Abstract
The factors underlying epilepsy are multifaceted, but recent research suggests that the brain’s neural circuits, which play a key role in controlling the balance between epileptic and antiepileptic factors, may lie at the heart of epilepsy. This article provides a comprehensive review of the neural mechanisms and potential treatment of intractable epilepsy from neural inflammatory responses, melanocortin circuits in brain and pedunculopontine tegmental nucleus. Further studies should be undertaken to elucidate the nature of neural circuits so that we may more effectively apply these new preventive and symptomatic therapies to the patient suffering from medically refractory seizures and its complications.
Keywords: Intractable epilepsy, neural inflammatory responses, melanocortin circuits, pedunculopontine tegmental nucleus
Introduction
It has long been known that epilepsy is a common chronic brain disorder. The factors underlying epilepsy are multifaceted, but recent research suggests that the brain’s neural circuits, which play a key role in controlling the balance between epileptic and antiepileptic factors, may lie at the heart of epilepsy. This article provides a comprehensive review of the neural mechanisms and potential treatment of intractable epilepsy from experimental and clinical studies.
Epilepsy and neural inflammatory responses
Increasing evidence shows that neural inflammatory responses in the epileptic focus contribute to the pathophysiology of seizure-induced brain damage. It is well known that there is a direct relationship between epileptic activity and CNS inflammation [1,2], which is characterized by accumulation, activation, and proliferation of microglia and astrocytes. Early studies of intractable epilepsy concentrated on astrocyte activation and regional changes, but recent work has emphasized its microglial function [3,4]. Najjar et al reported that microglial activation and proliferation were prevalent in resected human epilepsy tissue from a consecutive series of 319 surgically treated epilepsy cases, suggesting that microglia may initiate a cycle of inflammation-induced seizures and seizure-induced inflammation, and microglia-driven epilepsy may be a primary pathogenic process [5]. Studies from Mayo clinic health system support the pathogenic role of neuroinflammation in medically intractable epilepsy [6]. Further studies are needed to clarify the effects of neural inflammatory responses on intractable epilepsy and seizure-induced brain damage.
Epilepsy and melanocortin circuits in brain
A very close relationship between astrocyte activation and medically intractable epilepsy has attracted much scientific interest in the past few decades. The central melanocortin signaling is a key regulator of energy metabolism and glucose metabolism, and this effect is mainly mediated by the melanocortinergic receptor (MCR) expressed in the brain [7,8]. A number of studies have verified that MC4R in the central nervous system plays an important role in regulating the release of insulin via the activity of sympathetic neurons [9]. Otherwise, being the predominant MCR subtype in the brain, the MC4R is demonstrated to specifically express in astrocytes [10-12]. Because of its roles in controlling between astrocyte activity and energy balance in many brain regions, MC4R on astrocytes has been a focus of interest [10].
It was known that stimulation of the subthalamic nucleus was proposed as a therapeutic approach to alleviate refractory epilepsy [13-16]. Of interest, Accumulating evidence from functional imaging and clinical neurophysiology have demonstrated that therapeutic mechanisms of subthalamic nucleus stimulation are closely related to the changes in cerebral glucose metabolism and blood flow [17,18]. The understanding of neuroanatomical connections in subthalamic nucleus is very important for studying the possible mechanism of subthalamic nucleus (STN) stimulation to refractory epilepsy. We had characterized different neuronal populations of the subthalamic nucleus neurons in adult transgenic mouse line expressing green fluorescent protein (GFP) under the control of the MC4R promoter [19]. We observed the expression of glial fibrillary acidic protein (GFAP)-immunoreactive cells in the MC4R-GFP reporter mouse by using fluorescence immunohistochemical detection, and found that GFAP-positive neurons were mainly labeled in the dorsal STN and sparsely distributed in the ventral STN, suggesting the dorsal STN is the principal subregion to participate in the regulation of astrocytic activity. Supporting the hypothesis of STN activation is the observation that STN stimulation induced different changes of the local cerebral blood flow (rCBF) responses as assessed by [15O] H2O positron emission tomography during dorsal STN versus ventral STN stimulation by astrocyte activation, suggesting STN stimulation acts through distinct neuronal pathways dependent on stimulation location [20]. Meanwhile, we also found that MC4R-GFP was mostly co-localized with GFAP-positive cells in the dorsal STN but seldom coexpressed in the ventral STN. Supporting the hypothesis of STN activation is the observation that STN stimulation increased the regional cerebral metabolic rate of glucose (rCMRGlc) in the middle frontal gyrus and the right anterior lobe of the cerebellum by employing FDG-PET study [21]. These results showed the melanocortinergic receptor mechanism of the modulation of astrocyte activity in the subregions of subthalamic nucleus.
Epilepsy, rapid eye movement sleep and pedunculopontine tegmental nucleus
Many lines of evidence show that susceptibility to epilepsy is increased during nonrapid eye movement (NREM, slow-wave) sleep whereas rapid eye movement (REM) sleep suppresses seizure occurrence [22-25]. Shouse et al reported that the neural generators of different sleep components can provoke seizure discharge propagation during NREM sleep, and can suppress it during REM sleep [26], suggesting that REM sleep may be a natural antiepileptogenic system in the body during the wake-sleep cycle. Therefore, intervening REM sleep may exert anti-epileptogenic influence.
The pedunculopontine tegmental nucleus (PPTg), which is in the lower midbrain, is considered a part of the reticular activating system [27,28], and exhibits a wide heterogeneity in terms of the neurochemical properties (cholinergic, catecholaminergic, serotonergic, glutamatergic-containing neurons, and GABAergic interneurons) and connectivity (afferent and efferent connections to the thalamus, cerebrum and spinal cord) [29-33]. Report from Hayashi et al showed that acetylcholinergic neurons in the PPTg were involved in mental development, and disruption of neuronal nicotinic acetylcholine receptors led to epilepsy [34]. It is demonstrated that stimulation of unilateral PPTg can selectively promote nocturnal REM sleep [35-38], and PPTg has been recently highlighted as an effective target of deep brain stimulation for seizure treatment in patients with intractable epilepsy who are unsuitable candidates for epilepsy brain surgery [39,40]. Otherwise, data from Schwartz et al showed that cholinergic agonist microinjection to the pontine produced polygraphic features of REM sleep [25]. So, the activation of specific pontine focus by nerve stimulation may induce REM-like state and suppress seizure occurrence.
Transneuronal tracing with neurotropic pseudorabies viruses (PRV) has greatly advanced our understanding of multisynaptic circuits between the PPTg and peripheral tissue. It has been shown previously that there is strong cholinergic innervation from PPTg to the thalamus and pons, which is involved in the generation of muscle tone and REM sleep [34,39]. These neural bases may partly explain many clinical symptoms that there exist visual, auditory and gastrointestinal complaints in patients with intractable epilepsy (Figure 1). Knowledge on the neural bases of minor gastrointestinal symptoms may help to describe those systems associated with the gastrointestinal phenomena in epilepsy. The past two decades have witnessed an explosion in the recognition that the CNS cell groups that project to the gastric sympathetic preganglionic neurons were identified by the viral retrograde transneuronal labeling method [41-43]. Card et al used synapse-dependent retrograde transneuronal transport of pseudorabies virus (PRV) to trace autonomic emotional motor circuit development from the stomach wall to CNS [44]. Banihashemi et al also reported that repeated brief postnatal maternal separation enhanced hypothalamic gastric autonomic circuits in juvenile rats [41]. Gao et al used PRV-152 expressed EGFP to inject into the stomach wall, and found that neurons expressed EGFP 60-72 h subsequent to PRV-152 inoculation of vagal terminals in the stomach wall were targeted in transverse brainstem slices [42]. In our experiment, PRV-614 was injected into the stomach wall, and after 5 days survival, the animals were perfused and their brains processed for immunohistochemical detection of PRV-614 [31,45,46]. We found that neurons of the STN and PPTg were retrogradely labeled with PRV-614 (Figure 1B), suggesting that neurons in STN and PPTg are tightly linked to the regulation of gastric functions.
Figure 1.
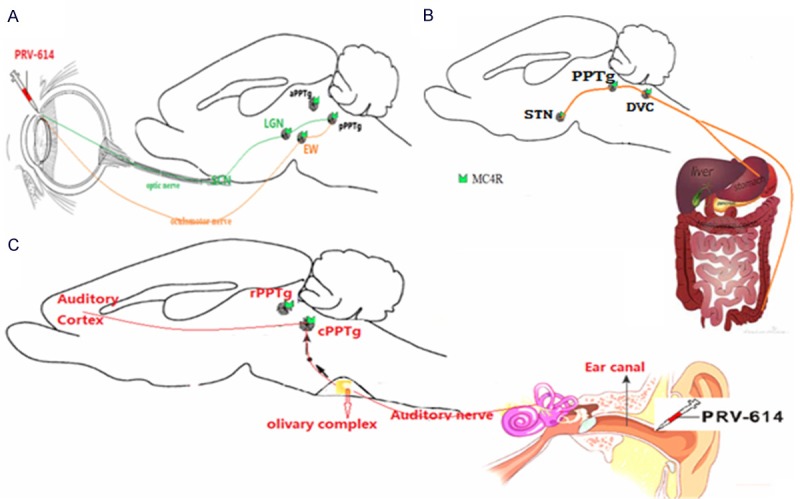
Summary diagram of the neural bases involving in visual, auditory and gastrointestinal complaints in patients with intractable epilepsy. A. Sagittal view of the mouse brain. PRV-614 spreads from infected retinal ganglion cells through the optic nerve to second-order neurons in the supracharismatic nucleus, dorsal and ventral aspects of the geniculate nuclei [dorsal aspect of the lateral geniculate nucleus (LGN) and ventral aspect of the lateral geniculate nucleus], intergeniculate nucleus, and pPPTg. Transport of PRV-614 is restricted to retrograde-only pathways. Although cells of the retinal ganglia are infected with PRV-614, this virus is restricted from anterograde spread through the optic nerve to retinorecipient neurons. Instead, retrograde spread of infection to first-order neurons in the ciliary ganglion leads to transport through the oculomotor nerve to second-order neurons in the Edinger-Westphal nucleus (EW) and pPPTg. Figure adapted from QX Hong (Epilepsy Behav, 2014); B. It is showing that the central circuits from the subthalamic nucleus to the stomach wall. PRV-614 injected into the ventral stomach wall is taken up by vagal terminals and enteric neurons, and then is retrogradely transported to the dorsal motor nucleus of the vagus (DMV), the nucleus of the solitary tract (NST) the area postrema (AP). Further replication and retrograde transsynaptic transport to regions of interest, including STN and most of the CNS including the STN, pedunculopontine tegmental nucleus (PPTg), paraventricular nuclei of the hypothalamus (PVN), and cortex thalamus. DVC, the dorsal vagal complex; STN, subthalamic nucleus. Figure adapted from HB Xiang (Brain, 2013; Parkinsonism Relat Disord, 2014). C. An overview of the binaural pathway from the outer ear to the auditory cortex via the eighth cranial nerve (the auditory nerve). The central auditory system receives the neural coding from the organ of Corti via the eighth cranial nerve (the auditory nerve). PRV-614 was injected into the ear canal in MC4R-GFP transgenic mouse, and the distribution patterns of PRV-614-positive neuronal labeling were analyzed in the auditory cortex, inferior colliculus, caudal PPTg (cPPTg), and olivary complex. PRV-614/MC4R-GFP dual labeled neurons were detected in the auditory cortex, inferior colliculus and cPPTg, suggesting direct melanocortinergic neuronal circuit from ear canal to the cPPTg. In contrast to the cPPTg, we didn’t detect PRV-614/MC4R-GFP neurons in the rostral PPTg (rPPTg).
Potential treatment of epilepsy
Collectively, the neural mechanisms play an important role in controlling the pathogenic development of pharmacologically resistant epilepsy. The past three decades have witnessed an explosion in the recognition of the safety and effectiveness of vagus nerve, STN and responsive cortical stimulation as an adjunctive therapy for partial onset seizures in adults with medically refractory epilepsy [47-49]. Further studies should be undertaken to elucidate the nature of vagus nerve, STN and responsive cortical stimulation so that we may more effectively apply these new preventive and symptomatic therapies to the patient suffering from medically refractory seizures and its complications.
Disclosure of conflict of interest
None to declare.
References
- 1.Aalbers MW, Rijkers K, Majoie HJ, Dings JT, Schijns OE, Schipper S, De Baets MH, Kessels A, Vles JS, Hoogland G. The influence of neuropathology on brain inflammation in human and experimental temporal lobe epilepsy. J Neuroimmunol. 2014;271:36–42. doi: 10.1016/j.jneuroim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Cai M, Choi SM, Song BK, Son I, Kim S, Yang EJ. Scolopendra subspinipes mutilans attenuates neuroinflammation in symptomatic hSOD1 (G93A) mice. J Neuroinflammation. 2013;10:131. doi: 10.1186/1742-2094-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzeng TT, Tsay HJ, Chang L, Hsu CL, Lai TH, Huang FL, Shiao YJ. Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid. J Biomed Sci. 2013;20:90. doi: 10.1186/1423-0127-20-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dambach H, Hinkerohe D, Prochnow N, Stienen MN, Moinfar Z, Haase CG, Hufnagel A, Faustmann PM. Glia and epilepsy: experimental investigation of antiepileptic drugs in an astroglia/ microglia co-culture model of inflammation. Epilepsia. 2014;55:184–92. doi: 10.1111/epi.12473. [DOI] [PubMed] [Google Scholar]
- 5.Najjar S, Pearlman D, Miller DC, Devinsky O. Refractory epilepsy associated with microglial activation. Neurologist. 2011;17:249–54. doi: 10.1097/NRL.0b013e31822aad04. [DOI] [PubMed] [Google Scholar]
- 6.Quek AM, Britton JW, McKeon A, So E, Lennon VA, Shin C, Klein C, Watson RE Jr, Kotsenas AL, Lagerlund TD, Cascino GD, Worrell GA, Wirrell EC, Nickels KC, Aksamit AJ, Noe KH, Pittock SJ. Autoimmune epilepsy: clinical characteristics and response to immunotherapy. Arch Neurol. 2012;69:582–93. doi: 10.1001/archneurol.2011.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDaniel FK, Molden BM, Mohammad S, Baldini G, McPike L, Narducci P, Granell S. Constitutive cholesterol-dependent endocytosis of melanocortin-4 receptor (MC4R) is essential to maintain receptor responsiveness to alpha-melanocyte-stimulating hormone (alpha-MSH) J Biol Chem. 2012;287:21873–90. doi: 10.1074/jbc.M112.346890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 9.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–9. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caruso C, Carniglia L, Durand D, Scimonelli TN, Lasaga M. Astrocytes: new targets of melanocortin 4 receptor actions. J Mol Endocrinol. 2013;51:R33–50. doi: 10.1530/JME-13-0064. [DOI] [PubMed] [Google Scholar]
- 11.Caruso C, Durand D, Schioth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. 2007;148:4918–26. doi: 10.1210/en.2007-0366. [DOI] [PubMed] [Google Scholar]
- 12.Catania A. Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci. 2008;31:353–60. doi: 10.1016/j.tins.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Ge Y, Hu W, Liu C, Zhang JG, Meng FG. Brain stimulation for treatment of refractory epilepsy. Chin Med J (Engl) 2013;126:3364–70. [PubMed] [Google Scholar]
- 14.Fridley J, Thomas JG, Navarro JC, Yoshor D. Brain stimulation for the treatment of epilepsy. Neurosurg Focus. 2012;32:E13. doi: 10.3171/2012.1.FOCUS11334. [DOI] [PubMed] [Google Scholar]
- 15.Capecci M, Ricciuti RA, Ortenzi A, Paggi A, Durazzi V, Rychlicki F, Provinciali L, Scerrati M, Ceravolo MG. Chronic bilateral subthalamic stimulation after anterior callosotomy in drug-resistant epilepsy: long-term clinical and functional outcome of two cases. Epilepsy Res. 2012;98:135–9. doi: 10.1016/j.eplepsyres.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Kase D, Inoue T, Imoto K. Roles of the subthalamic nucleus and subthalamic HCN channels in absence seizures. J Neurophysiol. 2012;107:393–406. doi: 10.1152/jn.00937.2010. [DOI] [PubMed] [Google Scholar]
- 17.Montaurier C, Morio B, Bannier S, Derost P, Arnaud P, Brandolini-Bunlon M, Giraudet C, Boirie Y, Durif F. Mechanisms of body weight gain in patients with Parkinson’s disease after subthalamic stimulation. Brain. 2007;130:1808–18. doi: 10.1093/brain/awm113. [DOI] [PubMed] [Google Scholar]
- 18.Batisse-Lignier M, Rieu I, Guillet C, Pujos E, Morio B, Lemaire JJ, Durif F, Boirie Y. Deep brain stimulation of the subthalamic nucleus regulates postabsorptive glucose metabolism in patients with Parkinson’s disease. J Clin Endocrinol Metab. 2013;98:E1050–4. doi: 10.1210/jc.2012-3838. [DOI] [PubMed] [Google Scholar]
- 19.Ke B, Liu TT, Liu C, Xiang HB, Xiong J. Dorsal subthalamic nucleus electrical stimulation for drug/treatment-refractory epilepsy may modulate melanocortinergic signaling in astrocytes. Epilepsy Behav. 2014;36:6–8. doi: 10.1016/j.yebeh.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Hill KK, Campbell MC, McNeely ME, Karimi M, Ushe M, Tabbal SD, Hershey T, Flores HP, Hartlein JM, Lugar HM, Revilla FJ, Videen TO, Earhart GM, Perlmutter JS. Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol. 2013;241:105–12. doi: 10.1016/j.expneurol.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagaoka T, Katayama Y, Kano T, Kobayashi K, Oshima H, Fukaya C, Yamamoto T. Changes in glucose metabolism in cerebral cortex and cerebellum correlate with tremor and rigidity control by subthalamic nucleus stimulation in Parkinson’s disease: a positron emission tomography study. Neuromodulation. 2007;10:206–15. doi: 10.1111/j.1525-1403.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- 22.Akman CI, Montenegro MA, Jacob S, Eck K, McBrian D, Chiriboga CA, Patterson MC. Subclinical seizures in children diagnosed with localization-related epilepsy: clinical and EEG characteristics. Epilepsy Behav. 2009;16:86–98. doi: 10.1016/j.yebeh.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Yi PL, Chen YJ, Lin CT, Chang FC. Occurrence of epilepsy at different zeitgeber times alters sleep homeostasis differently in rats. Sleep. 2012;35:1651–65. doi: 10.5665/sleep.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu TT, Feng J, Bu HL, Liu C, Guan XH, Xiang HB. Stimulation for the compact parts of pedunculopontine nucleus: An available therapeutic approach in intractable epilepsy. Epilepsy Behav. 2013;29:252–3. doi: 10.1016/j.yebeh.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Jaseja H. Purpose of REM sleep: endogenous anti-epileptogenesis in man -- a hypothesis. Med Hypotheses. 2004;62:546–8. doi: 10.1016/j.mehy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Shouse MN, Farber PR, Staba RJ. Physiological basis: how NREM sleep components can promote and REM sleep components can suppress seizure discharge propagation. Clin Neurophysiol. 2000;111(Suppl 2):S9–S18. doi: 10.1016/s1388-2457(00)00397-7. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Insola A, Padua L, Scarnati E, Valeriani M. Where are the somatosensory evoked potentials recorded from DBS leads implanted in the human pedunculopontine tegmental nucleus generated? Mov Disord. 2011;26:1573–4. doi: 10.1002/mds.23589. author reply 1574-5. [DOI] [PubMed] [Google Scholar]
- 29.Lee MS, Rinne JO, Marsden CD. The pedunculopontine nucleus: its role in the genesis of movement disorders. Yonsei Med J. 2000;41:167–84. doi: 10.3349/ymj.2000.41.2.167. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Ye DW, Guan XH, Li RC, Xiang HB, Zhu WZ. Stimulation of the pedunculopontine tegmental nucleus may affect renal function by melanocortinergic signaling. Med Hypotheses. 2013;81:114–116. doi: 10.1016/j.mehy.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Ye D, Guo Q, Feng J, Liu C, Yang H, Gao F, Zhou W, Zhou L, Xiang H, Li R. Laterodorsal tegmentum and pedunculopontine tegmental nucleus circuits regulate renal functions: Neuroanatomical evidence in mice models. J Huazhong Univ Sci Technolog Med Sci. 2012;32:216–20. doi: 10.1007/s11596-012-0038-2. [DOI] [PubMed] [Google Scholar]
- 32.Xiang HB, Liu C, Guo QQ, Li RC, Ye DW. Deep brain stimulation of the pedunculopontine tegmental nucleus may influence renal function. Med Hypotheses. 2011;77:1135–8. doi: 10.1016/j.mehy.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Holmstrand EC, Sesack SR. Projections from the rat pedunculopontine and laterodorsal tegmental nuclei to the anterior thalamus and ventral tegmental area arise from largely separate populations of neurons. Brain Struct Funct. 2011;216:331–45. doi: 10.1007/s00429-011-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi M, Nakajima K, Miyata R, Tanuma N, Kodama T. Lesions of acetylcholine neurons in refractory epilepsy. ISRN Neurol. 2012;2012:404263. doi: 10.5402/2012/404263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim AS, Moro E, Lozano AM, Hamani C, Dostrovsky JO, Hutchison WD, Lang AE, Wennberg RA, Murray BJ. Selective enhancement of rapid eye movement sleep by deep brain stimulation of the human pons. Ann Neurol. 2009;66:110–4. doi: 10.1002/ana.21631. [DOI] [PubMed] [Google Scholar]
- 36.Arnulf I, Ferraye M, Fraix V, Benabid AL, Chabardes S, Goetz L, Pollak P, Debu B. Sleep induced by stimulation in the human peduncu lopontine nucleus area. Ann Neurol. 2010;67:546–9. doi: 10.1002/ana.21912. [DOI] [PubMed] [Google Scholar]
- 37.Alessandro S, Ceravolo R, Brusa L, Pierantozzi M, Costa A, Galati S, Placidi F, Romigi A, Iani C, Marzetti F, Peppe A. Non-motor functions in parkinsonian patients implanted in the pedunculopontine nucleus: focus on sleep and cognitive domains. J Neurol Sci. 2010;289:44–8. doi: 10.1016/j.jns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Romigi A, Placidi F, Peppe A, Pierantozzi M, Izzi F, Brusa L, Galati S, Moschella V, Marciani MG, Mazzone P, Stanzione P, Stefani A. Pedunculopontine nucleus stimulation influences REM sleep in Parkinson’s disease. Eur J Neurol. 2008;15:e64–5. doi: 10.1111/j.1468-1331.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 39.Jaseja H. Pedunculopontine nucleus (PPN) stimulation in intractable epilepsy: evidence-related programming. Epilepsy Behav. 2014;31:56. doi: 10.1016/j.yebeh.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Jaseja H. Deep brain stimulation in intractable epilepsy: Pedunculopontine nucleus versus thalamic nuclei: A perspective. World Neurosurg. 2014;82:e568–9. doi: 10.1016/j.wneu.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Banihashemi L, Rinaman L. Repeated brief postnatal maternal separation enhances hypothalamic gastric autonomic circuits in juvenile rats. Neuroscience. 2010;165:265–77. doi: 10.1016/j.neuroscience.2009.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao H, Glatzer NR, Williams KW, Derbenev AV, Liu D, Smith BN. Morphological and electrophysiological features of motor neurons and putative interneurons in the dorsal vagal complex of rats and mice. Brain Res. 2009;1291:40–52. doi: 10.1016/j.brainres.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci. 2004;24:2782–6. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci. 2005;25:9102–11. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang HB, Zhu WZ, Guan XH, Ye DW. The cuneiform nucleus may be involved in the regulation of skeletal muscle tone by motor pathway: a virally mediated trans-synaptic tracing study in surgically sympathectomized mice. Brain. 2013;136:e251. doi: 10.1093/brain/awt123. [DOI] [PubMed] [Google Scholar]
- 46.Ye DW, Li RC, Wu W, Liu C, Ni D, Huang QB, Ma X, Li HZ, Yang H, Xiang HB, Zhang X. Role of spinal cord in regulating mouse kidney: a virally mediated trans-synaptic tracing study. Urology. 2012;79:745, e1–4. doi: 10.1016/j.urology.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 48.Liu TT, Guo QQ, An K, Zhang Y, Tian XB, Li RC, Xiang HB, Wang P. The optimal acupoint for acupuncture stimulation as a complementary therapy in pediatric epilepsy. Epilepsy Behav. 2014;31:387–9. doi: 10.1016/j.yebeh.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 49.Sun FT, Morrell MJ, Wharen RE Jr. Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5:68–74. doi: 10.1016/j.nurt.2007.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


