Abstract
Background: We investigated whether extracorporeal shock wave (ECSW) therapy can attenuate cyclophosphamide (CYP)-induced acute interstitial cystitis (AIC) in rats. Methods and Results: Eighteen male-adult Sprague-Dawley rats were equally divided into group 1 (sham control), group 2 (AIC induced by 150 mg/kg CYP by intra-peritoneal injection) and group 3 (AIC + ECSW 200 impulses at 0.11 mJ/mm2 to the urinary bladder at 3 and 24 h after CYP treatment). Smooth-muscle cells co-culture with menadione (25 µM) with and without ECSW treatment was performed. Western-blot results demonstrated that ECSW significant attenuated oxidative stress and inflammatory reactions in this in-vitro studies (all p < 0.001). 24-hour urine amount and microscopic findings of red-blood-cell count (i.e., hematuria) were higher in group 2 than in groups 1 and 3, and significantly higher in group 3 than in group 1 (all p < 0.001). The urine levels of albumin and interleukin-6 showed an identical pattern of hematuria among all three groups (all p < 0.001). The cellular and mRNA expressions of macrophage migration inhibitory factor (MIF)+, CD74+, CD68+, substance p+, and Cox-2+ cells in the bladder tissue exhibited an identical pattern of hematuria among all groups (all p < 0.0001). The integrity of epithelial layer and collagen-deposition area as stained by Sirius red displayed an opposite pattern of hematuria among the three groups (p < 0.0001). The protein expression of IL-12, iNOS, TNF-α, NF-κB, MMP-9, NOX-1, NOX-2, RANTES, and Oxyblot displayed an identical pattern of hematuria among all groups (all p < 0.01). Conclusion: ECSW therapy markedly attenuated CYP-induced AIC through inhibitions of the inflammation and oxidative stress.
Keywords: Acute interstitial cystitis, cyclophosphamide treatment, extra-corporeal shock wave, inflammatory biomarkers
Introduction
Interstitial cystitis (IC) is a syndrome of multifarious etiology that is characterized by pelvic and/or bladder pain associated with urinary urgency and frequency, nocturia and sterile urine [1-5]. Due to its symptom complex, IC has also been called painful bladder syndrome, leaky bladder syndrome, and irritative bladder syndrome [1-6]. IC is a chronic, noninfectious, probable inflammatory disorder of the urinary bladder that primarily affects women (approximately 90% of patients are female) [6,7]. The pathology of IC does not usually correspond to any other disease/syndrome, including urinary tract infections, carcinoma, or cystitis induced by radiation or medication.
It is believed that IC prevalence ranges from 1 in 100,000 to 5.1 in 1,000 of the general population worldwide [6,8,9] depending upon methodology used for the calculation, such as the index or scale used for the diagnosis. Studies have reported the incidence of IC to be as high as 52-67 per 100,000 cases in the United States [11] and a more recent report suggested that it may affect up to 2.7%-12.5% of the adult female population [11-13]. As IC progresses, pain usually increases in severity and becomes the most dominating and debilitating symptom, ultimately, significantly impacting the personal and professional lives of patients [1-13].
Since the etiology of IC is proposed to be multi-factorial, multiple therapies have been proposed to produce synergistic effects and better outcome [6,7,14]. However, not all therapies are effective in every individual. Additionally, complete remission is only attainable in some cases. Therefore, treatment of IC is a challenge for physicians and other health care providers who manage patients with this condition. Finding an effective therapeutic regimen for IC patients, especially those unresponsive to conventional therapies, is of utmost importance.
Extra-corporeal shock wave (ECSW) therapy was originally used for treating kidney or urethral stones. Recently, ECSW has been successfully applied for the treatment of shoulder tendinitis, plantar fasciitis [15,16], smooth muscle-cell proliferation [17] and chronic injuries of soft tissues and delayed fracture healing and nonunion [18] with safe and promising results [19]. The mechanisms of ECSW therapy for these diseases have been suggested to be mainly through relieving painful sensations [15,16,20], attenuating inflammatory responses [17] and inducing angiogenesis/vasculogenesis [21,22]. Shock wave therapy employs a sequence of transient pressure disturbances characterized by high peak pressure (100 MPa), fast pressure rise (< 10 ns), rapid propagation, and short lifecycle (10 µs) produced by an appropriate generator and directed to a specific target area with an energy density in the range of 0.003-0.890 mJ/mm2 [17,22-24]. Based on the reports of attenuation of inflammatory reactions and painful sensations by ECSW, we hypothesized that ECSW therapy might be a useful tool for the treatment of IC.
Materials and methods
Ethics
All animal experimental procedures were approved by the Institute of Animal Care and Use Committee at Chang Gung Memorial Hospital – Kaohsiung Medical Center (Affidavit of Approval of Animal Use Protocol No. 2008121108) and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, National Academy Press, Washington, DC, USA, revised 1996).
In vitro studies and experimental model of acute interstitial cystitis and rationale of ESCW energy dosage.
This study was designed to contain two parts of the experimental studies, including in vitro experimental study (i.e., Part I) and in vivo animal model study (i.e., Part II).
Part I study: to elucidate the impact of ECSW therapy on attenuating the generations of reactive oxygen species (ROS) and oxidative stress, the smooth muscle cell cline (A7R5) (1.4 x 105 cells for each experimental study) which was pretreated by ECSW [200 shots (0.12 mJmm2)] was cultured in DMEM-high glucose (4500 mg/L) 10% FBS culture medium for 3 hours, followed by co-cultured with menadione (i.e., an oxidative stimulator) 25 µM for 30 min. The menadione was then washed out and the cells were continuously cultured in DMEM culture medium for six hour prior to be collected for Western blot analysis. Additionally, to determine the impact of ECSW on ameliorating the inflammatory reaction, the smooth muscle cell cline (A7R5) which was pretreated by ECSW [200 shots (0.12 mJmm2)] was cultured in DMEM-high glucose (4500 mg/L) 10% FBS culture medium for 3 hours, followed by co-cultured with lipopolysaccharide LPS (500 ng/ml) (i.e., an inflammatory stimulation) for 3 hours. By the end of the six hours, the cells were collected for Western blot analysis.
In part II experimental study, pathogen-free, adult male Sprague-Dawley (SD) rats (n = 18) weighing 325-350 g (Charles River Technology, BioLASCO Taiwan, Taiwan) were randomized and equally divided into group 1 (sham control, intra-peritoneal injection of 2 cc normal saline), group 2 [acute interstitial cystitis (AIC) induced by intra-peritoneal injection of 150 mg/kg cyclophosphamide (CYP)], and group 3 (AIC + ECSW 200 impulses at 0.11 mJ/mm2). ECSW was applied to the skin surface above the urinary bladder at 3 and 24 h after CYP treatment. All animals were anesthetized by inhalational of 2.0% isoflurane, and placed supine on a warming pad at 37°C for ECSW therapy.
The application of ECSW energy dosage for the treatment of the AIC was based on our previous study with some modification [17].
Collection of 24 h urine for determining urine amount and the proteinuria at 72 h after AIC induction and sporadic urine for hematuria
24 h urine was collected in all animals at 72 h (i.e., from 48 to 72 h) after AIC induction prior to animal sacrifice to determine color of urine, inflammatory biomarkers, daily urine volume and proteinuria and that was leaked from the enhanced permeability of the urine bladder. Additionally, sporadic urine was collected at 72 h for analysis of hematuria among the three groups of animals.
Immunohistochemical and immunofluorescent studies
The procedures and protocols for immunohistochemical (IHC) and immunofluorescent (IF) examinations were also as described previously [25]. Briefly, for IHC staining, rehydrated paraffin sections were first treated with 3% H2O2 for 30 minutes and incubated with Immuno-Block reagent (BioSB; Santa Barbara, CA, USA) for 30 minutes at room temperature. Sections were then incubated with primary antibodies specifically against CD74 at 4°C overnight. Irrelevant antibodies and mouse control IgG were used as controls. IF staining was performed for the examination of CD68, Cox-2, macrophage migration inhibitory factor (MIF) and substance P (SP) using appropriate primary antibodies; irrelevant antibodies were used as controls. Three sections of bladder specimens were analyzed in each rat. For quantification, three randomly selected high power fields (HPFs, 200× for IHC and IF studies) were analyzed in each section. The percentage of positively-stained cells per HPFs for each animal was then determined. For negative control experiments, the primary antibodies were omitted. The sections were counterstained with 4’, 6-Diamidino-2-phenylindole (DAPI) (dilution 1/500) (Sigma-Alrich; St. Louis, Mo, USA) to identify cellular nuclei that reflected the cell number.
To analyze the intact of collagen synthesis and deposition, three bladder paraffin sections (4 µm) were stained with Picro-Sirius red (1% Sirius red in saturated picric acid solution) for one hour at room temperature using standard methods. The sections were then washed twice with 0.5% acetic acid. The water was physically removed from the slides by vigorous shaking. After dehydration in 100% ethanol three times, the sections were cleaned with xylene and mounted in a resinous medium. Ten low power fields (10x) of each section were used to identify Sirius red-positive area on each section. Image-pro plus 6.1 software (Media Cybernetics, Bethesda, MD, USA) was used to calculate the total cross-sectional area of the bladder and the total area of Sirius red-positive staining. The mean area of clump of condensed collagen deposition (an indicator of smooth muscle damage of urinary bladder) (A) was obtained by summation of Sirius red-positive areas on each section divided by the total numbers of sections. In addition, the mean cross-sectional area (B) of the bladder was obtained by dividing the sum of all cross sectional areas with the total number of sections examined. Finally, the percentage change in clump of condensed collagen deposition was obtained by dividing (A) by (B), followed by multiplication by 100.
Alcian Blue stain for distributive intensity of glycosaminoglycan (GAG) over the epithelial layer of urinary bladder was according to manufactory’s instructor. Additionally, histopathology scoring for the injury of epithelial layer based on the results of Alcian Blue stain was determined in urinary bladder specimen from all animals, sectioned at 4 mm under microscopic findings. The score reflected the grading of injured epithelial layer in 10 randomly chosen, non-overlapping fields (100x) for each animal as follows: 0 (none), 1 (≤ 10%), 2 (11-25%), 3 (26-45%), 4 (46-75%), and 5 (≥ 76%).
Real-time quantitative PCR analysis
Real-time polymerase chain reaction (RT-PCR) was conducted using LightCycler TaqMan Master (Roche, Germany) in a single capillary tube according to the manufacturer’s guidelines for individual component concentrations as previously reported [26]. Forward and reverse primers were each designed based on individual exons of the target gene sequence to avoid amplifying genomic DNA.
During PCR, the probe was hybridized to its complementary single-strand DNA sequence within the PCR target. As amplification occurred, the probe was degraded due to the exonuclease activity of Taq DNA polymerase, thereby separating the quencher from reporter dye during extension. During the entire amplification cycle, light emission increased exponentially. A positive result was determined by identifying the threshold cycle value at which reporter dye emission appeared above background.
Western blot analysis of bladder specimens
Equal amounts (10-30 μg) of protein extracts from ischemic kidneys of the animals (n = 10 for each group) were loaded and separated by SDS-PAGE using 7% or 12% acrylamide gradients. The membranes were incubated with monoclonal antibodies against matrix metalloproteinase (MMP)-9 (1:1000; Millipore, Billerica, MA, USA), and polyclonal antibodies against tumor necrosis factor (TNF)-α (1:1000; Cell Signaling, Danvers, MA, USA), nuclear factor (NF)-κB (1:600; Abcam, Cambridge, MA, USA), ADPH oxidase (NOX)-1 (1:1500; Sigma-Alrich, St. Louis, Mo, USA), NOX-2 (1:500; Sigma-Alrich, St. Louis, Mo, USA), interleukin (IL)-12 (1:500; Abcam, Cambridge, MA, USA), iNOS (1:250; Abcam Cambridge, MA, USA) and RANTES (1:1000, Cell signaling), MIF (1:1000; Abcam, Cambridge, MA, USA) and COX-2 (1:500; Abcam, Cambridge, MA, USA) were used. Signals were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse, goat anti-rat, or goat anti-rabbit IgG.
The Oxyblot Oxidized Protein Detection Kit was purchased from Chemicon (S7150). Derivatization with 2,4-dinitrophenylhydrazine (DNPH) was carried out on 6 μg of protein for 15 minutes according to the manufacturer’s instructions. One-dimensional electrophoresis was carried out on 12% SDS/polyacrylamide gel after DNPH derivatization. Proteins were transferred to nitrocellulose membranes which were then incubated in the primary antibody solution (anti-DNP 1:150) for two hours, followed by incubation with the second antibody solution (1:300) for one hour at room temperature. The washing procedure was repeated eight times within 40 minutes.
Immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences), which was then exposed to Biomax L film (Fuji). For quantification, ECL signals were digitized using Labwork software (UVP; Waltham, MA, USA). For oxyblot protein analysis, a standard control was loaded on each gel.
Statistical analysis
Quantitative data are expressed as means ± SD. Statistical analyses were performed using SAS statistical software for Windows version 8.2 (SAS Institute, Cary, NC) to conduct ANOVA followed by Bonferroni multiple-comparison post hoc test. A probability value of less than 0.05 was considered statistically significant.
Results
The effect of ECSW on inhibiting inflammatory reaction and the generations of reactive oxygen species and oxidative stress in smooth muscle cells (Figures 1 and 2)
Figure 1.
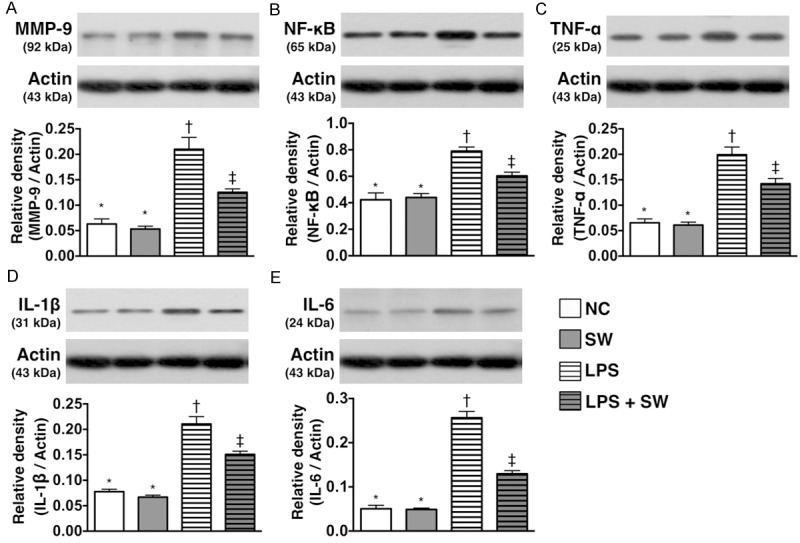
Protein expressions of inflammatory markers of smooth muscle cells under LPS stimulation and ECSW therapy (n = 6). A. The protein expression of matrix metalloproteinase (MMP)-9. *vs. other group with different symbols, p < 0.001. B. The protein expression of nuclear factor (NF)-κB. *vs. other group with different symbols, p < 0.001. C. The protein expression of tumor necrosis factor (TNF)-α. *vs. other group with different symbols, p < 0.001. D. The protein expression of interleukin (IL)-1β. *vs. other group with different symbols, p < 0.001. E. The protein expression of IL-6. *vs. other group with different symbols, p < 0.001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SW = shock wave; LPS = lipopolysaccharide.
Figure 2.
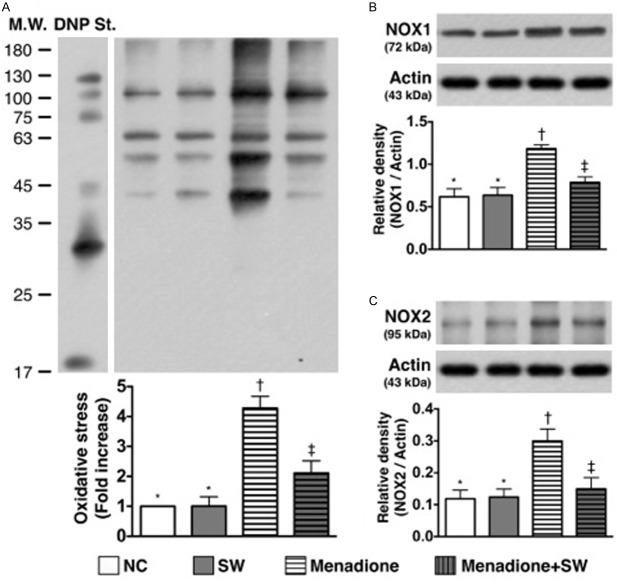
Protein expressions of reactive oxygen species and oxidative stress of smooth muscle cells under menadione stimulation and ECSW therapy (n = 6). A. Protein expression of oxidative index (protein carbonyls). *vs. other group with different symbols, p < 0.0001. (Note: left and right lanes shown on the upper panel represent protein molecular weight marker and control oxidized molecular protein standard, respectively). DNP = 1-3 dinitrophenylhydrazone. B. The protein expression of NOX-1. *vs. other group with different symbols, p < 0.001. C. The protein expression of NOX-2. *vs. other group with different symbols, p < 0.001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SW = shock wave; LPS = lipopolysaccharide.
As expected, the results of in vitro studies demonstrated that the protein expressions of MMP-9 (Figure 1A), TNF-α (Figure 1B), NF-κB (Figure 1C), IL-1β (Figure 1D), and IL-6 (Figure 1E), four indicators of inflammatory biomarkers did not differ between control group and control + ECSW-treated group. These findings suggest that ECSW therapy did not elicit the inflammatory reaction. However, these four biomarkers were significantly higher in LPS-treated group than the controls that were significantly reversed after ECSW therapy. These findings exhibited that ECSW had ability of anti-inflammation.
Additionally, the in vitro study showed that the oxidized protein (Figure 2A), an indicator of oxidative stress and protein expressions of NOX-1 (Figure 2B) and NOX-2 (Figure 2C), two indices of ROS and were similar between control group and control + ECSW-treated group. These findings once again proved that ECSW therapy did not augment the generation of ROS and oxidative stress. Conversely, these three parameters were significantly higher in menadione-treated group than the controls that were significantly reversed after ECSW therapy. These findings displayed that ECSW had property of attenuating the generation of ROS and oxidative stress.
Urine appearance, urine amount and proteinuria (Figure 3)
Figure 3.
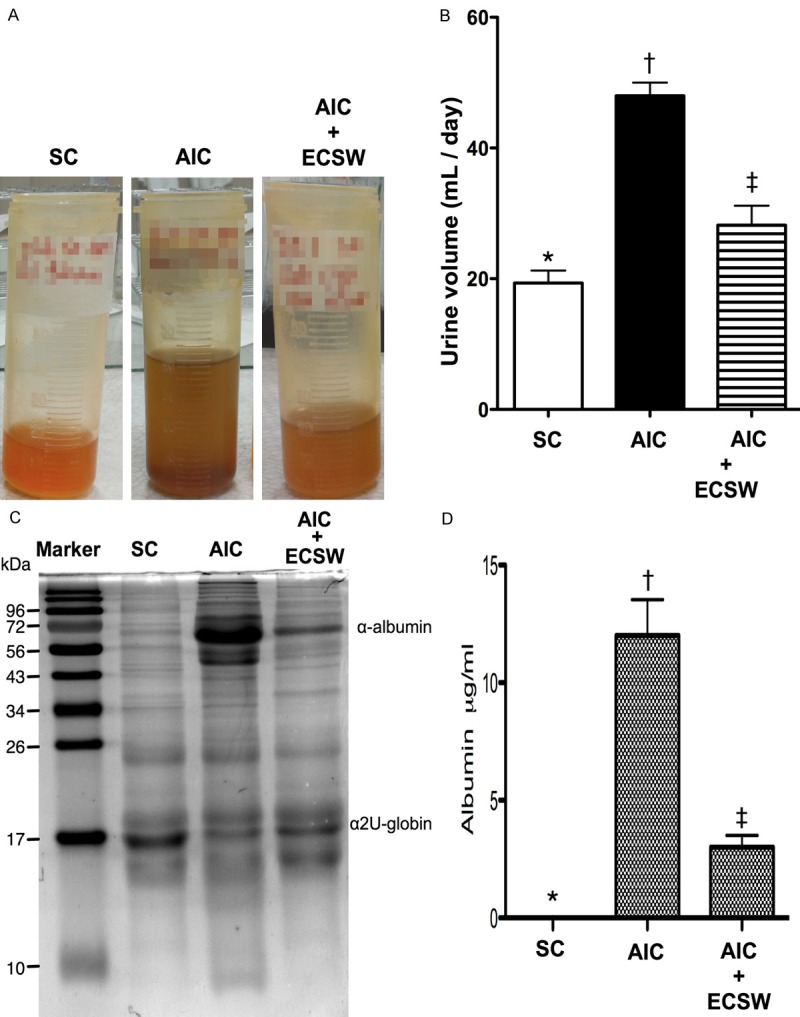
Urine appearance and 24 h urine amount and proteinuria (n = 6). A. Urine was noted more turbid in the acute interstitial cystitis (AIC) group than in the sham control (SC) and AIC-ECSW (extracorporeal shock wave) groups at 72 h after cyclophosphamide (CYP)-induced AIC. B. The 24 h urine volume by the end of 72 h among the three groups. *vs. other group with different symbols, p < 0.0001. C. The illustration of coomassie blue staining for identification of urine albumin. D. The statistically analytical results of 24 h proteinuria among the three groups. *vs. other group with different symbols, p < 0.0001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
Urine was more turbid in the AIC group than in the SC and AIC-ECSW groups at 72 h after AIC induction (Figure 3A). Additionally, the 24 h urine volume was significantly higher in the AIC group than the other groups, and significantly higher in the AIC-ECSW group than the SC group (Figure 3B). Moreover, the 24 h proteinuria showed a similar pattern of urine volume among the three groups (Figure 3C and 3D). These findings suggest that ECSW therapy may effectively attenuate proteinuria and abnormally increased urine amount resulting in urinary urgency and frequency, and nocturia.
Number of red blood cells in urine and the urine level of interleukin-6 (Figure 4)
Figure 4.
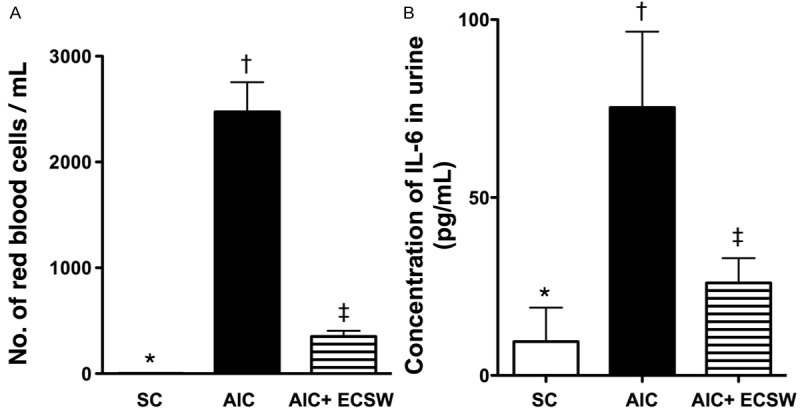
Number of red blood bells in sporadic urine and the urine Level of interleukin-6 by 72 h after cyclophosphamide (CYP)-induced AIC (n = 6). A. The number of red blood cells/ml among three groups. *vs. other group with different symbols, p < 0.0001. B. Urine level of interleukin (IL)-6 among three groups. *vs. other group with different symbols, p < 0.001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
The number of red blood cells/ml was significantly higher in the AIC group than in the other groups, and significantly higher in the AIC-ECSW group than in the SC group (Figure 4A). Additionally, the urine level of IL-6, an indicator of inflammation, exhibited an identical pattern to the number of red blood cells (Figure 4B). These findings suggest that ECSW therapy can inhibit hematuria and inflammatory reaction.
Microscopic findings of IHC and IF staining at 72 h after AIC induction (Figures 5, 6 and 7)
Figure 5.
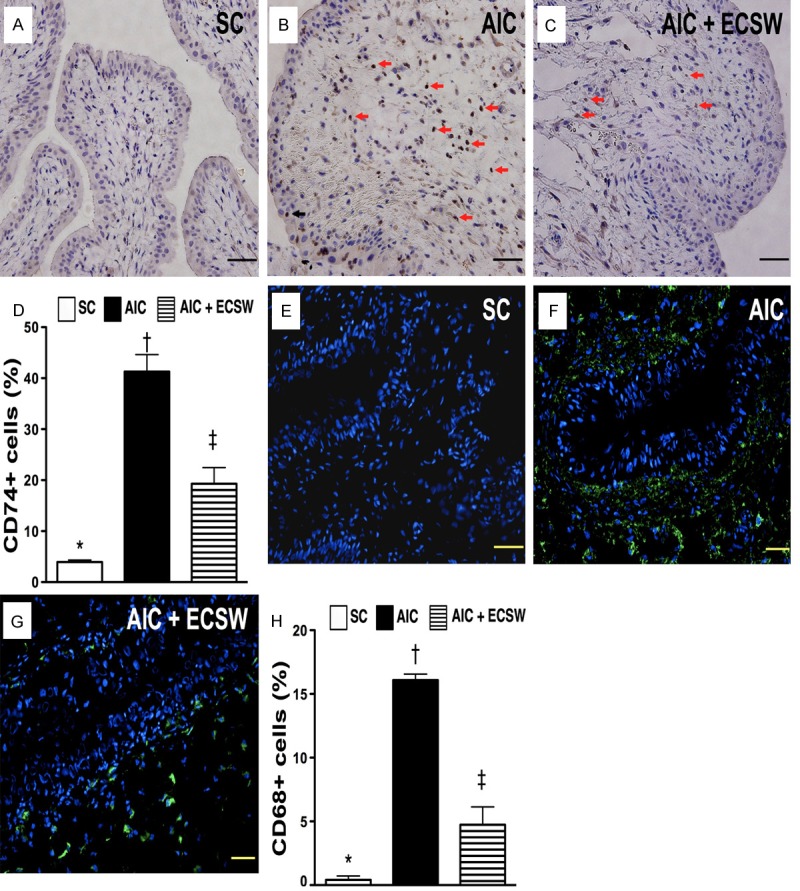
Immunohistochemical (IHC) for identification of CD74+ cells and immunofluorescent (IF) staining for identification of CD68+ cells in urinary bladder at 72 h after AIC induction (n = 6). A to C. Microscopic (200x) finding of IHC staining for number of CD74+ cells (black arrows) infiltrated in bladder among three groups. D. Analytical results: *vs. other group with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. E to G. Microscopic (200x) finding of IF staining for number of CD68+ cells infiltrated in bladder among three groups. H. Analytical results: *vs. other group with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
Figure 6.

Immunofluorescent (IF) staining for identification of MIF+ and Cox-2+ cells in urinary bladder at 72 h after AIC induction (n = 6). A to C. Microscopic (200x) finding of IF staining for number of macrophage inhibitor factor (MIF)+ cells infiltrated in bladder among three groups. D. Analytical results: *vs. other group with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. E to G. Microscopic (200x) finding of IF staining for number of Cox-2+ cells infiltrated in bladder among three groups. H. Analytical results: *vs. other group with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. I to K. Microscopic (200x) finding of IF staining for number of substance P+ cells infiltrated in bladder among three groups. L. Analytical results: *vs. other group with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
Figure 7.
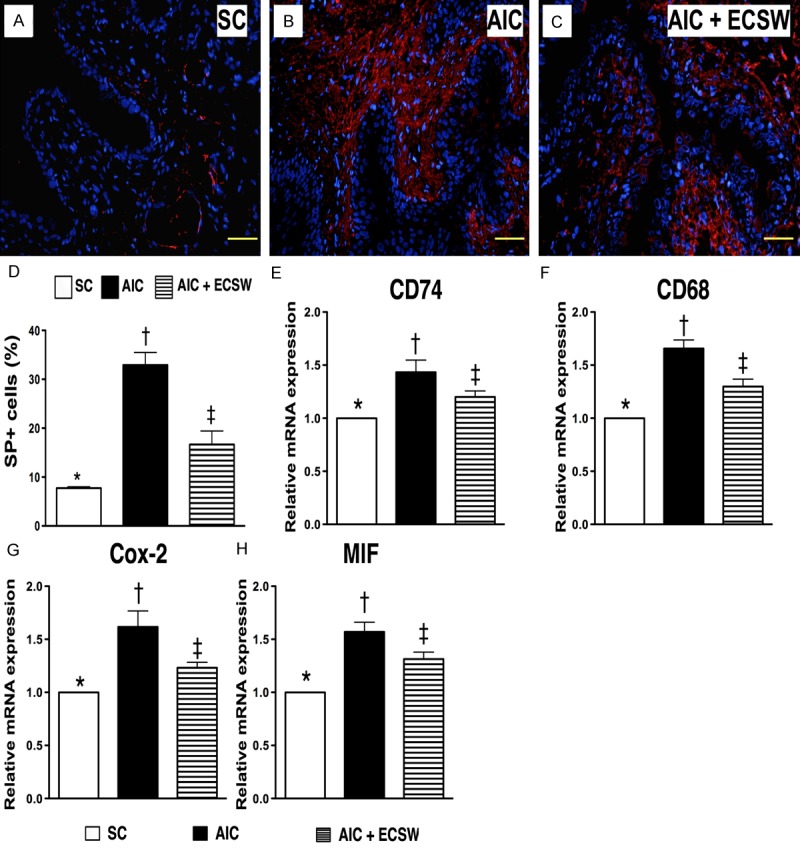
IF microscopic finding of substance p and mRNA expression of inflammatory biomarkers in urinary bladder at 72 h after AIC induction (n = 6). A to C. Microscopic (200x) finding of IF staining for number of substance P+ cells infiltrated in bladder among three groups. D. Analytical results: *vs. other group with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. E. The mRNA expression of CD74 in bladder among three groups. *vs. other group with different symbols, p < 0.001. F. The mRNA expression of CD68 in bladder among three groups. *vs. other group with different symbols, p < 0.001. G. The mRNA expression of Cox-2 in bladder among three groups. *vs. other group with different symbols, p < 0.0001. H. The mRNA expression of macrophage inhibitor factor (MIF) in bladder among three groups. *vs. other group with different symbols, p < 0.0001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
The results of IHC staining showed that the number of CD74+ cells infiltrated in bladder, a biomarker of inflammation, was significantly higher in the AIC group than in the other two groups, and significantly higher in AIC-ECSW group than in the SC group (Figure 5A-D). Consistently, the results of IF staining revealed that the expression of CD68+ cells in the bladder, a typical acute inflammatory cell, was similar to the expression of CD74+ cells in the bladder among the three groups (Figure 5E-H).
The results of IF microscopic findings showed a significantly higher number of infiltrated MIF+ cells, an indicator of inflammation, in the AIC group than in the other two groups, and a significantly higher number of infiltrated MIF+ cells in the AIC-ECSW group than in SC group (Figure 6A-D). Additionally, the IF microscopic findings showed that the expression of Cox-2+ cells (Figure 6E-H) and substance P+ cells (Figure 7A-D) in the bladder, another two inflammatory mediators, were identical to the expression of MIF+ cells in the bladder among the three groups. These findings suggest that ECSW inhibited the infiltration of inflammatory cells in the bladder.
mRNA expression of inflammatory biomarkers at 72 h after AIC induction (Figure 7)
To determine the transcription level of inflammation at 72 h after AIC induction with and without ECSW therapy, RT-PCR of bladder specimens was performed. Consistent with the expression of cellular level of inflammation, mRNA expression of CD74 (Figure 7E), CD68 (Figure 7F), Cox-2 (Figure 7G) and MIF (Figure 7H), four inflammatory biomarkers, were significantly higher in AIC only than other two groups and significantly higher in the AIC-ECSW group than in the SC group. These findings suggest that ECSW can reduce the inflammatory process after AIC induction.
Protein expression of inflammatory and ROS biomarkers, and oxidative stress at 72 h after aic induction (Figures 8, 9 and 10)
Figure 8.
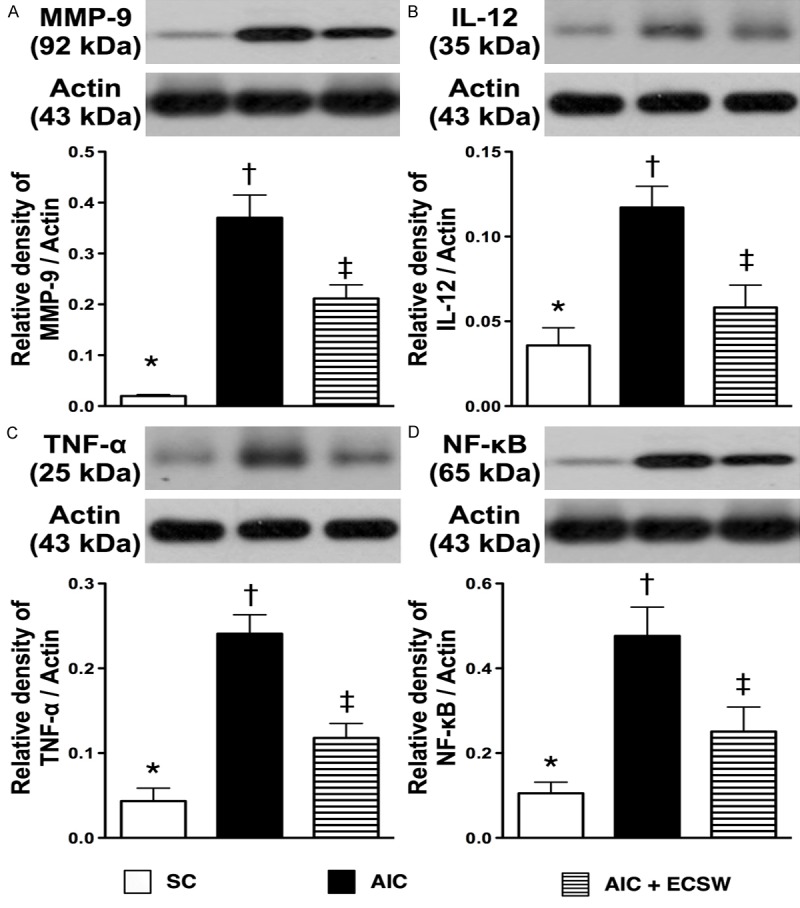
Protein expressions of inflammatory biomarkers in urinary bladder at 72 tissues of after AIC induction (n = 6). A. The protein expression of matrix metalloproteinase (MMP)-9. *vs. other group with different symbols, p < 0.001. B. The protein expression of interleukin (IL)-12. *vs. other group with different symbols, p < 0.001. C) The protein expression of tumor necrosis factor (TNF)-α. *vs. other group with different symbols, p < 0.001. D) The protein expression of nuclear factor (NF)-κB. *vs. other group with different symbols, p < 0.001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
Figure 9.
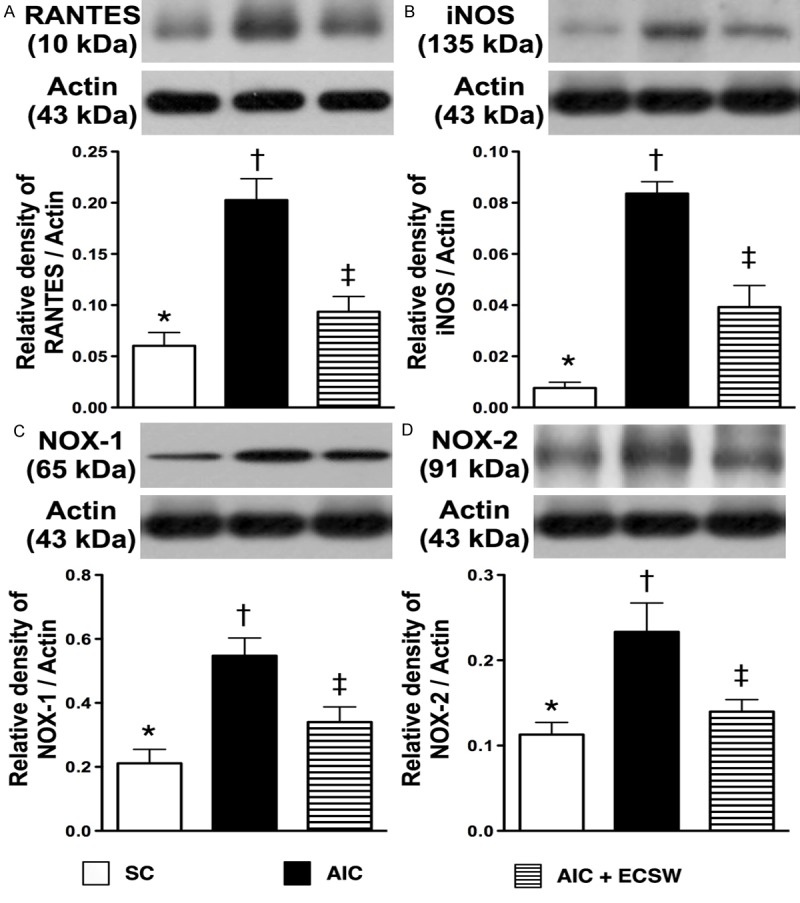
Protein expressions of inflammatory and reactive oxygen species biomarkers in urinary bladder at 72 h after AIC induction (n = 6). A. Protein expression of RANTES. *vs. other group with different symbols, p < 0.001. B. Protein expression of inducible nitric oxide synthase (iNOS). *vs. other group with different symbols, p < 0.0001. C. Protein expression of NOX-1. *vs. other group with different symbols, p < 0.001. D. Protein expression of NOX-2. *vs. other group with different symbols, p < 0.001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
Figure 10.
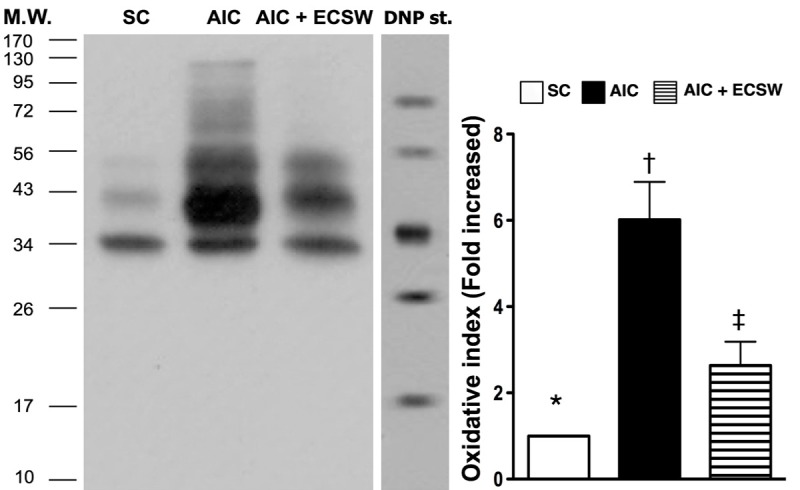
Protein expression of oxidative-stress biomarker in urinary bladder at 72 h after AIC induction (n = 6). Oxidative stress (i.e., oxidized protein) in urinary bladder at 72 h after AIC induction. DNP = 1-3 dinitrophenylhydrazone; M.W. = molecular weight (Note: Right lane and left lane shown on upper panel represent control oxidized molecular protein standard and protein molecular weight marker, respectively). *vs. other groups with different symbols, p < 0.001. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
To elucidate the translation levels of inflammation, ROS and oxidative stress at 72 h after AIC induction with and without ECSW therapy, the western blot of bladder tissue was performed. Mirroring the gene level expression profiles, the protein expression of MMP-9 (Figure 8A), IL-12 (Figure 8B), TNF-α (Figure 8C) and NF-κB (Figure 8D), four inflammatory biomarkers, were significantly higher in the AIC group than in the other two groups, and significantly higher in the AIC-ECSW group than in the SC group. Furthermore, the protein expression of RANTES (Figure 9A) and iNOS (Figure 9B), another two inflammatory biomarkers, and NOX-1 (Figure 9C) and NOX-2 (Figure 9D), two ROS biomarkers, showed identical expression patterns to the four inflammatory biomarkers above among the three groups. The expression of oxidized protein, an oxidative-stress index, also exhibited a pattern identical to that of ROS among the three groups (Figure 10). These findings, once again suggested that ECSW therapy ameliorated the generation of inflammation, ROS and oxidative stress in the AIC setting.
Impact of ECSW on preserving collagen expression in the smooth muscle layer of the urinary bladder and glycosaminoglycan expression in the epithelial layer, and on reducing the injury of epithelial layer (Figure 11)
Figure 11.
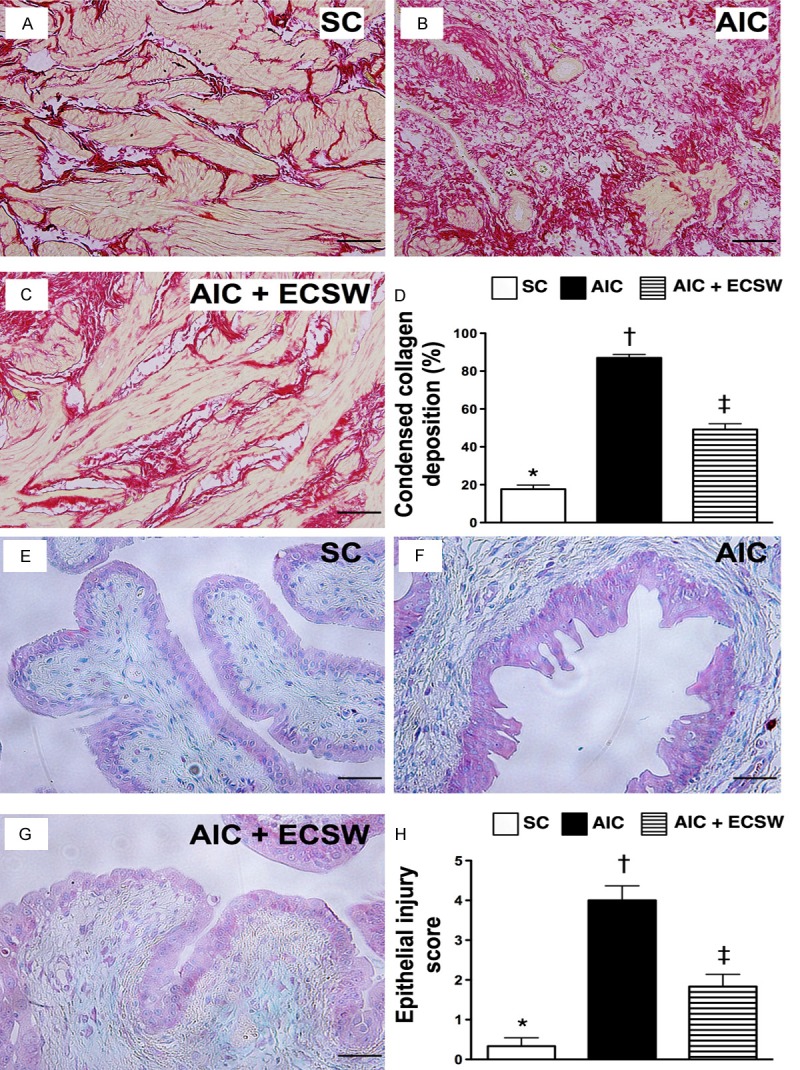
Collagen expression in the smooth muscle layer of the urinary bladder and the injury of epithelial layer (n = 6). A to C. Microscopic findings (100 x) Sirius red stain for identification of collagen deposition in urinary bladder. The area of condensed collagen deposition in urinary bladder (white color) was notably higher in AIC groups than in other two groups. D. Analytic results: *vs. other groups with different symbols, p < 0.0001. Scale bars in right lower corner represent 100 µm. E to G. Microscopic findings (200 x) of IHC stain for identification of intensive expression of GAG in the epithelial layer of the bladder (pink color) and the results showed no differ among the three groups. H. The analytical results of the injury score of epithelial layer: *vs. other groups with different symbols, p < 0.0001. Scale bars in right lower corner represent 50 µm. All statistical analyses using one-way ANOVA, followed by Bonferroni multiple comparison post hoc test. Symbols (*, †, ‡) indicate significance (at 0.05 level). SC = sham control; AIC = acute interstitial cystitis; ECSW = extracorporeal shock wave.
To investigate whether ECSW therapy preserved collagen expression in the smooth muscle layer of the urinary bladder, Sirius red stain was performed for each group of animals (Figure 11A-D). As expected, the integrity of collagen deposition area was substantially lower in the AIC group than in the other two groups, and significantly lower in AIC-ECSW group than in the SC group (Figure 11E-H). On the other hand, IHC stain demonstrated that the intensive expression of GAG in the epithelial layer of the bladder did not differ among the three groups. However, the injury score of epithelial layer was significantly higher in the AIC group than in the other two groups, and significantly higher in AIC-ECSW group than in the SC group (Figure 11E-H). These findings suggest that treatment with ECSW significantly preserved collagen deposition in the bladder and the integrity of the epithelial layer of bladder.
Discussion
The present study, which investigated the potential impact of ECSW on reducing AIC in rat, provided several valuable implications. First, the in vitro results showed that ECSW therapy remarkably inhibiting inflammatory reaction and the productions of ROS and oxidative stress. Second, ECSW treatment was substantially reduced the hematuria and the urine levels of proteinuria and IL-6. Third, the inflammatory response at the cellular, gene and protein levels were markedly reduced in the bladder after ECSW therapy. Finally, the generation of ROS and oxidative stress in bladder were suppressed, whereas the integrity of urinary bladder (i.e., including epithelial and smooth muscle layers) was preserved after ECSW therapy.
Treating IC is still a formidable challenge for clinicians. Several management strategies have been tried, including oral or intravesical pentosan polysulphate (PPS) [27,28], Cystoprotek therapy [29], intravesical hyaluronic acid administration [30], cyclosporine A therapy [31] and agents for inhibiting acute or chronic suburothelial inflammation [33-35]. However, these therapies remain problematic due to limited effectiveness and/or serious side effects. One important finding in the present study was that the urine amount was increased in AIC animals in comparison with SC animals and this symptom was remarkably reduced in AIC animals after receiving ECSW therapy. Additionally, not only the urine amount, but also hematuria and proteinuria and number of red blood cells in the urine were increased in AIC animals without treatment and these symptoms were significantly reduced in AIC animals after application of ECSW therapy. Furthermore, the inflammatory cytokine IL-6 level in urine was increased in AIC and significantly reduced after ECSW therapy. Urinary urgency and frequency associated with increase of urine amount and pathological findings of sub-urothelial inflammation, hematuria and proteinuria are common symptoms/signs in IC patients [1-14,28-39]. ECSW therapy significantly reduced these pathological processes in rat urinary bladder. Therefore, our findings extend the findings reported in previous studies [1-14,27-39].
Although the exact mechanisms of IC is not fully understood, both inflammation [6,7,38,39] and immune response [35-37,39] have been proposed to have key roles in disease pathogenesis. In this study inflammatory biomarkers, not only in gene level, but also at the protein and cellular levels were enhanced in AIC animals as compared with SC animals. Our findings, therefore, support the findings of clinical observational studies [6,7,35-39]. Of particular importance is that ESCW substantially suppressed the expressions of these inflammatory biomarkers in the urinary bladder. These findings, in addition to extending the findings of the previous studies, highlight the possibility of the clinical application of ECSW in IC patients who have a poor response to conventional therapy.
It is well recognized that ROS and oxidative stress, manifested as the production of free radicals, mitochondrial damage, organ damage, smooth muscle cell proliferation, cellular apoptosis and program of cell death frequently participate in many pathologies [17,25,26,40]. Two essential findings in the present study are that as compared with SC, ROS and oxidative stress were substantially higher in AIC animals. These results may, at least in part, explain why integrity of collagen deposition in the urinary bladder and the epithelial layer of urinary bladder were significantly reduced in AIC animals. Intriguingly, these parameters were notably preserved after ECSW therapy further supporting the benefits of ECSW in the AIC setting.
The principal finding in the in vitro study was that the ECSW therapy substantially ameliorated the inflammatory reaction and the generations of ROS and oxidative stress. Our findings not only delineated the mechanistic basis of ECSW therapy but also powerfully supported for why ECSW therapy was effective for AIC in rat.
Study limitations
The present study has several limitations. First, this study only investigated the effect of ECSW on an experimental model of AIC. Therefore, the long-term effect of ECSW on chronic IC is still unclear. Second, this study did not measure the bladder pressure or perform any urodynamic test. Thus, we cannot provide any urodynamic parameters such as the bladder function or urodynamic stress incontinency. Third, although the results are promising, the mechanisms underlying the benefit of ECSW therapy other than anti-inflammation and anti-oxidative stress on AIC remain unclear. The proposed mechanisms by which application of ECSW on improve the outcome of AIC are summarized in Figure 12.
Figure 12.
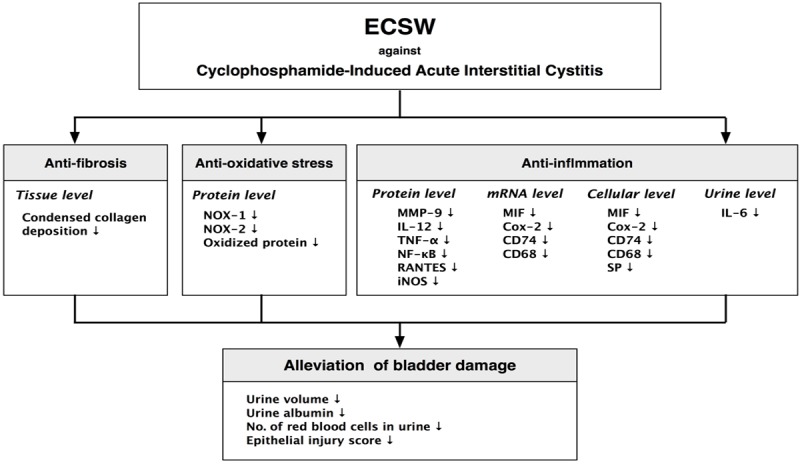
Proposed mechanisms underlying the effects of extracorporeal therapy on protecting the urinary bladder from cyclophosphamide injury in a rat AIC model based on the findings of the present study. ECSW = extracorporeal shock wave; NOX = NADPH oxidase; MMP-9 = matrix metalloproteinases-9; IL-12 = interleukin 12; TNF-α = tumor necrosis factor-α; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; iNOS = inducible nitric oxide synthase; MIF = macrophage migration inhibitory factor; SP = substance P.
In conclusion, ECSW therapy markedly improved rat AIC mainly through inhibition of inflammation, collagen deposition and fibrosis, as well as inhibition of the generation of ROS and oxidative stress in the urinary bladder which reduced hemturia, proteinuria and urine-rich potassium.
Acknowledgements
This study was supported by a program grant from Chang Gung Memorial Hospital, Chang Gung University (Grant number: CMRPG9C0372).
Disclosure of conflict of interest
None to declare.
References
- 1.Elbadawi A. Interstitial cystitis: A critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology. 1997;49(Suppl):14–40. doi: 10.1016/s0090-4295(99)80329-x. [DOI] [PubMed] [Google Scholar]
- 2.Curhan GC, Speizer FE, Hunter DJ, Curhan SG, Stampfer MJ. Epidemiology of interstitial cystitis: a population based study. J Urol. 1999;161:549–552. [PubMed] [Google Scholar]
- 3.Leppilahti M, Tammela TL, Huhtala H, Auvinen A. Prevalence of symptoms related to interstitial cystitis in women: a population based study in Finland. J Urol. 2002;168:139–143. [PubMed] [Google Scholar]
- 4.Nickle JC. Interstitial cystitis: A chronic pelvic pain syndrome. Med Clin North Am. 2004;88:467–481. doi: 10.1016/S0025-7125(03)00151-2. [DOI] [PubMed] [Google Scholar]
- 5.Kuo HC. Therapeutic Strategies for Interstitial Cystitis. Incont Pelvic Floor Dysfunct. 2007;2:33–36. [Google Scholar]
- 6.Phatak S, Foster HE Jr. The management of interstitial cystitis: an update. Nat Clin Pract Urol. 2006;3:45–53. doi: 10.1038/ncpuro0385. [DOI] [PubMed] [Google Scholar]
- 7.Nordling J. Interstitial cystitis: how should we diagnose it and treat it in 2004? Curr Opin Urol. 2004;14:323–327. doi: 10.1097/00042307-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2–9. doi: 10.1016/s0090-4295(99)80327-6. [DOI] [PubMed] [Google Scholar]
- 9.Hanno P, Keay S, Moldwin R, Van Ophoven A. International consultation on IC—Rome, September 2004/Forging an international consensus: progress in painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor dysfunct. 2005;16(Suppl 1):S2–S34. doi: 10.1007/s00192-005-1301-x. [DOI] [PubMed] [Google Scholar]
- 10.Curhan GC, Speizer FE, Hunter DJ, Curhan SG, Stampfer MJ. Epidemiology of interstitial cystitis: a population based study. J Urol. 1999;161:549–552. [PubMed] [Google Scholar]
- 11.Ueda , et al. Int J Urol. 2003;10:1–70. [Google Scholar]
- 12.Leppilahti M, Tammela TL, Huhtala H, Auvinen A. Prevalence of symptoms related to Interstitial Cystitis in women: a population based study in Finland. J Urol. 2002;168:139–143. [PubMed] [Google Scholar]
- 13.Rosenberg MT, Hazzard M. Prevalence of interstitial cystitis symptoms in women: a population based study in the primary care office. J Urol. 2005;174:2231–2234. doi: 10.1097/01.ju.0000181203.82693.95. [DOI] [PubMed] [Google Scholar]
- 14.Marshall K. Interstitial cystitis: understanding the uyndrome. Altern Med Rev. 2003;8:426–437. [PubMed] [Google Scholar]
- 15.Rompe JD, Zoellner J, Nafe B. Shock wave therapy versus conventional surgery in the treatment of calcifying tendinitis of the shoulder. Clin Orthop Relat Res. 2001;387:78–82. doi: 10.1097/00003086-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Rompe JD, Decking J, Schoellner C, Nafe B. Shock wave application for chronic plantar fasciitis in running athletes: a prospective, randomized, placebo-controlled trial. Am J Sports Med. 2003;31:268–275. doi: 10.1177/03635465030310021901. [DOI] [PubMed] [Google Scholar]
- 17.Shao PL, Chiu CC, Yuen CM, Chua S, Chang LT, Sheu JJ, Sun CK, Wu CJ, Wang CJ, Yip HK. Shock wave therapy effectively attenuates inflammation in rat carotid artery following endothelial denudation by balloon catheter. Cardiology. 2010;115:130–144. doi: 10.1159/000262331. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Qin L, Lu HB, Cheung WH, Yang H, Wong WN, Chan KM, Leung KS. Extracorporeal shock wave therapy in treatment of delayed bone-tendon healing. Am J Sports Med. 2008;36:340–7. doi: 10.1177/0363546507307402. [DOI] [PubMed] [Google Scholar]
- 19.van Leeuwen MT, Zwerver J, van den Akker-Scheek I. Extracorporeal shockwave therapy for patellar tendinopathy: a review of the literature. Br J Sports Med. 2009;43:163–8. doi: 10.1136/bjsm.2008.050740. [DOI] [PubMed] [Google Scholar]
- 20.Rompe JD, Meurer A, Nafe B, Hofmann A, Gerdesmeyer L. Repetitive low energy shock wave application without local anesthesia is more efficient than repetitive low-energy shock wave application with local anesthesia in the treatment of chronic plantar fasciitis. J Orthop Res. 2005;23:931–941. doi: 10.1016/j.orthres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–2830. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 22.Sun CK, Shao PL, Wang CJ, Yip HK. Study of vascular injuries using endothelial denudation model and the therapeutic application of shock wave: a review. Am J Transl Res. 2011;3:259–268. [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden JA, Tóth-Kischkat A, Schultheiss R. Principles of shock wave therapy. Clin Orthop Relat Res. 2001;387:8–17. doi: 10.1097/00003086-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Ciampa AR, de Prati AC, Amelio E, Cavalieri E, Persichini T, Colasanti M, Musci G, Marlinghaus E, Suzuki H, Mariotto S. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS Lett. 2005;579:6839–6845. doi: 10.1016/j.febslet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Sun CK, Yen CH, Lin YC, Tsai TH, Chang LT, Kao YH, Chua S, Fu M, Ko SF, Leu S, Yip HK. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun CK, Chang LT, Sheu JJ, Chiang CH, Lee FY, Wu CJ, Chua S, Fu M, Yip HK. Bone marrow-derived mononuclear cell therapy alleviates left ventricular remodeling and improves heart function in rat-dilated cardiomyopathy. Crit Care Med. 2009;37:1197–1205. doi: 10.1097/CCM.0b013e31819c0667. [DOI] [PubMed] [Google Scholar]
- 27.Nickel JC, Barkin J, Forrest J, Mosbaugh PG, Hernandez-Graulau J, Kaufman D, Lloyd K, Evans RJ, Parsons CL, Atkinson LE Elmiron Study Group. Randomized, double-blind, doseranging study of pentosan polysulfate sodium for interstitial cystitis. Urology. 2005;65:654–658. doi: 10.1016/j.urology.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 28.Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo- controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168–171. doi: 10.1046/j.1464-410x.1997.03384.x. [DOI] [PubMed] [Google Scholar]
- 29.Theoharides TC, Sant GR. A pilot open label study of Cystprotek in interstitial cystitis. Int J Immunopathol Pharmacol. 2005;18:183–188. doi: 10.1177/039463200501800119. [DOI] [PubMed] [Google Scholar]
- 30.Kallestrup EB, Jorgensen SS, Nordling J, Hald T. Treatment of interstitial cystitis with Cystistat: A hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143–147. doi: 10.1080/00365590410015876-1. [DOI] [PubMed] [Google Scholar]
- 31.Sairanen J, Forsell T, Ruutu M. Long-term outcome of patients with interstitial cystitis treated with low dose cyclosporine A. J Urol. 2005;171:2138–2141. doi: 10.1097/01.ju.0000125139.91203.7a. [DOI] [PubMed] [Google Scholar]
- 32.Hanno PM, Buehler J, Wein AJ. Use of amitriptyline in the treatment of interstitial cystitis. J Urol. 1989;141:846–848. doi: 10.1016/s0022-5347(17)41029-9. [DOI] [PubMed] [Google Scholar]
- 33.Soucy F, Gregoire M. Efficacy of prednisolone for severe refractory ulcerative interstitial cystitis. J Urol. 2005;173:841–843. doi: 10.1097/01.ju.0000153612.14639.19. [DOI] [PubMed] [Google Scholar]
- 34.Hanno PM, Buehler J, Wein AJ. Use of amitriptyline in the treatment of interstitial cystitis. J Urol. 1989;141:846–848. doi: 10.1016/s0022-5347(17)41029-9. [DOI] [PubMed] [Google Scholar]
- 35.Soucy F, Gregoire M. Efficacy of prednisolone for severe refractory ulcerative interstitial cystitis. J Urol. 2005;173:841–843. doi: 10.1097/01.ju.0000153612.14639.19. [DOI] [PubMed] [Google Scholar]
- 36.Van De Merwe JP, Arendsen HJ. Interstitial cystitis: a review of immunological aspects of the etiology and pathogenesis, with a hypothesis. BJU Int. 2000;85:995–999. doi: 10.1046/j.1464-410x.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 37.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69:34–40. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 38.Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, Vodovotz Y, Chancellor M. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2012;42:629–635. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 39.Chuang FC, Kuo HC. Increased urothelial cell apoptosis and chronic inflammation are associated with recurrent urinary tract infection in women. PLoS One. 2013;8:e63760. doi: 10.1371/journal.pone.0063760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YT, Yang CC, Zhen YY, Wallace CG, Yang JL, Sun CK, Tsai TH, Sheu JJ, Chua S, Chang CL, Cho CL, Leu S, Yip HK. Cyclosporine-assisted adipose-derived mesenchymal stem cell therapy to mitigate acute kidney ischemia-reperfusion injury. Stem Cell Res Ther. 2013;4:62. doi: 10.1186/scrt212. [DOI] [PMC free article] [PubMed] [Google Scholar]