Abstract
Large cell neuroendocrine carcinoma of the prostate (LCNEC), de novo in particular, is an extremely rare entity that has only been described in the literature in case reports. Historically, the majority of the cases of LCNEC reported in the literature represent typical prostatic adenocarcinomas that transformed after long standing androgen deprivation therapy (ADT). These cases were admixed with histological areas of usual adenocarcinoma and showed hybrid features of both neuroendocrine and usual adenocarcinoma. Here we present a case of an LCNEC without admixed areas of usual prostatic adenocarcinoma arising de novo in a patient without prior history of hormonal therapy. The tumor also shows morphologic evidence of neuroendocrine differentiation; composed of large sheets and nests of cells with moderate amphophilic cytoplasm with peripheral palisading, and vesicular clumpy chromatin with prominent nucleoli. The carcinoma’s prostatic origin is indicated by positive immunohistochemical staining for PSA, PAP, PSMA, racemase, and Nkx3.1. Diffusely positive staining for chromogranin and synaptophysin, as well as the presence of secretory granules in the cytoplasm of the tumor cells demonstrated by electron microscopy supports the NE differentiation. NE prostate cancer usually does not express AR and is refractory to ADT therapy while AR and ERG are positive in this case. In summary, we report a de novo LCNEC of the prostate with review of literature, in particular, clinical implications.
Keywords: Large cell, neuroendocrine, prostate cancer
Introduction
Prostate cancer is the most common noncutaneous cancer and the second leading cause of cancer death in men in the United States [1]. The annual incidence of prostate cancer continues to increase. In the United States, there will be an estimated 233,000 cases and 29,500 deaths in 2014 [2].
The clinical behavior of prostate cancer ranges from microscopic, well-differentiated tumors that may never be clinically significant to aggressive, high grade cancers ultimately cause metastases, morbidity, and death. The majority of prostate cancers are adenocarcinoma, which may, on rare occasions, demonstrate neuroendocrine differentiation.
A small subset of neuroendocrine cells is present in normal prostate. They arise from a putative stem cell with a basal cell phenotype and lack androgen receptor. They are thought to play a regulatory role in the proliferative and secretory activity of prostatic glandular epithelium [3]. Neuroendocrine cells have also been identified in prostatic neoplasms. In 2013, the Prostate Cancer Foundation developed a classification system for prostate cancer with neuroendocrine differentiation. It includes usual prostate adenocarcinoma with NE differentiation, adenocarcinoma with Paneth cell NE differentiation, carcinoid tumor, small cell carcinoma, large cell carcinoma and mixed NE carcinoma-acinar adenocarcinoma [4].
Neuroendocrine differentiation in prostatic carcinoma is most commonly seen in the form of scattered NE cells. There is a clear association between the numbers of neuroendocrine cells and the use of androgen-deprivation therapy (ADT), but the prognostic significance of this finding remains incompletely understood [5]. The least common manifestation of neuroendocrine differentiation in prostate cancer is the development of neuroendocrine tumors, either pure neuroendocrine tumors or mixed neuroendocrine and typical adenocarcinoma [6]. Pure neuroendocrine prostate cancer is a rare entity and encompasses various clinical scenarios [6-8]. Overall, these tumors behave aggressively, associate with frequent distant metastasis and correlate with poor prognosis [9]. Small-cell carcinoma is the most common neuroendocrine prostate cancer, whereas carcinoid and large-cell neuroendocrine prostate cancer (LCNEC) are exceedingly rare [4,10,11]. To date, the few LCNEC cases published occurred almost exclusively in the context of long-standing treatment with androgen deprivation therapy (ADT) [4,6,8,12,13]. Usually, neuroendocrine prostate carcinoma does not express the androgen receptor and is considered clinically hormone refractory. The degree of neuroendocrine differentiation of prostate adenocarcinoma increases with tumor progression.
De novo LCNEC is extremely rare. Until now, only three series of LCNEC have been published [6-8]. Evans et al described 7 cases of LCNEC, with only one pure de novo case. Azad et al studied two de novo LCNECs coexisting with poorly differentiated prostatic adenocarcinoma. Due to its rarity, it is important for pathologists to be able to recognize and accurately diagnose these tumors in order to be able to understand their prognostic and therapeutic implications. In this study, we present a rare case of pure de novo large cell neuroendocrine carcinoma of the prostate.
Case presentation
A 66-year-old African American man was evaluated in 2012 for an elevated prostate specific antigen (PSA) of 48 ng/mL. At the time the patient refused a prostate biopsy. He presented again in June of 2014 with bilateral hydronephrosis and was found to have a PSA level of 97 ng/mL. The ultrasound revealed a tumor covering the bladder neck, trigone, and floor with complete obstruction of the ureteral orifices. He underwent a channel transurethral resection of the prostate (TURP) to manage his symptoms suspected to be due to adenocarcinoma of the prostate. A subsequent non-contrast CT showed bulky retroperitoneal and pelvic lymphadenopathy, consistent with metastatic disease.
Pathological findings
A urine cytology sample was evaluated prior to TURP. The thin prep slide showed tumor cells in single cells and groups of three-dimensional cell clusters of variable size with the cell size ranging from medium-to-large, up to 22 µm (Figure 1A). The majority of the cells had a high nuclear cytoplasmic ratio with some cells demonstrating naked nuclei. Nuclear pyknosis, molding and mitosis were frequently present. These features are inconsistent with a usual urothelial carcinoma or prostatic adenocarcinoma. A diagnosis of large cell neuroendocrine carcinoma was rendered because of a combination of morphological features including pleomorphism, nuclear molding, pyknosis, high nucleocytoplasmic ratio, apoptosis and mitosis.
Figure 1.
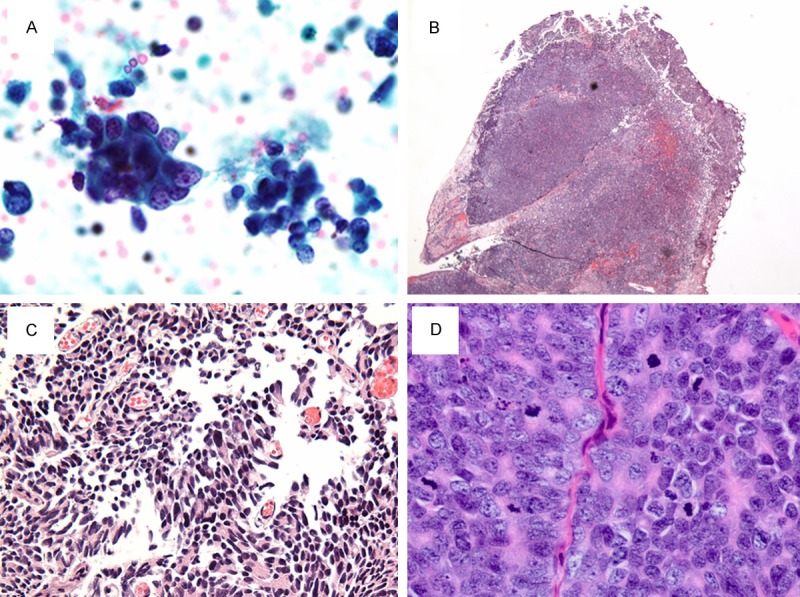
Histological features of the LCNEC of prostate. A. Cytological appearance of the large neuroendocrine cells from urine. B. Low power view showing diffuse solid sheets of infiltrating cells. C. High power view showing areas of poorly formed papillary structures. D. High power view of the cells showing sheets of cells with moderate amphophilic to pale cytoplasm and vesicular clumpy chromatin with prominent nucleoli.
The hematoxylin and eosin slides of the TURP specimen showed a high grade neoplasm composed of solid sheets and nests of polygonal cells diffusely infiltrating the prostatic parenchyma (Figure 1B-D). The nests of cells showed peripheral palisading focally. The cells were large in size with abundant amphophilic to pale cytoplasm. The nuclei showed vesicular clumped chromatin with prominent nucleoli. There were areas of necrosis and high mitotic count (up to 35/10 hpf). These features present in the current specimen are similar to the histologic description of LCNEC given by Evans et al in the largest series ever published on LCNEC. There are no areas of conventional adenocarcinoma throughout the specimen.
Immunohistochemistry (IHC) (Table 1) was performed to determine the origin of the cancer and the extent of neuroendocrine differentiation. IHC was diffusely positive for PSAP (Figure 2A), PSMA, racemase and focally positive for PSA. We also performed IHC for CK20, p63 and GATA-3 to rule out bladder cancer as some of the cancer areas showed ill-defined papillary structures (Figure 1C). CK20, p63 and GATA-3 were negative, ruling out a bladder urothelial origin of the cancer. We stained the tumor cells with NE markers including chromogranin (Figure 2B), synaptophysin and CD56. IHC showed strong diffuse cytoplasmic staining for all NE markers. Electron microscope images of the carcinoma cells showed medium to large cells (up to 20 µm) with clumpy chromatin and prominent nucleoli. The cytoplasm of the cells demonstrated abundant secretory granules, providing ultrastructural evidence of their neuroendocrine differentiation.
Table 1.
Results summary for molecular tests
| Marker | Test | Results |
|---|---|---|
| PSA | IHC | Focally positive |
| PAP | IHC | Diffusely positive |
| PSMA | IHC | Diffusely positive |
| AMACR | IHC | Diffusely positive |
| AR | IHC | Diffusely positive (nuclear) |
| ERG | IHC | Diffusely positive (nuclear) |
| Nkx3.1 | IHC | Diffusely positive (nuclear) |
| Chromogranin | IHC | Diffusely positive |
| Synaptophysin | IHC | Diffusely positive |
| CD56 | IHC | Diffusely positive |
| Ki67 | IHC | Up to 35% |
| CK7 | IHC | Negative |
| CK20 | IHC | Negative |
| TTF-1 | IHC | Negative |
| p63 | IHC | Negative |
| GATA-3 | IHC | Negative |
| Pten | FISH | Negative for hetero- and homozygotic deletion |
Figure 2.
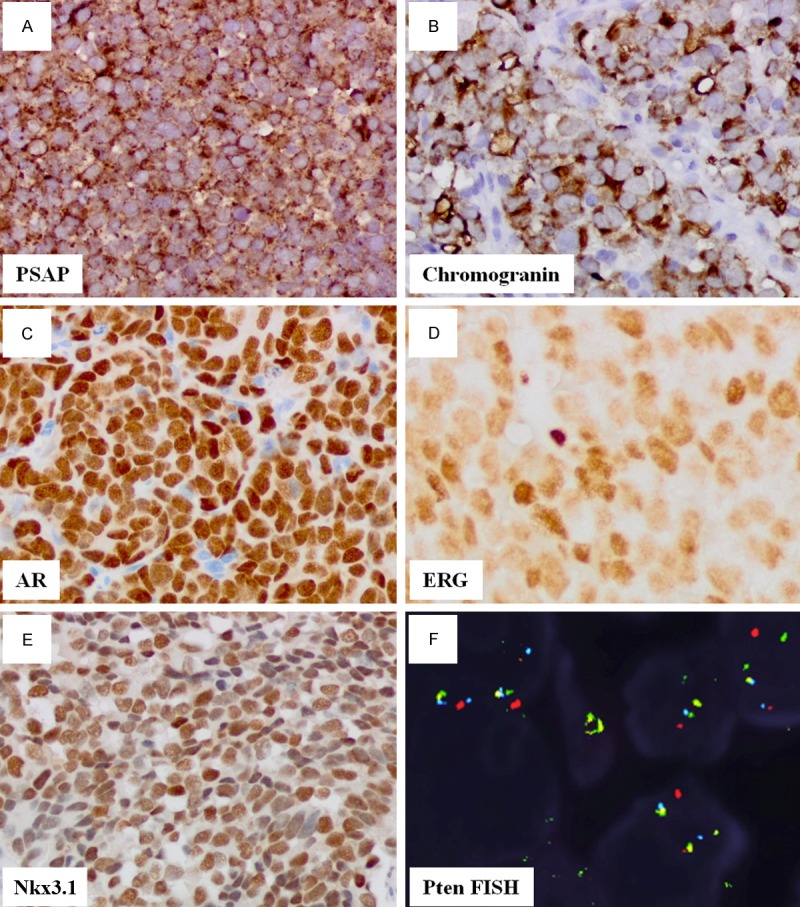
Molecular profile of the LCNEC. The carcinoma is positive for: PSAP (A), Synaptophysin (B), AR (C), ERG (D), Nkx3.1 (E) and negative for Pten deleteion (F).
We further characterized the cancer with androgen receptor (AR) and ERG expression as prostate cancer is regulated by the androgen signaling pathway. ERG is positive when fused to TMPRSS gene, regulated by androgen and estrogen [14]. Both AR (Figure 2C) and ERG (Figure 2D) were diffusely and strongly positive. Homeobox protein Nkx3.1, a prostate specific tumor suppressor with its expression in castration-resistant prostate cancer (Nkx3-1-expressing cells, CARNs) and implicated in stem cell function [15], was strongly positive (Figure 2E) in this case. Myc oncoprotein is often overexpressed in high grade prostate cancer, however, it was negative in our case. TTF-1 is sometimes positive in NE carcinoma other than lung cancer and was negative in this case.
Finally, we performed FISH studies to determine whether there was either hetero- or homozygotic deletion for Pten. Of 100 cells examined, there was no evidence of Pten deletion (Figure 2F).
Discussion
LCNEC is an exceedingly rare entity, especially when arising de novo. This entity is so rare that there are just a few case reports in the literature (Table 2). The largest series of LCNEC described only seven cases. These cases were identified using the histologic criteria of LCNEC of the lung described by Travis et al: large polygonal cells with low nuclear to cytoplasmic ratio, nuclei with prominent nucleoli and coarse chromatin, mitotic count greater than 10 mitosis per 10 high power fields, and evidence of neuroendocrine differentiation by immunohistochemistry or ultrastructurally. Six of these seven cases began their natural histories as typical adenocarinomas of the prostate and then progressed after long standing androgen deprivation therapy. Pathologic evaluation of all six of these cases demonstrated LCNEC admixed with typical adenocarinoma of the prostate. Only one case in this series arose de novo and demonstrated pure LCNEC on pathologic examination. All seven cases showed diffuse immunopositivity with at least one neuroendocrine marker (chromogranin, CD56, or synaptophysin) with variable expression of PSA, PSAP, CK7, and CK 20. Proliferation rate in the series was usually above 50% and all five cases tested for AR immunoreactivity were negative.
Table 2.
Literature review of LCNEC of the prostate
| Study | Clinicopathological features |
|---|---|
| Large Cell Neuroendocrine Carcinoma of Prostate: A Clinicopathologic Summary of 7 Cases of a Rare Manifestation of Advanced Prostate Cancer (6) | 7 cases were presented with 6 arose from prostatic adenocarcinoma following hormone therapy and one case as de novo. 6 of the 7 cases had foci of admixed conventional adenocarcinoma. The LCNEC component was comprised of sheets and ribbons of cells with areas of necrosis, abundant amphophilic cytoplasm, large nuclei with coarse chromatin and prominent nucleoli, and high mitotic activity. They proposed that LCNEC arises through clonal outgrowth associated with the selective pressure of ADT |
| Metastatic Large-Cell Neuroendocrine Prostate Carcinoma: Successful Treatment with Androgen Deprivation Therapy (7) | Two cases of metastatic poorly differentiated prostatic adenocarcinoma with coexisting foci of LCNEC were included. The NE component was composed of sheets of large hyperchromatic cells with prominent nucleoli, abundant amphophilic cytoplasm, and high mitotic activity. The NE component was strongly positive for synaptophysin and chromogranin. Both patients were treated with ADT with marked and durable response at 1 and 2 years after diagnosis. They concluded that although prostate cancer with NE differentiation is usually associated with more aggressive disease and failure of hormonal therapy, LCNEC can respond to ADT |
| Large-Cell Neuroendocrine Carcinoma of Prostate: A Case Report (8) | LCNEC that arose in a patient previously diagnosed prostatic adenocarcinoma and after 5 years of ADT. 5 years after diagnosis patient presented with rapidly progressive metastatic disease. Re-biopsy of the prostate at this time, showed LCNEC positive for TTF-1 and chromogranin. The Travis criteria for LCNEC of the lung were used for the morphologic diagnosis. They propose that LCNEC arises from clonal outgrowth associated with the selective pressure of ADT |
Since all but one case in the series by Evans et al some component of usual prostatic adenocarcinoma and arose in the setting of ADT, the authors suggested that NE carcinomas in the prostate arise from usual type adenocarcinomas through clonal outgrowth associated with the selective pressure of ADT. This theory was challenged by Azad et al. who presented two cases of LCNEC arising de novo with no prior history of ADT. Both cases were diagnosed on TRUS-guided prostate needle biopsy in patients with advanced, metastatic disease, at diagnosis with metastases and lymphadenopathy, and both pathologic specimens were admixed with areas of usual prostatic adenocarcinoma. Both patients responded well to treatment with ADT and remained alive without evidence of disease progression at 15 and 30 months. The authors concluded that LCNEC can arise de novo, independent of ADT, and may retain some level of AR expression and androgen dependence, although neuroendocrine prostate carcinoma is usually AR and PSA negative.
In 2013, the Prostate Cancer Foundation set out to better characterize and refine the diagnostic terminology of NE tumors and NE differentiation in the prostate. Their goal was to arrive at relevant pathological diagnoses that would encourage further molecular and clinical studies to identify improved treatments. They concluded that, since poorly differentiated usual prostatic adenocarcinoma (Gleason score 5 + 5 = 10) can diffusely express NE markers, the definition of LCNEC should be more restrictive than the one proposed by Evans et al. The Foundation’s panel reached the consensus that for a diagnosis of LCNEC to be rendered, the tumor cells should express at least one NE marker by IHC and should show morphologic evidence of NE differentiation characterized by large nests with peripheral palisading. Given these criteria, the diagnosis of LCNEC should be rare, and some LCNEC diagnosed in the past may represent poorly differentiated adenocarcinomas with diffuse NE expression.
Our case shows diffuse immunoreactivity for the NE markers chromogranin and synaptophysin with ultrastructural evidence of NE differentiation by electron microscopy and morphological evidence of NE differentiation characterized by the large nest of cells with moderate amount of amphophilic cytoplasm, vesicular clumpy chromatin with prominent nucleoli, high mitotic count, and focal peripheral palisading. No areas of usual prostatic adenocarcinoma were identified in the histologic sections and the patient had no prior history of hormonal therapy. The strong AR positivity was unusual, but the report by Azad et al. does suggest that de novo LCNEC may retain androgen dependence.
In conclusion, LCNEC is an extremely rare, poorly-studied tumor. The overwhelming majority of prior case reports suggest that LCNEC is often associated with long standing ADT. Generally, NE differentiation in the prostate has been associated with poor prognosis and aggressive disease. Molecular characterization of the de novo LCNEC may shed light into the biology of this rare tumor so that appropriate therapies can be identified.
Acknowledgements
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development). This work is supported by NIH UO1 (1U01CA149556-01), DOD PCRP PC111624 awards to PL and National Cancer Institute (UH3CA140233) to ZP.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12(Suppl 2):S141–144. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38:756–767. doi: 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fine SW. Variants and unusual patterns of prostate cancer: clinicopathologic and differential diagnostic considerations. Adv Anat Pathol. 2012;19:204–216. doi: 10.1097/PAP.0b013e31825c6b92. [DOI] [PubMed] [Google Scholar]
- 6.Evans AJ, Humphrey PA, Belani J, van der Kwast TH, Srigley JR. Large cell neuroendocrine carcinoma of prostate: a clinicopathologic summary of 7 cases of a rare manifestation of advanced prostate cancer. Am J Surg Pathol. 2006;30:684–693. doi: 10.1097/00000478-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Azad AA, Jones EC, Chi KN. Metastatic large-cell neuroendocrine prostate carcinoma: successful treatment with androgen deprivation therapy. Clin Genitourin Cancer. 2014;12:e151–153. doi: 10.1016/j.clgc.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Moratalla Charcos LM, Pastor Navarro T, Cortes Vizcaino V, Osca Garcia JM, Gil Salom M. Large-cell neuroendocrine carcinoma of prostate. Case report. Arch Esp Urol. 2013;66:368–371. [PubMed] [Google Scholar]
- 9.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM, Rubin MA, Nanus DM. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J. Clin. Oncol. 2012;30:e386–389. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–162. [PMC free article] [PubMed] [Google Scholar]
- 11.Humphrey PA. Histological variants of prostatic carcinoma and their significance. Histopathology. 2012;60:59–74. doi: 10.1111/j.1365-2559.2011.04039.x. [DOI] [PubMed] [Google Scholar]
- 12.Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, Huang J, True L, Gleave ME, Soule H, Logothetis C, Rubin MA. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20:2846–2850. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lima MV, Nogueira C, Oliveira JA, Muniz Neto FJ, Franco M, Tavora F. Prostatic carcinomas with neuroendocrine differentiation diagnosed in needle biopsies, a morphologic study of 7 cases among 465 sequential biopsies in a tertiary cancer center. Int Braz J Urol. 2011;37:598–604. doi: 10.1590/s1677-55382011000500005. [DOI] [PubMed] [Google Scholar]
- 14.Park K, Dalton JT, Narayanan R, Barbieri CE, Hancock ML, Bostwick DG, Steiner MS, Rubin MA. TMPRSS2:ERG gene fusion predicts subsequent detection of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. J. Clin. Oncol. 2014;32:206–211. doi: 10.1200/JCO.2013.49.8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]


