Abstract
Although significant progress in bypass surgery and catheter intervention against peripheral artery disease, the number of severe critical limb ischemia (CLI) patients is increasing. Thus, it is crucial to develop new, non-invasive therapeutic strategies. The purpose of this study was to determine the mechanism of therapeutic ultrasound (TUS) on ischemic angiogenesis using mouse model of hindlimb ischemia and the cellular/molecular mechanisms underlying TUS-related neovascularization. The hindlimb ischemic mice were exposed to extracorporeal TUS for 3, 6, 9 minute per day (1 MHz, 0.3 W/cm2) until day 14 after left femoral artery ligation. Increased blood perfusion and capillary density were determined following 9 min of TUS compared with ischemic group. Moreover, TUS treatment increased the protein levels of vascular endothelial growth factor (VEGF), hypoxic inducible factor-1α (HIF-1α), endothelial nitric oxide synthase (eNOS) and p-Akt in vivo. TUS promoted capillary-like tube formation, migration and motility of human umbilical venous endothelial cells (HUVECs). Furthermore, the protein expressions of VEGF, eNOS and p-Akt were increased after TUS treatment. In conclusion, TUS therapy promotes postnatal neovascularization through multiple angiogenic pathways in mice model of ischemic hindlimb.
Keywords: Therapeutic ultrasound, angiogenesis, critical limb ischemia, VEGF
Introduction
About one third of peripheral artery disease (PAD) would develop to critical limb ischemia (CLI) when the arterial blood flow to the part or entire foot markedly reduced, in most cases as a result of progressive obstructive atherosclerosis. Although bypass surgery or catheter intervention have made tremendous advance against refractory PAD, the long-term outcome of revascularization therapy for infrapopliteal lesions remains unsatisfactory [1]. According to a report of the American College of Cardiology Foundation/American Heart Association Task Force (ACCF/AHA) on practice guidelines [2], there will be approximately 500 to 1000 new cases of CLI every year within 1 million European/North American population, among which about 25% of the patients with CLI undergo primary foot amputation even in developed countries, and amputation seems to be the common first-line therapy in developing countries, where there is lack of specialized podiatry program [2]. Thus, non-invasive therapeutic strategies for severe CLI remain to be developed.
Ultrasound is a form of sound whose frequency is higher than the natural audible range for humans (> 20 kHz) and ultrasonography has been widely used as diagnostic devices for several decades. In addition to diagnostic purposes, ultrasound is clinically used for therapeutic applications, including tumor ablation, thrombolysis, bone regeneration, and facilitated drug delivery [3]. Recently, therapeutic angiogenic effects of low-intensity ultrasound have been reported in endothelial cells, chick chorioallantoic membrane, and a rat model of hind limb ischemia [4,5]. However, the underlying mechanism remains modest clarified. In the present study, we thus examined whether low intensity therapeutic ultrasound (TUS) (1 MHz, 0.3 W/cm2) amplifies endothelial functions in vitro and how TUS augments ischemia-induced angiogenesis using a mouse model of hindlimb ischemia in vivo.
Material and methods
Animals
All animal procedures were conducted with prior institutional ethical approval under the requirements of the Chinese Prevention of Cruelty to Animals Act and the institutional guidelines for health and care of experimental animals of Shanghai Jiaotong University School of Medicine. Male adult C57BL/6 mice weighing 18-22 g were randomly divided into 4 groups (n = 6 mice/group). All animals were subjected to left femoral artery resection to develop hindlimb ischemia. Post ischemic TUS-treated groups received TUS exposure 3, 6 or 9 minute per day. Ischemic group was treated equally without receiving TUS therapy. Mice were sacrificed on day 14.
Hind limb ischemia
Male C57BL/6 mice were subjected to unilateral femoral artery ligation as described [6,7]. Briefly, left femoral artery was ligated and excised between the inguinal ligaments and proximal to the branching into saphenous and popliteal artery using 7-0 polypropylene sutures (Ethicon, USA). For sample collection, the whole adductor muscle was harvested and split in half resulting in a proximal and distal portion. The middle part around the former femoral artery was used for analysis to avoid sampling variances of regions with greater or lesser ischemia.
TUS treatment
Animals received therapeutic ultrasound therapy immediately after excision of the femoral vessels and skin closure, still under anesthesia at the area above the adductor muscles. Common ultrasound gel was used for coupling. TUS were generated by a device with transducer designed and produced by Institute of Acoustics, Tongji University (Shanghai, China). The diameter of the transducer’s membrane is 2.0 centimeters. Ultrasound was delivered to the ischemic area with an energy flux density of 0.3 W/cm2 at a frequency of 1.0 MHz. Post ischemic TUS-treated groups were exposed to TUS for different time as indicated (3, 6 or 9 minute) per day, while the ischemic group received the same procedures without TUS exposure. The safety and the efficacy of low-frequency level of ultrasound wave to the organism have been examined in the pre-experiment.
Infrared thermal imaging analysis
The blood induced temperature variation of local shin area can be well measured with the development of infrared and even Terahertz detection technology [8,9]. The large blood flow will induce a high skin temperature near the blood vessel. Here the blood flow measurements were performed pre hind-limb ischemia, directly after femoral artery ligation (day 0) and 14 days after ischemic surgery by infrared thermal imaging analyzer (Prism-DS 50137, FLIR Systems, USA). To minimize data variables attributable to ambient temperature mice were kept on a heating plate at 37°C for 10 minutes before measurement. Blood perfusion is indirectly shown as changes in the local skin temperature using different color pixels representing the ratio of left (operated, ischemic leg) versus right (not operated, not-ischemic leg) limb blood flow. A ratio of 1 prior to surgery indicated equal blood perfusion in both legs, whereas after femoral artery excision this ratio drops to values between 0.2-0.3, indicating severe attenuation of leg blood supply in the operated hindlimb.
Necrosis score
Necrosis score was assessed as previously described [10]. Briefly, mice were investigated at 0 and 14 days after hind limb ischemia surgery and scored with 0 points if no necrosis or defect was observed, with 1 point if skin necrosis was present, with 2 points if below ankle amputation was present and with 3 points if above ankle amputation was observed. Two researchers evaluated the necrosis score in a blinded manner and the average scores for each animal were used for quantitative analysis.
Immunofluorescence staining
Immunofluorescence staining was performed as described previously [11]. Briefly, muscle samples were fixed in 4% paraformaldehyde and subsequently embedded in paraffin. Prior to the staining procedure, heat-mediated antigen retrieval was performed in sodium-citrate buffer (10 mM sodium-citrate, 0.05% Tween 20, pH 6.0) followed by fixation in methanol for 10 min at 4°C. After blocking for 30 min, with 2% BSA in PBS, samples were incubated with rabbit anti-mouse CD31 antibody (5 μg/ml, Becton-Dickinson Biosciences, Franklin Lakes, NJ, USA), and followed by a secondary antibody (Invitrogen, Carlsbad, CA, USA). Ten fields were randomly selected from each section for calculation of capillary density and the result was expressed as numbers of capillary/field (x 400 magnification) [12,13].
Western blotting
Ischemic muscle samples were homogenized and processed for western blotting as described [14,15]. HUVECs were resolved and prepared according to the protocol provided by the manufacturer. The primary antibodies used were as follows: anti-VEGF (Proteintech, Chicago, IL, USA); anti-eNOS (Sigma, St. Louis, MO, USA); anti-Akt, anti-p-Akt (Ser473), anti–hypoxic-inducible factor (HIF)-1α and anti-GAPDH (Cell Signaling Technology, Danvers, MA, USA). Bands were visualized with a FluorChem E data system (Cell Biosciences, Santa Clara, CA, USA) and quantified by densitometry using Quantity One 4.52 (Bio-Rad).
Cell culture
HUVECs (San Diego, CA, USA) in passage 6-8 were cultured in DMEM low-glucose medium supplemented with 10% inactivated fetal bovine serum and 100 U/ml penicillin and 100 μg/ml streptomycin under standard culture conditions (37°C, 95% humidified air and 5% CO2). HUVECs were reseeded into plates overnight and stimulated for different time of TUS exposure (3, 6 or 9 min per day, 1 MHz, 0.3 W/cm2) per day for 3 days. Supernatant and cell lysates were collected on day 3 after reseeding.
Tube formation assay
To examine the angiogenic effect of TUS in vitro, tube formation assay was performed as previously described [16]. Matrigel-Matrix (BD Biosciences, Franklin Lakes, New Jersey, USA) was pipetted into pre-chilled 96-well plates (50 µL matrigel per well) and polymerized for 45 min at 37°C. HUVECs (2 × 104 per well) in complete media were simultaneously seeded in Matrigel coated plates. Then culture plates were exposed to TUS for different time (3, 6 or 9 min, 1 MHz, 0.3 W/cm2). After 6 h of incubation, tubular structures were photographed. Images were acquired under a fluorescent microscope (IX-71; Olympus, Tokyo, Japan) with 12.8 M pixel recording digital color cooled camera (DP72; Olympus). The control sample was defined as 100% tube formation, and the increase or decrease in tube formation relative to the control was calculated for each sample. Each experiment was triply repeated under similar conditions.
Scratch assay
HUVECs were allowed to grow to 100% confluence in six-well plates. Cells were mechanically wounded by scraping with a 200 µl pipette tip at time zero, denuding a strip of the monolayer 300 µm wide. The boundary of the wound was marked. The cells were rinsed three times with PBS to remove dislodged cells and cellular debris and cultured with DMEM without FBS. Then, cells were treated with TUS for different time as indicated (3, 6 or 9 min, 1 MHz, 0.3 W/cm2). The wound was observed 24 h and 48h later with an Olympus microscope at x 40 magnification fitted with an ocular grid. Images were taken with an Olympus DP72 digital camera under phase contrast microscope. We measured the width of each denuded area and calculated the average. The migration ability was quantified by the formula: 100% - (width24 h or 48 h /width0 h) x 100%.
Transwell migration assay
The chemotactic motility of HUVECs was determined using Transwell migration chambers (BD Biosciences) with 6.5-mm-diameter polycarbonate filters (8-µm pore size) [17]. In brief, the bottom chambers were filled with 600 µL of DMEM media containing all supplements. HUVECs (3 × 104 per well) were seeded in top chambers in 100 µL DMEM media without serum. Thereafter, cells were treated with external TUS for different time as indicated (3, 6 or 9 minute, 1 MHz, 0.3 W/cm2). Cells were allowed to migrate for 8 h. Non-migrated cells were removed with cotton swabs, and migrated cells were fixed with ice cold ethanol and stained with 0.01% crystal violet. Images were captured with x 100 magnification and invasive cells were quantified by manual counting.
Statistic analysis
Data are expressed as means ± SEM. Multiple groups were analyzed by one-way ANOVA test followed by post hoc tests to determine statistical significance. Probability values < 0.05 were considered statistically significant. SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA) was used. All experiments were repeated at least in triplicate.
Results
TUS improve ischemia-induced angiogenesis
Blood perfusion of hind limbs was measured by infrared thermal imaging before ischemia, directly (day 0) as well as 14 days after femoral artery excision (day 14). Although untreated ischemic animals showed a notable self-regeneration potential, significant improvements of wound healing and blood perfusion could be observed in the 9 min-TUS treatment group on day 14 (Figure 1A and 1B). We also investigated the necrosis score of the ischemic limb on day 14, which showed a marked improvement in 9 min-TUS group to compare with untreated ischemic group (Figure 1C), which indicated that 9 min-TUS treatment effectively improved the blood perfusion after ischemic episode. This was further supported by the quantitative data of capillary density in TUS group, which indicated that the microcirculation was significantly improved in 9 min-TUS treated mice than in ischemic mice (Figure 2A and 2B). However, there was not significant difference in comparison with other 2 TUS-treated groups.
Figure 1.
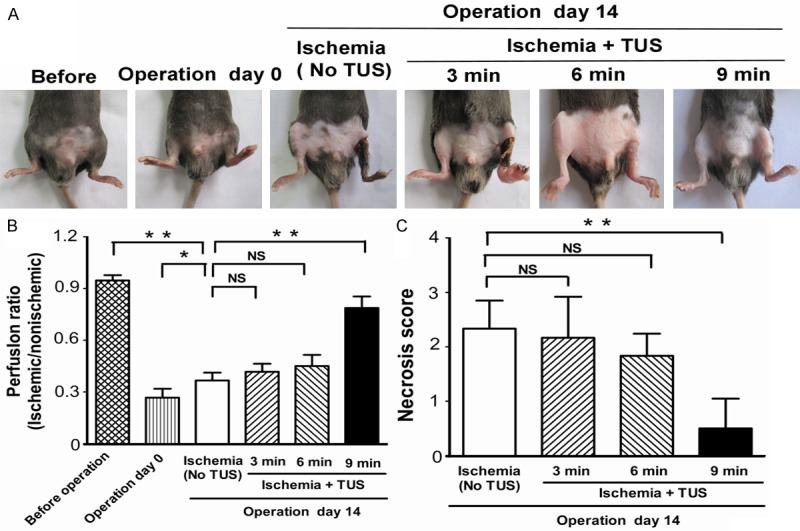
TUS improved blood perfusion and ameliorated hindlimb necrosis. Mice were separated to 4 groups, control group without TUS stimulation, and TUS treated groups with different time (3, 6 and 9 min, n = 6 per group). Mice were subjected to left femoral artery resection to develop hindlimb ischemia. A. Photographs of hindlimbs were observed on day 14 after induction of ischemia. B. Infrared thermal imaging data were analyzed and illustrated as an ischemic/normal hindlimb temperature ratio. C. Necrosis score was also assessed on day 14. All data are shown as means ± SEM, *P < 0.05, **P < 0.01, versus control group.
Figure 2.
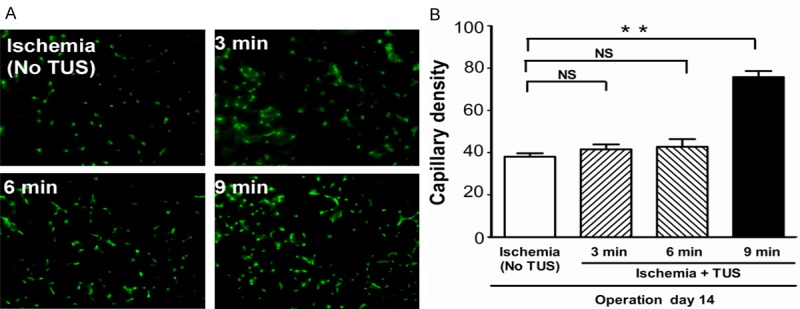
TUS increased capillary density in ischemic muscle. A. Representative images of microphotomicrographs of anti-CD31 immunohistochemical staining among all groups (magnification x 400, bars represent 50 μm). B. Quantitative data of capillary density (capillaries/field) among all groups. All data are shown as means ± SEM, **P < 0.01, versus control group.
TUS increase of angiogenic factors expressions in vivo
To further examine the mechanisms underlying the TUS-induced angiogenesis in mice, we evaluated levels of eNOS, VEGF, p-Akt and HIF-1α protein expressions in local tissue among the 4 groups of mice at day 14 after induction of ischemia. We found that all these protein level were significantly increased in response to 9 min of TUS treatment compared with other groups (Figure 3).
Figure 3.
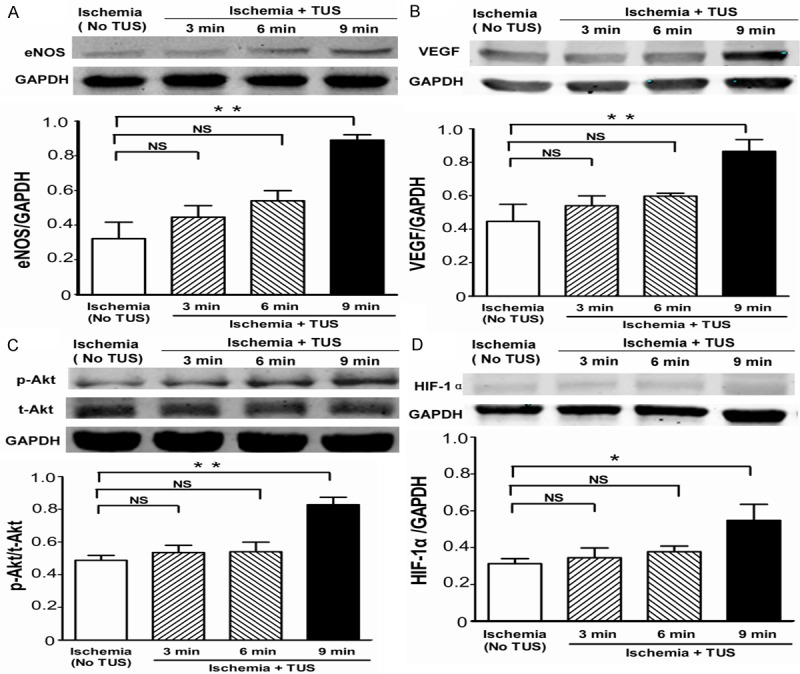
Angiogenic effect of TUS in ischemic hind limbs. Western blotting assay to determine the expression of (A) eNOS, (B) VEGF, (C) phospho-Akt (p-Akt), total Akt (t-Akt) and (D) HIF-1α contained in the ischemic muscle on day 14 (n = 6 per group). All data are shown as means ± SEM, *P < 0.05, **P < 0.01, versus control group.
TUS induced tube formation
We next determined the effect of TUS treatment on the tubulogenesis using HUVECs on Matrigel in vitro. Six hours after reseeded into dishes, the TUS-treated HUVECs exhibited more extensive interconnecting tubes than others groups (Figure 4A). TUS promoted HUVECs tube formation in a dose-dependent manner. The tube length of the HUVECs exposed to TUS was significantly extended in comparison with control group (Figure 4B).
Figure 4.
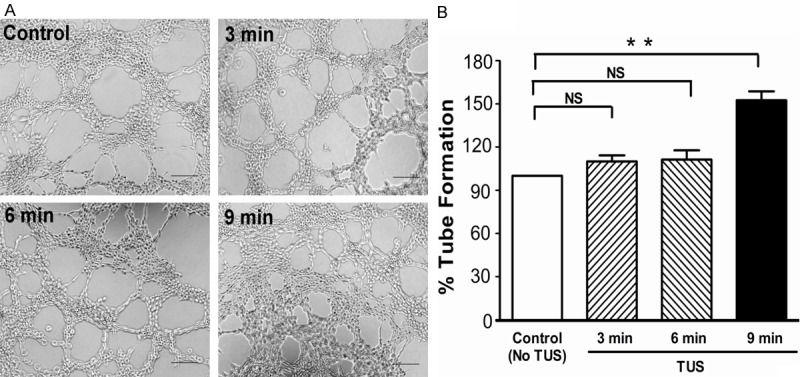
Effect of TUS on tube formation. Pro-angiogenic benefit of TUS on the tube formation of HUVEC was examined using Matrigel assay. Tubular structures were photographed at 100x magnification (A) and tube length was measured (B). All data are shown as means ± SEM, **P < 0.01, versus control group.
TUS enhances migration and motility in vitro
Tube formation in vitro requires both cell attachment to extracellular matrix (ECM) and cell migration. Cell motility was assessed with a scratch assay, in which migration was initiated in a confluent layer of cells by mechanical denuding. A wound healing assay was performed to evaluate endothelial migration under TUS treatment. After culture under different conditions, HUVEC monolayers were mechanically wounded by scraping with a pipette tip (Figure 5A) and wound healing was observed 24 h and 48 h later. TUS treatment increased wound healing in comparison with control in a dose-dependent manner (Figure 5B). Cell motility was assessed with transwell migration assay. After 8 h, we observed that the presence of TUS in the lower chambers results in higher invasion of HUVECs through polyester layer compared to control (Figure 5C). In the 9 min-TUS group, the number of HUVECs invasion through polyester layer was significantly increased (Figure 5D). Overall, these results clearly showed that TUS treatment promotes the migration and motility of HUVECs.
Figure 5.
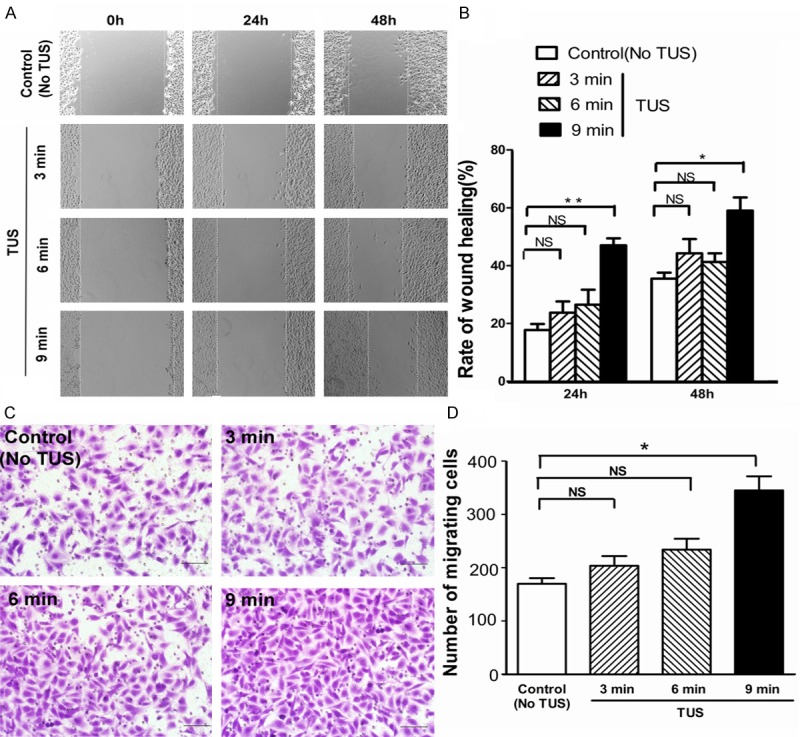
TUS accelerated migratory ability of HUVECs. A, B. An in vitro wound-healing assay was performed to evaluate the migration ability of HUVECs treated with different time of TUS treatment. The migration ability was determined by measuring the width of each denuded area and by calculating through the formula: 100%-(width24 h or 48 h/width0 h) x 100%. C, D. Effect of TUS on the migratory potential of HUVECs was examined using Transwell migration chambers. All data are shown as means ± SEM, *P < 0.05, **P < 0.01, versus control group. Scale bars = 200 μm.
TUS up-regulates angiogenic factors in vitro
To maintain the integrity of this study, the effect of TUS on eNOS, VEGF, t-Akt and p-Akt protein expression was detected by western blotting analysis in HUVECs (Figure 6A-C). Except for t-Akt, other protein expression was up-regulated in response to TUS following a dose-dependent manner. These results indicated that TUS stimulated eNOS expression in HUVECs and that may be a promoter of VEGF expression.
Figure 6.
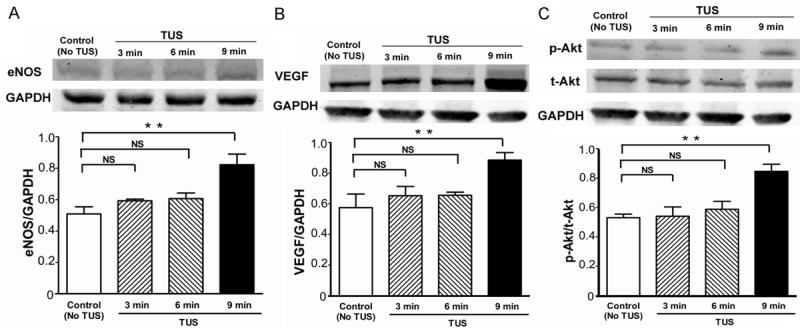
Pro-angiogenic effect of TUS on HUVECs. Quantitative results of protein expression of (A) eNOS, (B) VEGF, (C) p-Akt and total Akt. TUS stimulated vascular endothelial growth factors secretion concentration dependently. 9 min-TUS significantly promoted the expressions of eNOS, VEGF and p-Akt protein. Data of Western blotting were represented as fold of control. All data are shown as means ± SEM, *P < 0.05, **P < 0.01, versus control group.
Discussion
The most significant finding addressed in this manuscript is that TUS induces postnatal neovascularization in the ischemic muscle through enhancing endothelial cells migrate and enhancing multiple angiogenic pathways.
We demonstrated that TUS rescued necrotic limbs through augmenting microvascular growth and blood perfusion recovery after ischemic attack. Moreover, the protein expressions of VEGF, eNOS and p-Akt increased after the 9 min of TUS treatment in vivo and in vivo. Meanwhile, the extent of an increase of the three proteins expression by other dose (3 and 6 min) TUS therapy was rather small. These results suggest that sufficient TUS exposure triggers angiogenic action.
The cell-to-cell interaction between endothelial cells and other types of cells is important in enhancing multiple angiogenic pathways [18]. Previous study has demonstrated that ultrasound wave exposure might increase the possibility of cell-cell interaction so that triggered cell proliferation and regeneration [1]. Application of ultrasound wave exposure was reported to stimulate various regenerative cells such as bone marrow-derived progenitor cells and residential stem cells to regenerate damaged tissue [19]. Toyama et al. reported that application of ultrasound to circulating angiogenic cells augmented their generation and migration capacities [20]. Xu et al. reported that ultrasound stimulated hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro [5]. Thus, it is possible that the ultrasound directly and/or indirectly affects the function and dynamics of immature cells, such as bone marrow derived mononuclear cells and residential vascular regenerative cells [14,21]. Except for those, previous studies have shown that the low-energy short wave therapy exerts multiple effects, including angiogenic, anti-inflammatory, anti-oxidant and anti-apoptotic effects [22-25]. Thus, as in the case with the low-energy short wave therapy, the mechanisms other than angiogenesis may also contribute to the beneficial effects of ultrasound in the present study.
Previous reports demonstrated that TUS delivered mechanical energy to tissues, which plays a central role in regulating eNOS, to the damaged tissues [4,26]. Endothelial cells normally sustain several mechanical stresses, including shear tension and compression. Due to their unique location between tissues and flowing blood, any changes of these mechanical forces can alter their morphology, proliferative activity and secretion of vasoregulatory mediators including endothelin-1 [27], prostacyclin [28] and NO [29]. In particular, fluid shear stress causes a rapid, large and sustained increase in eNOS activity [30], stimulating both an early calcium-dependent activation as well as an Akt-dependent phosphorylation [31] and increased transcription [32]. Additionally, HIF-1α, one of the most potent angiogenic transcription factors [33-35], could be activated by intracellular NO which secondary to the PI3K-Akt-eNOS signaling pathway [13,36]. Moreover, Kuwabara et al. reported that NO increased the VEGF protein expression and secretion through HIF-1α regulation in cardiomyocytes [37]. Together with the current study that TUS could up-regulated the protein expression of eNOS, VEGF, p-Akt and HIF-1α, it could be summarized that TUS increasing eNOS might in part be a result of the cell signaling through PI3K-Akt, and also in part a result of the angiogenic signaling through HIF-1α-VEGF (Figure 3D).
Mechanical stimuli are known to affect endothelial cell function via mechanosensors embedded in endothelial cell membranes, such as integrins and caveolins [38]. Ultrasound irradiation has been reported to produce shear stress on endothelial cells [39]. In this study, using the well-established Matrigel assay, we demonstrated that TUS dramatically enhance tube formation of HUVECs. We carried out an in vitro wound healing experiment and transwell assay to evaluate the effects of TUS on endothelial cell function. Our results showed that TUS enhanced endothelial migration. Our data obtained here are in agreement with previous reports [40]. In addition, ultrasound has been shown to induce sonoporation on endothelial cell membrane leading to influx of calcium ion [41]. Increased intracellular Ca2+ produced by TUS may lead to increase of NO through the action of eNOS, which is dominant modulator to enhance revascularization in vivo. Thus, it is conceivable that several mechanisms, such as triggering of mechanosensors, activation of endothelial cell growth and sonoporation-activated alterations, contribute to the ultrasound induced angiogenesis.
In conclusion, our findings indicate that noninvasive extracorporeal TUS treatment enhances multiple angiogenic pathways, effectively normalizes blood perfusion, decreases necrosis and promotes angiogenesis. These findings suggest that TUS deserves further consideration of investigation in its regulation on the angiogenic signaling pathway and new clinical strategies for CLI.
Acknowledgements
This work was supported by the China National Natural Science Foundation (11374213) and Foundation of National Lab for Infrared Physics (200901).
Disclosure of conflict of interest
None to declare.
References
- 1.Serizawa F, Ito K, Kawamura K, Tsuchida K, Hamada Y, Zukeran T, Shimizu T, Akamatsu D, Hashimoto M, Goto H, Watanabe T, Sato A, Shimokawa H, Satomi S. Extracorporeal shock wave therapy improves the walking ability of patients with peripheral artery disease and intermittent claudication. Circ J. 2012;76:1486–1493. doi: 10.1253/circj.cj-11-1216. [DOI] [PubMed] [Google Scholar]
- 2.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline) Vasc Med. 2011;16:452–476. doi: 10.1177/1358863X11424312. [DOI] [PubMed] [Google Scholar]
- 3.ter Haar G. Therapeutic applications of ultrasound. Prog Biophys Mol Biol. 2007;93:111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Atar S, Siegel RJ, Akel R, Ye Y, Lin Y, Modi SA, Sewani A, Tuero E, Birnbaum Y. Ultrasound at 27 kHz increases tissue expression and activity of nitric oxide synthases in acute limb ischemia in rabbits. Ultrasound Med Biol. 2007;33:1483–1488. doi: 10.1016/j.ultrasmedbio.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Xu P, Gul-Uludag H, Ang WT, Yang X, Huang M, Marquez-Curtis L, McGann L, Janowska-Wieczorek A, Xing J, Swanson E, Chen J. Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol Lett. 2012;34:1965–1973. doi: 10.1007/s10529-012-0984-6. [DOI] [PubMed] [Google Scholar]
- 6.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–1679. [PMC free article] [PubMed] [Google Scholar]
- 7.Hao CN, Shintani S, Shimizu Y, Kondo K, Ishii M, Wu H, Murohara T. Therapeutic Angiogenesis by Autologous Adipose-Derived Regenerative Cells: Comparison With Bone Marrow Mononuclear Cells. Am J Physiol Heart Circ Physiol. 2014;307:H869–79. doi: 10.1152/ajpheart.00310.2014. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Li Z, Li N, Chen X, Chen P, Shen X, Lu W. High-polarization-discriminating infrared detection using a single quantum well sandwiched in plasmonic micro-cavity. Sci Rep. 2014;4:6332. doi: 10.1038/srep06332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Chen X, Yu A, Zhang Y, Ding J, Lu W. Highly sensitive and wide-band tunable terahertz response of plasma waves based on graphene field effect transistors. Sci Rep. 2014;4:5470. doi: 10.1038/srep05470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S, Wolf AM, Bahlmann FH, Patsch JR, Wolf D, Schratzberger P, Mahata SK, Kirchmair R. The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res. 2010;107:1326–1335. doi: 10.1161/CIRCRESAHA.110.219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blunder S, Messner B, Aschacher T, Zeller I, Turkcan A, Wiedemann D, Andreas M, Bluschke G, Laufer G, Schachner T, Bernhard D. Characteristics of TAV- and BAV-associated thoracic aortic aneurysms--smooth muscle cell biology, expression profiling, and histological analyses. Atherosclerosis. 2012;220:355–361. doi: 10.1016/j.atherosclerosis.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Duan J, Murohara T, Ikeda H, Sasaki K, Shintani S, Akita T, Shimada T, Imaizumi T. Hyperhomocysteinemia impairs angiogenesis in response to hindlimb ischemia. Arterioscler Thromb Vasc Biol. 2000;20:2579–2585. doi: 10.1161/01.atv.20.12.2579. [DOI] [PubMed] [Google Scholar]
- 13.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:III370–376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 14.Hao CN, Huang JJ, Shi YQ, Cheng XW, Li HY, Zhou L, Guo XG, Li RL, Lu W, Zhu YZ, Duan JL. Pulsed electromagnetic field improves cardiac function in response to myocardial infarction. Am J Transl Res. 2014;6:281–290. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang XH, Lei H, Liu AJ, Zou YX, Shen FM, Su DF. Increased oxidative stress is responsible for severer cerebral infarction in stroke-prone spontaneously hypertensive rats. CNS Neurosci Ther. 2011;17:590–598. doi: 10.1111/j.1755-5949.2011.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 17.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Shah AM. ROS signalling between endothelial cells and cardiac cells. Cardiovasc Res. 2014;102:249–257. doi: 10.1093/cvr/cvu050. [DOI] [PubMed] [Google Scholar]
- 19.Garin G, Mathews M, Berk BC. Tissue-resident bone marrow-derived progenitor cells: key players in hypoxia-induced angiogenesis. Circ Res. 2005;97:955–957. doi: 10.1161/01.RES.0000193566.65262.d8. [DOI] [PubMed] [Google Scholar]
- 20.Toyama Y, Sasaki K, Tachibana K, Ueno T, Kajimoto H, Yokoyama S, Ohtsuka M, Koiwaya H, Nakayoshi T, Mitsutake Y, Chibana H, Itaya N, Imaizumi T. Ultrasound stimulation restores impaired neovascularization-related capacities of human circulating angiogenic cells. Cardiovasc Res. 2012;95:448–459. doi: 10.1093/cvr/cvs173. [DOI] [PubMed] [Google Scholar]
- 21.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, Matsumoto Y, Kajihara N, Eto M, Matsuda T, Yasui H, Takeshita A, Sunagawa K. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110:3055–3061. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 23.Fukumoto Y, Ito A, Uwatoku T, Matoba T, Kishi T, Tanaka H, Takeshita A, Sunagawa K, Shimokawa H. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron Artery Dis. 2006;17:63–70. doi: 10.1097/00019501-200602000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Fukumoto Y, Shimokawa H. Extracorporeal shock wave therapy for ischemic cardiovascular disorders. Am J Cardiovasc Drugs. 2011;11:295–302. doi: 10.2165/11592760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Hanawa K, Ito K, Aizawa K, Shindo T, Nishimiya K, Hasebe Y, Tuburaya R, Hasegawa H, Yasuda S, Kanai H, Shimokawa H. Low-intensity pulsed ultrasound induces angiogenesis and ameliorates left ventricular dysfunction in a porcine model of chronic myocardial ischemia. PLoS One. 2014;9:e104863. doi: 10.1371/journal.pone.0104863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, Bonert M, Ojha M, Marsden PA, Cybulsky MI. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. 2007;171:1691–1704. doi: 10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J. 2009;73:1983–1992. doi: 10.1253/circj.cj-09-0583. [DOI] [PubMed] [Google Scholar]
- 29.Ghriallais RN, McNamara L, Bruzzi M. Comparison of in vitro human endothelial cell response to self-expanding stent deployment in a straight and curved peripheral artery simulator. J R Soc Interface. 2013;10:20120965. doi: 10.1098/rsif.2012.0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemeny SF, Figueroa DS, Clyne AM. Hypo- and hyperglycemia impair endothelial cell actin alignment and nitric oxide synthase activation in response to shear stress. PLoS One. 2013;8:e66176. doi: 10.1371/journal.pone.0066176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandrangi P, Sosa M, Shyy JY, Rodgers VG. Flow-dependent mass transfer may trigger endothelial signaling cascades. PLoS One. 2012;7:e35260. doi: 10.1371/journal.pone.0035260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Liu C, Bin J, Wang Y, Chen J, Xiu J, Pei J, Lai Y, Chen D, Fan C, Xie J, Tao Y, Wu P. Mutant hypoxia inducible factor-1alpha improves angiogenesis and tissue perfusion in ischemic rabbit skeletal muscle. Microvasc Res. 2011;81:26–33. doi: 10.1016/j.mvr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Hao C, Huang ZH, Song SW, Shi YQ, Cheng XW, Murohara T, Lu W, Su DF, Duan JL. Arterial baroreflex dysfunction impairs ischemia-induced angiogenesis. J Am Heart Assoc. 2014;3:e000804. doi: 10.1161/JAHA.114.000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng XW, Kuzuya M, Kim W, Song H, Hu L, Inoue A, Nakamura K, Di Q, Sasaki T, Tsuzuki M, Shi GP, Okumura K, Murohara T. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation. 2010;122:707–716. doi: 10.1161/CIRCULATIONAHA.109.909218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 37.Kuwabara M, Kakinuma Y, Ando M, Katare RG, Yamasaki F, Doi Y, Sato T. Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. J Physiol Sci. 2006;56:95–101. doi: 10.2170/physiolsci.rp002305. [DOI] [PubMed] [Google Scholar]
- 38.Heo KS, Fujiwara K, Abe J. Shear stress and atherosclerosis. Mol Cells. 2014;37:435–440. doi: 10.14348/molcells.2014.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizrahi N, Seliktar D, Kimmel E. Ultrasound-induced angiogenic response in endothelial cells. Ultrasound Med Biol. 2007;33:1818–1829. doi: 10.1016/j.ultrasmedbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Zaragoza C, Marquez S, Saura M. Endothelial mechanosensors of shear stress as regulators of atherogenesis. Curr Opin Lipidol. 2012;23:446–452. doi: 10.1097/MOL.0b013e328357e837. [DOI] [PubMed] [Google Scholar]
- 41.Hassan MA, Campbell P, Kondo T. The role of Ca(2+) in ultrasound-elicited bioeffects: progress, perspectives and prospects. Drug Discov Today. 2010;15:892–906. doi: 10.1016/j.drudis.2010.08.005. [DOI] [PubMed] [Google Scholar]


