Abstract
DKK1 is a secreted glycoprotein that inhibits Wnt/β-catenin signaling but may up-regulate the nonconanical Wnt signaling. Consistent with its inhibitory function in Wnt/β-catenin signaling, aberrant DKK1 expression has been observed in many types of human cancers, while contradicting findings have been reported in other studies. There are also several studies on serum DKK1 levels in various cancers with conflicting findings. In the present study, serum DKK1 was determined in 217 non- small cell lung cancer (NSCLC) patients, 35 small cell lung cancer (SCLC) patients and 286 matched healthy controls using a commercially available ELISA assay kit. Compared to healthy controls, serum DKK1 level was significantly lower in NSCLC (p < 10-28) and SCLC (p <10-4) patients. Interestingly, serum DKK1 level was higher in NSCLC patients in stage IV (p < 0.0005), with lymph node involvement (p < 0.0002) or with metastasis (p < 0.0001), suggesting that DKK1 may promote metastasis. After surgery and/or chemotherapy, serum DKK1 level is rapidly increased and reached levels observed in healthy controls in most patients. The degree of post therapeutic DKK1 increase varied in different treatment regimens. Our results thus provide strong evidence for the reduced levels of serum DKK1 in both types of lung cancer. However, in the context of all published studies, DKK1 appears to have a dichotomous role in cancer and its effect in a given cancer type or even a given cancer patient is likely to depend on the molecular context of the patient.
Keywords: DKK1, biomarker, lung cancer, wnt signaling
Introduction
Lung cancer is one of the leading causes of all cancer-related deaths worldwide, with a 5-year survival rate as low as 13% [1]. Despite some advances in early detection and recent improvements in its treatment, the prognosis of patients with lung cancer remains poor [2] because it exhibits high resistance to anticancer therapy [1]. Molecular diagnostics may offer precise, objective cancer classification. However, standard molecular markers for the prognostic classification of most solid tumors have yet to be identified [3]. The known markers such as carcinoembryonic antigen, cytokeratin 19 fragment (CYFRA 21-1), neuron-specific enolase (NSE) [4-6] are not satisfactory for diagnosis at an early stage or monitoring disease because of their relatively low sensitivity and specificity.
DKK1 is a secreted protein that plays a crucial role in head formation during vertebrate development and specifically inhibits Wnt/ β-catenin signaling [7], a critical pathway in cancer. DKK1 overexpression was reported in multiple myeloma [8], hepatoblastomas, Wilm’s tumors [9] and breast cancer [10,11]. More recently, increased DKK1 expression in tumor tissues has been observed in many types of human cancers [10,11]. However, these findings have been contradicted by other reports. In other studies, DKK1 expression in tumor tissues is reduced in colon cancer [12-14], in melanoma [12-14] and in renal clear cell carcinoma [15]. DKK1 expression increases early in prostate cancer development but decreases during progression [16].
Several studies have also measured serum levels of DKK1 in patients with multiple carcinomas and healthy controls; however, the findings are also controversial. Three different studies reported increased serum DKK1 levels in lung cancer patients than in controls and worse prognosis in patients with higher serum DKK1 levels [17-19]. Serum DKK1 was found to be elevated in multiple cancers including pancreas, stomach, liver, bile duct, breast, cervix [10,11], esophagus [17-19] and breast cancer with bone metastasis [20]. In contrast, serum DKK1 was also reported to be significantly lower in multiple cancers including stomach, colon, ovary and cervix [19] as well as kidney [15]. These controversial results suggested that DKK1 should further be studied in various types of cancers.
In the present study, we measured serum DKK1 levels in a large number of healthy controls and patients with NSCLC or SCLC and investigated the relationship between DKK1 and clinicopathological variables.
Materials and methods
Human subjects and serum samples
The selection criteria for patients with lung cancer were as follows: 1) pathologically confirmed patients (the diagnoses in all patients were confirmed each time by microscopic examination of the material obtained during bronchoscopy, biopsy, or surgery); 2) patients had no history of other carcinomas. A total of 252 diagnosed patients with lung cancer in Jiangsu Cancer Hospital between October 2011 and December 2012 and 286 healthy control subjects from Nanjing were used in the present study. The characteristics of the patients are summarized in Table 1. Blood samples were collected from patients at the time of diagnosis and before any treatment (surgery and/or chemotherapy). Furthermore, blood samples from patients treated with surgery and/or chemotherapy were also collected monthly. Samples were centrifuged for 10min at 3,000 rpm at 4°C, and serum was subsequently frozen at -80°C until use. This study has been approved by the human subject ethics committee of the Jiangsu Cancer Hospital and informed consent signed by the study subjects.
Table 1.
Clinical features of patients with lung cancer and controls
| Variables | Patients | Controls | ||
|---|---|---|---|---|
|
| ||||
| SCLC | NSCLC | Total | ||
| Age (year) | ||||
| Mean (SD) | 63.2 ± 9.57 | 61.0 ± 11.3 | 61.3 ± 11.1 | 41.8 ± 13.5 |
| < 50 | 2 | 30 | 32 | 217 |
| 50-70 | 24 | 133 | 157 | 55 |
| > 70 | 9 | 54 | 63 | 14 |
| Sex | ||||
| Male | 31 | 155 | 186 | 260 |
| Female | 4 | 62 | 66 | 26 |
Enzyme-linked immunosorbent assay
Serum DKK1 levels were measured by enzyme-linked immunosorbent assay (ELISA) with an immunoassay kit (R&D Systems, USA) according to the manufacturer’s directions. The optical density (OD) at 450 nm was determined.
Statistical analysis
Protein concentrations were estimated using a regression fit to the standard curve with known concentration included on each plate using a serial dilution series. The concentrations were logarithmically transformed prior to all statistical analyses to achieve normal distribution. The differences between two groups were examined using an unpaired t-test or Mann-Whitney test. The comparisons for ≥ 3 groups were made by ANOVA followed by pair-wise comparisons. The statistical significance of differences was set at P < 0.05. To examine the relationships between disease phenotype and the serum protein levels, logistic regression was used by including age and sex as co-variables. All statistical analyses were performed using the R language and environment for statistical computing (R version 2.15.1; R Foundation for Statistical Computing; www.r-project.org).
Results
Serum DKK1 levels are lower in patients with NSCLC or SCLC
Serum DKK1 was measured in 286 healthy controls and 252 lung cancer patients, of whom 217 had NSCLC and 35 had SCLC. Mean serum DKK1 levels were 4.85 ng/ml in the SCLC group, 4.67 ng/ml in the NSCLC group, and 9.10 ng/ml in the control group (Figure 1). Compared to controls, the mean serum DKK1 levels were approximately two-fold lower in patients with NSCLC (p = 3.6E-29) and SCLC (p = 1.7E-5) (Figure 1). The two lung cancer groups had similar serum DKK1 levels (P > 0.05).
Figure 1.
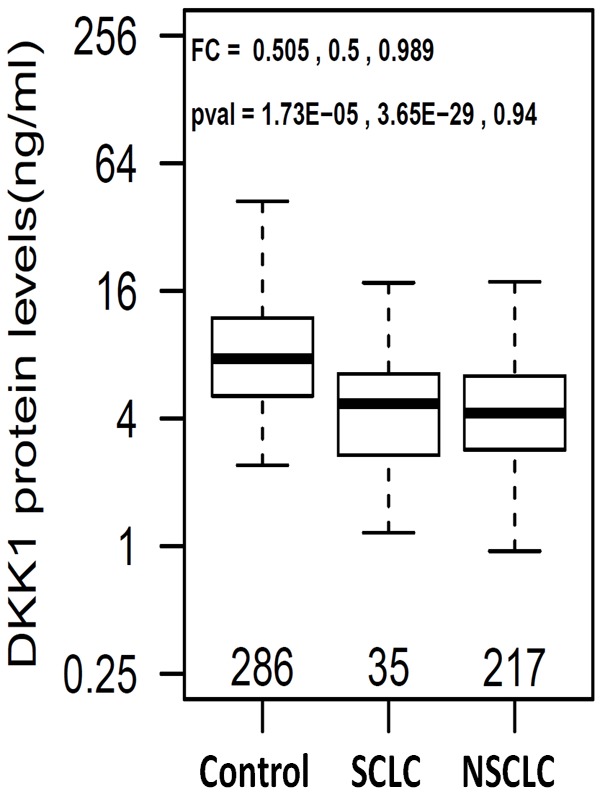
Boxplots of serum DKK1 in healthy controls and patients. Fold change (FC) and p-values are listed in the order of control vs SCLC, control vs NSCLC, and SCLC vs NSCLC.
Logistic regression was carried out using protein concentration as dependent variable and sex and age as covariates. After adjusting for age and sex, DKK1 remains significantly associated with both NSCLC (OR = 0.263, adjusted p value = 6.1E-13) and SCLC (OR = 0.262, adjusted p = 1.6E-5) (Table 2). However, no significant correlation was observed between serum DKK1 levels and age (P > 0.05) or gender (P > 0.05).
Table 2.
Logistic regression analysis of serum DKK1 before and after adjustment of sex and age as covariates
| Unadjusted | Adjusted (Age & Sex) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR | (95% CI) | P-value | OR | (95% CI) | P-value | |
| N vs NSCLC | 0.247 | (0.183-0.325) | 1.33E-21 | 0.263 | (0.179-0.371) | 6.17E-13 |
| N vs SCLC | 0.258 | (0.148-0.415) | 2.16E-07 | 0.262 | (0.135-0.461) | 1.64E-05 |
Early stage NSCLC patients have more severe reduction in serum DKK1
In the NSCLC patient group, there was sufficient number of patients to examine DKK1 differences according to various clinico-pathological characteristics. It was surprising to find that serum DKK-1 levels were significantly higher in stage IV patients than stage I-III patients (p = 4.9E-4, Table 3). Consistent with this finding, patients with distant metastasis have higher DKK1 levels than non-metastatic patients (p = 8.9E-5, Table 3, Figure 2). Patients with lymph node involvement also have higher DKK1 levels than patient without lymph node involvement (P = 1.6E-4, Table 3).
Table 3.
DKK1 differences according to clinico-pathological characteristics of NSCLC patients
| Phenotype | Groups | N | Mean (SD) | p-value |
|---|---|---|---|---|
| Gender | Male | 155 | 4.81 (2.88) | |
| Female | 62 | 4.59 (2.33) | 0.552 | |
| Age (year) | < 50 | 30 | 5.07 (3.39) | |
| 50-70 | 133 | 4.64 (2.56) | ||
| > 70 | 54 | 4.84 (2.76) | 0.854 | |
| TNM stage | I | 13 | 3.26 (1.7) | |
| II | 6 | 2.65 (1.44) | ||
| III | 31 | 3.98 (2.81) | ||
| IV | 122 | 5.24 (2.75) | 4.9e-04 | |
| Unknown | 5 | 4.03 (2.54) | ||
| Lymph node | Yes | 154 | 4.88 (2.81) | |
| No | 18 | 3 (1.59) | 1.6e-04 | |
| Unknown | 45 | 5.01 (2.58) | ||
| Distant metastasis | Yes | 116 | 5.29 (2.75) | |
| No | 62 | 3.66 (2.45) | 8.9e-05 | |
| Unknown | 39 | 4.89 (2.62) |
Figure 2.
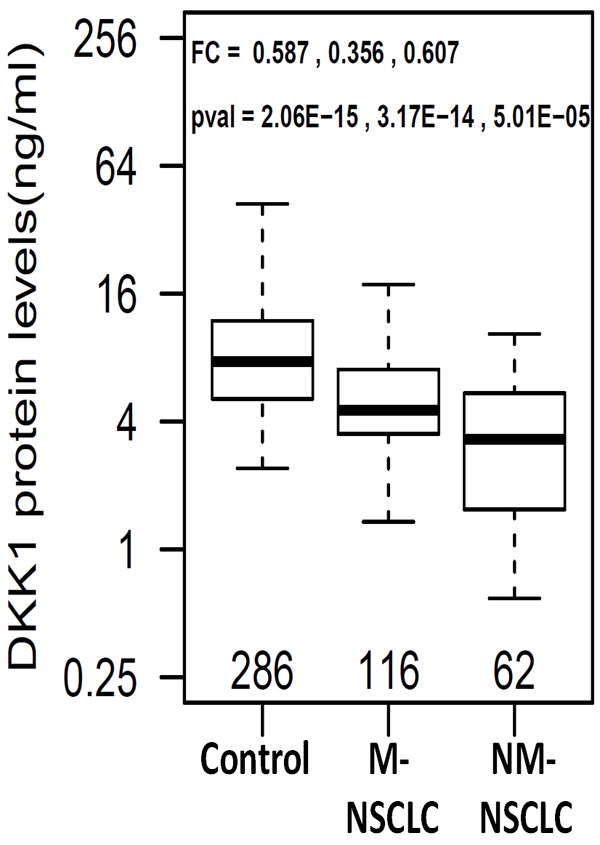
Boxplots of serum DKK1 in healthy controls and NSCLC patients with metastasis (M-NSCLC) and non-metastasis (NM-NSCLC). Fold change (FC) and p-values are listed in the order of control vs M-NSCLC, control vs NM-NSCLC, and M-NSCLC vs NM-NSCLC.
DKK1 increases after therapy in NSCLC patients
Among the 137 NSCLC patients treated with surgery and/or chemotherapy, serum samples were available at different time points post treatment. As shown in Figure 3A, serum DKK1 was significantly higher after treatment than pretreatment for samples obtained within one month of treatment (post/pretreatment fold change [FC] = 2.54, p = 1.3E-10), within two months (FC = 2.28, p = 4.7E-13) and within 3 months (FC = 1.97, p = 2.4E-10). The longitudinal change of serum DKK1 for each individual is plotted in Figure 3B, which suggests an immediate increase of DKK1 after treatment in most individuals. Interestingly, in some individuals DKK1 levels increased quickly (within one month) and then started decreasing, while in other individuals DKK1 remained high 3 months after treatment.
Figure 3.
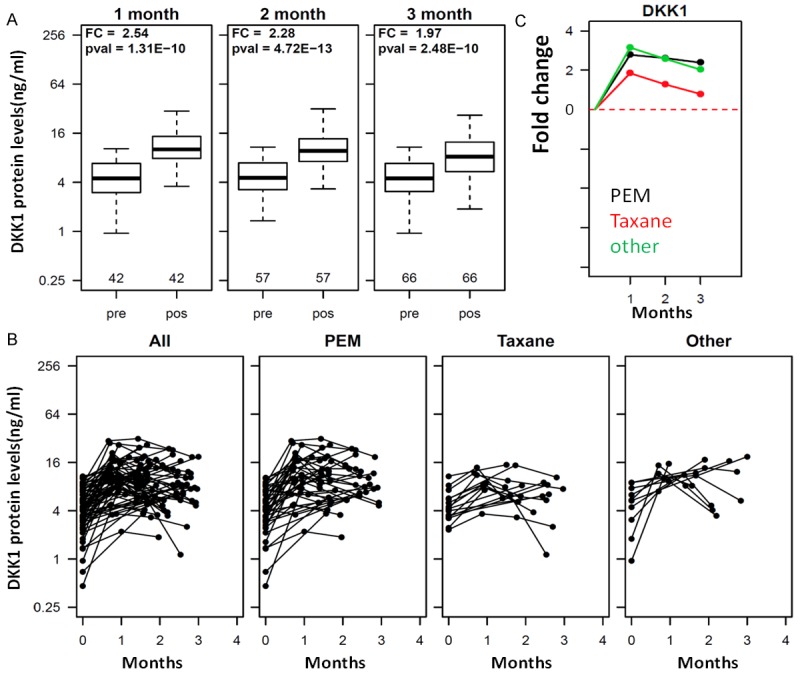
Influence of therapeutic regimens on serum DKK1 levels. A: Boxplots of DKK1 in serum samples from pre-therapy (Pre) and 1 month, 2-months and 3-months post therapy (pos). B: Longitudinal serum DKK1 in individual patients. Serum concentration of DKK1 is plotted on the Y axis and sampling time after therapy is plotted on the X axis. Each dot represents a sample and different time points for the same patient is linked by a line. All: all patients with longitudinal samples are included. PEM: patients treated with the PEM regimen are included. Taxane: patients with the taxane regimen are included. Other: patients treated with other regimens are plotted. PEM represents treatment with platinum plus PEM (Pemetrexed) combined with platinum (DDP, LBP, CBP or NDP); Taxane represents platinum plus taxane (PTX, TAX, Abraxane, TXT, DOC) combined with platinum (DDP, LBP, CBP or NDP). C: Mean change in the PEM, Taxane and other treatment regimens relative to the pre-treatment value. The fold change (FC) between post treatment values and pre-treatment values are calculated and the mean fold increase in each treatment group was plotted for three different time intervals (1, 2, and 3 month). For example, DKK1 is increased on average by 1.9 fold in the taxane group and by 2.9 fold in the PEM treatment group for the one month interval.
The NSCLC patients were treated with different regimens. All patients received platinum-based therapy with other drugs. In the PEM group, patients received platinum plus Pemetrexed (PEM), in the Taxane group patients received platinum plus taxane, and in the Other group patients received platinum plus various other drugs. From the longitudinal change on each individual patient in Figure 3B, it is clear that all treatment regimens appear to increase DKK1 levels after treatment. The mean increase in DKK1 levels in patients treated with the Taxane regimen is smaller compared to the PEM regimen (p = 0.06, 0.01 and 0.01 for 1, 2 and 3 month, respectively, Figure 3C).
Discussion
DKK1 is a 35-kDa glycoprotein that contains a signal peptide sequence and two cysteine-rich domains and a secreted protein that functions as a negative regulator of the Wnt signaling [21], which plays a critical role in cancer pathogenesis. In this study, serum DKK1 was measured in a large set of healthy controls and patients with NSCLC or SCLC. Our results showed that serum DKK1 in pre-therapy samples from patients with NSCLC or SCLC was significantly lower than in control samples. This observation is consistent with several previous reports showing decreased serum DKK1 in patients with gastric cancer, colorectal cancer, ovarian cancer, and cervical adenocarcinoma [19] as well as with renal clear cell cancer [15]. However, there are also several reports suggesting that serum DKK1 levels are elevated in lung cancer [17-19] and in esophagus cancer [17-19]. In a comprehensive study, serum DKK1 was found to be elevated in multiple cancers including pancreas, stomach, liver, bile duct, breast, and cervix [10,11].
The reasons responsible for these controversial findings are still unclear at this time and may be multifactorial. First, since these studies in different laboratories used different ELISA assays, some being commercially available and others being developed in house, it is possible that the assays are not measuring the same form of DKK1 protein. In this study, we used a validated commercial assay that other investigators can evaluate. It will be important in future studies to compare different assays using the same set of samples from multiple types of cancers.
Second, all serum DKK1 studies were primarily based on a case/control study design, which can be heavily influenced by the potential mismatching of cases and controls in confounding variables. Therefore, spurious associations may be found in studies based on small number of samples. The present study has the largest sample size for both patients and controls and therefore our findings may be more reliable. To avoid mismatching issues, longitudinal data provides more solid evidence. In all three published lung cancer studies, survival analysis suggested that higher DKK1 is associated with worse prognosis [17-19]. This is actually not surprising as NSCLC patients at Stage IV or with metastasis have higher serum DKK1 in all four studies on lung cancer including the present study. These results are consistent with the notion that DKK1 may promote metastasis. Another useful approach to examine the potential role of a protein on cancer is to compare data before and after treatment in the same patient set. This approach avoids all confounding variables including patient heterogeneity, which is expected to be very large in cancer. This approach was not used in other DKK1 studies. In the present study, we found that serum DKK1 was significantly increased 1-3 months after therapy (surgery and/or chemotherapy), suggesting that serum DKK1 deficiency is normalized by cancer removal and/or chemotherapy. Furthermore, the degree of DKK1 normalization seems to vary in different individuals and by treatment regimen.
Finally and perhaps most importantly, the controversial findings on DKK1 in cancer may reflect the functional dichotomy of DKK1 [22]. DKK1 is mainly known as a negative regulator of the canonical Wnt signaling [21] via blocking the interaction with Wnt-1 resulting in β-catenin degradation and effects on proliferation [23]. However, it has been shown that DKK1 functions not only as an antagonist of the Wnt/β-catenin pathway but also as an agent that can up-regulate noncanonical Wnt signaling pathway if the requisite Wnt/receptor combinations are available [24]. Consistent with these findings, over-expression of DKK1 in hepatocellular carcinoma (HCC) cell lines inhibited cell growth and increased apoptosis even though some HCC cell lines expressed DKK1 [25]. It has also been shown that conditioned media from mesenchymal stem cells, which contain high levels of DKK1, inhibits growth of MCF-7 breast cancer cells in vitro and in vivo via inhibiting Wnt signaling and down-regulation of β catenin. DKK1-nutralizing antibody is capable of nullifying the effect of the media [26]. A number of studies reported that DKK1 upregulation or over expression can inhibit tumor growth and induce apoptosis [22]. Cumulative evidence suggests that DKK1 may act as an antagonist of the canonical Wnt/β-catenin signaling pathway or as an agonist of the noncanonical Wnt pathway and the overall effect of DKK1 may depend on the molecular context of the cells, thus providing a viable molecular explanation for the widely controversial findings. In future studies, it will be important to examine all other proteins in the DKK1-Wnt pathway together to understand the role of DKK1 in each cancer type and perhaps in different patients.
Acknowledgements
This research is supported by the National Science Foundation of China (grant number 81272244), special fund for Discipline Construction Funds, School of Pharmaceutical Sciences, Nanjing Tech University and Jinfiniti Biosciences LLC, USA.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, Jiang H, Zhang C, Wang H, Yang L, Yu Y, Yu J, Shi B, Shen Z, Gao H. Dickkopf-1 autoantibody is a novel serological biomarker for non-small cell lung cancer. Biomarkers. 2010;15:128–134. doi: 10.3109/13547500903325662. [DOI] [PubMed] [Google Scholar]
- 4.Shinkai T, Saijo N, Tominaga K, Eguchi K, Shimizu E, Sasaki Y, Fujita J, Futami H, Ohkura H, Suemasu K. Serial plasma carcinoembryonic antigen measurement for monitoring patients with advanced lung cancer during chemotherapy. Cancer. 1986;57:1318–1323. doi: 10.1002/1097-0142(19860401)57:7<1318::aid-cncr2820570711>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Pujol JL, Grenier J, Daurès JP, Daver A, Pujol H, Michel FB. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993;53:61–66. [PubMed] [Google Scholar]
- 6.Ferrigno D, Buccheri G, Giordano C. Neuron-specific enolase is an effective tumour marker in non-small cell lung cancer (NSCLC) Lung Cancer. 2003;41:311–320. doi: 10.1016/s0169-5002(03)00232-0. [DOI] [PubMed] [Google Scholar]
- 7.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the β-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 8.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 9.Wirths O, Waha A, Weggen S, Schirmacher P, Kuhne T, Goodyer CG, Albrecht S, Von Schweinitz D, Pietsch T. Overexpression of human Dickkopf-1, an antagonist of wingless/WNT signaling, in human hepatoblastomas and Wilms’ tumors. Lab Invest. 2003;83:429–434. doi: 10.1097/01.lab.0000059926.66359.bd. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70:5326–5336. doi: 10.1158/0008-5472.CAN-09-3879. [DOI] [PubMed] [Google Scholar]
- 11.Forget M, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96:646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Sancho JM, Aguilera O, García JM, Pendás-Franco N, Peña C, Cal S, de Herreros AG, Bonilla F, Muñoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of β-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2004;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 13.Kuphal S, Lodermeyer S, Bataille F, Schuierer M, Hoang B, Bosserhoff A. Expression of Dickkopf genes is strongly reduced in malignant melanoma. Oncogene. 2006;25:5027–5036. doi: 10.1038/sj.onc.1209508. [DOI] [PubMed] [Google Scholar]
- 14.Mikata R, Yokosuka O, Fukai K, Imazeki F, Arai M, Tada M, Kurihara T, Zhang K, Kanda T, Saisho H. Analysis of genes upregulated by the demethylating agent 5‐aza‐2’‐deoxycytidine in gastric cancer cell lines. Int J Cancer. 2006;119:1616–1622. doi: 10.1002/ijc.21968. [DOI] [PubMed] [Google Scholar]
- 15.Guo CC, Zhang XL, Yang B, Geng J, Peng B, Zheng JH. Decreased expression of Dkk1 and Dkk3 in human clear cell renal cell carcinoma. Mol Med Rep. 2014;9:2367–73. doi: 10.3892/mmr.2014.2077. [DOI] [PubMed] [Google Scholar]
- 16.Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET. Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate. 2008;68:1396–1404. doi: 10.1002/pros.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;67:2517–2525. doi: 10.1158/0008-5472.CAN-06-3369. [DOI] [PubMed] [Google Scholar]
- 18.Dong LL, Qu LY, Chu LY, Zhang XY, Liu YY. Serum level of DKK-1 and its prognostic potential in non–small cell lung cancer. Diagn Pathol. 2014;9:52. doi: 10.1186/1746-1596-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Sheng S, Huang G, Yu B, Qin WX. Clinical significance and prognostic value of serum Dickkopf-1 concentrations in patients with lung cancer. Clin Chem. 2009;55:1656–1664. doi: 10.1373/clinchem.2009.125641. [DOI] [PubMed] [Google Scholar]
- 20.Voorzanger-Rousselot N, Goehrig D, Journe F, Doriath V, Body JJ, Clezardin P, Garnero P. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer. 2007;97:964–970. doi: 10.1038/sj.bjc.6603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedi P, Bafico A, Soria AN, Burgess WH, Miki T, Bottaro DP, Kraus MH, Aaronson SA. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274:19465–19472. doi: 10.1074/jbc.274.27.19465. [DOI] [PubMed] [Google Scholar]
- 22.Menezes ME, Devine DJ, Shevde LA, Samant RS. Dickkopf1: a tumor suppressor or metastasis promoter? Int J Cancer. 2012;130:1477–1483. doi: 10.1002/ijc.26449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 24.Koppen A, Ait-Aissa R, Hopman S, Koster J, Haneveld F, Versteeg R, Valentijn LJ. Dickkopf-1 is down-regulated by MYCN and inhibits neuroblastoma cell proliferation. Cancer Lett. 2007;256:218–228. doi: 10.1016/j.canlet.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Kwack MH, Hwang SY, Jang IS, Im SU, Kim JO, Kim MK, Kim JC, Sung YK. Analysis of cellular changes resulting from forced expression of Dickkopf-1 in hepatocellular carcinoma cells. Cancer Res Treat. 2007;39:30–36. doi: 10.4143/crt.2007.39.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008;269:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]


