Abstract
Introduction: Recent experimental studies have suggested that various coagulation-related molecules may be important players in the development and progression of pancreatic cancer. However, these findings have not yet been verified in a clinical setting. Methods: In this study, we comprehensively examined the levels of multiple hemostatic substances, including prothrombin, antithrombin, plasminogen, thrombin-anti-thrombin (TAT) and plasmin-anti-plasmin (PAP) complexes, as well as, soluble CD40 (sCD40) in patients diagnosed with pancreatic cancer (n = 37) or other tumors (neuroendocrine neoplasms - NEN [n = 7] or solid pseudopapillary tumors-SPT [n = 3]), and healthy individuals (n = 31). Results: We found significantly higher anti-thrombin, PAP and sCD40 levels in patients with pancreatic cancer compared to healthy controls and patients diagnosed with other types of pancreatic tumors (for all, at least p < 0.05). Cancer patients had lower plasminogen concentrations than individuals from the other analyzed groups (for both, p < 0.05). None of the examined coagulation-related parameters was significantly associated with neither systemic sCD40 concentrations nor clinical staging of malignancy. Levels of analyzed molecules were comparable between pancreatic cancer patients presenting with early and advanced disease. Moreover, our study identified a potential diagnostic value of prothrombin/TAT and anti-thrombin/TAT coefficients in detection of pancreatic cancer in humans. However, both of these were inferior to currently used marker-CA19.9. Conclusions: Subclinical hemostatic alterations (mainly in plasmin-related molecules) i) appear as soon as during the earliest stages of the pancreatic adenocarcinoma development in humans, ii) do not seem to alter within progression of the disease nor are associated with clinical staging, iii) are not observed in patients with other types of pancreatic tumors, as well as, iv) do not seem to be associated with elevated sCD40 concentrations in pancreatic cancer patients. Moreover, examined thrombin- and plasmin-related substances do not appear to possess a sufficient diagnostic value to serve as makers of pancreatic adenocarcinoma in humans.
Keywords: Hemostasis, neuroendocrine tumors, pancreatic cancer, plasmin, thrombin
Introduction
Pancreatic cancer is one of the leading causes of mortality among patients suffering from malignancy worldwide. According to the recent analyses [1], every year around 279,000 new cases of pancreatic cancer worldwide are reported. Almost all of those diagnosed with pancreatic cancer die because of this disease, and it is estimated that the incidence of pancreatic adenocarcinoma will increase even more in upcoming years. This dramatic epidemiological tendency is a quantitative reflection of several limitations in the current clinical management of patients suffering from pancreatic cancer, and those foremost include difficulties in its early diagnosis, ineffectiveness of currently used chemotherapeutics, as well as lack of comprehensive understanding of multiple pathomechanism, responsible for progression and systemic spread of this disease.
For many years it has been suspected that the constituents of coagulation system may be not only important players in the development and spread of pancreatic malignancy in humans, but also promising “molecular points”, which modulation may offer novel therapies. For example, an experimental study by Gasic and colleagues from 1968 [2] highlighted critical importance of platelets metabolism in the metastatic spread of cancer cells to the lungs shortly after their, cancer cells, introduction into circulation. Currently, this significance of thrombocytes in cancer progression is mainly attributed to their ability to secrete numerous growth factors that regulate neovascularization and increase cancer cell survival [3-6]. However, within time further experimental reports demonstrated that almost all constituents of the coagulation pathway may, at some point, significantly contribute to promotion of cancer development and progression (reviewed in detail in [7,8]), whereas application of inhibitors of the coagulation cascade (mainly of thrombin) strongly augments this process and reduces both-implantation of tumor cells, as well as metastasis formation in experimental animals [9]. Among all hemostatic parameters, the significance of thrombin- and plasmin-related molecules, such as (anti) thrombin, (anti) plasmin and their complexes (thrombin-anti-thrombin-TAT and plasmin-anti-plasmin-PAP) is especially highlighted, and is believed to be of crucial importance for the development of malignancy. In experimental studies it has been demonstrated that action of these substances may i) protect tumor cells from vascular shear stress, ii) enhance tumor cell survival through improvement of attachment to the endothelium iii) protect cancer cells from immune attack from the site of NK cells, as well as, iv) significantly modulate function of the whole immune system, for example via regulation of systemic levels of immune-stimulating molecules, such as soluble CD40 [3,7,8,10-13].
From the clinical standpoint abnormalities in the coagulation balance are relatively frequently observed among patients suffering from pancreatic cancer. These manifest mainly as venous thromboembolism (VT), and may be a first clinical symptom leading to the diagnosis of this malignancy (reviewed in detail in [14]). Studies demonstrated that such initial presentation of the disease reveals its advanced stage, is strongly associated with much worse prognosis and results in around 3-fold lower overall 1-year survival rate of these patients [15]. Interestingly, several authors demonstrated that in patients with pancreatic cancer presenting with VT indeed abnormalities in the hemostatic balance are observed [14]. However, little is known if i) such alterations are also observed in pancreatic cancer patients without clinical symptoms of coagulation abnormalities, ii) accompany pancreatic cancer development in humans from its earliest stages, or iii) if these are also present in patients with other types of pancreatic malignancies, such as neuroendocrine neoplasms (NEN) or solid pseudopapillary tumors (SPT). Therefore, taking all these facts into consideration, in this study, we decided to comprehensively analyze systemic levels of selected coagulation-related parameters in patients with pancreatic cancer, and compare their values with those detected in healthy individuals, and patients diagnosed with other pancreatic neoplasms. We also verified whether systemic levels of examined parameters have diagnostic clinical value for the detection of pancreatic cancer in humans. We hypothesized that patients suffering from pancreatic cancer would present a modulated hemostatic balance. We also posited that levels of some of the examined coagulation-related substances could potentially serve as novel markers for detection of pancreatic cancer in humans.
Material and methods
Patients and blood samples
A total of 78 individuals with generally good and stable health were included in the study. These patients were divided into groups: a “cancer” group (newly diagnosed pancreatic adenocarcinoma, n = 37), a “other” group (patients diagnosed with pancreatic neuroendocrine neoplasms [NEN], n = 7 or solid pseudopapillary tumors [SPT], n = 3), and a “control” group (healthy volunteers, n = 31).
The final diagnosis of pancreatic adenocarcinoma and other malignancies was based on biopsy specimen analysis. In order to establish disease staging, all patients underwent ultrasonography, computed tomography and/or endoscopic ultrasonography, as well as chest x-ray examinations. According to the international Tumor Node Metastasis classification system, in the pancreatic cancer group stage 1 pancreatic cancer was observed in 2 patients, stage 2 in 8 patients, 9 patients were classified as stage 3, and the rest presented with distal metastases to the liver and/or lungs. At the time of their inclusion in the study, none of the patients was on chemotherapy treatment, had received any cytotoxic agents or drugs within the last 12 months before the study, had been on any anti-platelet therapy nor received any medications interfering with coagulation system/parameters, or had presented any clinical signs of an active infectious disease. All patients were recruited from individuals hospitalized in the Department of Gastroenterology of the Pomeranian Medical University in Szczecin. The general characteristics of the individuals enrolled in the study, together with a statistical comparison of these features between the examined groups, are presented in Table 1.
Table 1.
General characteristics of analyzed patients and healthy individuals enrolled in the study (data presented as means ± SD or median [interquartile range])
| Parameters | Control group | Pancreatic cancer | Other |
|---|---|---|---|
| Age (years) | 60 ± 7 | 62 ± 10 | 48 ± 19 |
| Gender (M-men/W-women) | 13-M/18-W | 12-M/25-W | 3-M/7-F |
| BMI (kg/m2) | 25.69 ± 3.57 | 22.73 ± 7.83 | 24.02 ± 3.51 |
| RBC (×1012 cells/L) | 4.77 ± 0.58 | 4.19 ± 0.93 | 4.74 ± 0.38 |
| Hb (g/dL) | 14.08 ± 1.74 | 12.57 ± 1.89* | 13.77 ± 0.82 |
| Platelets count (×109 cells/L) | 224 ± 62 | 264 ± 106 | 231 ± 48 |
| CRP (mg/L) | 2.91 ± 1.84 | 12.40 [4.80; 70.20]**,# | 3.00 [1.85; 7.00] |
| CA19.9 (U/mL) | 12.41 ± 5.27 | 486.35 [112.35; 1675.75]** | 9.48 [3.73; 15.14]** |
BMI-body mass index; RBC-red blood cells; Hb-hemoglobin; CRP-C-reactive protein;
P < 0.05;
P < 0.01 (vs “control” group);
P < 0.05 (vs “other” group).
Peripheral blood samples (8-10 mL) were collected from all included individuals. Samples were processed immediately according to standard laboratory protocols, and plasma was separated, frozen, and stored at -80°C until further assessment.
Analysis of systemic levels of coagulation-related parameters and sCD40
The systemic levels of thrombin-related (prothrombin, anti-thrombin [AT] and TAT) and plasmin-related parameters (plasminogen and PAP), together with soluble CD40 (sCD40) were measured using commercially available, high-sensitivity ELISA kits (Uscn Life Science Inc., Wuhan, Hubei, China or eBioscience, San Diego, CA, USA) according to the manufacturer instructions.
Statistical analysis
Statistical analyses were performed similarly as in our previous studies [16-20]. Briefly, the Shapiro-Wilk test was used to determine the distribution of the continuous variables analyzed. The Student’s t-test was used to compare mean parameter values between the examined groups (for normally distributed variables). For variables that were not normally distributed, the values were log transformed. If a normal distribution was then achieved, these transformed variables were also compared using the Student’s t-test. However, if the transformation did not result in a normal distribution, a Mann-Whitney U-test was performed. Correlations between various analyzed parameters were calculated using the Pearson test or Spearman rank test, according to the normality of the distribution. To evaluate the effects of continuous variables on pancreatic cancer staging and levels of examined biochemical parameters, multivariate regression analyses were performed using a stepwise selection method. Variables excluded from the initial model were re-entered individually to exclude residual confounding. During development of multivariate regression models, the number of inserted independent variables did not exceed 10% of the total number of analyzed patients. Constructed models were verified using the Akaike information criterion (AIC), and wrongly constructed matrices resulted in rejection of the model. Receiver operating characteristic (ROC) curves were constructed for all parameters analyzed as diagnostic for pancreatic cancer, and the area under each ROC curve (AUC) was calculated. Statistical analysis was performed using SPSS statistical analysis software. P-values less than 0.05 were considered significant. The Bioethical Committee of the Pomeranian Medical University in Szczecin approved the study protocol, and all patients provided written informed consent prior to participation.
Results
Analysis of included individuals
Initial comparison of the analyzed groups of recruited individuals revealed significantly higher CA19-9 levels in patients with pancreatic adenocarcinoma compared to all other patient groups (Table 1). Moreover, cancer patients had significantly higher C-reactive protein levels than control individuals and subjects diagnosed with other pancreatic neoplasms.
Comparison of systemic levels of examined hemostatic parameters in patients diagnosed with pancreatic tumors and healthy individuals
The mean systemic concentrations of examined thrombin-related parameters in patients with pancreatic tumors and healthy individuals are depicted in Figure 1. We found that levels of AT were significantly higher in patients diagnosed with pancreatic cancer than in healthy individuals. No significant differences were observed in terms of prothrombin and TAT levels between cancer and control group. Also, we did not find any significant differences in levels of all examined thrombin-related parameters between patients diagnosed with NENs or SPTs and both-healthy and cancer individuals (Figure 1).
Figure 1.
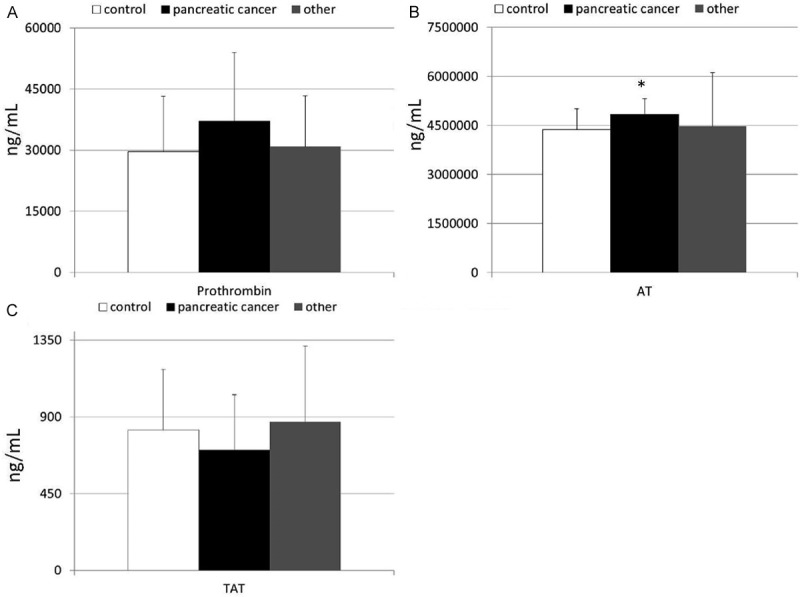
(A-C) Levels of thrombin-related parameters, that is (A) prothrombin, (B) anti-thrombin and (C) thrombin-anti-thrombin complex in healthy individuals and patients diagnosed with pancreatic cancer or other pancreatic tumors together with their statistical comparison (means ± standard deviation). AT-anti-thrombin; TAT-thrombin-anti-thrombin complex; *p < 0.005 (vs healthy individuals).
Interestingly, systemic mean levels of plasmin-related parameters significantly differed between patients diagnosed with pancreatic adenocarcinoma and both-control individuals and patients suffering from other pancreatic neoplasms (Figure 2). Namely, significantly lower plasminogen levels and higher PAP concentrations were observed in cancer patients than in both-healthy individuals and patients from the “other” group. No statistically significant differences in terms of plasminogen and PAP levels were found in comparison of the control and “other” group.
Figure 2.

(A, B) Levels of plasmin-related parameters, that is (A) plasminogen, and (B) plasmin-anti-plasmin complex in healthy individuals, and patients diagnosed with pancreatic cancer or other pancreatic tumors together with their statistical comparison (means ± standard deviation). PAP-plasmin-anti-plasmin complex; *p < 0.05 (vs pancreatic cancer group).
In order to more extensively examine the hemostatic balance in examined groups, we calculated a series of coefficients using examined thrombin- and plasmin-related parameters (presented in Table 2). These analyses further demonstrated that the biochemical balance between the examined coagulation-related parameters is significantly modulated in patients with pancreatic cancer in comparison to both-control individuals and patients diagnosed with NENs or SPTs.
Table 2.
Mean values of calculated coefficients of examined coagulation-related parameters, and their statistical comparison between groups of patients (data presented as means ± SD or median [interquartile range])
| Coefficient | Control group | Pancreatic cancer | Other |
|---|---|---|---|
| Prothrombin/TAT | 33.24 ± 14.38*** | 53.38 ± 24.38 | 33.79 ± 13.36** |
| AT/TAT | 5370.54 ± 1594.73* | 7480.70 ± 3858.89 | 5205.74 ± 3023.67 |
| Plasminogen/PAP | 2971.14 ± 720.46*** | 2168.95 ± 932.32 | 2995.10 [2266.13; 4234.56] |
AT-anti-thrombin; NET-neuroendocrine tumor; TAT-thrombin-anti-thrombin complex; PAP-plasmin-anti-plasmin complex;
p < 0.05;
p < 0.01;
p < 0.005 (vs pancreatic cancer group).
Coagulation-related parameters and sCD40 molecule
Next, we decided to verify if the altered hemostatic profile observed in patients diagnosed with pancreatic cancer is associated with systemic levels of immune-related molecules, such as sCD40. In our study significantly higher systemic concentrations of this substance were observed in cancer patients in comparison to both - “other” and control groups (Figure 3). Among all examined coagulation-related parameters only PAP levels were strongly correlating with higher sCD40 concentrations detected in patients with pancreatic cancer (r = 0.48; p = 0.02). No significant differences were observed during comparison of mean sCD40 values between healthy individuals and patients suffering from other pancreatic neoplasms.
Figure 3.
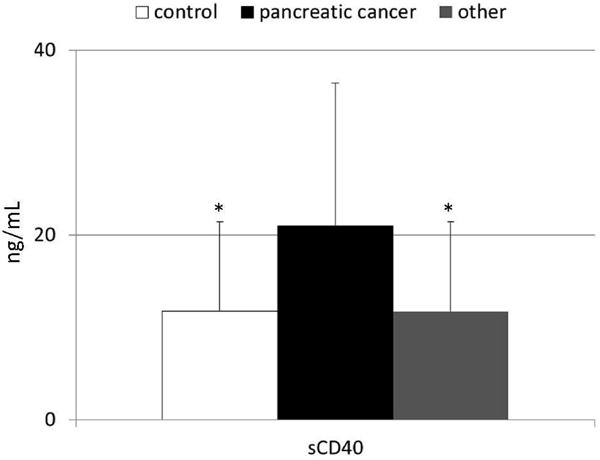
Levels of sCD40 in healthy individuals, and patients diagnosed with pancreatic cancer or other pancreatic tumors together with their statistical comparison (means ± standard deviation). *p = 0.02 (vs healthy individuals).
Clinical associations between hemostasis-related parameters and pancreatic cancer
Finally, we decided to verify potential associations between systemic levels of examined coagulation-related parameters and the clinical presentation of pancreatic cancer among our patients. Using correlation and multivariate regression analyses we found that none of all examined parameters were significantly associated with clinical staging of the pancreatic cancer, classified according to the TNM staging (unpublished observation). Also, there were no significant differences in terms of mean levels of examined parameters between patients diagnosed with early TNM I-II stage and those with an advanced disease (unpublished observation).
However, after noting differences in the levels of selected hemostatic-related parameters between the pancreatic cancer patients and other examined groups of individuals, we decided to preliminarily examine the potential diagnostic value of these parameters for the detection and discrimination of pancreatic cancer in humans. In order to realize this task we constructed ROC curves, and determined the approximate AUC values to assess the suitability of these parameters as potential diagnostic markers for pancreatic cancer. Among all examined parameters, only those with a 95% confidence interval (CI) lower bound value that exceeded 0.50 are presented (Figure 4). Our analysis demonstrated that among all examined parameters values of only AT/TAT and prothrombin/TAT coefficients fulfilled these criteria, and therefore possessed potential value for discrimination of pancreatic cancer patients from other individuals. However, all of these parameters seemed to be inferior to CA19.9 marker that is currently used in the clinical setting.
Figure 4.
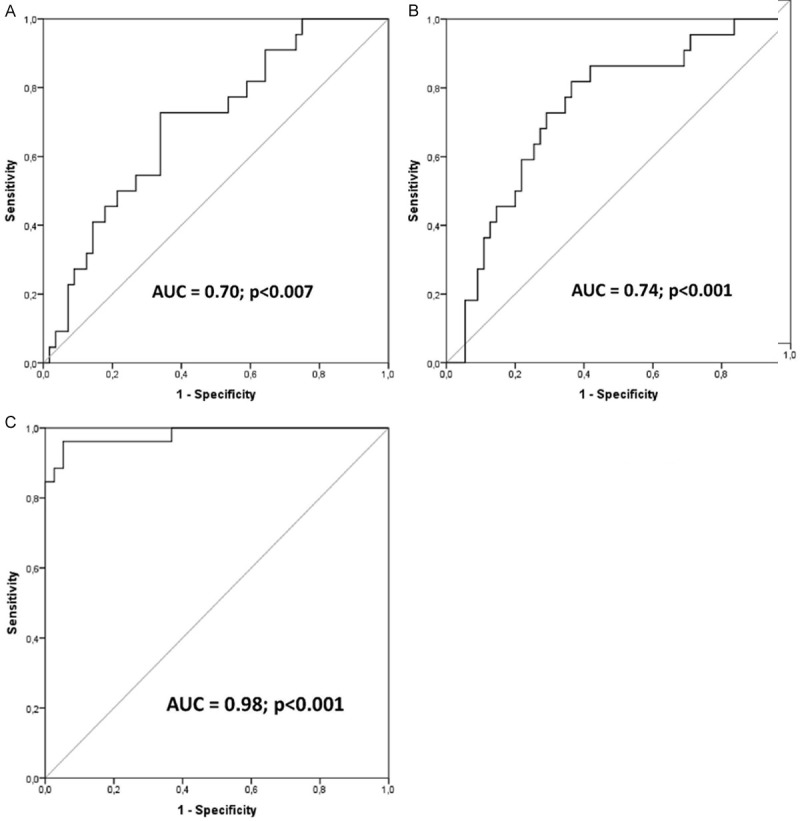
(A-C) Receiver operating characteristics (ROC) curves of examined parameters and CA19.9 as indicators of pancreatic cancer. Calculated sensitivity (y-axis) is plotted against 1-specificity formula (x-axis) for examined coefficients, that is (A) AT/TAT, (B) Prothrombin/TAT, and (C) CA19.9 as indicators of pancreatic cancer, together with precise description of their area under curve value and statistical significance. AT-anti-thrombin; AUC-area under curve; TAT-thrombin/anti-thrombin complex; p-level of significance.
Discussion
While it is well known that substances of the coagulation cascade regulate proper hemostatic profile, growing experimental evidence gradually starts to demonstrate that molecules responsible for hemostatic balance may in fact be involved into promotion and/or modulation of cancer survival and its systemic spread in humans. These data direct the scientific attention to potential significance of various hemostatic molecules in orchestration of also pancreatic cancer development and progression. Therefore, in this study we decided to comprehensively analyze selected coagulation-related substances in patients with pancreatic tumors, as well as verify their potential diagnostic value and associations with clinical presentation of this disease.
In our study we found that patients diagnosed with pancreatic cancer have significantly different hemostatic balance than healthy individuals and patients diagnosed with other types of pancreatic neoplasms, even though no clinical symptoms of abnormalities in the coagulation parameters were clinically present. Even though in multiple experimental studies various authors demonstrated that pancreatic cancer cells possess relatively high expression of functional thrombin receptors and of tissue factor that may lead to activation of thrombin [21,22], results of our analyses surprisingly revealed that the most profound alterations are mainly found not in thrombin-related parameters, but rather in terms of systemic levels of plasmin-related molecules, i.e. plasminogen and PAP. Interestingly, none of the examined parameters was significantly associated with clinical staging of the disease in our patients, and no significant differences in mean values of these substances were found between patients diagnosed on early stages of the disease, and those with much more advanced malignancy. Basing on our results we conclude that subclinical hemostatic alterations appear as soon as during the earliest stages of the pancreatic adenocarcinoma development in humans, and do not seem to alter within progression of the disease. Our data seem to be in agreement and may be indirectly supported by results derived from the recent multi-center trials, in which was observed that preventive application of medications interfering with thrombin generation, among general population of patients diagnosed with pancreatic adenocarcinoma, does not result in a significantly better overall outcome, whereas use of urokinase plasminogen activator inhibitors was a bit more successful therapy associated with a very low tendency to metastasis formation among patients with locally advanced pancreatic cancer [23,24].
Moreover, as in our previous study we have reported presence of an altered cytokine balance in patients with pancreatic cancer [25], in this study we were also interested in verifying whether modulated hemostatic balance in patients with pancreatic cancer may also be associated with abnormal levels of sCD40, a member of the tumor necrosis factor receptor superfamily that is a critical regulator of cellular and humoral immunity. Indeed, we found that in patients with pancreatic cancer significantly higher sCD40 levels are present in the peripheral blood, what is in agreement with previous studies [26]. However, those were not associated with any of the examined coagulation-related parameters. From the molecular standpoint, increased levels of sCD40 might be caused by several molecular mechanisms (reviewed in detail in [27,28]). Our study demonstrates, that in patients with pancreatic cancer other mechanisms than the hemostatic-dependent pathway of sCD40 generation/release seem to be associated with systemically elevated sCD40 concentrations.
Finally, we also conducted a preliminary analysis of the potential diagnostic value of examined hemostatic-related parameters for the detection of pancreatic cancer in our study population. In our study, we found that systemic levels of only calculated coefficients, that is AT/TAT and prothrombin/TAT, showed preliminary potential to be diagnostic markers for the detection of pancreatic adenocarcinoma. However, they seemed to be inferior to currently used CA19.9 marker. Therefore, even though in our study these parameters fulfilled all necessary criteria to be consider as potential markers of pancreatic cancer, still their clinical applicability seems to be very limited. Nevertheless, our analysis is based on a relatively small group of patients, and further clinical studies are needed to fully confirm these preliminary observations.
In summary, our study demonstrated that subclinical hemostatic alterations (mainly in plasmin-related molecules) i) appear as soon as during the earliest stages of the pancreatic adenocarcinoma development in humans, ii) do not seem to alter within progression of the disease nor are associated with clinical staging, as well as iii) are not observed in patients with other types of pancreatic tumors. Moreover, in our study elevated systemic sCD40 levels did not seem to be associated with the hemostatic imbalance observed in patients with pancreatic cancer. Finally, examined thrombin- and plasmin-related substances do not appear to possess sufficient clinical diagnostic value to serve as makers of pancreatic adenocarcinoma in humans.
Acknowledgements
WB is supported by the “START” stipend awarded by the Foundation for Polish Science.
Disclosure of conflict of interest
None.
References
- 1.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gasic GJ, Gasic TB, Stewart CC. Anti-metastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coupland LA, Chong BH, Parish CR. Platelets and p-selectin control tumor cell metastasis in an organ-specific manner and independently of NK cells. Cancer Res. 2012;72:4662–4671. doi: 10.1158/0008-5472.CAN-11-4010. [DOI] [PubMed] [Google Scholar]
- 4.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013;24:393–400. doi: 10.1016/j.ejim.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Ho-Tin-Noe B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68:6851–6858. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil-Barnabe AM, Lucotti S, Muschel RJ. Coagulation and metastasis: what does the experimental literature tell us? Br J Haematol. 2013;162:433–441. doi: 10.1111/bjh.12381. [DOI] [PubMed] [Google Scholar]
- 8.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11:223–233. doi: 10.1111/jth.12075. [DOI] [PubMed] [Google Scholar]
- 9.Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding and spontaneous metastasis. Blood. 2004;104:2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 10.Kirstein JM, Graham KC, Mackenzie LT, Johnston DE, Martin LJ, Tuck AB, MacDonald IC, Chambers AF. Effect of anti-fibrinolytic therapy on experimental melanoma metastasis. Clin Exp Metastasis. 2009;26:121–131. doi: 10.1007/s10585-008-9221-z. [DOI] [PubMed] [Google Scholar]
- 11.Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting nautral killer cell antitumor reactivity. Cancer Res. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- 12.Varo N, Libby P, Nuzzo R, Italiano J, Doria A, Schonbeck U. Elevated release of sCD40L from platelets of diabetic patients by thrombin, glucose and advanced glycation end products. Diab Vasc Dis Res. 2005;2:81–87. doi: 10.3132/dvdr.2005.014. [DOI] [PubMed] [Google Scholar]
- 13.Angelico F, Alessandri C, Ferro D, Pignatelli P, Del Ben M, Fiorello S, Cangemi R, Loffredo L, Violi F. Enhanced soluble CD40L in patients with the metabolic syndrome: Relationship with in vivo thrombin generation. Diabetologia. 2006;49:1169–1174. doi: 10.1007/s00125-006-0222-7. [DOI] [PubMed] [Google Scholar]
- 14.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–663. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]
- 15.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 16.Dołęgowska B, Błogowski W, Domański L. Clinical evidence of the association between serum perioperative changes in xanthine metabolizing enzymes activity and early post-transplant kidney allograft function. J Am Coll Surg. 2010;211:587–595. doi: 10.1016/j.jamcollsurg.2010.06.391. [DOI] [PubMed] [Google Scholar]
- 17.Dolegowska B, Blogowski W, Safranow K, Domanski L, Jakubowska K, Olszewska M. Lipoxygenase-derived hydroxyeicosatetraenoic acids--novel perioperative markers of early post-transplant allograft function? Nephrol Dial Transplant. 2010;25:4061–4067. doi: 10.1093/ndt/gfq320. [DOI] [PubMed] [Google Scholar]
- 18.Dołęgowska B, Błogowski W, Domański L. Association between the perioperative antioxidative ability of platelets and early post-transplant function of kidney allografts: a pilot study. PLoS One. 2012;7:e29779. doi: 10.1371/journal.pone.0029779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Błogowski W, Dołęgowska B, Sałata D, Budkowska M, Domański L, Starzynska T. Clinical analysis of perioperative complement activity during ischemia/reperfusion injury following renal transplantation. Clin J Am Soc Nephrol. 2012;7:1843–1851. doi: 10.2215/CJN.02200312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Błogowski W, Dolegowska B, Budkowska M, Sałata D, Domański L, Starzynska T. Perioperative release of pro-regenerative biochemical signals from human renal allografts subjected to ischemia-reperfusion injury. Innate Immun. 2014;20:126–132. doi: 10.1177/1753425913482018. [DOI] [PubMed] [Google Scholar]
- 21.Rudroff C, Schafberg H, Nowak G, Weinel R, Scheele J, Kaufmann R. Characterization of functional thrombin receptors in human pancreatic tumour cells (MIA PACA-2) Pancreas. 1998;16:189–194. doi: 10.1097/00006676-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda O, Egami H, Ishiko T, Ishikawa S, Kamohara H, Hidaka H, Mita S, Ogawa M. Expression of proteinase-activated receptor-2 in human pancreatic cancer: a possible relation to cancer invasion and induction of fibrosis. Int J Oncol. 2003;22:295–300. [PubMed] [Google Scholar]
- 23.Pelzer U, Hilbig A, Stieler JM, Bahra M, Sinn M, Gebauer B, Dorken B, Riess H. Intensified chemotherapy and simultaneous treatment with heparin in outpatients with pancreatic cancer-the CONKO 004 pilot trial. BMC Cancer. 2014;14:204. doi: 10.1186/1471-2407-14-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinemann V, Ebert MP, Laubender RP, Bevan P, Mala C, Boeck S. Phase II randomised proof-of-concept study of the urokinase inhibitor upamostat (WX-671) in combination with gemcitabine compared with gemcitabine alone in patients with non-resectable, locally advanced pancreatic cancer. Br J Cancer. 2013;108:766–770. doi: 10.1038/bjc.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Błogowski W, Deskur A, Budkowska M, Sałata D, Madej-Michniewicz A, Dąbkowski K, Dolegowska B, Starzynska T. Selected cytokines in patients with pancreatic cancer: a preliminary report. PLoS One. 2014;9:e97613. doi: 10.1371/journal.pone.0097613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He S, Zhao H, Fei M, Wu Y, Wang L, Zhu X, Li D. Expression of the co-signaling molecules CD40-CD40L and their growth inhibitory effect on pancreatic cancer in vitro. Oncol Rep. 2012;28:262–268. doi: 10.3892/or.2012.1790. [DOI] [PubMed] [Google Scholar]
- 27.Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol. 2008;83:1069–1078. doi: 10.1189/jlb.0907615. [DOI] [PubMed] [Google Scholar]
- 28.Kirk AD, Blair PJ, Tadaki DK, Xu H, Harlan DM. The role of CD154 in organ transplant rejection and acceptance. Philos Trans R Soc Lond B Biol Sci. 2001;356:691–702. doi: 10.1098/rstb.2001.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]


