min) | TnT (AUC 72 h after surgery) | 25% reduction of TnT, NS |
| Candilio et al., 2014 (115) | 90/90 surgery (CABG and/or valve surgery) | Cardiac lower limb 2 cycles I/R (5/5 min) | Upper and myocardial infarction (AUC 52 h after surgery) | Perioperative reduction of TnT Reduction of incidence of AF, renal failure, stay at ICU | 26% |
AF = atrial fibrillation; AUC = area under curve; CABG = coronary artery bypass grafting; CK = creatine kinase; CM-MB = creatine kinase-myocardial band; I = ischemia; NS = not significant, R = reperfusion; TnI = troponin I; TnT = troponin T.
Table 2.
Clinical Studies of RIC in Elective PCI
| Study | Number of Patients (control/RIC) | RIC Regimen | Endpoint | Outcome |
|---|---|---|---|---|
| Iliodromitis et al., (117) | 21/20 | Upper limb 3 cycles I/R (3/3 min) | TnI (12, 24, and 48 h after PCI) | No effect |
| Hoole et al., (118) | 98/104 | Upper limb 3 cycles I/R (3/3 min) | TnI (Proportion of patients with TnI <0.04 ng/mL) | Reduction of proportion of patients with elevated TnI Reduced cardiac events 6 months after PCI |
| Davies et al., 2013 (119) | 97/95 | Upper limb 3 cycles I/R (3/3 min) | MACCE at 6 years | 13% reduction of MACCE |
| Ahmed et al., 2013 (120) | 72/77 | Upper limb 3 cycles I/R (3/3 min) | TnT (Plasma level 16 h after PCI) | 57% reduction of TnT |
| Prasad et al., 2013 (121) | 48/47 | Upper limb 3 cycles I/R (3/3 min) | TnT (proportion of patients with TnT ≥0.03 ng/dL) | No effect |
| Luo et al., 2013 (122) | 104/101 | Upper limb 3 cycles I/R (3/3 min) | TnI (Plasma level 16 h after PCI) | 48% reduction of TnI |
| Xu et al., 2014 (123) | 98/102 | Upper limb 3 cycles I/R (3/3 min) | TnI (Plasma level 16 h after PCI) | 24% reduction of TnI in DM patients aged ≥65 years, NS |
| Liu et al., 2014 (124) | 102/98 | Upper limb 3 cycles I/R (3/3 min) | TnI (Plasma level 24 h after PCI) | 75% reduction of TnI Reduction of adverse events at 6 months |
| Zografos et al 2014 (125) | 47/47 | Upper limb 1 cycle I/R (5 /5 min) | TnI (Increase in TnI from baseline to 24 h after PCI) | 79% reduction in post- PCI TnI increment |
MACCE = major adverse cardiovascular and cerebral events. Other abbreviations as in
Importantly, recent larger studies have not only relied on surrogate markers of cardioprotection, but also included long-term clinical outcomes and demonstrated a reduction of major cardiovascular events by RIPC up to 4 years after CABG (114) and up to 6 years after elective PCI (119). A recent study randomized 1,280 patients scheduled for elective cardiac surgery to control or RIPC and remote postconditioning (95). RIC was given as 2 cycles of 5 min ischemia and 5 min of reperfusion on the upper arm before cardiopulmonary bypass or coronary anastomoses in those who had beating heart surgery, and repeated in the same sequence immediately after bypass. While the cardioprotective effect was not documented by a reduction of postoperative biomarker release, RIC did not reduce the primary endpoint, a composite of major adverse outcomes including death, myocardial infarction, arrhythmia, stroke, coma, renal damage, respiratory failure, gastrointestinal complications, and multiorgan failure, suggesting that this endpoint may have been too broad. Although RIC is thought to have systemic protective effects on various distal organs, the results are debatable because the composite endpoint differs from other studies yielding beneficial results. Moreover, the heterogeneity of the patient group, including CABG, cardiac valve surgery, and their combination, as well as ascending or transverse aortic surgery and congenital heart defect repair may have introduced bias. Recent meta-analyses demonstrated that RIPC reduces biomarkers in patients undergoing CABG (92,135). The 2 follow-up studies with clinical outcomes were single-center trials, not powered to demonstrate definitive answers about clinical outcome (114,120). The consistency of the beneficial clinical outcome in the studies adds credibility to a clinically relevant benefit of RIC in relation to CABG and elective PCI. However, larger multicenter studies are still required to clarify the extent to which these findings translate into clinical benefit. Future studies should include high-risk patients, who might benefit most from protection by RIC, and preferably avoid propofol in their anesthetic regimen when specific cardioprotective effects are addressed.
Effects of RIC on the heart: acute myocardial infarction
Although the incidence of AMI is declining in the Western World (136,137), ischemic heart disease is still the leading cause of death worldwide (138). Improvements in treatment have changed the epidemiology after AMI, with markedly improved 30-day survival, but less favorably influenced long-term survival (136,139). Consistent with this, due to remodeling and heart failure (140), nonfatal ischemic heart disease has increased more than ischemic heart disease deaths since 1990 (138). The declining incidence of heart failure after AMI has not reached the magnitude that we might have expected from clinical trial data (139) and the prevalence is increasing (138). Consequently, one of the potentially most important applications of RIC may be in patients with AMI (28,141–147) (Table 3).
Table 3.
Clinical studies of RIC in AMI.
| Study | No of patients (control/RIC) | RIC regimen | Endpoint | Outcome |
|---|---|---|---|---|
| Bøtker et al., 2010 (28) | 69/73 | Upper limb 4 cycles I/R (5/5 min) | Salvage index (SPECT) | 20% increase in salvage index |
| Munk et al., 2010 (141) | 110/108 | Upper limb 4 cycles I/R (5/5 min) | LVEF at 30 days | 5% increase in LVEF in anterior infarcts |
| Rentoukas et al., 2010 (142) | 30/33 | Upper limb 3 cycles I/R (5/5 min) | ST-segment resolution | 20% increase in proportion of patients achieving full ST-segment resolution |
| Crimi et al., 2013 (143) | 50/50 | Lower limb 3 cycles I/R (5/5 min) | CK-MB (AUC 72 h after PCI) | 20% reduction of CK-MB release |
| Prunier et al., 2014 (144) | 17/18 | Upper limb 4 cycles I/R (5/5 min) | CK-MB (AUC 72 h after PCI) | 31% reduction of CK-MB release |
| Sloth et al., 2014 (145) | 167/166 | Upper limb 4 cycles I/R (5/5 min) | MACCE at 4 years | 12% reduction in MACCE |
| Yellon et al., 2014 (147) | 260/260 | Upper limb 4 cycles I/R (5/5 min) | TnT (AUC 24 h after PCI) | 17% reduction of TnT release |
| White et al., 2014 (146) | 40/43 | Upper limb 4 cycles I/R (5/5 min) | CMR | 27% reduction of infarct size |
LVEF = left ventricular ejection fraction; SPECT = single photon emission computerized tomography. Other abbreviations as in Table 2.
Most clinical studies on infarct size after coronary revascularization have used indirect estimates of tissue damage, such as release of biomarkers and resolution of ST-segment elevation (148,149) . Direct visualization of the area-at-risk and final infarct size to calculate the salvage index (proportion of salvaged area-at-risk) can be achieved by myocardial perfusion imaging using 99technetium-sestamibi single-photon emission computerized tomography (150) or cardiac magnetic resonance (CMR) imaging (151–153). CMR quantification of the area-at-risk poses challenges because the optimal protocol to quantify edema, thought to represent area-at-risk, is not defined (154–156) and because any cardioprotective intervention that reduces final infarct size also may reduce edema (157,158), potentially underestimating salvage.
The first proof-of-concept study demonstrating that RIC can increase myocardial salvage investigated 333 patients undergoing primary PCI for STEMI, of whom 132 had available imaging data (28). A simultaneous study demonstrated that RIC increases the number of patients achieving complete ST-segment resolution and a statistically borderline reduction of troponin-T release (142). In the former study, RIC was applied as 4 cycles of 5 min upper arm ischemia and 5 min reperfusion and initiated in the ambulance during transportation to primary PCI. RIC increased salvage by 36% and tended to reduce final infarct size. In patients with anterior infarcts and patients with occluded culprit artery (TIMI 0-1) on admission, infarct size reduction, as measured by single photon emission computerized tomography, was 44% and 31%, respectively, indicating that patients at highest risk benefit more from RIC as an adjunctive therapy to primary PCI. The findings translated into an increment of left ventricular ejection fraction in anterior infarcts (141). Although not powered to evaluate clinical outcome, a follow-up study of the total cohort showed that the beneficial effect of RIC translated into a reduction of major cardiovascular events up to 4 years after the index event (145). In a recent study, 4 cycles of 5 min cuff inflation/5 min deflation on the upper arm reduced myocardial edema and reduced infarct size, reflected by troponin release and CMR (146).
The first window of protection lasts 2 to 3 h and onset appears to be instant, as RIC initiated immediately prior to revascularization also reduces infarct size in STEMI patients (144). In some protocol algorithms, remote preconditioning combined with local post-conditioning reduced infarct size in rats (71). This additive effect was not seen for remote preconditioning combined with local postconditioning in the clinical setting of patients with reperfused AMI (144). In a recent randomized study of 100 patients, remote postconditioning also reduced infarct size, assessed by the area under the curve of creatine kinase-myocardial band release (143). Infarct size was consistently reduced, as reflected by delayed gadolinium enhancement volume on CMR and ST-segment resolution >50% in twice as many patient in the treatment than in the control group. After 1-year follow-up, 1 patient in the control group (refractory heart failure) and none in the postconditioning group had died, and cardiovascular events were reduced in the treatment group. The beneficial effect was obtained by 3 cycles of 5 min/5 min blood pressure cuff inflation/deflation of the lower limb initiated at the time of reperfusion by balloon inflation or thrombectomy. Although a recent clinical study suggested that 1 occlusion cycle induces protection during elective PCI (125), experimental data from mice indicate that cardioprotective efficacy is determined by the number and duration of inflations (34).
Present reperfusion therapy is effective in the majority of patients undergoing primary PCI. It may be difficult to demonstrate additional clinical benefit from further intervention because this would require demonstration of further reduction in small myocardial infarcts and its translation into a clinical benefit. A subgroup of patients undergoing not only primary PCI, but also elective PCI and CABG, develops serious complications, including extensive myocardial injury, which is most frequently vascular in origin. Although preclinical human data indicate that RIC may modify thrombogenesis (159,160) and yield cardioprotection beyond an unequivocal reduction of infarct size (e.g. by anti-inflammatory mechanisms)(161), the clinical implications are yet unknown. However, some patients, predominantly those with large anterior infarcts, develop heart failure due to myocardial injury and subsequent left ventricular remodeling several months or years after the infarct, despite optimal medical treatment according to guidelines (140). Because RIC reduces final tissue necrosis, improved clinical outcome must be assessed by reduced post-infarction left ventricular dysfunction and heart failure, combined with mortality reduction (162). To achieve widespread clinical acceptance of RIC, focus should be kept on patients at risk of extensive myocardial injury and global tissue damage. Its potential clinical utility is far from fully explored.
An emerging concept, known as chronic conditioning, is the daily use of RIC for weeks. In rats, RIC administration daily for the first 28 days after MI had a dose-dependent effect on cardiac remodeling, heart failure, and even death rate in the absence of a significant reduction of infarct size (163). This effect demonstrates benefits beyond modification of acute ischemic effects. However, in a recent pilot study, this did not immediately translate into improved exercise capacity in heart failure patients (50).
Confounding factors in RIC
No recognized effective therapeutic intervention for protecting the myocardium against the detrimental effects of ischemia-reperfusion injury presently exists. A major reason for this unfortunate situation is the inability to take the relevance of confounding factors present in the majority of basic and clinical studies into account; RIC studies are no different in this regard (126).
Infarct location/patient selection
Only a quarter of all STEMI patients have infarcts of sufficient size to benefit from adjunctive therapy (164). Patients presenting with right and/or circumflex coronary artery occlusion, where the infarct is relatively small, do not benefit as much from cardioprotective therapy as those presenting with proximal left anterior descending coronary artery occlusion, where the infarct is significantly larger (28,165). “All-comer” trials will lead to the recruitment of far more patients with small infarcts and little additional myocardial salvage, which may actually dilute the positive effect elicited by any novel protective strategy. Alternatively, limiting recruitment to patients with large anterior infarcts is more challenging because they are the most ill (166); however, the benefit of proof-of-concept trials is that demonstration of a significant difference between treatment and placebo requires recruitment of fewer patients (167).
Control of TIMI flow prior to RIC
Some patients presenting with an acute myocardial infarction have already undergone spontaneous reperfusion prior to interventional reperfusion and are not likely to benefit from a therapy designed to protect against reperfusion injury (168). Therefore only those patients with TIMI scores <1 should be included in such studies (28).
Importance of Coronary Collaterals
The coronary collateral circulation’s ability to influence the size of an evolving myocardial infarction cannot be underestimated. In STEMI patients, substantial collateralization reduces the sizes of the area at risk and the evolving infarct. The extent of collateralization will thus negatively influence the ability to demonstrate an effect of any novel cardioprotective strategy. Patients with visible collaterals (Rentrop grade ≥1) should therefore be excluded (169).
Duration of chest pain and timing of intervention
Patients presenting with an AMI who receive interventional or thrombolytic reperfusion must do so within 12 h of the onset of chest pain (170,171). Given the crucial events that occur in the first few minutes of reperfusion (oxidative stress, calcium overload and mitochondrial permeability transition pore opening), any cardioprotective strategy must be applied prior to opening the infarct-related coronary artery. Accordingly, RIC given to patients in the ambulance whilst in transit to the interventional center demonstrated a beneficial effect (28).
With late presentation, the infarct will have been completed, and the patient will derive little benefit from either intervention or an adjunct to reperfusion. Early presentation and revascularization will lead to small myocardial infarcts, and this patient will have little advantage from adjunctive therapy. There is a “sweet spot,” probably between 3 and 8 h from time of symptom onset to time of reperfusion, for adjunctive therapies to demonstrate maximal benefit. Comorbidities and comedications: In preclinical studies, age (172) and comorbid diseases (126), such as hyperlipidemia, diabetes, hypertension, which require a more robust conditioning signal, raise the threshold for protection. This raised cardioprotective threshold reflects fundamental molecular alterations within the heart, affecting both sensitivity to ischemia/reperfusion injury and response to a particular cardioprotective strategy (126,172–174). Unfortunately, most experimental models use healthy young animals, free of any comorbidities (175). Experimental studies using human atrial muscle from patients undergoing CABG, from aged and diabetic patients and patients with heart failure (176–178) confirmed the effect of comorbidity on the conditioning threshold and demonstrated resistance to various conditioning strategies. Pharmacological therapy also impacts cardioprotection. Specific sulfonlyureas used to treat type 2 diabetes can attenuate the conditioning response (134). Conversely, insulin, metformin, some statins, ACE-inhibitors, anti-platelet agents, and opioids can themselves be cardioprotective and raise the threshold for an additional benefit (64,173,179–181). A number of pharmacological agents used during cardiopulmonary bypass surgery interfere with the cardioprotective efficacy of RIC. Volatile anesthetics, such as isoflurane, and the intravenous anesthetic, propofol, either themselves confer cardioprotection or interfere with RIC through down-regulation of cardioprotective signaling (46,48). Intravenous nitroglycerine, nitroprusside, and opioid analgesics, each protective in experimental settings, also interfere with the apparent cardioprotective efficacy of a study intervention (173,180).
Taking these confounders into consideration in the design of any clinical study investigating RIC is hugely important; either design a study which does not use these agents (which may be impractical) or ensure that it is adequately powered and properly randomized.
Effects of RIC on the blood and vasculature
Platelet activation is both a consequence and a driver of ischemia/reperfusion injury. Local ischemic preconditioning attenuates platelet activation and aggregation (182). In humans, marked systemic platelet activation has been demonstrated in patients with acute coronary syndromes (183) or acute limb ischemia (184). In animal models, the extent of platelet activation is related to the extent of subsequent tissue injury after reperfusion (185). Indeed, blockade of platelet aggregation alone can significantly attenuate reperfusion injury. In healthy male volunteers subjected to 20 min forearm ischemia (160), platelet activation (measured by increased circulating monocyte-platelet aggregates) persisted up to 45 min, but was completely abolished in subjects randomized to receive RIPC prior to the ischemic insult. In patients with known obstructive coronary artery disease (186), RIPC prior to exercise stress testing reduced ADP-stimulated platelet aggregation. Similarly attenuated platelet aggregation was seen in patients undergoing ablation for atrial fibrillation when receiving RIPC (187). However, the potential clinical benefit of any of these findings remains to be seen.
Circulating monocytes play a key role in ischemia/reperfusion injury. RIC down-regulated the expression of a broad portfolio of proinflammatory genes in circulating monocytes (161). The functional importance of these gene expression changes was demonstrated by reduced neutrophil adhesion over 10 days of daily RIC (188). Neutrophil phagocytosis was not significantly altered at 24 h, but was suppressed after 10 days of RIC. In patients undergoing CABG (189), RIC was not associated with any difference in circulating markers of inflammation (e.g., interleukins 6, 8, 10, or tumor necrosis factor α levels) but neutrophil kinase beta 1 and beta 2 receptor expression was significantly reduced, confirming similar results in healthy human volunteers subjected to RIC (190).
The RIC stimulus is associated with coronary vasodilation in animal models (191) and peripheral vasodilation in the contralateral limb of human subjects undergoing RIC (192). In Kharbanda’s original description (17), RIPC by 3 cycles of 5 min ischemia/5 min reperfusion in the forearm provided potent protection against the endothelial dysfunction induced by 20 min ischemia/reperfusion in the contralateral arm. Using the same model, RIPC was not only effective immediately, but also induced a second window of protection against endothelial dysfunction at 24 h (44). When the RIC protocol was performed on the contralateral arm during the ischemia phase, but prior to reperfusion, both RIPC and remote ischemic perconditioning were blocked by pretreatment with the ATP-dependent potassium channel blocker, glibenclamide (193). Compared to young volunteers, elderly hypertensives benefitted more from RIC, whereas basal levels of flow-mediated dilation were significantly greater in the younger population (194). RIC in healthy young subjects, repeated daily for 7 days (195), was associated with progressively improved flow-mediated dilation and cutaneous vascular conductance (as a measure of microcirculatory function), which was sustained at 8 days after the cessation of RIC. In a subsequent study (196), similar beneficial effects persisted after 8 weeks of repeated RIC treatments. This prolonged effect of RIC on endothelial function was also observed in patients with AMI undergoing PCI (197). Endothelial function was tested at baseline, within 3 h, and then on days 2 and 7 post-procedure in 48 patients randomized to PCI with or without RIPC. Endothelial function improved early after treatment and was sustained 7 days after the intervention. Whether this was a primary effect of sustained modification of endothelial function, or a secondary phenomenon, resulting from less systemic inflammatory reaction is unknown. Likewise, 1 week of twice daily limb RIC improved ATP-recruitable coronary blood flow velocity reserve in a small cohort of healthy volunteers and patients with heart failure (198).
Conclusions and perspective
Solid evidence from experimental and clinical studies supports protection by RIC from ischemia/reperfusion injury of the heart and other organs (16). Details of the mechanisms for local release of the protective signal at the remote site and the contributions of neuronal and humoral pathways are not yet clear, not only in signal release, but also in signal transfer to the target organ, and protective signal transduction within the target organ. Repeated brief inflation/deflation of a blood pressure cuff at the arm, leg, or both is easily feasible, noninvasive, inexpensive, effective, and safe. Ongoing trials will reveal whether the benefit in clinical outcome reported from small proof-of-concept trials where clinical outcome was not the primary endpoint (199) will really hold true (200,201).
Thus far, translation of cardioprotective strategies from successful experiments to the clinic has been somewhat disappointing, for reasons that have been highlighted elsewhere (64,166,167,202): premature enthusiasm for experimental data that were not unequivocal and not confirmed in larger mammalian models; poor clinical trial design; and lack of consideration for patients’ multiple comorbidities and comedications (126). The pharmaceutical industry has, understandably, largely given up development of cardioprotective agents, because they may need to be given only once in the situation of acute ischemia/reperfusion, but not as continuous therapy.
It appears reasonable to focus on mechanical protection of the heart and other organs by RIC and to optimize protocols. Apart from RIC algorithm optimization (number/duration of ischemia/reperfusion cycles), a better mechanistic understanding of the underlying signal transduction will be necessary to overcome the confounding impact of comorbidities and comedications. RIC may then, indeed, be the future of cardioprotection (203). Future investigations should explore the potential benefit of RIC, not only in patients with large evolving myocardial infarctions, but also in patients with cardiogenic shock and severe arrhythmias, including cardiac arrest and threatening global ischemia of the brain, heart, liver and kidney during organ transplantation and extensive cardiovascular surgery.
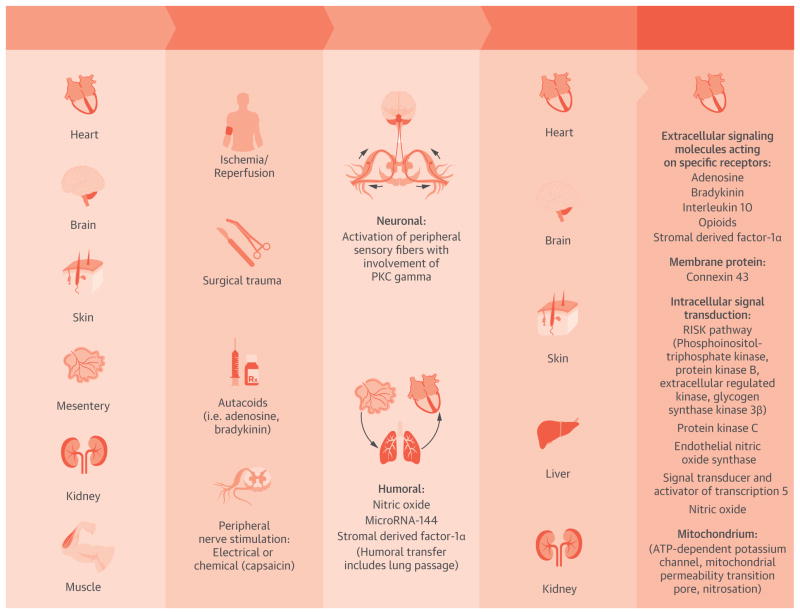
Figure.
Central Illustration. Signal Transduction of Remote Ischemic Conditioning From the Source Organ in Response to Several Stimuli Via Neuronal and/or Humoral Transfer to the Heart and Other Organs, Where a Protective Intracellular Signal Transduction Cascade is Activated
Akt = protein kinase B; Cx 43 = connexin 43; ERK = extracellular regulated kinase; eNOS = endothelial nitric oxide synthase; γPKC = protein kinase C γ; GSK3β = glycogen synthase kinase 3β; KATP = ATP-dependent potassium channel; mPTP = mitochondrial permeability transition pore; NO = nitric oxide; PI3-K = phosphoinositol-triphosphate kinase; RISK = reperfusion injury salvage kinase; SDF-1 α = stromal derived factor 1α.
Acknowledgments
Dr. Heusch was supported by the German Research Foundation (He 1320/18-1, 3). Dr. Bøtker was supported by the Novo Nordic Foundation, Fondation Leducq (06CVD), the Danish Research Council for Strategic Research (11-115818), and the Danish Research Council (11-108354). Dr. Przyklenk was partially supported by NIH-HL072684. Dr. Yellon was supported by the Medical Research Council (MR/K002066/1), the British Heart Foundation (RG/08/015/26411) and UCLH/UCL, which received partial funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Abbreviations
- AMI
acute myocardial infarction
- CABG
coronary artery bypass grafting
- CMR
cardiac magnetic resonance
- PCI
percutaneous coronary intervention
- RIC
remote ischemic conditioning
- RIPC
remote ischemic preconditioning
- RISK
reperfusion injury salvage kinase
- STEMI
ST-segment elevation myocardial infarction
- TIMI
thrombolysis in myocardial infarction
- TNF
tumor necrosis factor
Footnotes
Disclosures: Dr. Heusch serves as consultant to Servier. Dr. Bøtker is a shareholder of CellAegis Inc., Toronto, Canada. Dr. Przyklenk serves on the advisory board of Infarct Reduction Technologies, Inc. Dr. Redington is a shareholder and board member of CellAegis devices. Dr. Yellon has served on the advisory boards for BMS, AZ, and The Medicine Company and received research support from AZ, MSD, and The Medicine Company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Przyklenk K, Bauer B, Ovize M, et al. Regional ischemic “preconditioning” protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 2.Whittaker P, Przyklenk K. Reduction of infarct size in vivo with ischemic preconditioning: mathematical evidence for protection via non-ischemic tissue. Basic Res Cardiol. 1994;89:6–15. doi: 10.1007/BF00788673. [DOI] [PubMed] [Google Scholar]
- 3.Przyklenk K, Darling CE, Dickson EW, et al. Cardioprotection 'outside the box'. The evolving paradigm of remote preconditioning. Basic Res Cardiol. 2003;98:149–57. doi: 10.1007/s00395-003-0406-y. [DOI] [PubMed] [Google Scholar]
- 4.Przyklenk K, Whittaker P. Genesis of remote conditioning: action at a distance - 'hypotheses non fingo'? J Cardiovasc Med (Hagerstown ) 2013;14:180–6. doi: 10.2459/JCM.0b013e328358c8eb. [DOI] [PubMed] [Google Scholar]
- 5.Dickson EW, Lorbar M, Porcaro WA, et al. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–7. doi: 10.1152/ajpheart.1999.277.6.H2451. [DOI] [PubMed] [Google Scholar]
- 6.Serejo FC, Rodrigues LF, Jr, da Silva Tavares KC, et al. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007;49:214–20. doi: 10.1097/FJC.0b013e3180325ad9. [DOI] [PubMed] [Google Scholar]
- 7.Huffman LC, Koch SE, Butler KL. Coronary effluent from a preconditioned heart activates the JAK-STAT pathway and induces cardioprotection in a donor heart. Am J Physiol Heart Circ Physiol. 2008;294:H257–62. doi: 10.1152/ajpheart.00769.2007. [DOI] [PubMed] [Google Scholar]
- 8.Breivik L, Helgeland E, Aarnes EK, et al. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–45. doi: 10.1007/s00395-010-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimizu M, Tropak M, Diaz RJ, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 10.Leung CH, Wang L, Nielsen JM, et al. Remote cardioprotection by transfer of coronary effluent from ischemic preconditioned rabbit heart preserves mitochondrial integrity and function via adenosine receptor activation. Cardiovasc Drugs Ther. 2014;28:7–17. doi: 10.1007/s10557-013-6489-2. [DOI] [PubMed] [Google Scholar]
- 11.Jensen RV, Stottrup NB, Kristiansen SB, et al. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol. 2012;107:285. doi: 10.1007/s00395-012-0285-1. [DOI] [PubMed] [Google Scholar]
- 12.Gho BCG, Schoemaker RG, van den Doel MA, et al. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 13.Pell TJ, Baxter GF, Yellon DM, et al. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275:H1542–7. doi: 10.1152/ajpheart.1998.275.5.H1542. [DOI] [PubMed] [Google Scholar]
- 14.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000;278:H1571–6. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 15.Liem DA, Verdouw PD, Ploeg H, et al. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29–37. doi: 10.1152/ajpheart.01031.2001. [DOI] [PubMed] [Google Scholar]
- 16.Candilio L, Malik A, Hausenloy DJ. Protection of organs other than the heart by remote ischemic conditioning. J Cardiovasc Med (Hagerstown ) 2013;14:193–205. doi: 10.2459/JCM.0b013e328359dd7b. [DOI] [PubMed] [Google Scholar]
- 17.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–3. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 18.Jones WK, Fan GC, Liao S, et al. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross GJ, Baker JE, Moore J, et al. Abdominal surgical incision induces remote preconditioning of trauma (RPCT) via activation of bradykinin receptors (BK2R) and the cytochrome P450 epoxygenase pathway in canine hearts. Cardiovasc Drugs Ther. 2011;25:517–22. doi: 10.1007/s10557-011-6321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross ER, Hsu AK, Urban TJ, et al. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res Cardiol. 2013;108:381. doi: 10.1007/s00395-013-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redington KL, Disenhouse T, Strantzas SC, et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol. 2012;107:241. doi: 10.1007/s00395-011-0241-5. [DOI] [PubMed] [Google Scholar]
- 22.Redington KL, Disenhouse T, Li J, et al. Electroacupuncture reduces myocardial infarct size and improves post-ischemic recovery by invoking release of humoral, dialyzable, cardioprotective factors. J Physiol Sci. 2013;63:219–23. doi: 10.1007/s12576-013-0259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merlocco AC, Redington KL, Disenhouse T, et al. Transcutaneous electrical nerve stimulation as a novel method of remote preconditioning: in vitro validation in an animal model and first human observations. Basic Res Cardiol. 2014;109:406. doi: 10.1007/s00395-014-0406-0. [DOI] [PubMed] [Google Scholar]
- 24.Przyklenk K, Maynard M, Greiner DL, et al. Cardioprotection with postconditioning: loss of efficacy in murine models of type-2 and type-1 diabetes. Antioxid Redox Signal. 2011;14:781–90. doi: 10.1089/ars.2010.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinten-Johansen J, Shi W. Perconditioning and postconditioning: current knowledge, knowledge gaps, barriers to adoption, and future directions. J Cardiovasc Pharmacol Ther. 2011;16:260–6. doi: 10.1177/1074248411415270. [DOI] [PubMed] [Google Scholar]
- 26.Kerendi F, Kin H, Halkos ME, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–12. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt MR, Smerup M, Konstantinov IE, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–90. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- 28.Bøtker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 29.Andreka G, Vertesaljai M, Szantho G, et al. Remote ischaemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–52. doi: 10.1136/hrt.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gritsopoulos G, Iliodromitis EK, Zoga A, et al. Remote postconditioning is more potent than classic postconditioning in reducing the infarct size in anesthetized rabbits. Cardiovasc Drugs Ther. 2009;23:193–8. doi: 10.1007/s10557-009-6168-5. [DOI] [PubMed] [Google Scholar]
- 31.Sachdeva J, Dai W, Gerczuk PZ, et al. Combined remote perconditioning and postconditioning failed to attenuate infarct size and contractile dysfunction in a rat model of coronary artery occlusion. J Cardiovasc Pharmacol Ther. 2014;19:567–73. doi: 10.1177/1074248413518967. [DOI] [PubMed] [Google Scholar]
- 32.Heusch G, Schulz R. Preservation of peripheral vasodilation as a surrogate of cardioprotection? The mechanistic role of ATP-dependent potassium channels and the mitochondrial permeability transition pore. Eur Heart J. 2011;32:1184–6. doi: 10.1093/eurheartj/ehq511. [DOI] [PubMed] [Google Scholar]
- 33.Whittaker P, Przyklenk K. From ischemic conditioning to 'hyperconditioning': clinical phenomenon and basic science opportunity. Dose Response. 2014:10. doi: 10.2203/dose-response.14-035.Whittaker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnsen J, Pryds K, Salman R, et al. Optimizing the cardioprotective effects of remote ischemic preconditioning. Eur Heart J. 2014;35(S1):444. (abstr) [Google Scholar]
- 35.Basalay M, Barsukevich V, Mastitskaya S, et al. Remote ischaemic pre- and delayed postconditioning - similar degree of cardioprotection but distinct mechanisms. Exp Physiol. 2012;97:908–17. doi: 10.1113/expphysiol.2012.064923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu SB, Liu Y, Zhu Y, et al. Remote preconditioning, perconditioning, and postconditioning: a comparative study of their cardio-protective properties in rat models. Clinics (Sao Paulo) 2013;68:263–8. doi: 10.6061/clinics/2013(02)OA22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel HH, Moore J, Hsu AK, et al. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34:1317–23. doi: 10.1006/jmcc.2002.2072. [DOI] [PubMed] [Google Scholar]
- 38.Weinbrenner C, Nelles M, Herzog N, et al. Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res. 2002;55:590–601. doi: 10.1016/s0008-6363(02)00446-7. [DOI] [PubMed] [Google Scholar]
- 39.Steensrud T, Li J, Dai X, et al. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–603. doi: 10.1152/ajpheart.00396.2010. [DOI] [PubMed] [Google Scholar]
- 40.Donato M, Buchholz B, Rodriguez M, et al. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischemic preconditioning. Exp Physiol. 2012;98:425–34. doi: 10.1113/expphysiol.2012.066217. [DOI] [PubMed] [Google Scholar]
- 41.Wong GT, Lu Y, Mei B, Xia Z, et al. Cardioprotection from remote preconditioning involves spinal opioid receptor activation. Life Sci. 2012;91:860–5. doi: 10.1016/j.lfs.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 42.Rassaf T, Totzeck M, Hendgen-Cotta UB, et al. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–10. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 43.Southerland EM, Milhorn DM, Foreman RD, et al. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2007;292:H311–7. doi: 10.1152/ajpheart.00087.2006. [DOI] [PubMed] [Google Scholar]
- 44.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, et al. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–6. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 45.Mastitskaya S, Marina N, Gourine A, et al. Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res. 2012;95:487–94. doi: 10.1093/cvr/cvs212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kottenberg E, Thielmann M, Bergmann L, et al. Protection by remote ischaemic preconditioning during coronary artery bypass grafting with isoflurane but not with propofol anesthesia - a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–8. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 47.Bautin A, Datsenko S, Tashkhanov D, et al. Influence of the anesthesia technique on the cardioprotective effects of the remote ischemic preconditioning in the patients undergoing the aortic valve replacement. Heart. 2013;99:A40–A41. [Google Scholar]
- 48.Kottenberg E, Musiolik J, Thielmann M, et al. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;147:376–82. doi: 10.1016/j.jtcvs.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Konstantinov IE, Li J, Cheung MM, et al. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a KATP channel-dependent mechanism. Transplantation. 2005;79:1691–5. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 50.McDonald MA, Braga JR, Li J, et al. A randomized pilot trial of remote ischemic preconditioning in heart failure with reduced ejection fraction. PLoS One. 2014;9:e105361. doi: 10.1371/journal.pone.0105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hepponstall M, Ignjatovic V, Binos S, et al. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS ONE. 2012;7:e48284. doi: 10.1371/journal.pone.0048284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tota B, Quintieri AM, Angelone T. The emerging role of nitrite as an endogenous modulator and therapeutic agent of cardiovascular function. Curr Med Chem. 2010;17:1915–25. doi: 10.2174/092986710791163948. [DOI] [PubMed] [Google Scholar]
- 53.Siddiqi N, Neil C, Bruce M, et al. NIAMI Investigators. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) Eur Heart J. 2014;35:1255–62. doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi M. Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J. 2010;74:418–23. doi: 10.1253/circj.cj-09-1021. [DOI] [PubMed] [Google Scholar]
- 55.Davidson SM, Selvaraj P, He D, et al. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol. 2013;108:377. doi: 10.1007/s00395-013-0377-6. [DOI] [PubMed] [Google Scholar]
- 56.Norata GD, Sala F, Catapano AL, et al. MicroRNAs and lipoproteins: a connection beyond atherosclerosis? Atherosclerosis. 2013;227:209–15. doi: 10.1016/j.atherosclerosis.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–44. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 59.Yellon DM, Davidson SM. Exosomes: nanoparticles involved in cardioprotection? Circ Res. 2014;114:325–32. doi: 10.1161/CIRCRESAHA.113.300636. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Rohailla S, Gelber N, et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol. 2014;109:423. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 61.Vicencio JM, Boi-Doku C, Das D, et al. Protecting the heart at a distance: exosomes for nano-sized cardioprotection. Heart. 2014;100:A9. (abstr) [Google Scholar]
- 62.Giricz Z, Varga ZV, Baranyai T, et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol. 2014;68:75–8. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–9. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 64.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–75. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 65.Wolfrum S, Schneider K, Heidbreder M, et al. Remote preconditioning protects the heart by activating myocardial PKCε-isoform. Cardiovasc Res. 2002;55:583–9. doi: 10.1016/s0008-6363(02)00408-x. [DOI] [PubMed] [Google Scholar]
- 66.Weinbrenner C, Schulze F, Sárváry L, et al. Remote preconditioning by infrarenal aortic occlusion is operative via δ1-opioid receptors and free radicals in vivo in the heart. Cardiovasc Res. 2004;61:591–9. doi: 10.1016/j.cardiores.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Michelsen MM, Stottrup NB, Schmidt MR, et al. Exercise-induced cardioprotection is mediated by a bloodborne, transferable factor. Basic Res Cardiol. 2012;107:260. doi: 10.1007/s00395-012-0260-x. [DOI] [PubMed] [Google Scholar]
- 68.Surendra H, Diaz RJ, Harvey K, et al. Interaction of δ and κ and opioid receptors with adenosine A1 receptors mediates cardioprotection by remote ischemic preconditioning. J Mol Cell Cardiol. 2013;60:142–50. doi: 10.1016/j.yjmcc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Cai ZP, Parajuli N, Zheng X, et al. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res Cardiol. 2012;107:277. doi: 10.1007/s00395-012-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hausenloy DJ, Iliodromitis EK, Andreadou I, et al. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012;26:87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- 71.Xin P, Zhu W, Li J, et al. Combined local ischemic postconditioning and remote perconditioning recapitulate cardioprotective effects of local ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2010;298:H1819–31. doi: 10.1152/ajpheart.01102.2009. [DOI] [PubMed] [Google Scholar]
- 72.Cai Z, Luo W, Zhan H, et al. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–7. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalakech H, Tamareille S, Pons S, et al. Role of hypoxia inducible factor-1alpha in remote limb ischemic preconditioning. J Mol Cell Cardiol. 2013;65:98–104. doi: 10.1016/j.yjmcc.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Skyschally A, van Caster P, Boengler K, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–8. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 75.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein C activation. Am J Physiol Heart Circ Physiol. 1994;266:H1145–52. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 76.Vahlhaus C, Schulz R, Post H, et al. No prevention of ischemic preconditioning by the protein kinase C inhibitor staurosporine in swine. Circ Res. 1996;79:407–14. doi: 10.1161/01.res.79.3.407. [DOI] [PubMed] [Google Scholar]
- 77.Lincoff AM, Roe M, Aylward P, et al. PROTECTION AMI Investigators. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: results of the PROTECTION AMI Randomized Controlled Trial. Eur Heart J. 2014;35:2516–23. doi: 10.1093/eurheartj/ehu177. [DOI] [PubMed] [Google Scholar]
- 78.Albrecht M, Zitta K, Bein B, et al. Remote ischemic preconditioning regulates HIF-1α levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol. 2013;108:314. doi: 10.1007/s00395-012-0314-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhou C, Li L, Li H, et al. Delayed remote preconditioning induces cardioprotection: role of heme oxygenase-1. J Surg Res. 2014;191:51–7. doi: 10.1016/j.jss.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 80.Schulz R, Gres P, Skyschally A, et al. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17:1355–7. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- 81.Brandenburger T, Huhn R, Galas A, et al. Remote ischemic preconditioning preserves Connexin 43 phosphorylation in the rat heart. J Transl Med. 2014;12:228. doi: 10.1186/s12967-014-0228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdul-Ghani S, Heesom KJ, Angelini GD, et al. Cardiac phosphoproteomics during remote ischemic preconditioning: a role for the sarcomeric Z-disk proteins. Biomed Res Int. 2014;2014:767812. doi: 10.1155/2014/767812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pepe S, Liaw NY, Hepponstall M, et al. Effect of remote ischemic preconditioning on phosphorylated protein signaling in children undergoing tetralogy of Fallot repair: a randomized controlled trial. J Am Heart Assoc. 2013;2:e000095. doi: 10.1161/JAHA.113.000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heusch G, Musiolik J, Kottenberg E, et al. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res. 2012;110:111–5. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 85.Gedik N, Thielmann M, Kottenberg E, et al. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS One. 2014;9:e96567. doi: 10.1371/journal.pone.0096567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Contractor H, Stottrup NB, Cunnington C, et al. Aldehyde dehydrogenase-2 inhibition blocks remote preconditioning in experimental and human models. Basic Res Cardiol. 2013;108:343. doi: 10.1007/s00395-013-0343-3. [DOI] [PubMed] [Google Scholar]
- 87.Slagsvold KH, Rognmo O, Hoydal M, et al. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microRNA expression in atrial myocardium during coronary bypass surgery. Circ Res. 2014;114:851–9. doi: 10.1161/CIRCRESAHA.114.302751. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt MR, Stottrup NB, Michelsen MM, et al. Remote ischemic preconditioning impairs ventricular function and increases infarct size after prolonged ischemia in the isolated neonatal rabbit heart. J Thorac Cardiovasc Surg. 2014;147:1049–55. doi: 10.1016/j.jtcvs.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 89.Duan X, Ji B, Wang X, et al. Expression of microRNA-1 and microRNA-21 in different protocols of ischemic conditioning in an isolated rat heart model. Cardiology. 2012;122:36–43. doi: 10.1159/000338149. [DOI] [PubMed] [Google Scholar]
- 90.Pierri MD, Capestro F, Zingaro C, et al. The changing face of cardiac surgery patients: an insight into a Mediterranean region. Eur J Cardiothorac Surg. 2010;38:407–13. doi: 10.1016/j.ejcts.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 91.Biancari F, Onorati F, Faggian G, et al. Determinants of outcome after isolated coronary artery bypass grafting in patients aged ≤50 years (from the Coronary aRtery diseAse in younG adultS study) Am J Cardiol. 2014;113:275–8. doi: 10.1016/j.amjcard.2013.08.038. [DOI] [PubMed] [Google Scholar]
- 92.Brevoord D, Kranke P, Kuijpers M, et al. Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. PLoS ONE. 2012;7:e42179. doi: 10.1371/journal.pone.0042179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Günaydin B, Cakici I, Soncul H, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–6. doi: 10.1006/phrs.1999.0611. [DOI] [PubMed] [Google Scholar]
- 94.Young PJ, Dalley P, Garden A, et al. A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res Cardiol. 2012;107:256. doi: 10.1007/s00395-012-0256-6. [DOI] [PubMed] [Google Scholar]
- 95.Hong DM, Lee EH, Kim HJ, et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J. 2014;35:176–83. doi: 10.1093/eurheartj/eht346. [DOI] [PubMed] [Google Scholar]
- 96.Rahman IA, Mascaro JG, Steeds RP, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation. 2010;122:S53–9. doi: 10.1161/CIRCULATIONAHA.109.926667. [DOI] [PubMed] [Google Scholar]
- 97.Karuppasamy P, Chaubey S, Dew T, et al. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic Res Cardiol. 2011;106:511–9. doi: 10.1007/s00395-011-0185-9. [DOI] [PubMed] [Google Scholar]
- 98.Lee JH, Park YH, Byon HJ, et al. Effect of remote ischaemic preconditioning on ischaemic-reperfusion injury in pulmonary hypertensive infants receiving ventricular septal defect repair. Br J Anaesth. 2012;108:223–8. doi: 10.1093/bja/aer388. [DOI] [PubMed] [Google Scholar]
- 99.Pavione MA, Carmona F, de Castro M, et al. Late remote ischemic preconditioning in children undergoing cardiopulmonary bypass: A randomized controlled trial. J Thorac Cardiovasc Surg. 2012;144:178–83. doi: 10.1016/j.jtcvs.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 100.Lucchinetti E, Bestmann L, Feng J, et al. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: Lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012;116:296–310. doi: 10.1097/ALN.0b013e318242349a. [DOI] [PubMed] [Google Scholar]
- 101.Ahmad AM, Ali GS, Tariq W. Remote ischemic preconditioning is a safe adjuvant technique to myocardial protection but adds no clinical benefit after on-pump coronary artery bypass grafting. Heart Surg Forum. 2014;17:E220–3. doi: 10.1532/HSF98.2014391. [DOI] [PubMed] [Google Scholar]
- 102.McCrindle BW, Clarizia NA, Khaikin S, et al. Remote ischemic preconditioning in children undergoing cardiac surgery with cardiopulmonary bypass: a single-center double-blinded randomized trial. J Am Heart Assoc. 2014;3:e000964. doi: 10.1161/JAHA.114.000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung MM, Kharbanda RK, Konstantinov IE, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–82. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 104.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet. 2007;370:575–9. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 105.Venugopal V, Hausenloy DJ, Ludman A, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–71. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 106.Hong DM, Mint JJ, Kim JH, et al. The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off-pump coronary artery bypass graft surgery. Anaesth Intensive Care. 2010;38:924–9. doi: 10.1177/0310057X1003800518. [DOI] [PubMed] [Google Scholar]
- 107.Thielmann M, Kottenberg E, Boengler K, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–64. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 108.Li L, Luo W, Huang L, et al. Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J Surg Res. 2010;164:e21–6. doi: 10.1016/j.jss.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 109.Zhou W, Zeng D, Chen R, et al. Limb ischemic preconditioning reduces heart and lung injury after an open heart operation in infants. Pediatr Cardiol. 2010;31:22–9. doi: 10.1007/s00246-009-9536-9. [DOI] [PubMed] [Google Scholar]
- 110.Wagner R, Piler P, Bedanova H, et al. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interact Cardiovasc Thorac Surg. 2010;11:758–62. doi: 10.1510/icvts.2010.243600. [DOI] [PubMed] [Google Scholar]
- 111.Ali N, Rizwi F, Iqbal A, et al. Induced remote ischemic pre-conditioning on ischemia-reperfusion injury in patients undergoing coronary artery bypass. J Coll Physicians Surg Pak. 2010;20:427–31. [PubMed] [Google Scholar]
- 112.Wu Q, Gui P, Wu J, et al. Effect of limb ischemic preconditioning on myocardial injury in patients undergoing mitral valve replacement surgery—A randomized controlled trial. Circ J. 2011;75:1885–9. doi: 10.1253/circj.cj-10-1130. [DOI] [PubMed] [Google Scholar]
- 113.Xie JJ, Liao XL, Chen WG, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing heart valve surgery: randomised controlled trial. Heart. 2012;98:384–8. doi: 10.1136/heartjnl-2011-300860. [DOI] [PubMed] [Google Scholar]
- 114.Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 115.Candilio L, Malik A, Ariti C, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2014 Sep 24; doi: 10.1136/heartjnl-2014-306178. [E-pub ahead of print], http://dx.doi:10.1136/heartjnl-2014-306178. [DOI] [PubMed]
- 116.Holmberg FE, Ottas KA, Andreasen C, et al. Conditioning techniques and ischemic reperfusion injury in relation to on-pump cardiac surgery. Scand Cardiovasc J. 2014;48:241–8. doi: 10.3109/14017431.2014.923930. [DOI] [PubMed] [Google Scholar]
- 117.Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA, et al. Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning? Heart. 2006;92:1821–6. doi: 10.1136/hrt.2006.089060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–7. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 119.Davies WR, Brown AJ, Watson W, et al. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–51. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 120.Ahmed RM, El-Haddad AM, Ashraf M, et al. Effect of remote ischemic preconditioning on serum troponin T level following elective percutaneous coronary intervention. Catheter Cardiovasc Interv. 2013;82:E647–53. doi: 10.1002/ccd.24825. [DOI] [PubMed] [Google Scholar]
- 121.Prasad A, Gossl M, Hoyt J, et al. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013;81:930–6. doi: 10.1002/ccd.24443. [DOI] [PubMed] [Google Scholar]
- 122.Luo SJ, Zhou YJ, Shi DM, et al. Remote ischemic preconditioning reduces myocardial injury in patients undergoing coronary stent implantation. Can J Cardiol. 2013;29:1084–9. doi: 10.1016/j.cjca.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 123.Xu X, Zhou Y, Luo S, et al. Effect of remote ischemic preconditioning in the elderly patients With coronary artery disease with diabetes mellitus undergoing elective drug-eluting stent implantation. Angiology. 2014;65:660–6. doi: 10.1177/0003319713507332. [DOI] [PubMed] [Google Scholar]
- 124.Liu Z, Wang YL, Xu D, et al. Late remote ischemic preconditioning provides benefit to patients undergoing elective percutaneous coronary intervention. Cell Biochem Biophys. 2014;70:437–42. doi: 10.1007/s12013-014-9936-1. [DOI] [PubMed] [Google Scholar]
- 125.Zografos TA, Katritsis GD, Tsiafoutis I, et al. Effect of one-cycle remote ischemic preconditioning to reduce myocardial injury during percutaneous coronary intervention. Am J Cardiol. 2014;113:2013–7. doi: 10.1016/j.amjcard.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 126.Ferdinandy P, Hausenloy DJ, Heusch G, et al. Interaction of risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 127.Ibanez B, Macaya C, Sanchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment elevation myocardial infarction patients undergoing primary PCI: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 128.Zhou C, Liu Y, Yao Y, et al. Beta-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: a meta-analysis of 15 randomized trials. J Cardiothorac Vasc Anesth. 2013;27:305–11. doi: 10.1053/j.jvca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 129.Kocsis GF, Pipis J, Fekete V, et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol. 2008;294:H2406–9. doi: 10.1152/ajpheart.00862.2007. [DOI] [PubMed] [Google Scholar]
- 130.Kristiansen SB, Lofgren B, Stottrup NB, et al. Ischaemic preconditioning does not protect the heart in obese and lean animal models of type 2 diabetes. Diabetologia. 2004;47:1716–21. doi: 10.1007/s00125-004-1514-4. [DOI] [PubMed] [Google Scholar]
- 131.Tsang A, Hausenloy DJ, Mocanu MM, et al. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–4. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 132.Povlsen JA, Lofgren B, Dalgas C, et al. Protection against myocardial ischemia-reperfusion injury at onset of type 2 diabetes in Zucker diabetic fatty rats is associated with altered glucose oxidation. PLoS One. 2013;8:e64093. doi: 10.1371/journal.pone.0064093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sloth AS, Schmidt MR, Munk K, et al. No effect modification by cardiovascular risk factors and medication on the efficacy of remote ischemic conditioning in patients with ST-elevation myocardial infarction. Eur Heart J. 2014;35(S1):323. doi: 10.1093/eurheartj/eht369. (abstr) [DOI] [PubMed] [Google Scholar]
- 134.Kottenberg E, Thielmann M, Kleinbongard P, et al. Myocardial protection by remote ischaemic pre-conditioning is abolished in sulphonylurea-treated diabetics undergoing coronary revascularisation. Acta Anaesthesiol Scand. 2014;58:453–62. doi: 10.1111/aas.12278. [DOI] [PubMed] [Google Scholar]
- 135.D'Ascenzo F, Cavallero E, Moretti C, et al. Remote ischaemic preconditioning in coronary artery bypass surgery: a meta-analysis. Heart. 2012;98:1267–71. doi: 10.1136/heartjnl-2011-301551. [DOI] [PubMed] [Google Scholar]
- 136.Schmidt M, Jacobsen JB, Lash TL, et al. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. Br Med J. 2012;344:e356. doi: 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moran AE, Forouzanfar MH, Roth GA, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1483–92. doi: 10.1161/CIRCULATIONAHA.113.004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moran AE, Forouzanfar MH, Roth GA, et al. The global burden of ischemic heart disease in 1990 and 2010: the global burden of disease 2010 study. Circulation. 2014;129:1493–501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–9. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Heusch G, Libby P, Gersh B, et al. Cardiovascular remodeling in coronary artery disease and heart failure. Lancet. 2014;383:1933–43. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Munk K, Andersen NH, Schmidt MR, et al. Remote Ischemic Conditioning in Patients With Myocardial Infarction Treated With Primary Angioplasty: Impact on Left Ventricular Function Assessed by Comprehensive Echocardiography and Gated Single-Photon Emission CT. Circ Cardiovasc Imaging. 2010;3:656–62. doi: 10.1161/CIRCIMAGING.110.957340. [DOI] [PubMed] [Google Scholar]
- 142.Rentoukas I, Giannopoulos G, Kaoukis A, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. J Am Coll Cardiol Intv. 2010;3:49–55. doi: 10.1016/j.jcin.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 143.Crimi G, Pica S, Raineri C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. J Am Coll Cardiol Intv. 2013;6:1055–63. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 144.Prunier F, Angoulvant D, Saint Etienne C, et al. The RIPOST-MI study, assessing remote ischemic perconditioning alone or in combination with local ischemic postconditioning in ST-segment elevation myocardial infarction. Basic Res Cardiol. 2014;109:400. doi: 10.1007/s00395-013-0400-y. [DOI] [PubMed] [Google Scholar]
- 145.Sloth AD, Schmidt MR, Munk K, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–75. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 146.White SK, Frohlich GM, Sado DM, et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patientswith ST-segment elevation myocardial infarction. J Am Coll Cardiol Intv. 2014 Sep 17; doi: 10.1016/j.jcin.2014.05.015. [E-pub ahead of print, http://dx.doi:10.1016/j.jcin.2014.05.015. [DOI] [PubMed]
- 147.Hauslenoy D. [Accessed November 1, 2014];Effect of Remote Ischaemic Conditioning in Heart Attack Patients (ERIC-LYSIS) 2014 Jul; Available at: http://clinicaltrials.gov/show/NCT02197117.
- 148.van 't Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–6. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 149.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–67. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 150.Miller TD, Christian TF, Hopfenspirger MR, et al. Infarct size after acute myocardial infarction measured by quantitative tomographic 99mTc sestamibi imaging predicts subsequent mortality. Circulation. 1995;92:334–41. doi: 10.1161/01.cir.92.3.334. [DOI] [PubMed] [Google Scholar]
- 151.Sorensson P, Saleh N, Bouvier F, et al. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart. 2010;96:1710–5. doi: 10.1136/hrt.2010.199430. [DOI] [PubMed] [Google Scholar]
- 152.Lonborg J, Vejlstrup N, Kelbaek H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–9. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 153.Erlinge D, Gotberg M, Lang I, et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction (The CHILL-MI trial): a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol. 2014;63:1857–65. doi: 10.1016/j.jacc.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 154.Bøtker HE, Kaltoft AK, Pedersen SF, et al. Measuring myocardial salvage. Cardiovasc Res. 2012;94:266–75. doi: 10.1093/cvr/cvs081. [DOI] [PubMed] [Google Scholar]
- 155.Ubachs JF, Engblom H, Koul S, et al. Myocardium at risk can be determined by ex vivo T2-weighted magnetic resonance imaging even in the presence of gadolinium: comparison to myocardial perfusion single photon emission computed tomography. Eur Heart J Cardiovasc Imaging. 2013;14:261–8. doi: 10.1093/ehjci/jes142. [DOI] [PubMed] [Google Scholar]
- 156.Langhans B, Nadjiri J, Jahnichen C, et al. Reproducibility of area at risk assessment in acute myocardial infarction by T1- and T2-mapping sequences in cardiac magnetic resonance imaging in comparison to Tc99m-sestamibi SPECT. Int J Cardiovasc Imaging. 2014;30:1357–63. doi: 10.1007/s10554-014-0467-z. [DOI] [PubMed] [Google Scholar]
- 157.Mewton N, Rapacchi S, Augeul L, et al. Determination of the myocardial area at risk with pre- versus post-reperfusion imaging techniques in the pig model. Basic Res Cardiol. 2011;106:1247–57. doi: 10.1007/s00395-011-0214-8. [DOI] [PubMed] [Google Scholar]
- 158.Thuny F, Lairez O, Roubille F, et al. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:2175–81. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 159.Pedersen CM, Barnes G, Schmidt MR, et al. Ischaemia-reperfusion injury impairs tissue plasminogen activator release in man. Eur Heart J. 2012;33:1920–7. doi: 10.1093/eurheartj/ehr380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pedersen CM, Cruden NL, Schmidt MR, et al. Remote ischemic preconditioning prevents systemic platelet activation associated with ischemia-reperfusion injury in humans. J Thromb Haemost. 2011;9:404–7. doi: 10.1111/j.1538-7836.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- 161.Konstantinov IE, Arab S, Kharbanda RK, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–50. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 162.Hausenloy DJ, Baxter G, Bell R, et al. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–86. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wei M, Xin P, Li S, et al. Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res. 2011;108:1220–5. doi: 10.1161/CIRCRESAHA.110.236190. [DOI] [PubMed] [Google Scholar]
- 164.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–13. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 165.Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–81. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 166.Hausenloy DJ, Bøtker HE, Condorelli G, et al. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- 167.Ovize M, Baxter GF, Di Lisa F, et al. Postconditioning and protection from reperfusion injury: where do we stand? Cardiovasc Res. 2010;87:406–23. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 168.Roubille F, Mewton N, Elbaz M, et al. No post-conditioning in the human heart with thrombolysis in myocardial infarction flow 2–3 on admission. Eur Heart J. 2014;35:1675–82. doi: 10.1093/eurheartj/ehu054. [DOI] [PubMed] [Google Scholar]
- 169.Rentrop KP, Cohen M, Blanke H, et al. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985;5:587–92. doi: 10.1016/s0735-1097(85)80380-6. [DOI] [PubMed] [Google Scholar]
- 170.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Lancet. 1994;343:311–22. [PubMed] [Google Scholar]
- 171.Schömig A, Mehilli J, Antoniucci D, et al. Beyond 12 hours Reperfusion AlternatiVe Evaluation (BRAVE-2) Trial Investigators. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: a randomized controlled trial. JAMA. 2005;293:2865–72. doi: 10.1001/jama.293.23.2865. [DOI] [PubMed] [Google Scholar]
- 172.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–61. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 173.Ferdinandy P, Szilvassy Z, Baxter GF. Adaptation to myocardial stress in disease states: is preconditioning a healthy heart phenomenon? Trends Pharmacol Sci. 1998;19:223–8. doi: 10.1016/s0165-6147(98)01212-7. [DOI] [PubMed] [Google Scholar]
- 174.Whittington HJ, Babu GG, Mocanu MM, et al. The diabetic heart: too sweet for its own good? Cardiol Res Pract. 2012;2012:845698. doi: 10.1155/2012/845698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ludman AJ, Yellon DM, Hausenloy DJ. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech. 2010;3:35–8. doi: 10.1242/dmm.003855. [DOI] [PubMed] [Google Scholar]
- 176.Loubani M, Ghosh S, Galinanes M. The aging human myocardium: tolerance to ischemia and responsiveness to ischemic preconditioning. J Thorac Cardiovasc Surg. 2003;126:143–7. doi: 10.1016/s0022-5223(02)73601-5. [DOI] [PubMed] [Google Scholar]
- 177.Hassouna A, Loubani M, Matata BM, et al. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc Res. 2006;69:450–8. doi: 10.1016/j.cardiores.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 178.Sivaraman V, Hausenloy DJ, Wynne AM, et al. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14:1740–6. doi: 10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roubille F, Lairez O, Mewton N, et al. Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol. 2012;107:275. doi: 10.1007/s00395-012-0275-3. [DOI] [PubMed] [Google Scholar]
- 180.Przyklenk K. Efficacy of cardioprotective 'conditioning' strategies in aging and diabetic cohorts: the co-morbidity conundrum. Drugs Aging. 2011;28:331–43. doi: 10.2165/11587190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 181.Cohen MV, Downey JM. Combined cardioprotectant and antithrombotic actions of platelet P2Y12 receptor antagonists in acute coronary syndrome: just what the doctor ordered. J Cardiovasc Pharmacol Ther. 2014;19:179–90. doi: 10.1177/1074248413508465. [DOI] [PubMed] [Google Scholar]
- 182.Linden MD, Whittaker P, Frelinger AL, 3rd, et al. Preconditioning ischemia attenuates molecular indices of platelet activation-aggregation. J Thromb Haemost. 2006;4:2670–7. doi: 10.1111/j.1538-7836.2006.02228.x. [DOI] [PubMed] [Google Scholar]
- 183.Sarma J, Laan CA, Alam S, et al. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–71. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- 184.Burdess A, Nimmo AF, Campbell N, et al. Perioperative platelet and monocyte activation in patients with critical limb ischemia. J Vasc Surg. 2010;52:697–703. doi: 10.1016/j.jvs.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 185.Xu Y, Huo Y, Toufektsian MC, et al. Activated platelets contribute importantly to myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;290:H692–9. doi: 10.1152/ajpheart.00634.2005. [DOI] [PubMed] [Google Scholar]
- 186.Battipaglia I, Scalone G, Milo M, et al. Upper arm intermittent ischaemia reduces exercise-related increase of platelet reactivity in patients with obstructive coronary artery disease. Heart. 2011;97:1298–303. doi: 10.1136/hrt.2011.226415. [DOI] [PubMed] [Google Scholar]
- 187.Stazi A, Scalone G, Laurito M, et al. Effect of remote ischemic preconditioning on platelet activation and reactivity induced by ablation for atrial fibrillation. Circulation. 2014;129:11–7. doi: 10.1161/CIRCULATIONAHA.113.005336. [DOI] [PubMed] [Google Scholar]
- 188.Shimizu M, Saxena P, Konstantinov IE, et al. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158:155–61. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 189.Saxena P, Aggarwal S, Misso NL, et al. Remote ischaemic preconditioning down-regulates kinin receptor expression in neutrophils of patients undergoing heart surgery. Interact Cardiovasc Thorac Surg. 2013;17:653–8. doi: 10.1093/icvts/ivt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saxena P, Shaw OM, Misso NL, et al. Remote ischemic preconditioning stimulus decreases the expression of kinin receptors in human neutrophils. J Surg Res. 2011;171:311–6. doi: 10.1016/j.jss.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 191.Shimizu M, Konstantinov IE, Kharbanda RK, et al. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol (Oxf) 2007;190:103–9. doi: 10.1111/j.1748-1716.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 192.Enko K, Nakamura K, Yunoki K, et al. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J Physiol Sci. 2011;61:507–13. doi: 10.1007/s12576-011-0172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a KATP channel-dependent mechanism. Circulation. 2007;116:1386–95. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 194.Moro L, Pedone C, Mondi A, et al. Effect of local and remote ischemic preconditioning on endothelial function in young people and healthy or hypertensive elderly people. Atherosclerosis. 2011;219:750–2. doi: 10.1016/j.atherosclerosis.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 195.Jones H, Hopkins N, Bailey TG, et al. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. 2014;27:918–25. doi: 10.1093/ajh/hpu004. [DOI] [PubMed] [Google Scholar]
- 196.Jones H, Nyakayiru J, Bailey TG, et al. Impact of eight weeks of repeated ischaemic preconditioning on brachial artery and cutaneous microcirculatory function in healthy males. Eur J Prev Cardiol. 2014 Aug 21; doi: 10.1177/2047487314547657. [E-pub ahead of print], http://dx.doi:10.1177/2047487314547657. [DOI] [PubMed]
- 197.Manchurov V, Ryazankina N, Khmara T, et al. Remote ischemic preconditioning and endothelial function in patients with acute myocardial infarction and primary PCI. Am J Med. 2014;127:670–3. doi: 10.1016/j.amjmed.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 198.Kono Y, Fukuda S, Hanatani A, et al. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Devel Ther. 2014;8:1175–81. doi: 10.2147/DDDT.S68715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Remote Preconditioning Trialists' Group. Healy DA, Khan WA, Wong CS, et al. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta-analysis. Int J Cardiol. 2014;176:20–31. doi: 10.1016/j.ijcard.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 200.Hausenloy DJ, Candilio L, Laing C, et al. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol. 2011;101:339–48. doi: 10.1007/s00392-011-0397-x. [DOI] [PubMed] [Google Scholar]
- 201.Meybohm P, Zacharowski K, Cremer J, et al. Remote ischaemic preconditioning for heart surgery. The study design for a multi-center randomized double-blinded controlled clinical trial--the RIPHeart-Study. Eur Heart J. 2012;33:1423–6. [PubMed] [Google Scholar]
- 202.Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: Recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–9. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Heusch G. Remote conditioning: the future of cardioprotection? J Cardiovasc Med (Hagerstown ) 2013;14:176–9. doi: 10.2459/JCM.0b013e328358e507. [DOI] [PubMed] [Google Scholar]