Abstract
BACKGROUND
Functional decline in stem cell-mediated regeneration contributes to aging associated with cellular senescence in c-kit+ cardiac progenitor cells (CPCs). Clinical implementation of CPC-based therapy with elderly patients would benefit tremendously from understanding molecular characteristics of senescence to antagonize aging. Nucleostemin (NS) is a nucleolar protein regulating stem cell proliferation and pluripotency.
OBJECTIVES
The goal is to demonstrate that NS preserves characteristics associated with “stemness” in CPCs and antagonizes myocardial senescence and aging.
METHODS
CPCs isolated from human fetal (FhCPC) and adult failing (AhCPC) hearts, as well as young (YCPC) and old mice (OCPC), were studied for senescence characteristics and NS expression. Heterozygous knockout mice with one functional allele of NS (NS+/−) were used to demonstrate that NS preserves myocardial structure and function and slows characteristics of aging.
RESULTS
NS expression is decreased in AhCPCs relative to FhCPC, correlating with lowered proliferation potential and shortened telomere length. AhCPC characteristics resemble OCPCs, which have a phenotype induced by NS silencing, resulting in cell flattening, senescence, multinucleated cells, decreased S phase progression, diminished expression of stemness markers and up-regulation of p53 and p16. CPC senescence resulting from NS loss is partially p53 dependent and is rescued by concurrent silencing of p53. Mechanistically, NS induction correlates with Pim-1 kinase-mediated stabilization of c-Myc. Engineering OCPCs and AhCPCs to overexpress NS decreases senescent and multinucleated cells, restores morphology, and antagonizes senescence, thereby preserving phenotypic properties of “stemness.” Early cardiac aging with decline in cardiac function, increase in senescence markers p53 and p16, telomere attrition, and accompanied CPC exhaustion is evident in NS+/− mice.
CONCLUSIONS
Youthful properties and antagonism of senescence in CPCs and the myocardium is consistent with a role for NS downstream from Pim-1 signaling that enhances cardiac regeneration.
Keywords: Senescence, aging, senescence, signal transduction
Introduction
Cardiac aging is a heterogeneous process accompanied by loss of tissue homeostasis and decreased organ function. Decline in stem cell function and regenerative capacity is a leading factor contributing to aging (1). The adult heart is home to resident c-kit+ cardiac progenitor cells (CPCs) that are clonogenic, self-renewing and can differentiate into cardio-myogenic lineages (2), but aging alters CPC properties, leading to progressive accumulation of senescent stem cells with decreased proliferative and regenerative potential (1). Identifying “stem cell associated molecules” and their mechanism of regulation is essential to enhance the therapeutic potential of stem cell-based repair. As cardiac stem cell therapy would be targeted to a predominantly geriatric population, defining molecular antagonism of senescence in a stem cell context would be both clinically relevant and highly significant.
A stem cell-related, nucleolar protein required for cell cycle progression and proliferation (3, 4), nucleostemin (NS) delays senescence, with mouse embryonic fibroblasts isolated from NS heterozygous knockout mice (NS+/−) exhibiting decreased proliferation and increased senescent cells upon passaging in culture (5). NS is also associated with maintenance of stem cell pluripotency, survival, and commitment (4, 6, 7). Expression of NS drops rapidly prior to stem cell differentiation and its loss increases differentiation in multiple stem cells (4, 7). Associated with increased proliferation of cardiac myocytes and CPCs, cardiac NS expression declines with aging (8). NS also functions as a nucleolar stress sensor in response to cardiac stress and regulates p53 (6), a cell cycle inhibitor typically associated with increased senescence (9). NS-mediated regulation of p53 is cell-type specific (4, 10, 11) such that consequences of NS action in selected cell-type populations remain obscure. The role of NS in antagonizing senescence in adult stem cells, including CPCs, remains speculative and requires investigation.
NS expression in the heart coincides with Pim-1, a serine threonine kinase that enhances regenerative potential by exerting pro-survival and pro-proliferative effects (8, 12, 13). Coincident functions of Pim-1 and NS suggest that NS may be regulated by Pim-1, which phosphorylates and stabilizes transcription factor c-Myc and, via c-Myc, regulates multiple functions in cancer and stem cells, including CPCs (14–17). Collectively these observations prompt the hypothesis that Pim-1 regulates NS via c-Myc in CPCs. Delineating this molecular pathway on a mechanistic level provides substantial insight into the underlying basis for regulation of senescence and suggests therapeutic intervention strategies that might promote youthfulness in the CPC population. This study delineates the role of NS in CPC “stemness” as defined by morphological and functional characteristics and expression of molecular markers and establishes a relationship to Pim-1 by: 1) determining phenotypic characteristics of senescent CPCs; 2) establishing NS antagonism of senescence in CPCs; 3) delineating upstream molecular signaling regulating NS; 4) demonstrating NS overexpression as an effective strategy to enhance ‘youthful’ characteristics of senescent CPCs; and 5) validating that NS is required to maintain myocardial youth.
METHODS
Cell culturing involved c-kit+ CPCs isolated from 3- and 13-month-old male FVB mice hearts, human patients receiving a left ventricular assist device (LVAD), and spontaneously aborted human fetuses at 16–17 weeks of age. The cardiac region from nonfailing human hearts included the mid left ventricle free wall with full thickness from epicardium to endocardium. Human fetal and adult failing myocardium were obtained, as previously described (13). The adult and fetal whole heart tissue was used either for isolation of adult human CPCs (AhCPCs) or fetal human CPCs (FhCPCs) or for biochemical analyses. Each sample was isolated from a different patient; tissue from individual patients was not combined. Experimental values obtained from multiple patients of a sample category (fetal, adult failing, adult nonfailing) were averaged for statistical analyses.
The Online Supplement contains a more extensive explanation of the methods.
RESULTS
CELLULAR SENESCENCE IN CPCS CORRELATES WITH NS LOSS
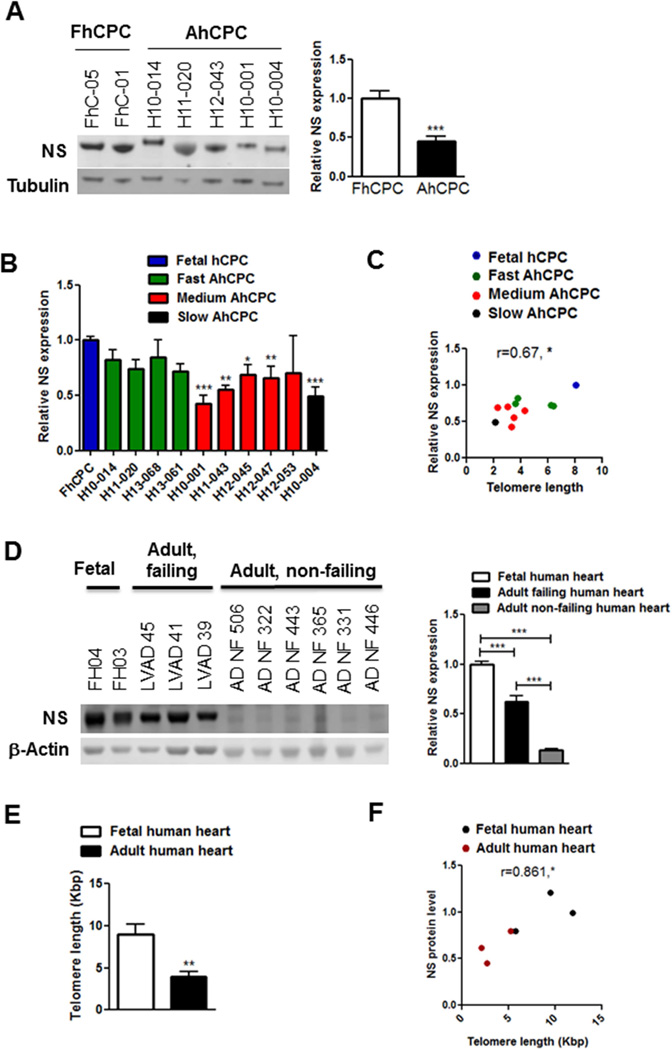
AhCPCs were compared with FhCPCs, which served as a reference for “youthful phenotypic characteristics” (13). NS is significantly decreased in multiple AhCPC lines relative to FhCPCs (−55%, Figure 1A) and NS loss correlates with impaired proliferative potential of AhCPCs (Figure 1B) consistent with prior reports assessing AhCPC growth characteristics (13). NS expression strongly correlates with telomere length (Figure 1C) as determined previously (13). The adult human heart under normal conditions expresses relatively low levels of NS (Figure 1D), consistent with nominal cell growth and proliferation under basal conditions. Corroborating findings in CPC lines, specifically human myocardial lysates of adult failing hearts, revealed significant reduction in NS expression (−43%, Figure 1D) and telomere length (−56%, Figure 1E) relative to fetal human hearts, reinforcing a significant correlation between NS expression and telomere length (Figure 1F). Collectively the results demonstrate reduced NS levels associated with increased senescence and aging.
FIGURE 1. NS Expression in Human CPCs and Myocardium.
(A) Immunoblot and densitometric analyses of nucleostemin (NS) expression in multiple adult human cardiac progenitor cells (AhCPC) lines relative to fetal human CPCs (FhCPC). Line numbers are indicated above the blot. (N = 3 FhCPC lines and 9 AhCPC lines) (B) NS expression in AhCPC lines classified as fast, medium, or slow growers. (C) Pearson correlation coefficient between NS expression and telomere length (Kbp) in hCPCs (r = 0.67). (B–C: N = 10 AhCPCs [green, red, black bars/dots], mean of 3 FhCPCs [blue bar/dot]). (D) Immunoblot and densitometric analyses shows NS expression in adult failing (LVAD) and non-failing (ADNF) hearts relative to fetal hearts (FH; N = 3 fetal, 3 failing and 6 NF hearts). (E) Telomere length (Kbp) decreases with aging. N = 4 fetal, 6 LVAD hearts. (F) Pearson correlation coefficient between NS expression and telomere length in human hearts (r = 0.86) is shown. hCPCs were at mid passages (p6–8).
*p < 0.05; †p < 0.01; ‡p < 0.001
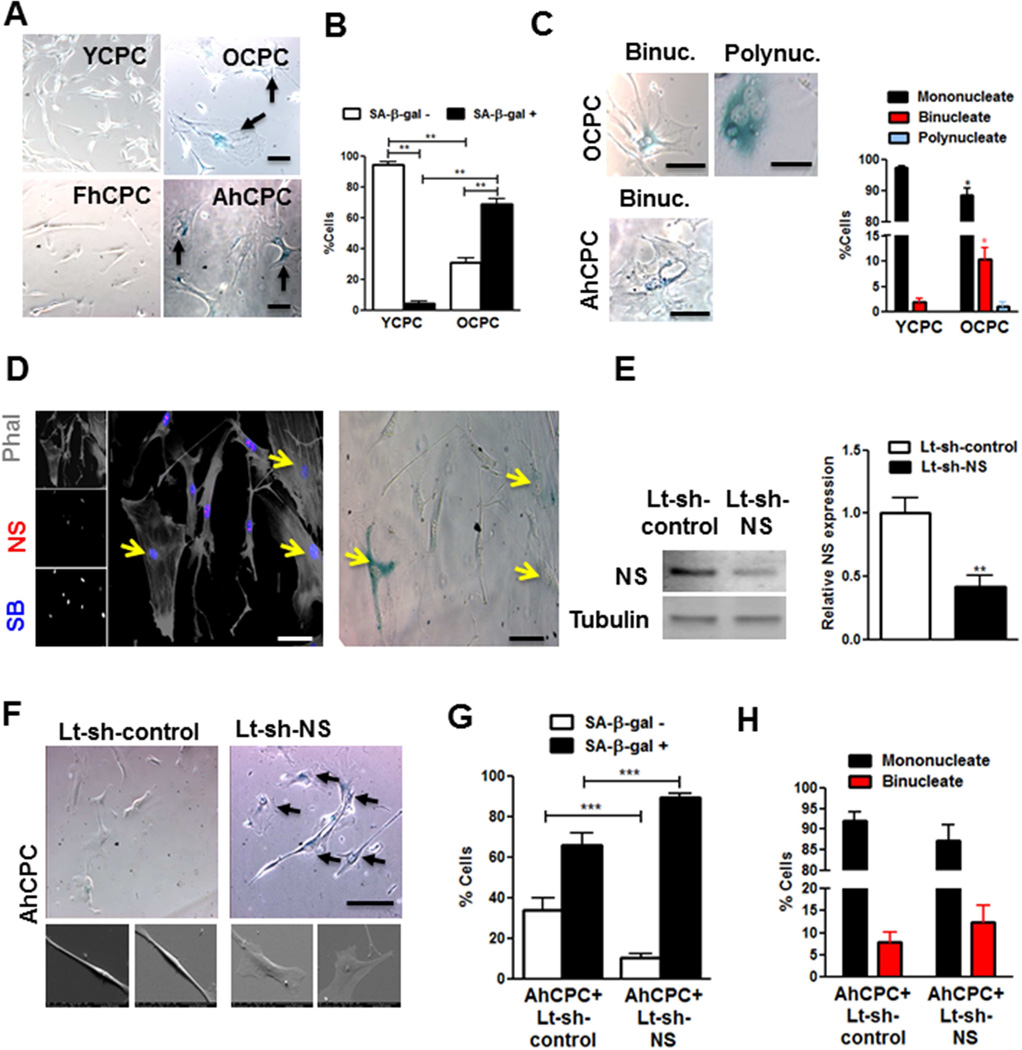
Cellular senescence was evaluated in AhCPCs, FhCPCs, and CPCs from old (13 months, OCPCs) and young mice (3 months, YCPCs) to better define NS’s cell-specific role in senescence as evidenced by phenotypic and molecular characteristics. Morphological changes associated with “cell flattening,” observed as increased cross-sectional area and roundness and decreased length/width ratio, were evident in OCPCs and AhCPCs relative to YCPCs and FhCPCs (Figure 2A), consistent with previous findings (13). Cell flattening was also evident 3-dimensionally as thinner cells (Online Supplement Figure 1). Senescent cell frequency increased in OCPCs (15-fold) and AhCPCs relative to YCPC as revealed by senescence-associated beta-galactosidase (SA-β-gal, Figure 2AB). Decreased proliferation (−75%;) consistent with increased G1 (28%) and diminished S (−62%) and G2 (−59%) cell cycle phases was evident in OCPCs (Online Supplement Figure 2AB). Cellular senescence in CPCs was also associated with multinucleation (Figure 2C), predominantly by OCPCs and AhCPCs becoming increasingly binucleated with polynucleate cells (>2 nuclei) detected only in mouse OCPCs. Furthermore, SA-β-gal+ binucleate and polynucleate cell number was increased in OCPCs (Online Supplement Figure 2C), thereby linking cell-autonomous multinucleation with senescence. Corroborating in vivo findings with myocardial sections reveal senescent CPCs, identifiable as large c-kit+ cells concurrently labeled for γ-H2AX, a deoxyribonucleic acid (DNA) damage response and senescence marker (18), were significantly more abundant (35%) in sections from 12-month-old versus 3-month-old mouse hearts (Online Supplement Figure 2DE). Binucleated CPCs were increased by 3-fold in 12-month-old hearts and 67% of binucleated CPCs were γ-H2AX+ (Online Supplement Figure 2FG), reinforcing the relationship between binucleation and senescence. Concurrence of lowered NS expression in OCPCs with flattened morphology and senescence occurs in conjunction with binucleated SA-β-gal+ cells (Figure 2D, Online Supplement Figure 2H). Flattened and binucleate AhCPCs consistently display dramatically reduced NS levels relative to FhCPCs (Online Supplement Figure 2I), demonstrating a cell autonomous role for decline in NS and cellular senescence.
FIGURE 2. NS Loss Causes Altered Morphology During Senescence.
(A) Phase contrast microscopy of young (YCPC) and old (OCPC) mouse CPCs, and FhCPC and AhCPC (lines FH-05 and H10-004) stained with senescence associated beta-galactosidase (SA-β-gal). Arrows indicate blue, senescent cells. (B) Graph indicates senescent cell counts. (C) Phase contrast images of SA-β-gal+ binucleate (2 nuclei, binuc.) and polynucleate (>2 nuclei, polynuc.) cells; and percentage of multinucleate YCPC/OCPCs are shown. (D) NS declines in flat, senescent OCPCs (arrows). Confocal microscopy of OCPCs stained with Phalloidin (Phal, grey), NS (red), and Sytox blue (SB, blue) (left). Phase contrast image of the same cells treated with SA-β-gal (right). (E) Immunoblot and densitometric analyses showing NS knockdown in YCPCs (N = 12). (F) Phase contrast (top panel, Line H10-005) and electron micrographs (bottom panel, Line H11-020) of AhCPCs with NS knockdown demonstrating flattened, SA-β-gal+ cells (arrows). (G–H) Senescent (G) and binucleated cell count (H) (N>200 cells). Scale bar: 100µm (A,C,F); 50 µm (D).
*p < 0.05; **p < 0.01; ***p < 0.001 (relative to each corresponding group in YCPCs/AhCPC+Lt-sh-control in C)
Lt-sh = Lentivirus harboring short hairpin ribonucleic acid; Other abbreviations as in Figure 1.
Striking morphologic changes demonstrate functional impact of NS silencing in multiple AhCPC and mouse CPC isolates (−75%) prompting cell flattening and increases in senescent (37%) and binucleated (48%) cells (Figure 2E–H). Similar findings of flattening, increased SA-β-gal activity (3.5-fold), and multinucleation also were observed following NS silencing in mouse CPCs (Online Supplement Figure 3), further reinforcing a causal role for NS in regulating CPC morphology and senescence.
PROLIFERATION AND MAINTENANCE OF ‘STEMNESS’ IN CPCS REQUIRES NS
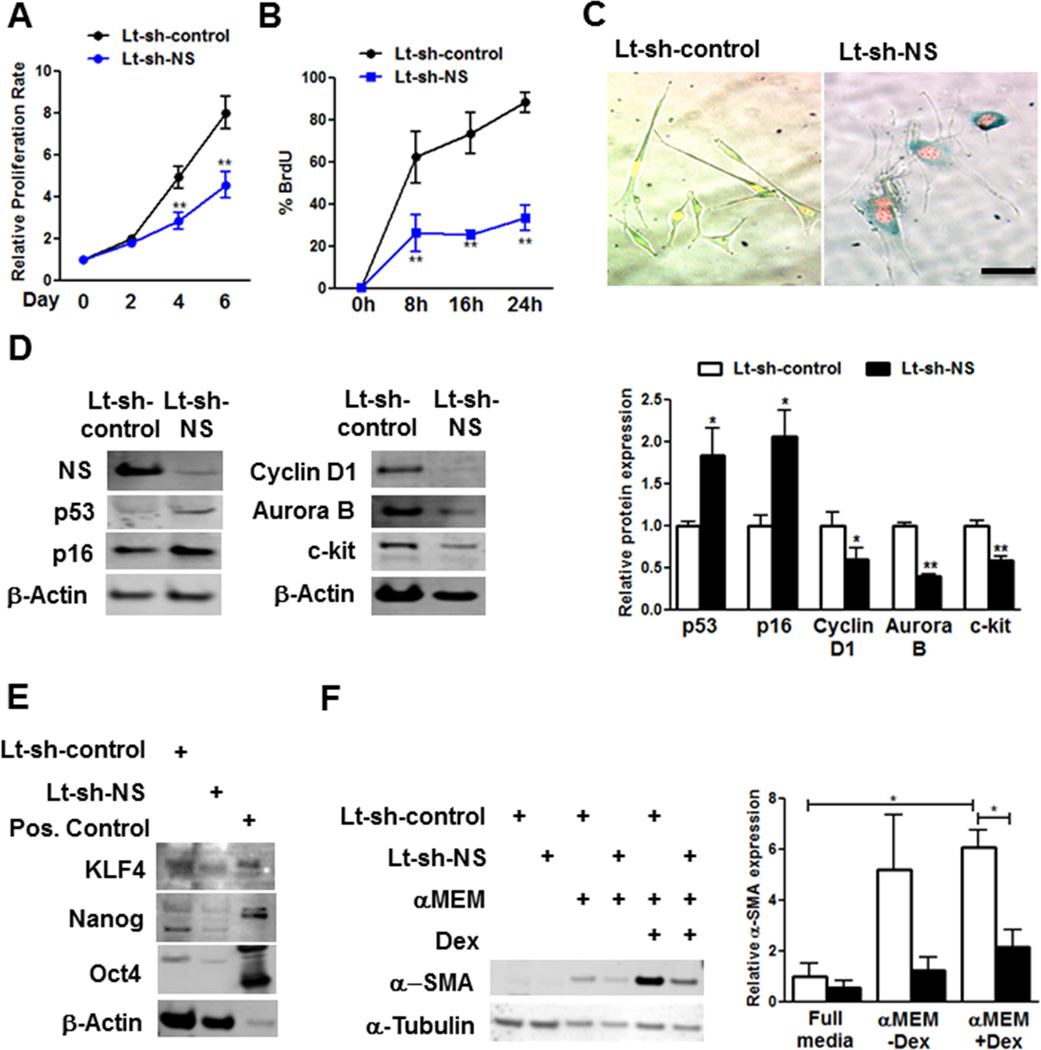
Decline in proliferation, (−42%, Figure 3A) correlates with increased G1 (7%) concomitant with decreases in S (−69%) and G2 phases (−47%; Online Supplement Figure 4A) in YCPCs upon NS silencing. Reduction in S phase upon NS knockdown was confirmed by BrdU incorporation (−62%, Figure 3B). Cell cycle stage relative to NS-mediated regulation was revealed using nuclear-localized fluorescent ubiquitination-based cell cycle indicator that shows G1 phase by expression of truncated Cdt1 fused to Kusabira-Orange (mKO), S to M phase by expression of truncated geminin fused to Azami green (AzG), with G1-S phase transition expressing both and appearing yellow (19). NS knockdown-induced senescence prompts cell cycle arrest as evidenced by decreased S to M phase with fewer Azami green + cells (−42%, Online Supplement Figure 4B) together with G1 phase accumulation revealed by mKO+ seen in SA-β-gal+ senescent cells (Figure 3C).
FIGURE 3. NS Silencing Causes Cell Cycle Arrest and Senescence in YCPCs.
(A) Proliferation rate determined by Cyquant assay (N = 5) (B) BrdU incorporation measured by fluorescence activated cell sorting (FACS) (N = 3). (C) Images of YCPCs expressing fluorescence ubiquitination cell cycle indicator constructs stained with SA-β-gal. (D) Immunoblot and densitmetric analyses of p53 (N = 13), p16 (N = 5), Cyclin D1 (N = 3), Aurora B (N = 3), and c-kit (N = 6) in YCPCs. (E) Immunoblots of multipotency markers KLF4, Nanog, and Oct 4 in YCPCs. Positive (Pos.) control: mouse embryonic stem cell lysate. (F) Immunoblot and densitometric analyses of α-smooth muscle actin (α-SMA) expression in YCPCs cultured in normal or minimal essential media (αMEM) with or without dexamethasone (Dex) (N = 3). Scale bar: 100γm.
*p < 0.05; **p < 0.01
Cell cycle inhibitors associated with senescence (9) were increased by NS silencing in YCPCs (Figure 3D) and AhCPCs (Online Supplement Figure 4C) as exemplified by higher p53 (1.9-fold) and p16 (2-fold) expression without affecting basal cell death (Online Supplement Figure 4D). Decreases in Cyclin D1 (−41%) and Aurora B kinase (−60%), Figure 3D) required for progression through G1 and cytokinesis respectively followed NS knockdown (20). Reduction in c-kit protein (−42%) and messenger ribonucleic acid (mRNA) expression (−46%) were observed after NS knockdown (Figure 3D, Online Supplement Figure 4E). Other markers of multipotency such as KLF4, Nanog, and Oct4 were also decreased upon NS knockdown, consistent with loss of stemness (Figure 3E). Early mRNA markers for lineage commitment including cardiomyogenic genes SM-22, α-smooth muscle actin (α-SMA) and myocyte enhancer-binding factor-2c were significantly decreased upon NS silencing in YCPCs (Online Supplement Figure 4E) Commitment of CPCs by dexamethasone (Dex), a non-specific inducer of differentiation, was similarly blunted as determined by lowered α-SMA protein expression (Figure 3F). Collectively, these results indicate that the senescence resulting from NS does not operate via initiation of enhanced lineage commitment.
CPC SENESCENCE INDUCED BY NS KNOCKDOWN IS PARTLY P53 DEPENDENT
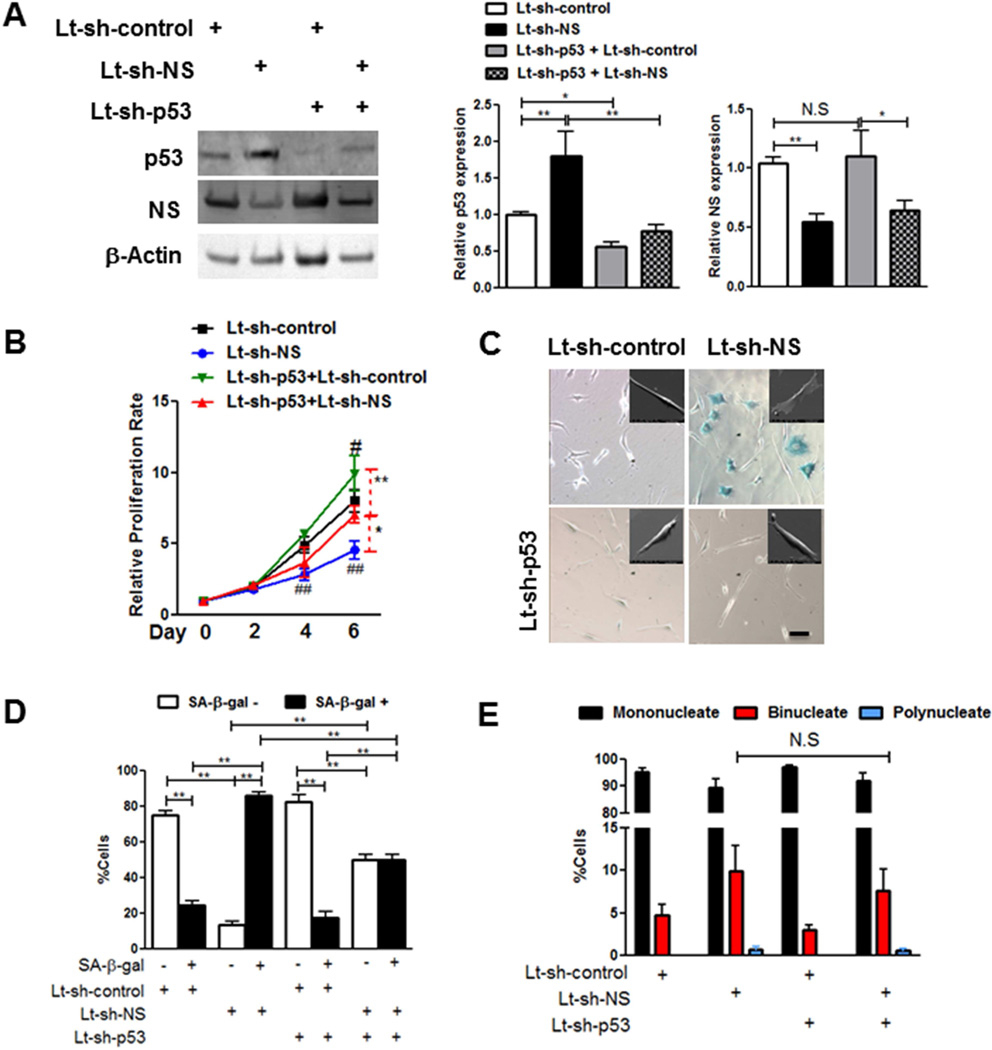
Mechanistic involvement of p53 in NS silencing-induced senescence was determined by stably silencing p53 (−46%) prior to NS knockdown in YCPCs and AhCPCs (Figure 4A). p53 knockdown increased proliferation of YCPCs both at baseline (25%) and after NS silencing (53.5%) (Figure 4B). NS silencing-induced morphological flattening was inhibited by concurrent p53 knockdown in AhCPCs similar to findings in YCPCs (Figure 4C, Online Supplement Figure 5A–C). Furthermore, loss of p53 significantly attenuated NS knockdown-induced increase in SA-β-gal+ cells (−43%) (Figure 4CD). However, NS silencing-induced multinucleation is p53 independent, as concurrent loss of p53 and NS does not decrease the percentage of multinucleate cells (Figure 4E). Thus, functional effects of p53 knockdown upon senescence characteristics were primarily evident in the mononucleate cell population, supporting the contention that NS silencing-induced senescence is partially p53 dependent.
FIGURE 4. NS Silencing-mediated CPC Senescence Is Partly p53 Dependent.
NS is silenced in YCPCs following stable knockdown of p53. (A) Immunoblots and densitometric analyses (N = 10 each). (B) Proliferation rate measured by Cyquant assay (N = 3). (C) Phase contrast micrographs of YCPCs stained with SA-β-gal. Insets represent electron micrographs of AhCPCs (Line H10-005, mid passage) transduced with indicated viruses. (D–E) Senescent (D) and multinucleate (E) cell counts in YCPCs (N = 700 cells). Scale bar: 100µm.
*p < 0.05;**p < 0.01; #p < 0.05 and ##p < 0.01 relative to each day Lt-sh-control values
REGULATION OF NS EXPRESSION DEPENDS UPON PIM-1-MEDIATED STABILIZATION OF C-MYC
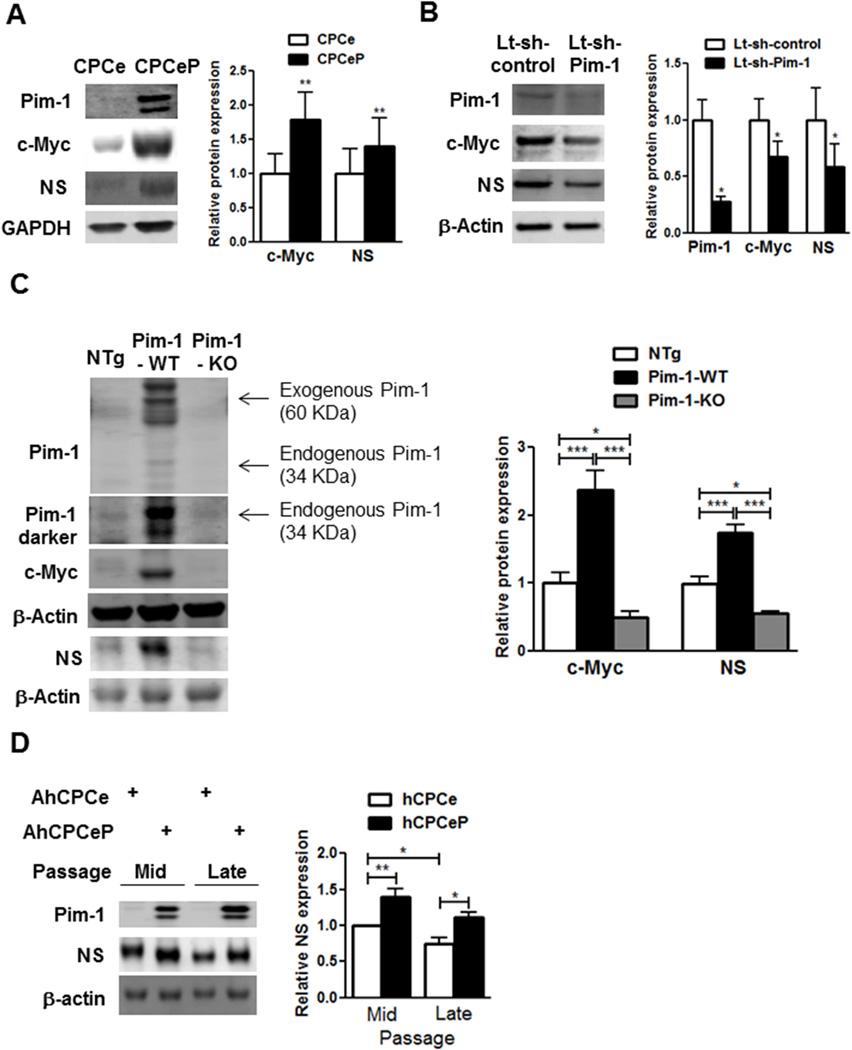
Establishing that NS influences CPC proliferation and senescence (Figures 1–4) prompted examination of the relationship between NS and Pim-1, since initial NS studies showed correlation with Pim-1 in the pathologically injured heart (8). Indeed, Pim-1 overexpression in YCPCs (CPCeP) induces significant increases in c-Myc and NS expression (79% and 40%, respectively, Figure 5A). In contrast, Pim-1 silencing decreases c-Myc (−33%) and NS protein levels (−41%; Figure 5B). Pim-1 regulates c-Myc and NS in mouse hearts as evidenced by depressed c-Myc and NS expression in Pim-1 knockout mice (Pim-1-KO; −50% and −44% respectively) and increased c-Myc and NS expression in transgenic mice with cardiac-specific overexpression of Pim-1 (Pim-1-WT; 2.3- and 1.7-fold respectively, Figure 5C). Collectively, these findings in CPCs and heart lysates reveal Pim-1-dependent regulation of NS that tracks with c-Myc levels.
FIGURE 5. Pim-1 Regulates c-Myc and NS.
Pim-1 is necessary and sufficient for c-Myc and NS regulation. Immunoblots and densitometric analyses of Pim-1, c-Myc, and NS in (A) YCPCs expressing express green fluorescent protein (eGFP) (CPCe) or eGFP and Pim-1 (CPCeP; N = 7) (B) YCPCs with knockdown of Pim-1 (Lt-sh-Pim-1, N = 4) (C) Protein lysates from hearts of 3-month-old transgenic mice with cardiac specific overexpression of Pim-1 (Pim-1-WT), global Pim-1 knockout mice (Pim-1-KO), and nontransgenic (NTg) mice (N = 4 each). Exogenous Pim-1 fused to GFP as well as endogenous Pim-1 levels are indicated at 2 different exposure levels. (D) Immunoblots and denistometric analyses of NS in AhCPCs (Line H10-001) with overexpression of Pim-1 (AhCPCeP) or eGFP (AhCPCe) at mid (p5–6) and late (p10) passages (N = 4).
*p < 0.05; **p < 0.01; ***p < 0.001
Relating murine findings to human CPCs, AhCPC lines were genetically modified to overexpress Pim-1 and green fluorescent protein or GFP (hCPCeP) or express GFP alone (hCPCe) (13). Serial culture passaging of hCPCe lines led to decreased NS expression (−23%, Figure 5D) that coincides with acquisition of replicative senescence in vitro. Conversely, Pim-1 overexpression significantly increased NS expression in all AhCPC lines (60%, Figure 5D, Online Supplement Figure 6AB). Collectively, these results are consistent with Pim-1 modification of AhCPCs extending culture time and serial passaging prior to achieving replicative senescence (13).
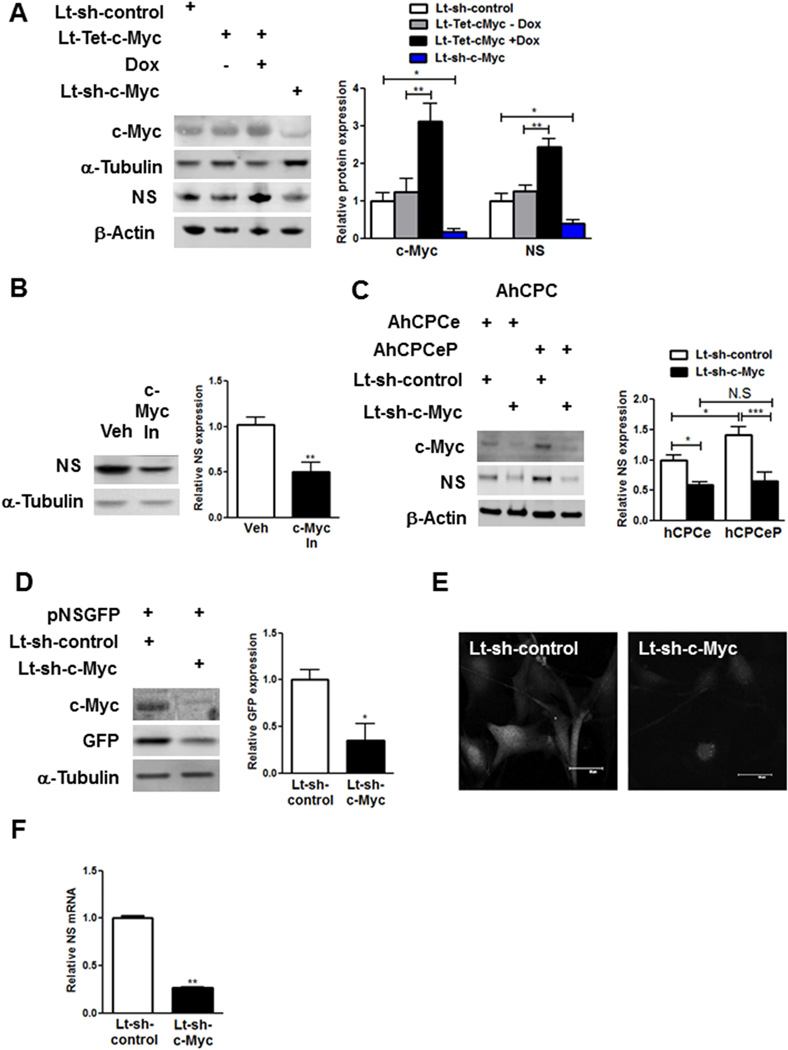
Effects on c-Myc are important given that c-Myc is necessary and sufficient for NS regulation, shown by modification of YCPCs to overexpress c-myc (increased 3.1-fold) that elevates NS expression (2.5-fold); conversely, knockdown or pharmacologic inhibition of c-Myc significantly decreases NS expression (−61%, −49%, Figure 6AB). c-Myc participation in NS induction by Pim-1 was confirmed in AhCPCs, where c-Myc silencing significantly decreased NS expression in hCPCe (−41%) and hCPCeP lines (−54%, Figure 6C), consistent with a role for c-Myc as a crucial intermediate for Pim-1-mediated activation of NS. c-Myc-dependent regulation of NS gene expression was confirmed using CPCs expressing GFP under control of the NS promoter (pNSGFP) isolated from transgenic mouse hearts (21), with GFP-reporter expression and fluorescence decreased 65% following silencing of c-Myc as well as a 73% decrease in NS mRNA (Figure 6DEF).
FIGURE 6. c-Myc in NS Regulation.
c-Myc is a critical intermediate in Pim-1-mediated NS regulation. Immunoblot and densitometric analyses of (A) YCPCs transduced with lentiviruses harboring tetracycline inducible c-Myc (Lt-Tet-c-Myc) and Tet-on transactivator in the presence/absence of doxycycline (Dox, 1µg/ml) and c-Myc knockdown (Lt-sh-c-Myc, N = 3). (B) YCPCs with pharmocological inhibition of c-Myc (c-Myc In, N = 4). (C) AhCPCe and AhCPCeP CPCs (Line H13-061, p6) transduced with Lt-sh-c-Myc (N = 4). (D–E) CPCs are isolated from transgenic mice expressing GFP under the NS promoter (pNSGFP). Immunoblots, densitometric analyses (D) and confocal microscopic images (E) of pNSGFP CPCs with c-Myc knockdown (N = 3). (F) q-PCR analyses in YCPCs with c-Myc knockdown (N = 3). Scale bar: 50µm.
*p < 0.05; **p < 0.01; ***p < 0.001.
REJUVENATION OF SENESCENT CPCS
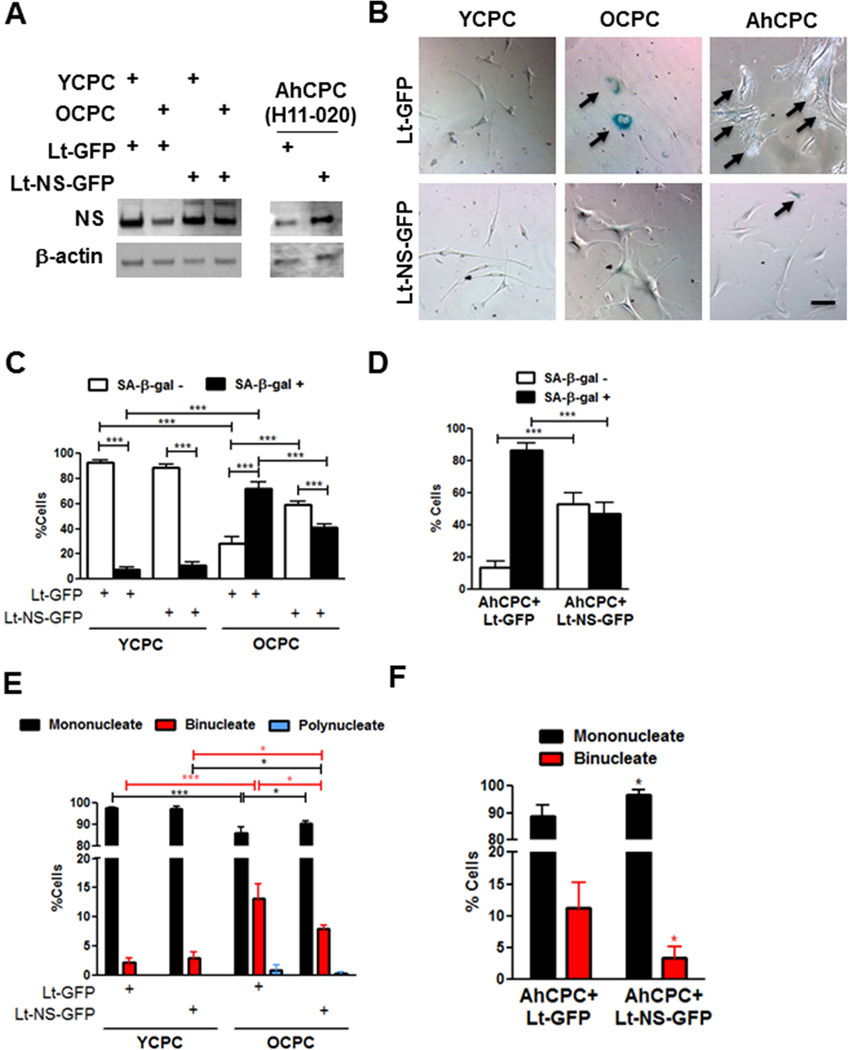
The senescent phenotype can be mitigated by NS overexpression in OCPCs and AhCPCs subjected to serial passaging in vitro (Figure 7A). Alteration in morphology and reduction in the percentage of senescent and multinucleated cells, consistent with restoration of “youthful” phenotype, are evident in senescent cells overexpressing NS (Figure 7B–F). Evidently NS overexpression is an effective strategy to potentiate rejuvenation of OCPC and AhCPCs.
FIGURE 7. Rejuvenated Senescent CPCs.
NS overexpression rejuvenates senescent CPCs. (A) Immunoblots of YCPCs, OCPCs, and AhCPCs after transduction with lentivirus overexpressing NS (Lt-NS-GFP) or GFP (Lt-GFP; N = 3). (B) Phase contrast microscopy of YCPCs, OCPCs, and AhCPCs (H11-020, late passage p10) stained with SA-β-gal. Arrows indicate senescent cells. (C–F) Senescent (C,D) and multinucleate cell (E,F) counts in mouse (C,E; N = 150 cells) and AhCPCs (D,F; N >100 cells). Scale bar: 100 µm. *p < 0.05; **p < 0.01; ***p < 0.001 relative to corresponding Lt-GFP group in F, red and black lines and * in E indicate comparison between binucleate or mononucleate groups.
STEM CELL EXHAUSTION AND CARDIAC AGING
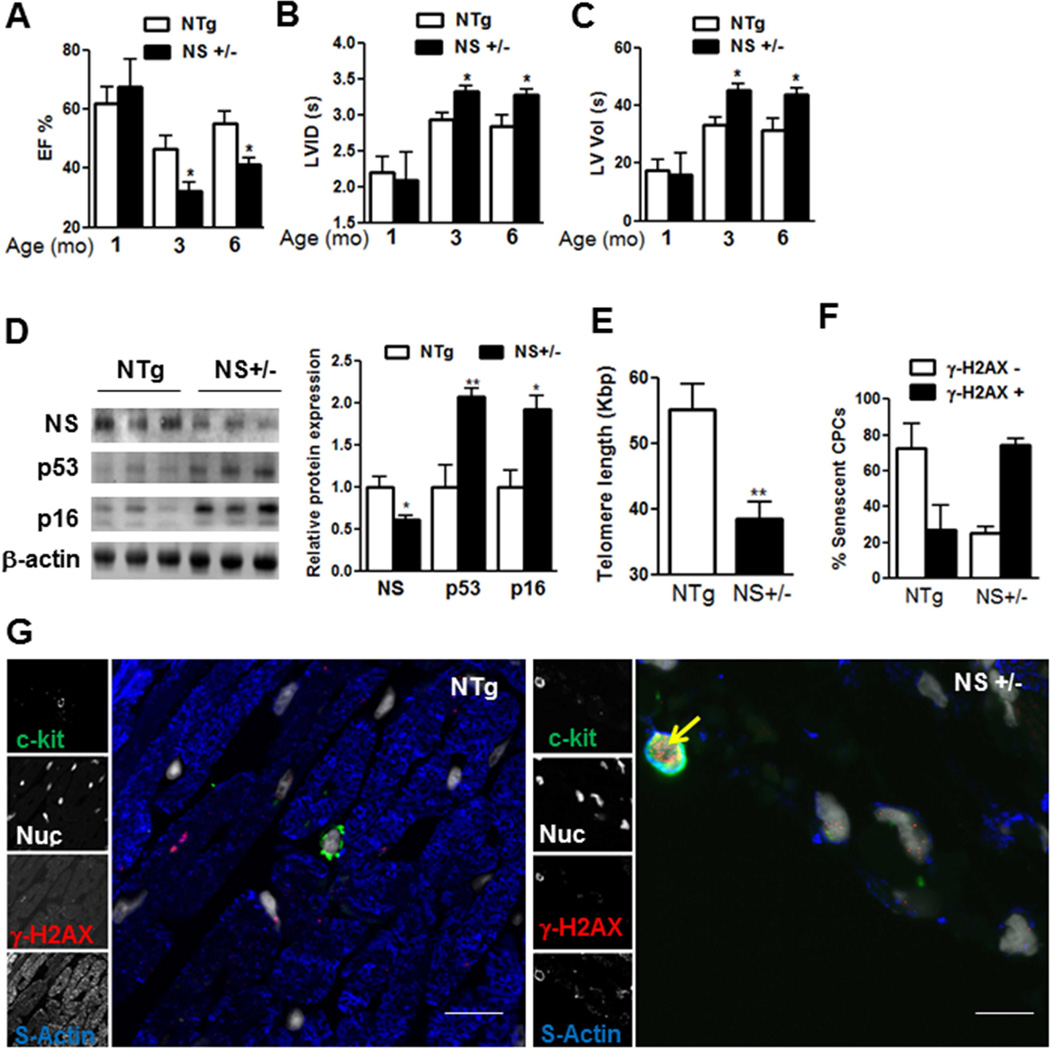
The Central Illustration and Figure 8 demonstrate that NS is required to maintain myocardial youth and prevent CPC exhaustion. That NS extends influences beyond cultured CPCs and impacts upon myocardial aging is evident in NS+/− mice hearts demonstrating dramatic decline in ejection fraction (−44%) associated with increases in left ventricular inner diameter and volume at systole by 3 months of age, consistent with a heart failure phenotype (Figure 8A–C, Online Supplement Table 1). p53 expression increased in NS+/− mice hearts at 1 month (2.4-fold) remains elevated with age (2.1-fold at 3 months, Figure 8D, Online Supplement Figure 7A. Coincident with loss of function, p16 expression is elevated at 3 months (1.8-fold) but not at 1 month, indicating predisposition to early cardiac senescence and associated functional decline (Figure 8D, Online Supplement Figure 7A). Furthermore, loss of NS also causes telomere attrition (−30%, Figure 8E) accompanied by accumulation of senescent γ-H2AX+ CPCs within 1 month after birth Figure 8FG, suggesting that CPC exhaustion is likely to contribute to the early cardiac aging phenotype evident in NS+/− mice, thus providing a compelling role for NS in maintaining myocardial youth and preserving CPC stemness and function.
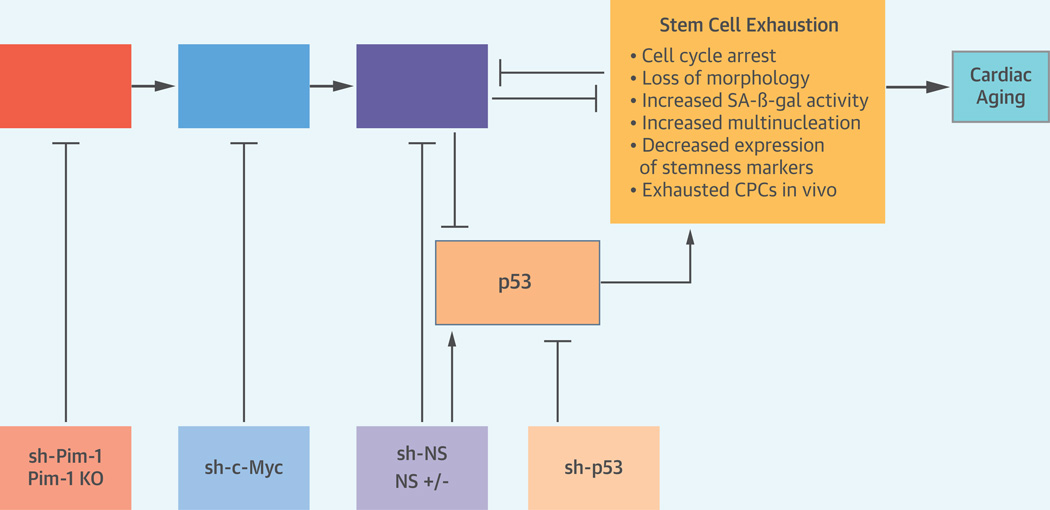
CENTRAL ILLUSTRATION. Schematic of Hypothetical Signaling Cascade.
Pim-1 stabilizes c-Myc that in turn transcriptionally activates NS. Loss of NS up-regulates p53 and regulates cellular senescence, characterized by cell cycle arrest, loss of morphology, increased SA-β-gal activity, increased multinucleation, and decreased expression of stemness markers. Loss of NS also leads to accumulation of exhausted CPCs in vivo causing cardiac aging. Inhibition mediated by knockout mice and lentivirus based gene knockdown is indicated in boxes below.
FIGURE 8. NS and Cardiac Aging.
NS haploinsufficiency causes cardiac aging preceded by stem cell exhaustion. (A–C) Echocardiographic measurements of ejection fraction (EF%; A), left ventricular inner diameter systolic (LVID [s]; B), and left ventricular volume systolic (LV vol [s]; C) in male NS+/− and NTg mice at 1 (N = 4 NTg; 3 NS+/−), 3 (N= 6 NTg; 5 NS+/−), and 6 months of age (N = 6 NTg; 5 NS+/−). (D) Immunoblots and densitometric analyses of p53 and p16 levels in NS+/− and NTg mice hearts at 3 months of age (N = 3 each). (E) Telomere length (Kbp) measured by qPCR in NS+/− and NTg mice at 10 days after birth (N = 6 NTg; 5 NS+/−). (F–G) Counts of senescent CPCs at 1 month of age identified as c-kit+, γ-H2AX+ CPCs (F) determined by staining paraffin heart sections with c-kit (green), γ-H2AX (red), sytox blue (white, Nuc) and sarcomeric actin (blue, s-actin) using confocal microscopy (G).
DISCUSSION
Organ aging and senescence at the cellular level occur differentially with select cells possessing advanced senescence characteristics and impaired function relative to others (1). Senescent stem cells affect their microenvironment by decreasing regenerative potential of the entire stem cell pool, while also impacting neighboring myocytes and vasculature. Therefore, identifying molecular characteristics of cellular senescence and specifically selecting for a “youthful” population of stem cells bereft of senescent cells would ideally enhance stem cell-mediated regeneration, accompanied by genetic engineering strategies to promote retention of youthful characteristics.
Results of our study delineate a novel, evolutionarily conserved role for NS to impact senescence of c-kit+ CPCs isolated from mice as well as fetal and adult human hearts. Molecular characteristics of senescence concomitantly associated with morphological changes including cellular “flattening” (13) and acquisition of 2 or more nuclei are found in OCPCs and AhCPCs (Figure 2) and seen in many proliferating cell types including other mammalian cell systems, suggesting that polyploidy and/or binucleation induces cell cycle arrest eventually leading to senescence or quiescence (9, 22). Nonsenescent binucleate CPCs account for a minimal basal percentage of the population in YCPCs (2% -to 3%); however, pro-senescent stimuli such as increased age or NS knockdown prompt concurrent acquisition of binucleation and senescence with 96% of binucleate CPCs expressing SA-β-gal+. Polynucleation observed only in mouse CPCs correlates 100% with senescence; all polynucleate cells are senescent and induction of polynucleation occurs only under a pro-senescent stress stimulus (Online Supplement Figures 2,3). To our knowledge, this is the first study in CPCs demonstrating multinucleation, a general accepted indicator of senescence in noncardiac cells. Collectively, our data indicate multinucleation and acquisition of cell-autonomous senescence occur concomitantly such that mononucleated CPCs are likely to possess enhanced proliferative and regenerative potential. The multinucleated progenitors’ role may be to confer cell cycle arrest and senescence upon neighboring mononucleate cells through a bystander effect via a paracrine signaling mechanism (1). Interestingly, both multinucleation and senescence are causally linked to loss of NS, which declines in morphologically altered senescent OCPCs and AhCPCs (Figure 2 and Online Supplement Figure 2).
NS’s pivotal role in regulating proliferation (3, 4) is evident in NS homozygous knockout mice that suffer from embryonic lethality and abort spontaneously at the early blastocyst stage owing to impaired proliferation (3, 5, 7). NS regulates cell cycle at both G1/S and G2/M phases in a cell-type dependent manner (5, 7, 10, 11, 23), with cell cycle analyses in YCPCs upon NS knockdown showing decreased S phase measured by both BrdU incorporation assays and fluorescent imaging (Figure 3). NS silencing increases cell cycle arrest markers p53 and p16 in YCPCs and AhCPCs, portraying a collective scenario wherein NS loss prompts increased senescence with cell cycle arrest in G1/S phase. Dependence upon p53 for loss of NS-mediated cell cycle arrest remains controversial (4, 10, 11), but our findings show that senescence mediated by NS silencing in YCPCs and AhCPCs is at least partly p53-dependent (Figure 4). Depletion of p53 prior to NS knockdown partly attenuates short hairpin-NS-induced senescence associated with cell cycle arrest and loss of morphology. Future studies will be needed to determine whether cardiac-specific p53 inhibition can rescue the senescent phenotype observed within weeks after birth in NS+/− (Figure 8). NS-mediated regulation of cytokinesis occurs independently of p53, consistent with selective inhibition of Aurora B kinase causing multinucleation in tumor cells independent of p53 (24). Although the exact mechanism for NS-mediated regulation of cytokinesis is unknown, Aurora B kinase (a critical regulator of cytokinesis) (20) appears to be influenced by NS silencing (Figure 3). Just as we require NS to maintain CPC function and prevent stem cell exhaustion, our data demonstrate that NS overexpression holds the potential to rejuvenate old mouse and human CPCs (Figure 7), corroborating previous findings demonstrating that transgenic NS overexpression decreases senescent mouse embryonic fibroblasts isolated from NS+/− mice (5).
Maintaining telomere length is an important facet of NS-mediated senescence regulation; a strong positive correlation between NS expression and telomere length is evident in human CPCs and hearts (Figure 1). Consistently NS+/− show dramatic reduction in cardiac telomere length within 10 days after birth (Figure 8). NS prevents telomeric shortening via well-studied mechanisms involving degradation of telomeric repeat binding factor-1, a negative regulator of telomere length, and by promoting recruitment and interaction of DNA repair protein complexes to telomeric ends (5, 25, 26). NS localization and interaction with telomeric proteins to maintain telomere length may also play an important role in cytokinesis, considering dysfunctional telomeres cause cytokinesis failure leading to accumulation of binucleate cells (27). NS overexpression increases telomerase reverse transcriptase expression and increases telomere length in CPCs (8). Consistent with preservation of telomere integrity, Pim-1 overexpression maintains telomeres in CPCs (16), but the connection to NS in mediating Pim-1-driven preservation of telomeric integrity remains a topic for future investigation.
Stem cell commitment is tied to NS as inferred from down-regulation of NS upon differentiation and, conversely, acquisition of lineage commitment phenotype following NS-knockdown (5, 7, 8). However, in an in vitro environment devoid of appropriate commitment cues, NS silencing in CPCs leads to phenotypic changes predominantly consistent with senescence without promotion of differentiation (Figure 3). As a quintessential marker of “stemness” and pluripotency in embryonic stem cells (7), NS is similarly associated with maintenance of CPC morphology and expression of markers associated with stemness, while being required to prevent CPC exhaustion in vivo (Central Illustration). NS expression identifies CPCs with enhanced regenerative potential, decreased senescence characteristics, and increased stemness, thus differentiating NS from markers such as p16 and telomere length used in published clinical trials with CPCs to determine senescence.
In the myocardium, NS expression decreases with age as seen in mouse (8) and human hearts, and deletion of one allele of NS in a murine system can cause early cardiac aging, associated with exhaustion of CPCs, telomere attrition, up-regulation of molecular markers of senescence, and decline in cardiac function resembling a heart failure phenotype. Understanding NS regulation and mechanism of action is essential to improve strategies to preserve the regenerative potential of aging stem cells, particularly with regard to upstream mediators of NS expression and stabilization such as Pim-1 and c-Myc (Central Illustration). Antagonism of senescence by NS may contribute to enhanced myocardial regeneration mediated by Pim-1, providing a novel mechanistic molecular direction for future studies.
Molecular characteristics of senescence acquired by CPCs as shown herein, including down-regulation of NS expression, serve as markers to identify and distinguish senescent AhCPCs isolated from patients. Thus, using NS expression as a prognostic indicator of regenerative potential will provide a rational basis for identifying those individuals who would benefit most from genetic engineering of their CPCs with Pim-1 to enhance youthful phenotypic traits, antagonize aging, and augment regeneration (13).
Supplementary Material
PERSPECTIVES.
Competency in Medical Knowledge
Nucleostemin (NS) is associated with maintenance of cardiac stem/progenitor cells (CPCs). Decline in NS is associated with and causal to senescence of CPCs and early myocardial aging accompanied by transition to heart failure.
Translational Outlook
NS can be used as a molecular marker to identify stem cells with enhanced regenerative potential and distinguish senescent CPCs isolated from patients. Targeting NS could be a promising strategy to antagonize cardiac senescence and maintain myocardial youth.
Acknowledgements
We thank Drs. Jian Qu and Michael J. Bishop at University of California, San Francisco for providing us with NS+/− mice.
Funding Sources
This work was supported by National Institutes of Health (1R37HL091102, 1R01HL105759, 5R01HL067245, 1R01HL113656, 1R01HL117163, 1R01HL113647 to M.A.S), Foundation Leducq Transatlantic Network Consortium, American Heart Association (12POST12060191 to N.H).
ABBREVIATIONS
- AhCPC
adult human CPC
- CPC
cardiac progenitor cell
- FhCPC
fetal human CPC
- NS
nucleostemin
- NS+/−
nucleostemin heterozygous knockout mice
- OCPC
old CPC
- SA-β-gal
senescence associated beta-galactosidase
- YCPC
young CPC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Sussman, CardioCreate, Inc. No other authors declared relationships with industry or financial information.
References
- 1.Beltrami AP, Cesselli D, Beltrami CA. Stem cell senescence and regenerative paradigms. Clin Pharmacol Ther. 2012;91:21–29. doi: 10.1038/clpt.2011.262. [DOI] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Beekman C, Nichane M, De Clercq S, et al. Evolutionarily conserved role of nucleostemin: controlling proliferation of stem/progenitor cells during early vertebrate development. Mol Cell Biol. 2006;26:9291–9301. doi: 10.1128/MCB.01183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991–3003. doi: 10.1101/gad.55671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Q, Yasumoto H, Tsai RY. Nucleostemin delays cellular senescence and negatively regulates TRF1 protein stability. Mol Cell Biol. 2006;26:9279–9290. doi: 10.1128/MCB.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avitabile D, Bailey B, Cottage CT, et al. Nucleolar stress is an early response to myocardial damage involving nucleolar proteins nucleostemin and nucleophosmin. Proc Natl Acad Sci U S A. 2011;108:6145–6150. doi: 10.1073/pnas.1017935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qu J, Bishop JM. Nucleostemin maintains self-renewal of embryonic stem cells and promotes reprogramming of somatic cells to pluripotency. J Cell Biol. 2012;197:731–745. doi: 10.1083/jcb.201103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi S, Gude N, Hosoda T, et al. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu R, Zhang Z, Xu Y. Downregulation of nucleostemin causes G1 cell cycle arrest via a p53-independent pathway in prostate cancer PC-3 cells. Urol Int. 2010;85:221–227. doi: 10.1159/000315968. [DOI] [PubMed] [Google Scholar]
- 11.Ma H, Pederson T. Depletion of the nucleolar protein nucleostemin causes G1 cell cycle arrest via the p53 pathway. Mol Biol Cell. 2007;18:2630–2635. doi: 10.1091/mbc.E07-03-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer KM, Cottage CT, Wu W, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohsin S, Khan M, Nguyen J, et al. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–1179. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang Z, Li X, Magnuson NS. Pim kinase-dependent inhibition of c-Myc degradation. Oncogene. 2008;27:4809–4819. doi: 10.1038/onc.2008.123. [DOI] [PubMed] [Google Scholar]
- 15.Cottage CT, Bailey B, Fischer KM, et al. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cottage CT, Neidig L, Sundararaman B, et al. Increased mitotic rate coincident with transient telomere lengthening resulting from pim-1 overexpression in cardiac progenitor cells. Stem Cells. 2012;30:2512–2522. doi: 10.1002/stem.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwolinska AK, Heagle Whiting A, Beekman C, Sedivy JM, Marine JC. Suppression of Myc oncogenic activity by nucleostemin haploinsufficiency. Oncogene. 2012;31:3311–3321. doi: 10.1038/onc.2011.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 19.Sakaue-Sawano A, Kurokawa H, Morimura T, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Wu Q, Zhang W, Mu T, Song T, Li D. Aurora B kinase is required for cytokinesis through effecting spindle structure. Cell Biol Int. 2013;37:436–442. doi: 10.1002/cbin.10057. [DOI] [PubMed] [Google Scholar]
- 21.Ohmura M, Naka K, Hoshii T, et al. Identification of stem cells during prepubertal spermatogenesis via monitoring of nucleostemin promoter activity. Stem Cells. 2008;26:3237–3246. doi: 10.1634/stemcells.2008-0506. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi A, Ohtani N, Yamakoshi K, et al. Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol. 2006;8:1291–1297. doi: 10.1038/ncb1491. [DOI] [PubMed] [Google Scholar]
- 23.Dai MS, Sun XX, Lu H. Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol Cell Biol. 2008;28:4365–4376. doi: 10.1128/MCB.01662-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair JS, Ho AL, Tse AN, et al. Aurora B kinase regulates the postmitotic endoreduplication checkpoint via phosphorylation of the retinoblastoma protein at serine 780. Mol Biol Cell. 2009;20:2218–2228. doi: 10.1091/mbc.E08-08-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu JK, Lin T, Tsai RY. Nucleostemin prevents telomere damage by promoting PML-IV recruitment to SUMOylated TRF1. J Cell Biol. 2012;197:613–624. doi: 10.1083/jcb.201109038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng L, Hsu JK, Zhu Q, Lin T, Tsai RY. Nucleostemin inhibits TRF1 dimerization and shortens its dynamic association with the telomere. J Cell Sci. 2011;124:3706–3714. doi: 10.1242/jcs.089672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pampalona J, Frias C, Genesca A, Tusell L. Progressive telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells. PLoS Genet. 2012;8:e1002679. doi: 10.1371/journal.pgen.1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.