Abstract
MicroRNAs were recently found to participate in oncogenesis and growth of various tumors. We hypothesized that microRNA-223 (miR-223) plays a role in endometrial carcinoma growth. In this study, we transfected RL95-2 cells with lentivirus containing miR-223 precursor to establish a miR-223 over-expression model. Proliferation of the cells was greatly inhibited when miR-223 was over-expressed, and cell cycle progress was blocked in G0/G1 phase. To investigate the mechanisms involved, we scanned the putative target genes of miR-223 using bioinformatics, and confirmed that insulin-like growth factor-1 receptor (IGF-1R) was a functional target of miR-223 using quantitative PCR, Western blot and luciferase reporter assay. Meanwhile, over-expressed miR-223 was found to regulate the expression of IGF-1R by repressing protein translation. Silencing IGF-1R with small interfering RNA resulted in similar effect as miR-223 overexpression. Therefore, our data suggest that miR-223 regulates RL95-2 cells proliferation and cell cycle progress by targeting IGF-1R.
Keywords: MiR-223, insulin-like growth factor-1 receptor, cell proliferation, in vitro, endometrial carcinoma cell
Introduction
Endometrial cancer is the most common gynecology cancer in developed countries, with endometrioid endometrial cancer (EEC) being the most dominant subtype that represents approximately 80% of all cases [1-3]. MicroRNAs (miRNAs) are single-stranded, small non-coding RNAs consisting of 20-22 nucleotides [4]. They can regulate gene expression by hybridizing the target sites with complementary sequences and result in translational repression, mRNA cleavage, or destabilization [5,6]. As posttranscriptional regulators, miRNAs participate in many biologic processes, such as cell proliferation, differentiation, apoptosis, and oncogenesis [5]. Several studies have reported the expression profiles of tissue miRNAs in EEC, and numerous miRNAs were aberrantly expressed [7-10]. A recent report claimed that four serum miRNAs were significantly increased in endometrial cancer patients compared with control, including miR-223 [11]. MiR-223 has been shown to have an important role in the proliferation of venous smooth muscle cells, Hela cells, and hematopoietic cells [12]. The functions of miR-223 have been linked to the suppression of a variety of target genes, with currently validated targets including Rasa1, NFI-A, STMN1, FOXO1, and IGF-1R. However, roles of miR-223 in endometrial cancer cells have not been deciphered.
The insulin-like growth factor-1 receptor (IGF-1R) system, which plays a key role in normal growth of the cells, is comprised of ligands (IGF-1, IGF-2), cell surface receptors (IGF-1R, IGF-2R, and insulin receptor) and at least six binding proteins (IGFBPs) [13]. The IGFs regulate cell proliferation mainly by binding to IGF-1R and activating downstream PI3K/Akt signal pathway [14]. Previous studies showed a pivotal role for IGF-1R action in endometrial cancer and emphasized the importance of altered IGF-1R gene expression in the development of a malignant phenotype [15-17]. IGF-1R inhibition techniques were investigated as promising therapeutic tools against endometrial carcinoma.
Using bioinformatics predictions, we identified that there were putative binding sites in the 3’-untranslated regions (3’UTR) of IGF-1R, STAT3, FOXO1, and IKKα mRNA, to which miR-223 seed sequence may bind (Table 1). Based on previous data, we hypothesized that miR-223 modulated cell proliferation via IGF-1R. In this study, we over-expressed miR-223 in RL95-2 cells and further investigated the potential regulation of miR-223 on IGF-1R.
Table 1.
Potential interactions between miR-223 and the 3’UTR of target genes
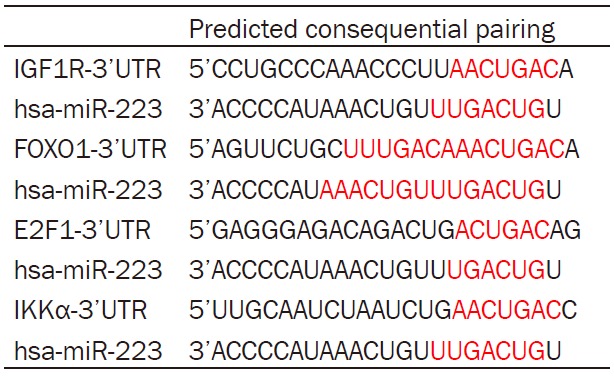
|
Materials and methods
Cell culture, lentivirus transduction
RL95-2 cells (China center for type culture collection of Wuhan) were cultured in DMEM/F12 and HEK-293 cells (ATCC), supplemented with 10% fetal bovine serum (Invitrogen) and 1% penicillin/streptomycin (Invitrogen).
Lentiviral particles for hsa-miR-223 (Cat. #: LP-HmiR0379-MR03-C0005) and scramble control (Cat. #: LP-CmiR0001-MR03-0200) were purchased from GeneCopoeia. Lentivirus transduction was performed according to the manufacturer’s instruction. Briefly, RL95-2 cells were seeded onto a 24-well plate and cultured for 24 h to achieve 70% confluence. RL95-2 cells were then transfected with indicated lentivirus in the presence of 8 μg/ml polybrene overnight. The efficiency of infection was measured under a fluorescent microscope 72 h after the transfection and the cells were sorted using a flow cytometry. The sorted cells were used in the following experiments.
Cell proliferation assays
Cell proliferation was determined with the Cell Counting kit-8 (Beyotime Biotech) assay. In brief, RL95-2 cells were seeded onto 96-well plates at an initial number of 3,000 cells per well. At the time point of 24, 48, and 72 h, 10 µl of the kit reagent was added to each well, and 1 h later all plates were scanned by a microplate reader (Thermo Fisher Scientific) at 450 nm. Each experiment was performed 3 times independently.
Luciferase reporter assay
HEK-293 cells were seeded in 96-well plates. After 24 hour’s incubation, cells were cotransfected with either IGF-1R 3’UTR clone or negative control clone (GeneCopoeia) and miR-223 vector or scramble control. Forty-eight hours after transfection, the cells were assayed by both firefly and renilla luciferase using the dual luciferase assay system (GeneCopoeia) according to manufacturer’s instructions. All transfection experiments were conducted in triplicate and repeated 3 times independently.
RNA extraction and quantitative RT-PCR
Total RNA was extracted from cells with TRIzol reagent (Ambion) and reverse transcription reaction was performed using a RT Kit (Takara) according to the manufacturer’s protocol. The RT products (cDNA) were amplified by real-time quantitative PCR with SYBR green Master Mix (Takara). The primers sequences are shown in Table 2. For sample analysis, the threshold was set based on the exponential phase of products, and the 2-ΔΔCT method was performed to analyze the data, as described previously [18]. The expression level of IGF-1R was normalized to β-actin mRNA. All reactions were run in triplicate and all experiments were performed 3 times independently.
Table 2.
Primer sequences (all 5’-3’) used in real-time RT-PCR
| Genes | GeneBank accession no | Forward primer | Reverse primer |
|---|---|---|---|
| IGF1R | NM_000875 | GGACAGGTCAGAGGGTTTC | CTCGTAACTCTTCTCTGTGCC |
| FOXO1 | NM_002015 | TGGACATGCTCAGCAGACATC | TTGGGTCAGGCGGTTCA |
| E2F1 | NM_005225 | CAGAGCAGATGGTTATGGT | TATGGTGGCAGAGTCAGT |
| IKKα | NM_001278 | TGGAGTTAGAGGCTGTGA | GTCATCTGCTCGCTGTAT |
| ACTB | NM_001101 | GTCCACCGCAAATGCTTCTA | TGCTGTCACCTTCACCGTTC |
miRNA PCR
Mature miR-223 was detected with the All-in-One miRNA qRT–PCR Detection Kit (Gene-Copoeia). Briefly, total RNA was polyadenylated and reverse transcribed using a poly dT-adaptor primer. Quantitative RT–PCR was performed using a miRNA-specific forward primer and a universal reverse primer. The following forward primers were synthesized by GeneCopoeia: 5’-UGUCAGUUUGUCAAAUACCCCA-3’ for hsa-miR-223-3p and 5’-CAAATTCGTGAAGCGTTCCATAT-3’ for U6 small nuclear RNA (U6). The universal reverse primer was purchased from the same company. U6 small nuclear RNA was used as an internal control.
Western blot
Cells were lysed in RIPA lysis buffer supplemented with a protease inhibitor cocktail solution. Proteins and pre-stained molecular weight markers were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. After being blocked in 5% no-fat milk or bovine serum albumin, the membranes were incubated with antibodies against human IGF-1R (1:1000, ABclonal), phospho-IGF-1R (1:500, ABclonal), FOXO1 (1:1000, ABclonal), E2F1 (1:800, ABclonal), IKK-α (1:1000, ABclonal), or β-actin (1:800, Santa Cruz) overnight at 4°C, and then followed by 1 hour’s incubation with the appropriate peroxidase-conjugated secondary antibody. Immunoreactive bands were visualized by enhanced chemiluminescence according to the manufacturer’s protocol (ECL kit, Advansta). To evaluate the expression of target proteins, the bands were scanned using a GS-800 scanning densitometer (BioRad). The intensity of each protein band was quantified with BioRad Quantity One software analysis system.
Flow cytometric analysis
Cell cycle distributions were assayed using flow cytometry. Briefly, RL95-2 cells were trypsinized, collected, washed, and stained with propidium iodide (PI, Sigma-Aldrich) and RNase A (Sigma) for 30 min at 4°C according to the manufacturer’s protocol (Sigma).
RNA interference
RL95-2 cells were plated into 6-well plate 24 h prior to transfection. After the replacement of fresh culture medium, the cells were transfected with IGF-1R siRNA (5’-GATTGAGTTTCTCAACGAA-3’) or the negative control siRNA (RiboBio Co., Ltd.) using lipofectamine 2000 (Invitrogen, CA) following the manufacturer’s protocol. Knockdown of IGF-1R was identified by quantitative PCR and western blot 48 hours after expression of the siRNAs. β-actin was used as an internal control.
Statistical analysis
Results were expressed as the mean ± SD. Statistical analysis was performed on raw data by Student’s t-test after testing for normal distribution (Skewness and Kurtosis test) and homoscedasticity (Levene test) of variance using Statistical Package for the Social Sciences 13.0 (SPSS, Chicago, IL, USA). In cases where data did not show a normal distribution or homoscedasticity of variance, values were log transformed prior to further statistical analysis. A P value < 0.05 was considered statistically significant.
Results
MiR-223 suppression of RL95-2 cell proliferation
To observe the biological role of miR-223 in the proliferation of endometrial carcinoma cell, RL95-2 cells were transfected with lentivirus particles expressing a mature miR-223-3p sequence, and the sorted miR-223 transfected RL95-2 cells were used as a stable miR-223 over-expression model. The expression of miR-223 was significantly increased in the over-expression model (Figure 1A). The proliferation rate of miR-223 over-expression cells was markedly reduced as compared with RL95-2 cells transfected with scramble control (Figure 1B). To investigate the effect of miR-223 on cell cycle progression, flow cytometry following staining with propidium iodide were then performed. The cell population in G0/G1 phase was higher in miR-223 overexpression group (miR-223 group) than in scramble control group (SC group). In contrast, the cell population in G2/M was lower, indicating a block in the G0/G1 phase transition of the cell cycle (Figure 1C-E).
Figure 1.
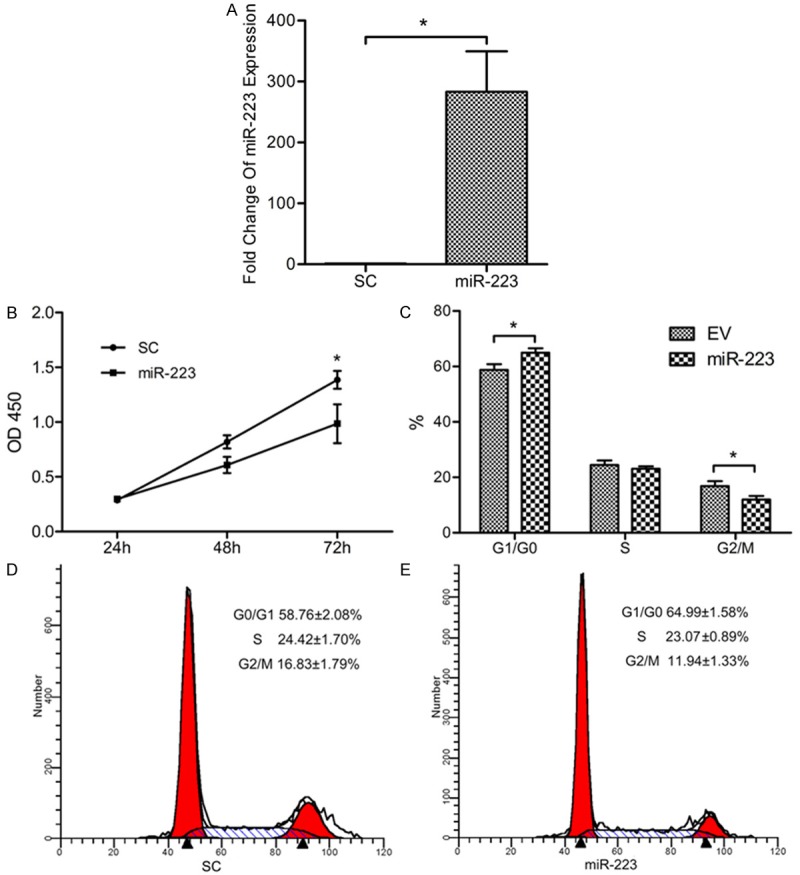
MiR-223 regulates RL95-2 cells proliferation. A. MiR-223 was over-expressed inRL95-2 cells and confirmed by quantitative PCR. *P < 0.05. B. Growth curves of miR-223 and EV infected RL95-2 cells were conducted by CCK-8 assay. *P < 0.05. C-E. Cell cycle distribution for SC and miR-223 groups were assayed by flow cytometry. Cells were treated with RNaseA and stained with PI. *P < 0.05.
IGF-1R is regulated by miR-223
To further investigate the mechanism how miR-223 inhibits the proliferation of RL95-2 cells, we searched for potential targets of miR-223 through the online algorithm of Targetscan 5.1. Hsa-miR-223 was identified having potential binding sites at the 3’UTR of IGF-1R, FOXO1, STAT3 and IKK-α mRNA. Results by quantitative PCR suggested that all potential targets did not reduce significantly at mRNA levels in the miR-223 group (Figure 2A). However, we found that both IGF-1R and phosphorylated IGF-1R proteins were significantly reduced in miR-223 group compared with SC group (Figure 2B, 2C). To determine whether miR-223 acts directly on IGF-1R 3’UTR, we conducted a series of luciferase reporter assays by co-transfection of miR-223 or scramble control and luciferase constructs containing IGF-1R 3’UTR or control clone. Luciferase activity was decreased approximately 70% in miR-223 and IGF-1R 3’UTR clone co-transfection group, compared with the other 3 groups (Figure 2D).
Figure 2.
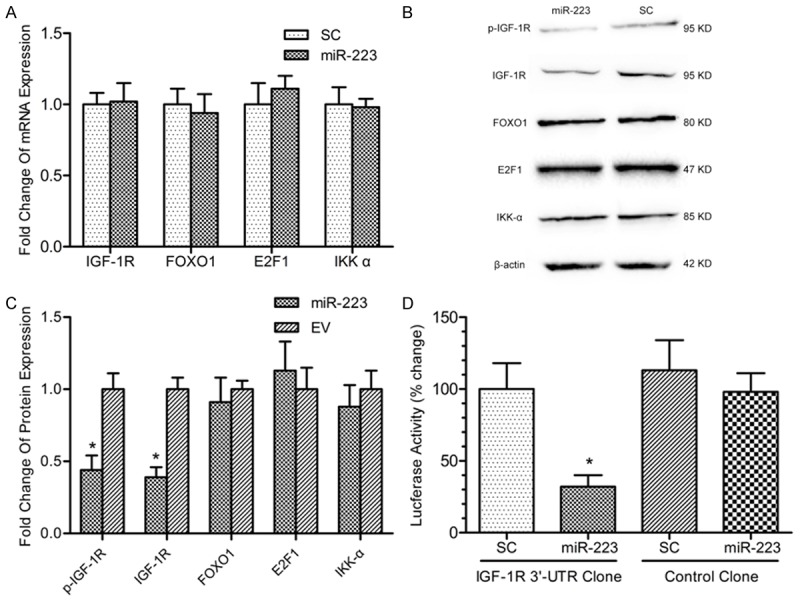
MiR-223 directly targets IGF-1R. A. Quantitative PCR was conducted to detect the mRNA expression levels of IGF-1R, FOXO1, E2F1 and IKK-α after miR-223 over-expression in RL95-2 cells. B. Western blot was performed to detect the protein levels of IGF-1R, phosphorylated IGF-1R, FOXO1, E2F1 and IKK-α after miR-223 over-expression in RL95-2 cells. C. Protein bands were scanned to measure the integral density in B experiments and normalized to those of β-actin. The relative expression was then compared. *P < 0.05. D. Scramble control or miR-223 lentiviral vector was co-transfected with IGF-1R 3’UTR clone or control clone in HEK-293 cells. After 48 hours, luciferase activity was assayed. *P < 0.05.
SiRNA mimicked the effect of miR-223 on downregulation of IGF-1R
Our results showed that miR-223 suppressed IGF-1R in protein level, but not in mRNA level. To further prove that inhibition of IGF-1R is sufficient to reduce cell proliferation and alter cell cycle distribution, siRNA of IGF-1R were conducted and transfected into RL95-2 cells. The expression of IGF-1R was significantly decreased after siRNA transient transfection (Figure 3A-C). Similar suppression of cell proliferation and cell cycle distribution as in miR-223 overexpression cells were observed (Figure 3D-F).
Figure 3.
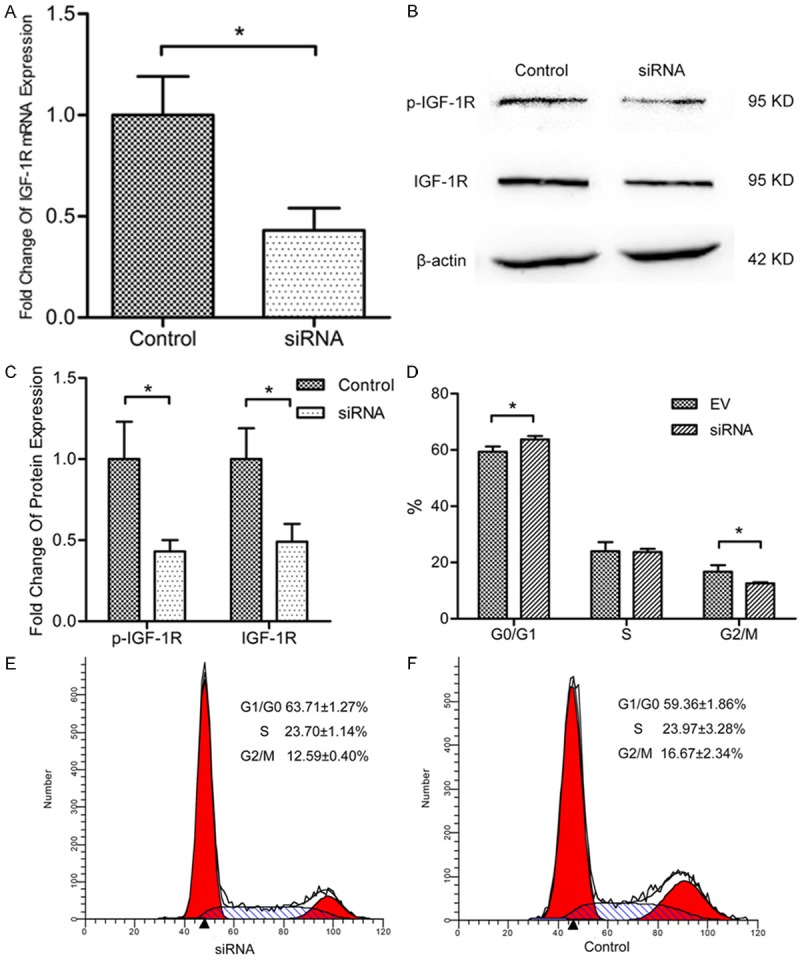
Interference of IGF-1R mimics the suppression of growth by miR-223. A. Quantitative PCR was conducted to detect the mRNA expression levels of IGF-1R after transfection with siRNA to RL95-2 cells. *P < 0.05. B. Western blot was performed to detect the protein levels of IGF-1R and phosphorylated IGF-1R after transfection with siRNA to RL95-2 cells. C. Protein bands were scanned to measure the integral density in B experiments and normalized to those of β-actin. The relative expression was then compared. *P < 0.05. D-F. Cell cycle distribution for negative control and siRNA groups were assayed by flow cytometry. Cells were treated with RNaseA and stained with PI. *P < 0.05.
Discussion
Previous studies have reported that miR-223 has an effect on different cellular processes, ranging from hematopoietic differentiation [19-21] and immune cell function [22,23] to carcinogenesis [24-27]. Wang et al. previously reported that miR-223 can inhibit cell proliferation and serve as a tumor suppressor [28]. However, Laios et al. found that high levels of miR-223 were correlated with recurrent ovarian cancer [25]. In the present study, a miR-223 over-expression model was established and the role of miR-223 in the proliferation and cell cycle distribution of endometrial carcinoma cell was investigated.
We hypothesized that miR-223 may be a pivotal component in regulation of RL95-2 cells proliferation. Lentiviral transduction was performed to achieve over-expression of miR-223, and CCK-8 assays revealed that over-expression of miR-223 inhibited the proliferation of RL95-2 cells. Furthermore, over-expression of miR-223 was found to increase the cells in the G0/G1 phase and decrease the cells in the G2/M phase. Together with previous reports [29], current study indicated that over-expression of miR-223 was able to block cell cycle progression by induce G0/G1 arrest. All these results suggested that miR-223 functioned as a negative regulator for the cell growth, which was consistent with previous studies [28,30].
In order to find out the target genes of miR-223, we detected several putative targets of miR-223 including IGF-1R, FOXO1, STAT3, IKK-α and found that all their mRNA levels changed slightly after miR-223 over-expression. However, protein of IGF-1R was found to be significantly down-regulated by miR-223. In the reporter assay with the vector containing 3’UTR of IGF-1R, the luciferase activity was greatly inhibited after co-transfection of miR-223 vector. All these results suggested that miR-223 interacted with 3’UTR of IGF-1R and suppressed IGF-1R protein translation. The effect may be derived from the mode by which miRNAs regulate gene expression when miRNAs and targeted mRNA sequences are incompletely complementary, namely, miRNAs inhibit protein translation but not degrade mRNA [31].
IGF-1R was involved in the development and tumorigenesis of many malignant human tumors, including endometrial carcinoma [32]. Hirano et al. [33] reported high expression of IGF-1R in cancers originating from the genital tract, with protein expression in 91.3% endometrial cancers. Likewise, McCampbell et al. [17] reported a significant increase in IGF-1R expression in biopsies from endometrial carcinoma compared with proliferative endometrium. Meanwhile, down-regulation of IGF-1R by a variety of techniques can inhibit endometrial cancer cells proliferation in vitro and in vivo [16,34,35]. In the present study, we confirmed that down-regulation of IGF-1R by siRNA suppressed proliferation of RL95-2 cells, which was similar as miR-223 overexpression.
Taken together, our data suggested that miR-223 suppressed the proliferation and cell cycle progression of endometrial carcinoma cells in vitro. The effect was obtained through its target gene IGF-1R.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81170619, No. 81372804).
Disclosure of conflict of interest
None.
References
- 1.Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 2.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 3.Di Saia PJ, Creasman WT. Philadelphia, PA: Elsevier/Saunders; 2012. Clinical gynecologic oncology. [Google Scholar]
- 4.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 7.Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Wei Z, Kamath S, Chen DT, Dressman H, Lancaster JM. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol. 2008;110:206–215. doi: 10.1016/j.ygyno.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Chung TK, Cheung TH, Huen NY, Wong KW, Lo KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, Yu MY, To KF, Mok SC, Wang VW, Li C, Cheung AY, Doran G, Birrer MJ, Smith DI, Wong YF. Dysregulated microRNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women. Int J Cancer. 2009;124:1358–1365. doi: 10.1002/ijc.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn DE, Fabbri M, Valeri N, Alder H, Ivanov I, Liu CG, Croce CM, Resnick KE. Comprehensive miRNA profiling of surgically staged endometrial cancer. Am J Obstet Gynecol. 2010;202:656.e651–658. doi: 10.1016/j.ajog.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratner ES, Tuck D, Richter C, Nallur S, Patel RM, Schultz V, Hui P, Schwartz PE, Rutherford TJ, Weidhaas JB. MicroRNA signatures differentiate uterine cancer tumor subtypes. Gynecol Oncol. 2010;118:251–257. doi: 10.1016/j.ygyno.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia W, Wu Y, Zhang Q, Gao G, Zhang C, Xiang Y. Identification of four serum microRNAs from a genome-wide serum microRNA expression profile as potential non-invasive biomarkers for endometrioid endometrial cancer. Oncol Lett. 2013;6:261–267. doi: 10.3892/ol.2013.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haneklaus M, Gerlic M, O’Neill LA, Masters SL. miR-223: infection, inflammation and cancer. J Intern Med. 2013;274:215–226. doi: 10.1111/joim.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeRoith D, Werner H, Beitner-Johnson D, Roberts CT Jr. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 14.Bruchim I, Werner H. Targeting IGF-1 signaling pathways in gynecologic malignancies. Expert Opin Ther Targets. 2013;17:307–320. doi: 10.1517/14728222.2013.749863. [DOI] [PubMed] [Google Scholar]
- 15.Amichay K, Kidron D, Attias-Geva Z, Schayek H, Sarfstein R, Fishman A, Werner H, Bruchim I. BRCA1 is expressed in uterine serous carcinoma (USC) and controls insulin-like growth factor I receptor (IGF-IR) gene expression in USC cell lines. Int J Gynecol Cancer. 2012;22:748–754. doi: 10.1097/IGC.0b013e318254011f. [DOI] [PubMed] [Google Scholar]
- 16.Attias-Geva Z, Bentov I, Kidron D, Amichay K, Sarfstein R, Fishman A, Bruchim I, Werner H. p53 Regulates insulin-like growth factor-I receptor gene expression in uterine serous carcinoma and predicts responsiveness to an insulin-like growth factor-I receptor-directed targeted therapy. Eur J Cancer. 2012;48:1570–1580. doi: 10.1016/j.ejca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 17.McCampbell AS, Broaddus RR, Loose DS, Davies PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res. 2006;12:6373–6378. doi: 10.1158/1078-0432.CCR-06-0912. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 20.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–831. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 21.Ramkissoon SH, Mainwaring LA, Ogasawara Y, Keyvanfar K, McCoy JP Jr, Sloand EM, Kajigaya S, Young NS. Hematopoietic-specific microRNA expression in human cells. Leuk Res. 2006;30:643–647. doi: 10.1016/j.leukres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O’Neill LA, Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 23.Zhuang G, Meng C, Guo X, Cheruku PS, Shi L, Xu H, Li H, Wang G, Evans AR, Safe S, Wu C, Zhou B. A novel regulator of macrophage activation: miR-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125:2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 24.Fazi F, Racanicchi S, Zardo G, Starnes LM, Mancini M, Travaglini L, Diverio D, Ammatuna E, Cimino G, Lo-Coco F, Grignani F, Nervi C. Epigenetic silencing of the myelopoiesis regulator microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell. 2007;12:457–466. doi: 10.1016/j.ccr.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B, O’ Leary J. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z, Han S, Nie Y, Chen X, Zhao Q, Ding J, Wu K, Daiming F. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 27.Masciarelli S, Fontemaggi G, Di Agostino S, Donzelli S, Carcarino E, Strano S, Blandino G. Gain-of-function mutant p53 downregulates miR-223 contributing to chemoresistance of cultured tumor cells. Oncogene. 2014;33:1601–1608. doi: 10.1038/onc.2013.106. [DOI] [PubMed] [Google Scholar]
- 28.Jia CY, Li HH, Zhu XC, Dong YW, Fu D, Zhao QL, Wu W, Wu XZ. MiR-223 suppresses cell proliferation by targeting IGF-1R. PLoS One. 2011;6:e27008. doi: 10.1371/journal.pone.0027008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulikkan JA, Dengler V, Peramangalam PS, Peer Zada AA, Muller-Tidow C, Bohlander SK, Tenen DG, Behre G. Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood. 2010;115:1768–1778. doi: 10.1182/blood-2009-08-240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song L, Duan P, Guo P, Li DW, Li ST, Xu Y, Zhou Q. Downregulation of miR-223 and miR-153 mediates mechanical stretch-stimulated proliferation of venous smooth muscle cells via activation of the insulin-like growth factor-1 receptor. Arch Biochem Biophys. 2012;528:204–211. doi: 10.1016/j.abb.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee D, Slack F. Control of developmental timing by small temporal RNAs: a paradigm for RNA-mediated regulation of gene expression. Bioessays. 2002;24:119–129. doi: 10.1002/bies.10046. [DOI] [PubMed] [Google Scholar]
- 32.Pavelic J, Radakovic B, Pavelic K. Insulin-like growth factor 2 and its receptors (IGF 1R and IGF 2R/mannose 6-phosphate) in endometrial adenocarcinoma. Gynecol Oncol. 2007;105:727–735. doi: 10.1016/j.ygyno.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Hirano S, Ito N, Takahashi S, Tamaya T. Clinical implications of insulin-like growth factors through the presence of their binding proteins and receptors expressed in gynecological cancers. Eur J Gynaecol Oncol. 2004;25:187–191. [PubMed] [Google Scholar]
- 34.Shu S, Li X, Yang Y, Zhang Y, Li T, Liang C, Wan J. Inhibitory effect of siRNA targeting IGF-1R on endometrial carcinoma. Int Immunopharmacol. 2011;11:244–9. doi: 10.1016/j.intimp.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Shu S, Yang Y, Li X, Li T, Zhang Y, Xu C, Liang C, Wang X. Down-regulation of IGF-1R expression inhibits growth and enhances chemosensitivity of endometrial carcinoma in vitro. Mol Cell Biochem. 2011;353:225–233. doi: 10.1007/s11010-011-0790-9. [DOI] [PubMed] [Google Scholar]


