Abstract
Introduction: Mesenchymal stem cells (MSCs) contribute to the engraftment of transplanted hematopoietic stem cells (HSCs). MSCs also accelerate hematological recovery by secreting SDF-1 and enabling HSCs to enter the bone marrow (BM) via the SDF-1/CXCR4 axis. HOXB4 has been shown to stimulate HSC self-renewal. In this study, we examined whether SDF-1 and HOXB4 expression in MSCs co-transplanted with HSCs could synergistically improve hematopoietic recovery in irradiated mice. Methods: Using recombinant adenoviruses, we generated genetically modified BM-MSCs that expressed SDF-1, HOXB4, and an SDF-1/HOXB4 fusion gene. We then co-transplanted these modified MSCs with HSCs and investigated blood cell counts, BM cellularity, degree of human HSC engraftment, and survival rate in irradiated mice. Results: We found that co-culturing the SDF-1/HOXB4 fusion gene-modified MSCs (SDF-1/HOXB4-MSCs) and human umbilical cord blood CD34+ cells significantly improved HSC cell expansion in vitro. More importantly, co-transplantation of CD34+ cells and SDF-1/HOXB4-MSCs markedly increased the hematopoietic potential of irradiated mice as evidenced by the rapid recovery of WBC, PLT and HGB levels in peripheral blood and of BM cellularity. Co-transplantation also markedly improved engraftment of human CD45+ cells in mouse BM. Conclusions: Our study demonstrates that SDF-1/HOXB4-MSCs markedly accelerate hematopoietic recovery and significantly improve survival among mice treated with a lethal dose of irradiation. Therefore, SDF-1/HOXB4-MSCs could have therapeutic value by improving the efficacy of clinical transplantations in patients with defective hematopoiesis.
Keywords: Mesenchymal stem cells, hematopoietic stem cells, SDF-1 gene, HOXB4 gene, irradiation, hematopoietic reconstitution, NOD/SCID mice
Introduction
It is well accepted that transplantation of hematopoietic stem cells (HSCs) is one of the most effective therapies for patients with hematopoietic disorders and with various forms of ischemic anemia resulting from chemoradiation [1]. Recent studies have found that the bone marrow engraftment of transplanted HSCs is improved when they are co-transplanted with umbilical cord-derived or bone marrow-derived mesenchymal stem cells (MSCs) [2-9]. These studies have not only uncovered an adjuvant role for MSCs [10], but they have also suggested that co-transplantation of HSCs with MSCs may improve overall clinical outcomes [5].
An early and critical step for engraftment involves the stable homing of transplanted HSCs to the bone marrow (BM). Masuda et al. have shown that co-transplantation with MSCs improves HSC migration and homing to the BM [7]. MSCs express high amounts of stromal cell-derived factor-1 (SDF-1), also known as chemokine (C-X-C motif) ligand 12 (CXCL12), which binds to its cognate receptor C-X-C motif receptor 4 (CXCR4) in HSCs [11]. This interaction mediates the proliferation, migration and homing of HSCs (3, 21, 22). These observations suggest that HSC engraftment and hematological recovery might be enhanced if SDF-1 expression is upregulated in MSCs [11-13]. In addition to external factors, it is known that reprogramming transcription factors, such as homeobox B4 (HOXB4), can effectively enhance the self-renewal of HSCs [14-16]. The reinforced expression of HOXB4 has been found to increase the efficiency of renewal and produce the most effective HSCs (reviewed in [17]).
In this study, we transduced human BM-MSCs with recombinant adenovirus expressing a SDF-1/HOXB4 fusion gene, and co-transplanted these modified MSCs with human cord blood CD34+ HSCs (CB-HSCs) into total body irradiated NOD-SCID mice. The hematopoietic reconstitution of these experimental mice was analyzed, and a potential application of this enhanced transplantation procedure is discussed in the context of acute irradiation injury and other hematopoietic disorders.
Materials and methods
Animals and human specimens
Four- to six-week-old female NOD/SCID/IL2rγnull mice from Jackson Laboratory (Bar Harbor, ME, USA), weighing 18-20 g, were kept in a sterile hood. The feed was sterilized with 60Co radiation. All animal studies were approved by the Institutional Animal Care and Use Committee at the Third Military Medical University (Chongqing, China).
Umbilical cord blood samples were collected from healthy, full-term newborn infants at the Department of Gynecology and Obstetrics. Bone marrow samples were collected from patients who underwent a bone marrow aspiration/biopsy procedure for suspected hematologic disorders at the Department of Hematology, the Southwest Hospital (Chongqing, China). Around 2-4 ml of bone marrow was collected from each patient. All the bone marrow cells used in this study were examined by routine morphologic and immunophenotypic assays and classified as normal. Written and informed consent was obtained from all study participants prior to enrollment. This study was approved by the Ethics Committee of the Third Military Medical University.
Preparation of recombinant adenovirus
Full length SDF-1 and HOXB4 genes, as well as a SDF-1-(GlySer) 3-HOXB4 fusion gene were synthesized within unique Xho I and EcoR I sites. These genes were inserted into the adenovirus vector pIRES2-EGFP (Foregene, Beijing, China) to generate the recombinant adenovirus expression plasmids pAD-SDF-1-IRES-GFP, pAD-HOXB4-IRES-GFP and pAD-SDF-1-(GlySer) 3-HOXB4-IRES-GFP. After digestion with Pac I, the linearized recombinant plasmids were transfected into 293A cells (Jingmei Biotech Co.Ltd., Shenzhen, China), which were subsequently maintained by routine cell culture. When 80% cytopathic effect (CPE) was achieved, the supernatants containing recombinant adenovirus AD-SDF-1-IRES-GFP, AD-HOXB4-IRES-GFP and AD-SDF-1-(GlySer) 3-HOXB4-IRES-GFP, hereafter called AD-SDF-1, AD-HOXB4 and AD-SDF-1/HOXB4 respectively, were harvested and titered in 293A cells. The titers of recombinant adenoviruses (rADs) were adjusted to a final of 1 × 1011 infectious units/ml (IFU) and stored in -80°C.
MSC preparation and transfection with rADs
Human BM-MSCs were isolated from BM aspirates based on previously published methods [7,10]. Briefly, the harvested BM aspirate was digested with ACK buffer (155 mM NH4Cl, 10 mM KHCO3, and 0.1 m Methylenediamine tetraacetic acid; Wako, Osaka, Japan) to lyse red blood cells and then subjected to Ficoll separation to obtain the nucleated cell fraction. MSCs were isolated by allowing them to adhere to plastic for 1 hour, and then they were cultured for 2 to 3 weeks in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum and penicillin/streptomycin at 37°C with 5% CO2.
MSCs, at 80% confluence, were transfected with 20 µl of the rAds with 10 multiplicity of infection (MOI). After 5 days, the transfected cells were observed under fluorescence microscope for GFP expression. The immunophenotype of MSCs transfected with various rAds were analyzed by flow cytometry (FCM) using PE labeled anti-human CD34, and FITC labeled anti-human CD45, CD44, and CD90 monoclonal antibodies (mAb). The expression levels of transduced genes were confirmed by RT-PCR and Western blot analysis.
Isolation of HSCs
Umbilical cord blood CD34+ cells were isolated according to our previously published method [18]. Briefly, umbilical cord blood samples were collected from healthy, full-term newborns immediately at birth. Mononuclear cells were isolated by using Isopaque-Ficoll gradients. The CD34+ cells were obtained using a mini magnetic cell sorting system (mini-MACS; Miltenyi Biotec, Germany) according to the manufacturer’s instructions. The purity of the isolated CD34+ cells was analyzed with FCM using PE labeled mouse anti-human CD34 mAb (eBioscience, USA).
In vitro co-culture of recombinant MSCs and HSCs
Recombinant Ads-transfected MSCs (1 × 104 cells) were seeded in single wells of a 24-well plate in triplicate. These MSCs were exposed to a 30 Gy dose of 60Co γ-radiation at 80% confluence, which were taken as the trophocytes. Purified CD34+ cells (5 × 104) were then plated into each well and co-cultured for 7 days in serum-free culture media supplemented with SFC (100 ng/ml; Behringwerke, Marburg, Germany), IL-3 (50 ng/ml; Boehringer Mannheim, Mannheim, Germany) and GM-CSF (25 ng/ml; Boehringer Mannheim). After 8 days, nonadherent cells in each well were counted and analyzed by FCM using PE labeled mouse anti-human CD34 mAb (eBioscience, USA).
Co-transplantation of recombinant MSCs and HSCs
After a 3.5 Gy dose of 60Co total radiation, the animals were provided with food supplemented with antibiotics and sterile, acidized drinking water containing amphotericin B (80 mg/l) and ciprofloxacin (80 mg/l). Within 12 hours of irradiation, 12 mice in each experimental group were transplanted or co-transplanted with the following amounts of cells via the tail vein: (1) SDF-1 group: 8 × 105 SDF-1/MSCs+ 1 × 105 CD34+ cells; (2) HOXB4 group: 8 × 105 HOXB4/MSCs+ 1 × 105 CD34+ cells; (3) S-H group: 8 × 105 SDF1-HOXB4/MSCs+ 1 × 105 CD34+ cells; (4) MSC-HSC group: 8 × 105 MSCs+ 1 × 105 CD34+ cells; (5) HSC group: 1 × 105 CD34+ cells; (6) IR group: 0.3 ml physiological saline per mouse (no treatment control).
The general status and survival of animals were recorded daily after transplantation. At 1, 2, 3 and 4 weeks after transplantation, tail vein blood was harvested and the leukocyte and platelet counts and the hemoglobin levels were examined. At 2 and 4 weeks after transplantation, 2 mice from each group were anesthetized with isoflurane and sacrificed by cervical dislocation. The BM cells from their tibia and femur were harvested for cytology analysis with Wright staining. Engraftment of human cells was evaluated by FCM immunophenotyping with FITC-labeled anti-human CD45 antibody (eBioscience, USA).
Reverse transcriptase polymerase chain reaction (RT-PCR)
The expression levels of SDF-1, HOXB4 or SDF-1/HOXB4 mRNA were analyzed by RT-PCR. Briefly, total RNA was isolated from rADs-transfected MSCs, reversely transcribed into cDNA, and subjected to PCR using a kit (Promega, USA). PCR primers, designed with Primer Premier 5.0 software (Premier, Canada), are as follows: for SDF-1, 5’-GCCTGAGCTACAGATGCC-3’ (forward) and 5’-AGCTTTCTCCAGGTACTCCT-3’ (reverse); for HOXB4, 5’-AGCGATTACCTACCCAGCGACC-3’ (forward) and 5’-AGGAGCCCGAGGGGACAGAC-3’ (reverse); for SDF-1/HOXB4 fusion gene, 5’-GCCTAGGCTACAGATGCC-3’ (forward) and 5’-AGGAGCCCGAGGGGACAGAC-3’ (reverse). Human β-actin was used as the internal control.
Western blotting
Total protein was prepared from MSCs transfected with various rAds. After separation by SDS-PAGE, proteins were transferred onto PVDF membranes and incubated at 4°C overnight in 1:1000 diluted goat anti-human SDF-1 (Santa Cruz Biotechnology, Inc., USA) or 1:800 diluted goat anti-human HOXB4 (Santa Cruz Biotechnology, Inc.). This was followed by incubation with rabbit anti-goat IgG HRP (Santa Cruz Biotechnology, Inc.) and detection of target bands by an enhanced chemiluminescence assay. Human β-actin was used as a control.
Statistical analysis
Statistical analysis was performed with SPSS 10.0 software. Measurement data were expressed as means ± standard deviation (SD). Measured data among groups were compared with single-factor analysis of variance. The survival rate among groups was compared by chi-square test. A difference was considered significant at P < 0.05.
Results
Expression levels of transduced genes in MSCs
Expression levels of transduced genes in human BM-MSCs were examined by RT-PCR and Western blotting assays. BM-MSCs transduced by rAd-SDF-1 simultaneously expressed endogenous SDF-1 and exogenous SDF-1 (Figure 1A). As expected, mRNA and protein levels of endogenous HOXB4 were low and undetectable, respectively, in BM-MSCs. In contrast, transduced MSCs expressed high levels of exogenous HOXB4. MSCs transduced with the SDF-1/HOXB4 fusion gene also produced stable levels of mRNA and protein product (Figure 1A). GFP expression, as detected by fluorescence microscopy, confirmed that the genetically modified MSC strains were transduced with similar efficiencies (Figure 1B).
Figure 1.
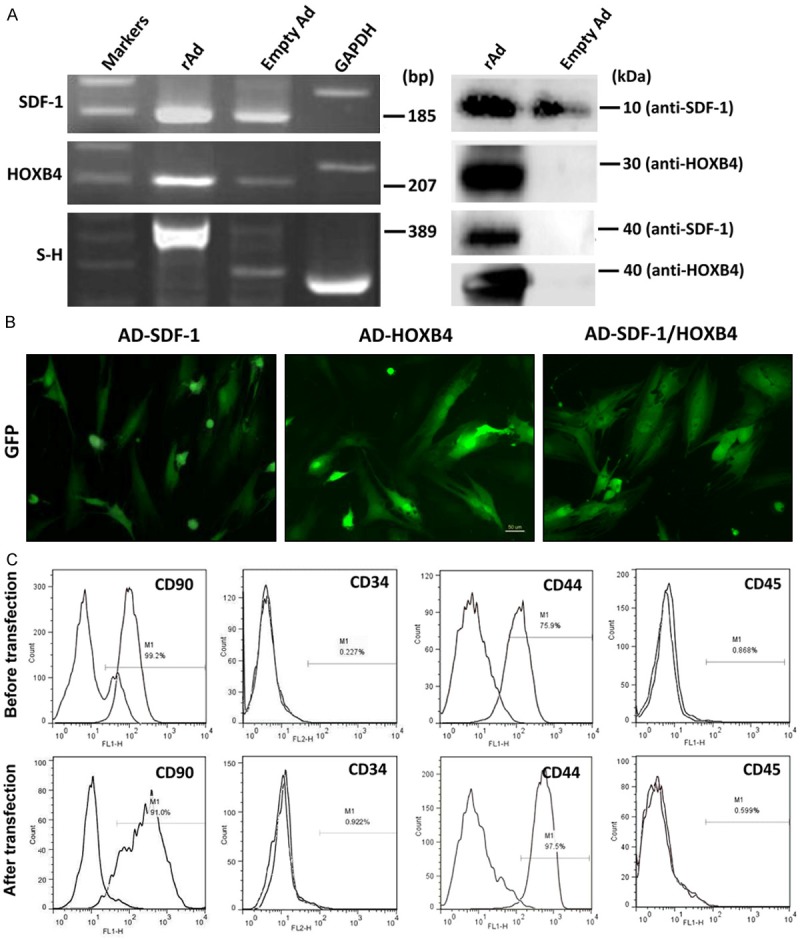
The properties of transfected MSCs. A and B. Expression of the transgenes in MSCs. The MSCs were prepared from bone marrow cells and transfected with the recombinant adenovirus Ad-SDF-1, Ad-HOXB4 and Ad-SDF-1/HOXB4. Five days later, the transfected cells were examined for expression of SDF-1, HOXB4 and SDF-1/HOXB4 genes by RT-PCR and Western blotting assays. The empty virus and the GAPDH gene were used as controls A. The transfected MSCs were further examined for GFP expression by fluorescence microscopy. B, C. The expression of surface markers of MSCs before and after transfection with rAD-SDF-1/HOXB4 as analyzed by FCM assay using PE labeled anti-human CD34 and FITC labeled anti-human CD45, CD44, and CD90 mAbs.
After confirming that the transgenes were being expressed, the modified MSCs were immunophenotyped using well-characterized surface markers. As expected, untransfected MSCs expressed CD90 and CD44 (MSC markers) and did not express CD34 and CD45 (hematopoietic cell markers) [19,20]. Importantly, these expression patterns were maintained in transfected MSCs (Figure 1C), indicating the transfection process did not affect the basic characteristics of MSCs.
SDF-1/HOXB4-MSCs efficiently expand isolated HSCs
Umbilical cord blood CD34+ cells, isolated with a mini magnetic cell sorting system, were composed of 2.87% HSCs prior to purification by MACS, which ultimately yielded a population of 99.2% pure HSCs. Remarkably, co-culturing of highly purified HSCs and modified MSCs, transduced with either SDF-1 or SDF-1/HOXB4, resulted in a dramatic increase in the number of expanded HSCs in vitro (Figure 2). However, this increase was not detected in HSCs co-cultured with HOXB4-transduced MSCs, suggesting that HOXB4 expression in MSCs does not directly affect HSC expansion. Among all the experimental groups, the SDF-1/HOXB4 (S-H) group yielded the highest increase in the number of expanded CD34+ cells, which was almost 2-fold higher than the cell counts in the MSC-HSC and HOXB4 groups (Figure 2).
Figure 2.
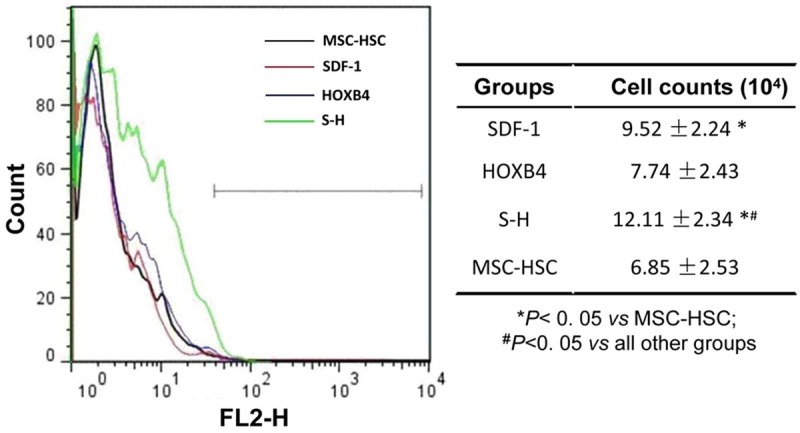
Effects of the transfected MSCs on HSCs. Recombinant Ads-transfected MSCs (1 × 104 cells) were exposed to a 30 Gy dose of 60Co γ-radiation, which were taken as the trophocytes. Then purified CD34+ cells (5 × 104 cells) were co-cultured with modified MSCs or unmodified MSCs for 7 days. The following day (day 8), nonadherent CD34+ cells in each well were detected by FCM using a PE labeled mouse anti-human CD34 antibody. The left panel shows a representative result of three independent experiments. The statistical analyses are displayed in the right panel. SDF-1: SDF-1/MSCs+ CD34+ cells; HOXB4: HOXB4/MSCs+ CD34+ cells; S-H: SDF1-HOXB4/MSCs+ CD34+ cells; MSC + HSC: untreated MSCs+ CD34+ cells.
Co-transplantation of HSCs and SDF-1/HOXB4-MSCs improved the general status and survival of irradiated mice
At 24 h post irradiation, mice in all experimental groups exhibited frayed fur, reduced activity, fastidiousness and gradually decreasing body weight. However, beginning at 7-10 days post irradiation, these indexes started to return to normal in mice from all groups except the IR group. As shown in Figure 3, at 14 days post irradiation, the survival rate in the IR group was significantly lower compared to other groups. The highest survival rate was observed in the S-H group, wherein all mice were viable 14 days post irradiation. Moreover, we also observed that the survival rate was higher in the SDF-1 group than in the HSC, MSC+HSC and HOXB4 groups. At 28 days post irradiation, we observed similar trends for the survival rates in each group. Specifically, all 12 mice died in IR group (0/12 survived), while most mice in the S-H group were viable (11/12 survived). Again, the survival rate in S-H group was the highest among all groups (Figure 3).
Figure 3.
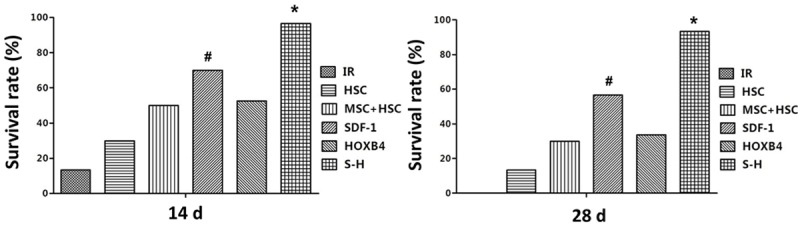
Survival rate in irradiated mice under different transplantation regimens. Within 12 hours of receiving a 3.5 Gy dose of 60Co total irradiation, 12 mice in each experimental group were transplanted with the indicated cell types via the tail vein. The survival rate of animals were recorded daily after transplantation. SDF-1: SDF-1/MSCs+ CD34+ cells; HOXB4: HOXB4/MSCs+ CD34+ cells; S-H: SDF1-HOXB4/MSCs+ CD34+ cells; MSC-HSC: MSCs+ CD34+ cells; HSC: CD34+ cells alone; IR: irradiated mice injected with 0.3 ml physiological saline. *P < 0.05 vs all other groups; #P < 0.05 vs IR, HSC, MSC + HSC, and HOXB4 groups, respectively.
Co-transplantation of CD34+ hematopoietic cells with genetically modified MSCs rapidly and effectively restored hematopoiesis in irradiated mice
As shown in Figure 4, levels of WBC, HGB and PLT in peripheral blood of NOD-SCID mice of all experimental groups dramatically decreased after receiving a 3.5 Gy dose of 60Co total body irradiation. All the mice in the IR group died 2-3 weeks post irradiation. Although the mice transplanted with HSCs and/or MSCs (genetically modified or wildtype) also experienced dramatically decreased WBC, PLT and HGB levels after irradiation, their levels recovered 7 to 28 days post irradiation. Remarkably, the WBC, PLT and HGB levels in the S-H group, which were transplanted with HSCs + SDF-1/HOXB4-MSCs, were completely restored to normal levels by day 28. A complete recovery was not observed in mice co-transplanted with HSCs + SDF-1-MSCs, although WBC, PLT and HGB levels in these mice recovered more quickly than in mice transplanted with HSCs alone, HSCs + unmodified MSCs, and HOXB4-MSCs.
Figure 4.
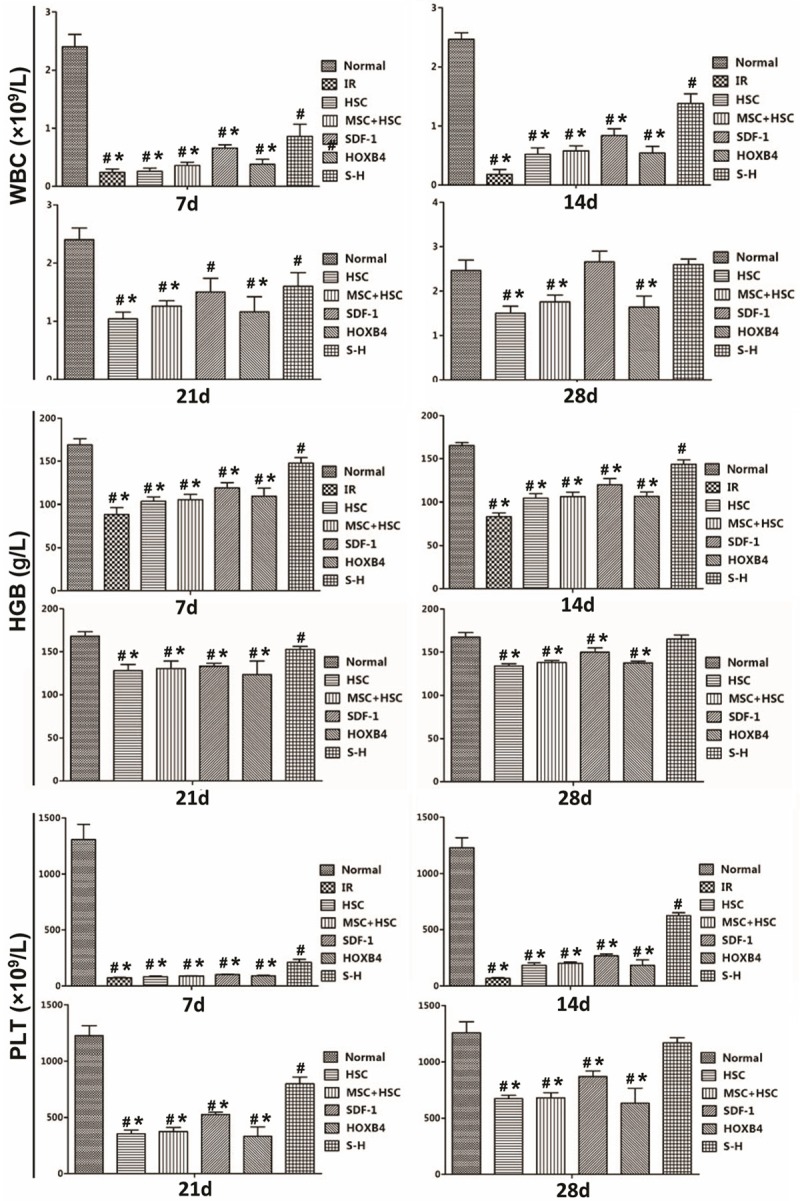
Recovery of peripheral hematogenesis in irradiated mice following transplantations. At 1, 2, 3 and 4 weeks post transplantation with the indicated cell types, mice from each group were examined for leucocyte, platelet and hemoglobin levels in blood (harvested from the tail vein). All IR group mice died between 2 and 3 weeks post irradiation and thus no data were collected thereafter. #P < 0.05 vs normal control, i.e., the naïve NOD-SCID mice; *P < 0.05 vs S-H group.
The results above suggest that the co-transplantation of HSCs with genetically modified MSCs could contribute to the recovery of levels of WBC, PLT and HGB by regulating the hematopoietic functions of bone marrow cells. To address this point, we investigated the bone marrow cytology of mice following transplantation. On days 14 and 28 post irradiation, bone marrow cells were harvested from each experimental group and subjected to Wright staining. As shown in Figure 5, on day 14 post transplantation, hematopoietic cells in mice of the IR group displayed marked hypocellularity, with empty particles. The bone marrow cytology in all other groups was comparably favorable. The cellularity was significantly higher in the SDF-1 and S-H groups, and the S-H group had the largest number of hematopoietic cells among all groups.
Figure 5.
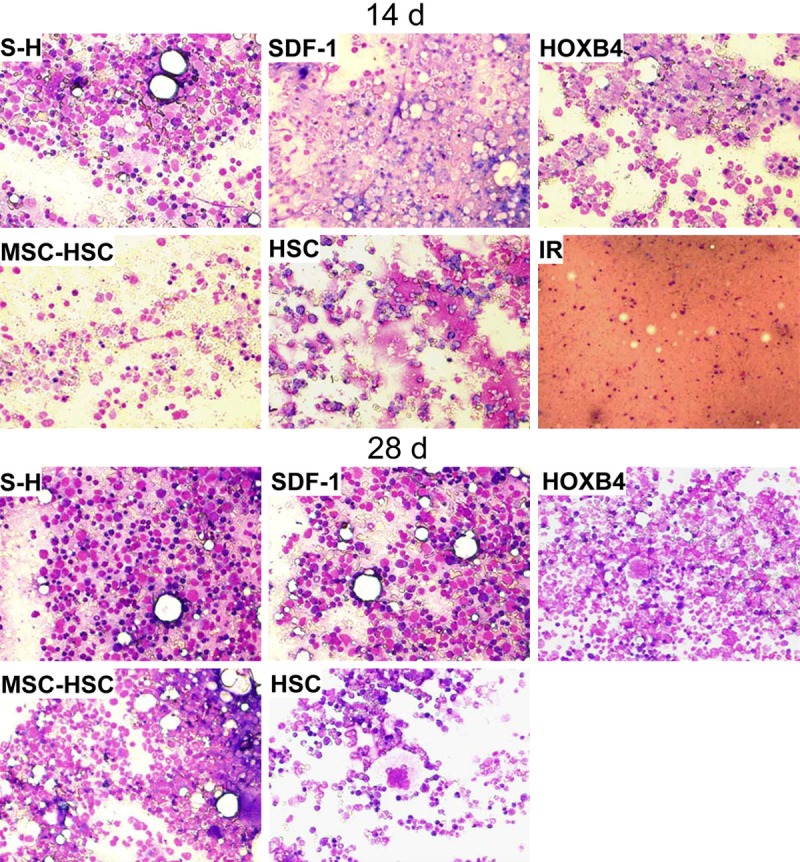
Cytology of bone marrow of mice transplanted with various cell types. At 2 and 4 weeks after transplantation, 2 mice from each group were anesthetized and sacrificed by cervical dislocation. The BM cells from the tibia and femur were harvested for cytological examination following Wright staining. IR group (no treatment control) mice died 2 to 3 weeks post irradiation and thus no data were collected after these time points. The image shown is a representative from two mice in each group (400× magnification).
On day 28 post transplantation, the hematopoietic cells in BM of HSC, MSC-HSC, SDF-1 and HOXB4 group mice had largely, but not completely, recovered. However, the BM cells in S-H group were almost completely restored to normal cellularity, as evidenced by the dense appearance of the BM and the increased amount of megakaryocytes (Figure 5).
Transplanted human CD45+ cells are engrafted in bone marrow of mice
We further investigated the engraftment and the migration of human CD45+ cells into the hematopoietic microenvironment. BM cells were harvested from mice at 4 weeks after transplantation and were analyzed by FCM for expression of human CD45. The results showed that the percentage of human CD45+ cells in BM cells from the SDF-1 and S-H groups was significantly higher than in other groups (Figure 6). Among all groups, the S-H group had the highest number of engrafted human CD34+ cells, comprising almost half of the total BM cells in mice (Figure 6).
Figure 6.
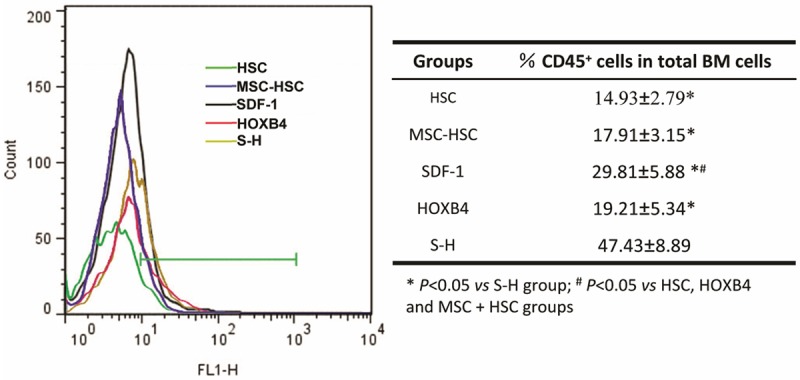
The engraftment of human CD45+ cells in irradiated mouse BM. At 6 weeks post transplantation, the BM cells from the indicated groups were evaluated for the presence of human CD45+ cells by FCM using a FITC-labeled anti-human CD45 antibody. The left panel shows a representative result of three independent experiments. The statistical analyses are displayed in the right panel. IR group mice died between 2-3 weeks and thus no data for this group was collected at this time point.
Discussion
Several studies have shown that MSCs possess the ability to promote engraftment and to accelerate hematological recovery after HSC transplantation [21]. In this study, we investigate whether exogenous expression of SDF-1 and HOXB4 in MSCs could augment its beneficial effects on hematopoietic recovery following HSC transplantation. Using recombinant adenoviruses, we genetically modified BM-MSCs to express SDF-1, HOXB4, and fused SDF-1/HOXB4 genes. We found that all modified MSCs effectively expressed the transgenes and that the neither the expression nor the transduction procedure altered MSC characteristics. Moreover, co-culturing of all genetically modified MSCs led to a significant expansion of transplanted HSCs in vitro. More importantly, co-transplantation of the CD34+ cells, isolated from human cord blood, with modified MSCs markedly increased hematopoietic recovery in irradiated mice, as evidenced by the rapid recovery of BM cellularity and WBC, PLT and HGB levels in peripheral blood. We speculate that these enhanced effects were likely due to improved engraftment efficiency of transplanted HSCs. Indeed, we detected an increased number of human CD45+ cells in mouse BM in our co-transplantation assays. Overall, our observations in vitro and in vivo demonstrate that the SDF-1/HOXB4 fusion transgene had the most favorable effect on HSC expansion.
MSCs are key components of the hematopoietic microenvironment and play important regulatory roles during hematopoiesis [22,23]. It is hypothesized that MSCs support hematopoiesis by mediating diverse mechanisms including directing cell-to-cell contacts, paracrine signaling, extracellular matrix scaffold dynamics, BM homing, and the functions of endogenous immunologic and nonimmunologic metabolites, all of which involve interactions between MSCs and HSCs [21,23,24]. In the context of HSC transplantation, HSCs can effectively expand and contribute to hematopoiesis and ultimately immunogenic function only upon engraftment into the BM cavity. It has been shown that chemokines play important roles during HSC engraftment. SDF-1, derived from MSCs and other stromal cells, couples with CXCR4, expressed by HSCs, to form one of the most important axes in the homing and engraftment of HSCs [12,25]. During this process, SDF-1 directly mediates the migration of HSCs through the endothelium (via E-selectin and P-selectin interactions) and the homing of HSCs to the stem cell niche. In line with previous studies, our results revealed that genetically modified MSCs, expressing exogenous SDF-1, markedly induced hematopoietic recovery and survival in lethally irradiated mice. Furthermore, our findings suggest that modified MSCs contribute to hematopoietic recovery through a mechanism that likely involves homing and engraftment of HSCs.
Aside from external signaling factors, expression of protein factors, such as the transcription factor HOXB4, within HSCs are also involved in the regulation of HSC proliferation and self-renewal [14-17]. In umbilical cord blood and adult BM, the enhanced expression of HOXB4 has been found to effectively increase proliferation of CD34+ cells without inducing leukemia or other deleterious processes [14,15]. In these studies, exogenous HOXB4 expression was genetically reinforced in HSCs, similar to our study. Several other studies have demonstrated that HOXB4 protein expression could enhance HSC activity. Krosl et al. and Tang et al. found that the expression of HOXB4 fused with the signal transduction domain of HIV transactivating protein (TAT-HOXB4) enhanced the expansion of HSCs from mice and humans [16,26]. In these cases, the ‘tat’ sequence of recombinant TAT-HOXB4 fusion protein facilitated its translocation into HSCs, where HOXB4 function is most evident [16]. Taken together, these studies indicate that exogenous HOXB4 expression, through the transgene method or the recombinant protein strategy, specifically contributes to HSC function. Indeed, in our co-transplantation assays, MSCs transduced with HOXB4 alone had a similar effect as untransduced MSCs on HSC function in mice.
In this study, we found that the SDF-1/HOXB4 fusion gene transduced in MSCs had the most beneficial effects on HSC expansion and bone marrow reconstitution. Moreover, this particular cell line most significantly improved survival of mice receiving a lethal dose irradiation. In fact, all mice transplanted with SDF-1/HOXB4 MSCs survived 4 weeks post irradiation, while none of the mice in the non-transplanted group were viable just 2 weeks post irradiation. Hematopoiesis in these mice at 4 weeks was also completely restored, as evidenced by the normal levels of BM cellularity and WBC, PLT and HGB levels in peripheral blood. We speculate that a synergistic effect of SDF-1 and HOXB4 contributed to these enhanced effects. Since SDF-1 is a chemokine and is actively extruded from MSCs, the fusion protein SDF-1/HOXB4 might similarly be secreted and be taken up by HSCs, which bind to SDF-1. In this way, the fusing of SDF-1 with HOXB4 might function to deliver HOXB4 to HSCs. Therefore, the SDF-1/HOXB4 fusion protein might represent a novel therapeutic strategy to treat hematopoietic deficiencies.
In conclusion, this study demonstrated that SDF-1/HOXB4-MSCs significantly increase HSC growth in vitro and engraftment in vivo. As a consequence, we detected significantly enhanced survival and hematopoietic recovery in lethally irradiated mice. Our results suggest that genetically modified MSCs expressing SDF-1/HOXB4 could potentially be a promising strategy to improve the clinical outcome of HSCs transplantation for patients with hematopoietic failure.
Acknowledgements
We would like to thank Dr. Jennifer C. van Velkinburgh (van Velkinburgh Initiative for Collaboratory BioMedical Research, USA) for helpful discussions and for polishing this manuscript. This work was supported by a grant from the General Program of National Natural Science Foundation of China (No. 30970868).
Disclosure of conflict of interest
None.
References
- 1.Guezguez B, Bhatia M. Transplantation of human hematopoietic repopulating cells: mechanisms of regeneration and differentiation using human-mouse xenografts. Curr Opin Organ Transplant. 2008;13:44–52. doi: 10.1097/MOT.0b013e3282f42486. [DOI] [PubMed] [Google Scholar]
- 2.Fliedner TM, Powles R, Sirohi B, Niederwieser D. Radiologic and nuclear events: the METREPOL severity of effect grading system. Blood. 2008;111:5757–5758. doi: 10.1182/blood-2008-04-150243. author reply 5758-5759. [DOI] [PubMed] [Google Scholar]
- 3.Isern J, Martin-Antonio B, Ghazanfari R, Martin AM, Lopez JA, del Toro R, Sanchez-Aguilera A, Arranz L, Martin-Perez D, Suarez-Lledo M, Marin P, Van Pel M, Fibbe WE, Vazquez J, Scheding S, Urbano-Ispizua A, Mendez-Ferrer S. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep. 2013;3:1714–1724. doi: 10.1016/j.celrep.2013.03.041. [DOI] [PubMed] [Google Scholar]
- 4.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, Alousi A, Saliba R, McMannis JD, Kaur I, Kebriaei P, Parmar S, Popat U, Hosing C, Champlin R, Bollard C, Molldrem JJ, Jones RB, Nieto Y, Andersson BS, Shah N, Oran B, Cooper LJ, Worth L, Qazilbash MH, Korbling M, Rondon G, Ciurea S, Bosque D, Maewal I, Simmons PJ, Shpall EJ. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda S, Izpisua Belmonte JC. Cotransplantation of MSCs and HSCs. Transplantation. 2013;95:e62–63. doi: 10.1097/TP.0b013e318290b0b1. [DOI] [PubMed] [Google Scholar]
- 6.Wu KH, Sheu JN, Wu HP, Tsai C, Sieber M, Peng CT, Chao YH. Cotransplantation of umbilical cord-derived mesenchymal stem cells promote hematopoietic engraftment in cord blood transplantation: a pilot study. Transplantation. 2013;95:773–777. doi: 10.1097/TP.0b013e31827a93dd. [DOI] [PubMed] [Google Scholar]
- 7.Masuda S, Ageyama N, Shibata H, Obara Y, Ikeda T, Takeuchi K, Ueda Y, Ozawa K, Hanazono Y. Cotransplantation with MSCs improves engraftment of HSCs after autologous intra-bone marrow transplantation in nonhuman primates. Exp Hematol. 2009;37:1250–1257. e1251. doi: 10.1016/j.exphem.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Sui W, Hou X, Che W, Chen J, Ou M, Xue W, Dai Y. Hematopoietic and mesenchymal stem cell transplantation for severe and refractory systemic lupus erythematosus. Clin Immunol. 2013;148:186–197. doi: 10.1016/j.clim.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Kim DS, Lee MW, Noh YH, Jang IK, Kim DH, Yang HM, Kim SJ, Choi SJ, Oh W, Yang YS, Chueh HW, Son MH, Jung HL, Yoo KH, Sung KW, Koo HH. A strategy for enhancing the engraftment of human hematopoietic stem cells in NOD/SCID mice. Ann Hematol. 2013;92:1595–602. doi: 10.1007/s00277-013-1830-1. [DOI] [PubMed] [Google Scholar]
- 10.Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–1486. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 11.Sharma M, Afrin F, Satija N, Tripathi RP, Gangenahalli GU. Stromal-derived factor-1/CXCR4 signaling: indispensable role in homing and engraftment of hematopoietic stem cells in bone marrow. Stem Cells Dev. 2011;20:933–946. doi: 10.1089/scd.2010.0263. [DOI] [PubMed] [Google Scholar]
- 12.Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 13.Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. Mobilization and homing of hematopoietic stem cells. Adv Exp Med Biol. 2012;741:152–170. doi: 10.1007/978-1-4614-2098-9_11. [DOI] [PubMed] [Google Scholar]
- 14.Bonde S, Dowden AM, Chan KM, Tabayoyong WB, Zavazava N. HOXB4 but not BMP4 confers self-renewal properties to ES-derived hematopoietic progenitor cells. Transplantation. 2008;86:1803–1809. doi: 10.1097/TP.0b013e31818fe741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu SJ, Feng Q, Ivanova Y, Luo C, Li T, Li F, Honig GR, Lanza R. Recombinant HoxB4 fusion proteins enhance hematopoietic differentiation of human embryonic stem cells. Stem Cells Dev. 2007;16:547–559. doi: 10.1089/scd.2007.0002. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, Chen J, Young NS. Expansion of haematopoietic stem cells from normal donors and bone marrow failure patients by recombinant hoxb4. Br J Haematol. 2009;144:603–612. doi: 10.1111/j.1365-2141.2008.07509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrester LM, Jackson M. Mechanism of action of HOXB4 on the hematopoietic differentiation of embryonic stem cells. Stem Cells. 2012;30:379–385. doi: 10.1002/stem.1036. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Guo C, Zhang H, Dong S. Synergistic protecting effect of cord blood CD34+ cells over-expressing both interleukin-3 and Flt3 ligand on lethally irradiated mice. Int J Hematol. 2009;90:64–73. doi: 10.1007/s12185-009-0348-8. [DOI] [PubMed] [Google Scholar]
- 19.Nogami M, Tsuno H, Koike C, Okabe M, Yoshida T, Seki S, Matsui Y, Kimura T, Nikaido T. Isolation and characterization of human amniotic mesenchymal stem cells and their chondrogenic differentiation. Transplantation. 2012;93:1221–1228. doi: 10.1097/TP.0b013e3182529b76. [DOI] [PubMed] [Google Scholar]
- 20.Calloni R, Viegas GS, Turck P, Bonatto D, Pegas Henriques JA. Mesenchymal stromal cells from unconventional model organisms. Cytotherapy. 2014;16:3–16. doi: 10.1016/j.jcyt.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Chou SH, Lin SZ, Day CH, Kuo WW, Shen CY, Hsieh DJ, Lin JY, Tsai FJ, Tsai CH, Huang CY. Mesenchymal stem cell insights: prospects in hematological transplantation. Cell Transplant. 2013;22:711–721. doi: 10.3727/096368912X655172. [DOI] [PubMed] [Google Scholar]
- 22.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045–1056. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pontikoglou C, Deschaseaux F, Sensebe L, Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7:569–589. doi: 10.1007/s12015-011-9228-8. [DOI] [PubMed] [Google Scholar]
- 24.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 25.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9:1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]


