Abstract
The optimal radiation schedule for the curative treatment of prostate cancer is not known. The dose-response of tumors and normal tissues to fractionated irradiation can be described according to a parameter called the alpha-beta ratio (α/β). In the past several years numerous reports have been published that suggest that the alpha-beta ratio for prostate cancer may be quite low; between 1 and 3. If this hypothesis is true, then a radiation therapy schedule that employs less frequent and larger fractions, termed hypofractionation, may be more efficacious. Multiple randomized trials have been conducted comparing moderate (less than 5 Gy/day) hypofractionated radiation therapy and standard radiation therapy in men with prostate cancer. In the majority of these studies the moderate hypofractionated arm had equivalent efficacy with a similar or improved side effect profile. One area to use caution may be in patients with compromised (IPSS > 12) urinary function at baseline due to an increase in urinary toxicity observed in patients treated with hypofractionated radiation in one study. Extreme hypofractionation (greater than or equal to 5 Gy/day), is currently being compared in a randomized trial. Early prospectively collected data from multiple institutions demonstrates efficacy and toxicity that compares favorably with historical controls. The cost savings from hypofractionation could be profound on a national level and only increases the necessity of testing hypofractionated treatment schedules. Long term data and future trials will help radiation oncologists determine the ideal fractionation scheme based on cost, efficacy, and toxicity.
Keywords: Hypofractionated, prostate cancer, radiation therapy, IMRT, protons, cyberknife
Introduction: biology of radiation-induced cell death
The optimal radiation schedule for the curative treatment of prostate cancer is not known [1]. Prostate cancer patients receiving external beam radiation therapy (EBRT) typically are treated 5 days per week for 7-8 weeks [2]. Based on recent data some clinicians have increased the total dose of radiation by increasing the number of treatment sessions or “fractions” and it is now the standard at some centers to treat men for 8-10 consecutive weeks [3]. Despite the potential for better control, this prolongation of treatment time increases health care costs and is less convenient for patients. In the past several years, preclinical and clinical evidence suggests that a long treatment regimen with greater than 35 small (1.8-2 Gy) fractions may not represent the optimal schedule [4-7].
Radiation-induced death for mammalian cells is historically described according to the linear quadratic equation (LQE). According to this model, the survival rate of a given cell will depend on the overall radiation dose, the dose per fraction, and the overall treatment time [8]. The dose-response of tumors and normal tissues to fractionated irradiation can be described according to a parameter called the alpha-beta ratio (α/β). The alpha-beta ratio is an indication of the fractionation sensitivity of a particular cell type. In general the alpha-beta ratio is high (≥ 10 Gy) for early-responding normal tissues such as skin and mucosa and low (< 5 Gy) for later responding normal tissues such as the spinal cord and bone [8]. Most tumors are believed to have a high α/β, similar to early responding normal tissues due to their frequent cell division. One implication of differing alpha-beta ratios for tumor cells and normal tissue is that it may be possible to increase the therapeutic ratio by using unconventional fractionation schedules. In the past several years numerous reports have been published that suggest that the alpha-beta ratio for prostate cancer may be quite low, between 1 and 3 [4-7]. If this hypothesis is true, then a radiation therapy schedule that employs less frequent and larger fractions, termed hypofractionation, may be more efficacious.
Hypo-fractionated radiation therapy in prostate cancer
Kupelian recently reported the 5-year freedom from biochemical recurrence (FFBR) and morbidity in the first 100 patients treated with hypofractionated EBRT [9]. In this series men were treated with intensity-modulated radiation therapy (IMRT). Patients were treated to 70 Gy delivered in 28 fractions (2.5 Gy/fraction) with median follow-up of 66 months. Fifty-one patients (51%) received androgen deprivation therapy for a period not greater than 6 months. Given the larger dose per fraction compared to conventional fractionation (1.8-2 Gy/day), the margin around the prostate gland to account for daily setup variation was reduced. This is referred to as the clinical target volume (CTV) to planning target volume (PTV) expansion or margin. The CTV-PTV margin was 4 mm posteriorly and 5-8 mm elsewhere to minimize the amount of normal tissue receiving the full prescription dose. The 4 mm posterior margin was used to specifically reduce rectal toxicity. To ensure that the gland was within the treatment field on any given day, daily prostate localization was performed with a trans-abdominal ultrasound system. The ASTRO Consensus Definition (ACD) and the RTOG Phoenix definition (PSA nadir + 2 ng/mL) were used to report FFBR [10] and the RTOG Morbidity System was used to report gastrointestinal (GI) and genitourinary (GU) morbidity. The estimated rate of FFBR 5 years following treatment was 85% according to the ACD and 88% according to the RTOG Phoenix definition. This biochemical result was similar to a group of patients treated contemporaneously with 3D-CRT to 78 Gy/39 fractions. In the 100 men treated with hypo-fractionated IMRT, the rate of combined grade 2/3 late rectal morbidity was 11% at 5 years. This low level of reported morbidity may be explained by two factors: the daily target localization allowed for very tight CTV-PTV margins, and the use of IMRT resulted in decreased volumes of normal tissue receiving high doses. These promising results led the M.D. Anderson group to conduct a prospective, randomized trial discussed below.
The results from five randomized trials examining the benefit of moderate (less than 5 Gy/day) hypofractionation schedules for prostate cancer are shown in Table 1 [11-17]. An Australian trial compared 64 Gy/32 fractions (conventional schedule) to 55 Gy/20 fractions (hypofractionated schedule) in men with favorable risk T1-2 prostate cancer [11]. The primary endpoint of this trial was morbidity. The sample size of 220 men (110 each arm) was determined to detect a difference in the frequency of mild late radiation morbidity of 20% (40% vs. 20%) with 90% power. Efficacy was a secondary endpoint. The median follow-up is 7.5 years. Two-dimensional EBRT was used in each arm; no 3D or IMRT was used. Three- or four-field techniques were used with 6-23 MV photons. Morbidity was measured with the LENT-SOMA questionnaires. GI morbidity measured with these questionnaires emphasizes six symptoms (stool frequency, stool consistency, rectal pain, mucus discharge, urgency of defecation, and rectal bleeding). GU morbidity includes four symptoms (urinary frequency, urgency, dysuria, and hematuria). Treatment efficacy was determined clinically and biochemically. PSA nadir and three consecutive rises were examined to estimate efficacy. Of the ten symptoms measured, only the prevalence of rectal bleeding was different between the treatment arms. Upon early analysis, the prevalence of rectal bleeding 2 years following treatment was 42% in the hypo-fractionated arm and 27% in the conventionally fractionated arm (p < .05) [18]. The prevalence of rectal bleeding is somewhat higher than expected and may be the result of the two-dimensional methods employed. However, upon final report there was no difference in late GI toxicity between the treatment arms. Late grade 4 urinary toxicity was higher in the standard fractionation arm than the hypofractionation arm (HR 1.58; p < 0.05). The authors also reported on treatment efficacy. Freedom from biochemical failure was significantly better (53% vs. 34%; p < .05) in the hypofractionated arm when using the Phoenix definition of biochemical failure. This advantage was not appreciated when using the ACD of biochemical failure (FFBF 44% vs. 44%).
Table 1.
Randomized phase III trials with outcome data in patients receiving moderate hypofractionation radiotherapy directed at an intact prostate
| Study Center | Patients | Median Follow-up (years) | Technique | Regimen | Outcome | Late Toxicity |
|---|---|---|---|---|---|---|
| Australia [11] | 217 | 7.5 | 2D/3DCRT | 55 Gy/20 fx vs. 64 Gy/32 fx | Phoenix definition 7.5 yr FFBF 53% vs. 34% (p < .05) | GU symptoms favor HFX at 4 yrs HR |
| ASTRO definition 44% vs. 44% | 1.58 (p < .05) | |||||
| No difference in late GI | ||||||
| Fox Chase Cancer Center [14] | 303 | 5.7 | IMRT | 70.2 Gy/26 fx vs. 76 Gy/38 fx | 5 yr BCF 23% vs. 21% (NS) | Gr ≥ 3 GI 2% vs. 2% |
| Gr ≥ 3 GU 4% vs. 3% | ||||||
| MD Anderson [16,17] | 204 | 4.6 efficacy 6.0 toxicity | IMRT | 72 Gy/30 fx vs. 75.6 Gy/42 fx | ASTRO definition 5 yr FFBF 96% vs. 92% | Gr ≥ 3 GI 2% vs. 1% |
| Gr ≥ 3 GU 1% vs. 0% | ||||||
| Ontario [13] | 936 | 5.7 | 3DCRT | 52.5 Gy/20 fx vs. 66 Gy/33 fx | 5 yr BCF 60% vs 53% | Gr ≥ 3 GI/GU toxicity equal (3.2%) |
| Italy [15,19] | 168 | 5.8 | 3DCRT | 62 Gy/20 fx vs. 80 Gy/40 fx | Phoenix definition 5 yr FFBF 85% vs. 79% (p = .065) | Gr ≥ 3 GI 1% vs. 0% |
| Gr ≥ 3 GU 1% vs. 2% |
FFBF = freedom from biochemical failure, BCF = Biochemical or clinical failure, HFX = hypofractionated.
A large randomized trial conducted at the Fox Chase Cancer Center was recently reported [14]. Three hundred and three men were randomized between 70.2 Gy in 26 fractions and 76 Gy in 38 fractions. These patients were all treated with IMRT with the help of MRI for target delineation. The PTV margins were tighter in the hypofractionated arm (3 mm posteriorly, 7 mm elsewhere versus 5 mm posteriorly, 8 mm elsewhere). Transabdominal ultrasound was used daily to account for variations in bladder and rectal filling. With a median follow-up of 5.7 years there was no significant difference in biochemical or clinical failure (23% vs. 21%). There were no statistically significant differences in late gastrointestinal or urinary toxicity between the treatment arms. However, in subgroup analysis, patients with compromised urinary function (IPSS > 12) before enrollment had significantly worse urinary function when treated with the hypofractionated regime.
Based on the promising results of their earlier work, the group at M.D. Anderson Cancer Center conducted a randomized trial comparing 75.6 Gy in 42 fractions versus 72 Gy in 30 fractions [16,17]. All patients were treated with IMRT with the planning constraint that less than 20% of the rectum receives greater than 70 Gy in the standard fractionation arm and greater than 60 Gy in the hypofractionation arm. At 5 years the freedom from biochemical failure using the ACD was 96% in the hypofractionated arm and 92% in the standard fractionation arm. This difference was not found to be statistically significant. The rates of late grade 3 or greater GI and GU toxicity were low and statistically equivalent in both arms. The rate of late GI toxicity was found to correlate with the amount of rectum receiving moderate and high dose, once again emphasizing the importance of limiting the dose to the rectum as much as possible. Overall, the hypofractionated treatment was effective and well tolerated.
A Canadian trial comparing 66 Gy in 33 fractions (Long arm) to 52.5 Gy in 20 fractions (Short arm) in men with low- and intermediate-risk prostate cancer has been completed [13]. The dose was prescribed to the isocenter and the prostate/seminal vesicle to edge of the radiation treatment field was 15 mm (could be reduced to 10 mm posteriorly at the discretion of the investigator). Four-field arrangement, radiation fields from origination from the front, the back, the left and the right of the patient, was required unless a prosthetic hip mandated a three-field approach. Most patients were treated using 3 dimensional planning with CT simulation, but IMRT was not used. The study was established as a non-inferiority study to exclude an absolute difference in biochemical or clinical failure (BCF) of 7.5%. In this trial the 5 year rate of failure (biochemical or clinical) is higher in the Short arm compared to the Long arm (60% vs. 53%). The difference was -7.0% (90% CI, -12.58% to -1.42%). Because the lower bound was less than the predefined tolerance of -7.5%, they could not exclude the possibility of the short arm being inferior. At first glance this would appear to suggest that hypo-fractionated regimens are inferior compared to a conventionally fractionated regimen, but the two arms were not designed to be isoeffective (same anticipated effect for given treatment schedule). In fact, the biologically effective dose of the Short arm is consistently less than the Long arm until the alpha beta ratio reaches a value of < 1. The results of the Canadian trial, therefore, are not inconsistent with an alpha-beta ratio for prostate cancer of 1.5. At a median follow-up of 5.7 years there is no difference in 5-year actuarial rate of late grade 3 or greater GI/GU toxicity between the two arms.
Recently, an Italian study compared the toxicity and efficacy of hypo-fractionated (62 Gy/20 fractions/5 weeks, 4 fractions per week) vs. conventional fractionation radiotherapy (80 Gy/40 fractions/8 weeks) in patients with high-risk prostate cancer [15,19]. One hundred sixty eight patients were randomized to receive either hypo-fractionated or conventional fractionated schedules of three-dimensional conformal radiotherapy to the prostate and seminal vesicles. All patients received a 9-month course of total androgen deprivation (TAD), and radiotherapy started 2 months thereafter. No significant difference was found for late toxicity between the two treatment groups, with 3-year Grade 2 rates of 17% and 16% for gastrointestinal and 14% and 11% for genitourinary in the hypo-fractionation and conventional fractionation groups, respectively. The 5-year freedom from biochemical failure (FFBF) rates were 85% and 79% in the hypo-fractionation and conventional fractionation groups, respectively (p = 0.065). The investigators concluded that that late toxicity is equivalent between the two treatment groups and that the hypo-fractionated schedule used in this trial is equivalent and possibly superior to the conventional fractionation in terms of FFBF.
With the alpha/beta ratio likely being around 1.5 there could be an advantage to extreme hypofractionation with doses of 5 Gy per day or higher. There have been no prospective, randomized trials completed to date testing this hypothesis. The Proton Collaborative Group is actively recruiting participants for a randomized trial of 79.2 Gy in 44 fractions versus 38 Gy in 5 fractions (ClinicalTrials.gov Identifier: NCT01230866). This trial takes advantage of the steep dose fall-off associated with particle therapy to attempt to limit dose to nearby normal tissue. Even in the absence of randomized trials many institutions are treating with extreme hypofractionation. Table 2 summarizes prospectively collected studies with at least 80 patients published in manuscript form.
Table 2.
Prospectively collected studies of extreme hypofractionation for intact prostate with at least 80 patients that are published in manuscript form
| Study Center | Patients | Median Follow-up (years) | Technique | Regimen | Outcome | Toxicity |
|---|---|---|---|---|---|---|
| Georgetown [20] | 100 | 2.3 | CK | 35-36.25/5 fx | 2 yr FFBF 99% | Gr 1/2/3 GI 11%/0%/0% |
| Gr 1/2/3 GU 26%/17%/1% | ||||||
| Italy [21] | 100 | 3 | CK | 35 Gy/5 fx | 3 yr FFBF 96% | Gr 1/2/3 GI 2%/1%/0% |
| Gr 1/2/3 GU 4%/3%/1% | ||||||
| Toronto [22] | 84 | 4.6 | IMRT | 35 Gy/5 fx | 5 yr FFBF 98% | 7 Gr 1 and 1 Gr 4 GI toxicity at last f/up |
| Quebec [23] | 80 | 2.8 | 3DCRT | 45 Gy/9 fx | 3 yr FFBF 97% | Gr 1/2/3 GI 22%/4%/0% |
| Gr 1/2/3/4 GU 4%/11%/0%/4% |
FFBF = freedom from biochemical failure.
Technical considerations
Advancements in radiation delivery have allowed for greater implementation of hypofractionated treatment. This is because newer techniques such as IMRT or stereotactic radiation therapy (SRT) can deliver the high dose per fraction to the prostate gland and limit normal organs such as the bladder and rectum, effectively exposing these normal tissues to less dose per day. An example of a hypofractionated treatment for prostate cancer is shown in Figure 1, taken from an ongoing Phase I Study at New York University Langone Medical Center (NYULMC) where the prostate is treated with doses of 3-3.3 Gy per day using IMRT. Note that the bladder and rectal doses are well below the prostate dose.
Figure 1.
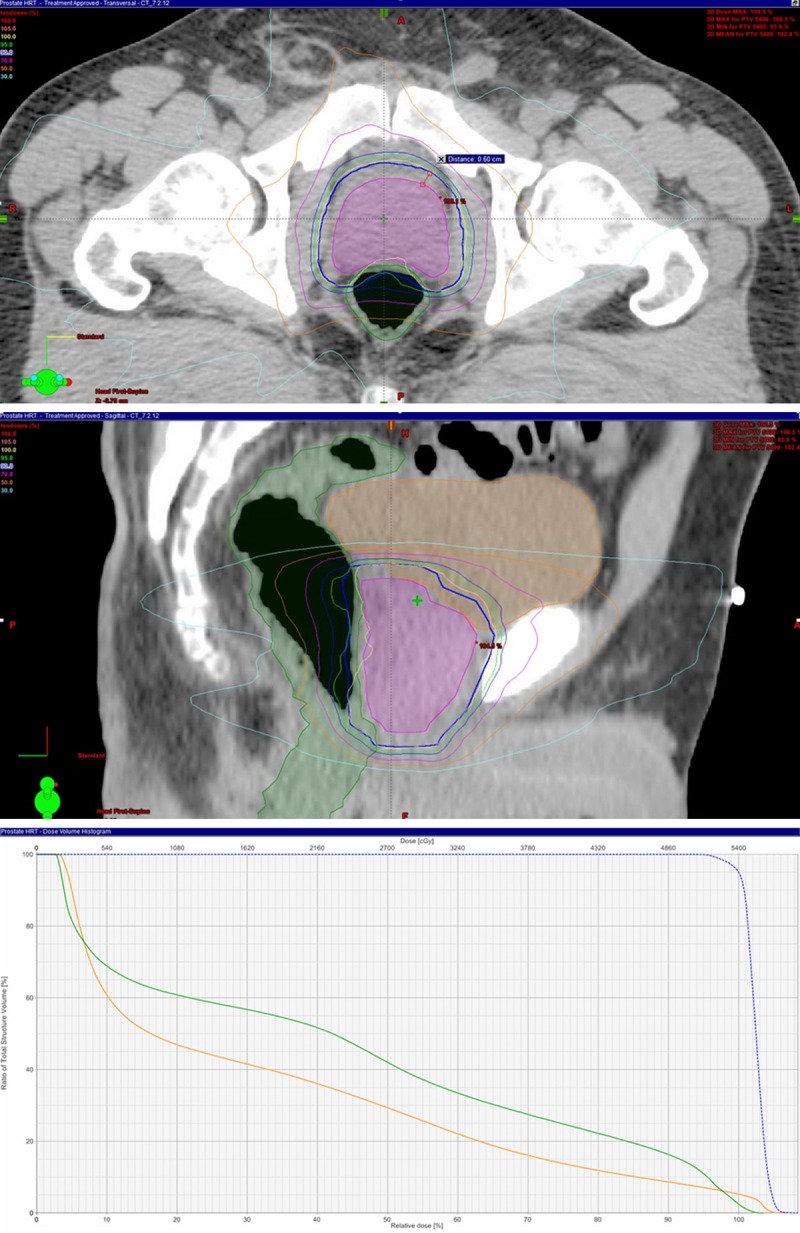
Treatment plan for a patient treated at 3 Gy per day for 18 fractions in the axial (top) and sagittal (middle) planes. There is a 6 mm CTV (magenta) to PTV(blue) expansion. The dose volume histogram (bottom) for the treatment plan shown above. Notice the PTV in blue, the rectum in green, and the bladder in orange.
SRT with devices such as CyberKnife® (Accuray, Inc., Sunnyvale, CA) [24] have been used in more dramatic hypofractionated regimens where doses to the prostate are 6-7.25 Gy per day. In these cases, reducing normal tissue exposure is even more important since large fractions contribute more to late toxicity.
Proton beam therapy is another radiation modality that may be used for hypofractionated treatment. The physical principle that governs proton therapy is that it deposits dose in a certain range of tissue and then stops, giving no dose beyond a certain point. This is called the Bragg Peak. A recent study from Uppsala University employed proton beam therapy for a boost of 20 Gy in 5 fractions after 50 Gy in 2 Gy fractions with conventional EBRT [25]. A perineal boost was employed where the proton beam is directed at the prostate through the perineum.
Economic considerations
Prostate cancer is the most common type of cancer in men in the United States, with 186,000 new cases in 2008 and 28,600 deaths, and this number is expected to rise by 55% by 2030 [26]. Mariotto and colleagues estimate that costs related to prostate cancer treatment will increase by 42% from 2010 to 2020 to a total of approximately $16.5 billion [27].
However, while incidence and costs project higher, resources available for such medical treatment may not be available. In its 2008 annual report to Congress, the Medicare Board of Trustees reported that the program’s hospital insurance trust fund could run out of money by 2017 [28]. The fundamental problem is that the ratio of workers paying Medicare taxes to retired people drawing benefits is shrinking, and at the same time, the price of health care services per person is increasing. Currently there are 3.9 workers paying taxes into Medicare for every older American receiving service (http://www.publicagendaarchives.org/charts/fewer-workers-projected-hi-beneficiary). By 2030, as the baby boom generation retires, that is projected to drop to 2.4 workers for each beneficiary. Medicare spending is expected to grow by about 7 percent per year for the next 10 years. As a result, the financing of the program is out of actuarial balance, presenting serious challenges in both the short-term and long-term. The context of these fiscal dilemmas is even more serious. As of 2009, total national debt (households, businesses and governments) exceeded $50.7 trillion, or approximately 350% of gross domestic product (GDP) of the United States [29].
Hypofractionated regimens for prostate cancer treatment may have an important role in reducing health care expenditures since the overall amount of administered treatment is greatly reduced. If longer follow up demonstrates equivalent rates of cancer control with low morbidity, hypofractionated EBRT may become widely accepted as the standard of care as it will provide excellent oncologic outcomes with the added benefit of cost savings and convenience.
Conclusions
Debate exists over the optimal degree of hypofractionation that should be used for prostate cancer treatment. SRT studies, as noted above, employ dramatically high doses per fraction (6-7.25 Gy) for only 5 fractions while completed proton studies have used hypofractionation only for the “boost” portion of the treatment with standard fractionation was used for the majority of the RT course. Still others, including NYULMC, have initiated prospective studies with moderate hypofractionation (3-3.5 Gy per fraction) for the entire schedule. Only future studies will determine the optimal dose schedule.
Disclosure of conflict of interest
The authors have no real or potential conflicts of interest.
References
- 1.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 2.Zietman A, Moughan J, Owen J, Hanks G. The Patterns of Care Survey of radiation therapy in localized prostate cancer: similarities between the practice nationally and in minority-rich areas. Int J Radiat Oncol Biol Phys. 2001;50:75–80. doi: 10.1016/s0360-3016(00)01569-8. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, Amols H, Venkatraman ES, Leibel SA. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–6. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 4.Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza WD, Thames HD. Thames, Is the alpha/beta ratio for prostate cancer low? Int J Radiat Oncol Biol Phys. 2001;51:1–3. doi: 10.1016/s0360-3016(01)01650-9. [DOI] [PubMed] [Google Scholar]
- 7.King CR, Fowler JF. A simple analytic derivation suggests that prostate cancer alpha/beta ratio is low. Int J Radiat Oncol Biol Phys. 2001;51:213–4. doi: 10.1016/s0360-3016(01)01651-0. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ, AG . Radiobiology for the Radiologist. 7th ed. Philadelphia: Lippincott Willimas and Wilkins; 2012. [Google Scholar]
- 9.Kupelian PA, Thakkar VV, Khuntia D, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 gy at 2.5 Gy per fraction) for localized prostate cancer: long-term outcomes. Int J Radiat Oncol Biol Phys. 2005;63:1463–8. doi: 10.1016/j.ijrobp.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 10.Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–41. [PubMed] [Google Scholar]
- 11.Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–8. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 12.Arcangeli G, Saracino B, Gomellini S, Petrongari MG, Arcangeli S, Sentinelli S, Marzi S, Landoni V, Fowler J, Strigari L. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:11–8. doi: 10.1016/j.ijrobp.2009.07.1691. [DOI] [PubMed] [Google Scholar]
- 13.Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, Levine M, Sathya J, Choo R, Prichard H, Brundage M, Kwan W. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J. Clin. Oncol. 2005;23:6132–8. doi: 10.1200/JCO.2005.06.153. [DOI] [PubMed] [Google Scholar]
- 14.Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, Stoyanova R, Movsas B, Greenberg RE, Uzzo RG, Ma C, Buyyounouski MK. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J. Clin. Oncol. 2013;31:3860–8. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcangeli S, Strigari L, Gomellini S, Saracino B, Petrongari MG, Pinnarò P, Pinzi V, Arcangeli G. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–8. doi: 10.1016/j.ijrobp.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman KE, Voong KR, Pugh TJ, Skinner H, Levy LB, Takiar V, Choi S, Du W, Frank SJ, Johnson J, Kanke J, Kudchadker RJ, Lee AK, Mahmood U, McGuire SE, Kuban DA. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. Int J Radiat Oncol Biol Phys. 2014;88:1074–84. doi: 10.1016/j.ijrobp.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Kuban DA, et al. Preliminary Report of a Randomized Dose Escalation Trial for Prostate Cancer using Hypofractionation. Int J Radiat Oncol Biol Phys. 2010;78(Supplement):S58–S59. [Google Scholar]
- 18.Yeoh EE, Fraser RJ, McGowan RE, Botten RJ, Di Matteo AC, Roos DE, Penniment MG, Borg MF. Evidence for efficacy without increased toxicity of hypofractionated radiotherapy for prostate carcinoma: early results of a Phase III randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:943–55. doi: 10.1016/s0360-3016(02)04146-9. [DOI] [PubMed] [Google Scholar]
- 19.Arcangeli G, Fowler J, Gomellini S, Arcangeli S, Saracino B, Petrongari MG, Benassi M, Strigari L. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79:1013–21. doi: 10.1016/j.ijrobp.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 20.Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, Hanscom HN, Laing S, Kim JS, Lei S, Batipps GP, Kowalczyk K, Bandi G, Pahira J, McGeagh KG, Collins BT, Krishnan P, Dawson NA, Taylor KL, Dritschilo A, Lynch JH, Collins SP. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown University experience. Radiat Oncol. 2013;8:58. doi: 10.1186/1748-717X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolzicco G, Favretto MS, Satariano N, Scremin E, Tambone C, Tasca A. A single-center study of 100 consecutive patients with localized prostate cancer treated with stereotactic body radiotherapy. BMC Urol. 2013;13:49. doi: 10.1186/1471-2490-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loblaw A, Cheung P, D’Alimonte L, Deabreu A, Mamedov A, Zhang L, Tang C, Quon H, Jain S, Pang G, Nam R. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107:153–8. doi: 10.1016/j.radonc.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Menkarios C, Vigneault É, Brochet N, Nguyen DH, Bahary JP, Jolicoeur M, Beauchemin MC, Villeneuve H, Van Nguyen T, Fortin B, Lambert C. Toxicity report of once weekly radiation therapy for low-risk prostate adenocarcinoma: preliminary results of a phase I/II trial. Radiat Oncol. 2011;6:112. doi: 10.1186/1748-717X-6-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler JR Jr, Murphy MJ, Chang SD, Hancock SL. Image-guided robotic radiosurgery. Neurosurgery. 1999;44:1299–306. [PubMed] [Google Scholar]
- 25.Johansson S, Aström L, Sandin F, Isacsson U, Montelius A, Turesson I. Hypofractionated proton boost combined with external beam radiotherapy for treatment of localized prostate cancer. Prostate Cancer. 2012;2012:654861. doi: 10.1155/2012/654861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J. Clin. Oncol. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 27.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CMS, editor. 2008 Annual Report of the Boards of Trustees of The Federal Hospital Insurance And Federal Supplementary Medical Insurance Trust Funds. 2008. [Google Scholar]
- 29.Reserve F, editor. Flow of Funds Accounts of the United States. 2010. [Google Scholar]


