Abstract
Among the methods of the peripheral nerve repair, artificial conduit bridging surgery is superior to epineurium and perineurium neurorrhaphy because of supplying enough space for nerve regeneration. Artificial conduit provides important microenvironment for peripheral nerve regeneration, especially for nerve amplification effect. Amplification phenomenon has been demonstrated in many studies using artificial conduit. When a finer nerve is used as a donor to connect to a distal nerve after injury, the donor nerve regenerates more lateral buds than its own fibers, which grow into distal endoneurial tubes and finally dominate the target organs. In this study, we used artificial conduit to investigate the amplification phenomenon in rats treated with Lumbricus extract as adjuvant treatment. The rats were divided into three groups at random. In the surgical groups, the proximal common peroneal nerve was used as a donor nerve to connect the distal tibial nerve. Rats in the normal group were not performed surgery. Postoperatively, the treatment group was administered Lumbricus extract as adjuvant treatment, while the model group and normal group were not given treatment. The results showed that the nerve conduction velocity, the morphometric measurements, the histological analysis and the amplification ratio in the treatment group were better than in the model group.
Keywords: Traditional Chinese medicine, lumbricus extract, amplification effect, lateral bud, peripheral nerve regeneration, nerve conduction velocity
Introduction
The application of artificial conduit for peripheral nerve repair is a big step forward in the exploration of the treatment of peripheral nerve injury, not only conducive to the regeneration of peripheral nerve, but also contributing to the generation of the amplification effect during peripheral nerve regeneration. According to the previous study, after a nerve was transected, single axon from the proximal stump of damaged nerve can grow several lateral buds during nerve regeneration, and the lateral buds grown by the proximal axons were significantly more than the distal endoneurial tubes of the damaged nerve [1], this phenomenon is known as “amplification phenomenon”. In the case of application of artificial conduit, using a finer nerve as a donor to connect the distal stump of damaged nerve can promote generation of amplification phenomenon during nerve regeneration [2]. The microenvironment for nerve regeneration supplied by artificial conduit can promote regeneration of nerve fibers [3], and the distal endoneurial tubes whose number is greater than that of the proximal donor axons can supply enough space for the newborn lateral buds to grow into [4]. However, the new nerve fibers grown by amplification are not enough for recovery of neurological structure and function because of limitations of cell metabolism, there is not an adequate supply of necessary constituents for too many lateral buds to grow from a single axon [4]. In order to better play the role of conduit to promote the functional recovery, we used Lumbricus Extract a preparation prepared from a Traditional Chinese Medicine called Lumbricus [5] as an adjuvant treatment after surgical operation, investigating the promoting effect on amplification effect via inducing more lateral buds growth from proximal axons. Previous study has demonstrated that Lumbricus extract could effectively promote peripheral nerve regeneration in nerve clamping injury model, obviously promoting the recovery of nerve function [6]. In this study, we further investigated the role of Lumbricus extract in the peripheral nerve amplification effect during regeneration.
Materials and methods
Ethics statement
The experimental procedures were carried out in accordance with the Chinese guidelines for the care and use of laboratory animals. The use of the animals was approved by the ethics committee and Experimental Animal Center of Peking University People’s Hospital. All animal protocols were approved by the ethics committee of Peking University People’s Hospital (Permit Number: 2011-16).
Drug preparation
Lumbricus was produced in Guangdong Province in China, and was purchased from Beijing, China. 1100 grams of dried lumbricus were boiled according to a traditional decocting method [7], as follows. They were first decocted with 11000 milliliter of distilled water for 2 h, the supernatant was decanted and saved, then 11000 milliliter of new water was used for the second decoction for 1 h, the two supernatant prepared were then mixed, following which the mixed solution was concentrated into 1 g/ml (equivalent to dry weight of crude drug) by high-pressure distillation and stored at 4°C condition for use.
Animals
Male Sprague-Dawley rats weighing 200-250 g were maintained under specific pathogen-free laboratory conditions on a 12 h light/dark cycle with free access to pellet food and water. The rats were separated into three groups at random (six animals in each group). Every effort was made to minimize animal suffering and reduce the number of animals used, according to the Chinese guidelines for the care and use of laboratory animals.
Materials
The materials used include: chitin biological absorbable tubes (ether-free chitin biological tubes; length, 8 mm; wall thickness, 0.2 mm; inner diameter, 1.5 mm); a Synergy electrophysiological instrument; a Leica tissue embedding machine; a Leica tissue microtome; a Leica image collection and analysis system; and a Leica dissecting microscope.
Surgical procedures
Rats in the surgical groups were anesthetized with sodium pentobarbital (30 mg/kg i.p.). After anesthesia, the right limbs of the animals were given sterile processing. Then the sciatic nerve and its two branches (the tibial nerve and the common peroneal nerve) were exposed. The tibial nerve and the common peroneal nerve were transected at 5 mm distal to the bifurcation. The distal stump of the common peroneal nerve and the proximal stump of the tibial nerve were ligated and stitched to the adjacent muscle. The proximal stump of the common peroneal nerve served as the donor nerve and was connected to the distal stump of the tibial nerve. Chitin biological absorbable conduits were used to create a necessary small gap, the conduits consist of a polysaccharide shell that showed satisfactory biocompatibility and degradation characteristics. The gap between the two nerve segments was kept at 2 mm. Subsequently, 4-0 nylon sutures were used to suture the muscle incision and close the wound (Figure 1A and 1B). The unprocessed tibial nerves served as the normal group. The nerves in all groups were removed for examination 12 weeks postoperative.
Figure 1.
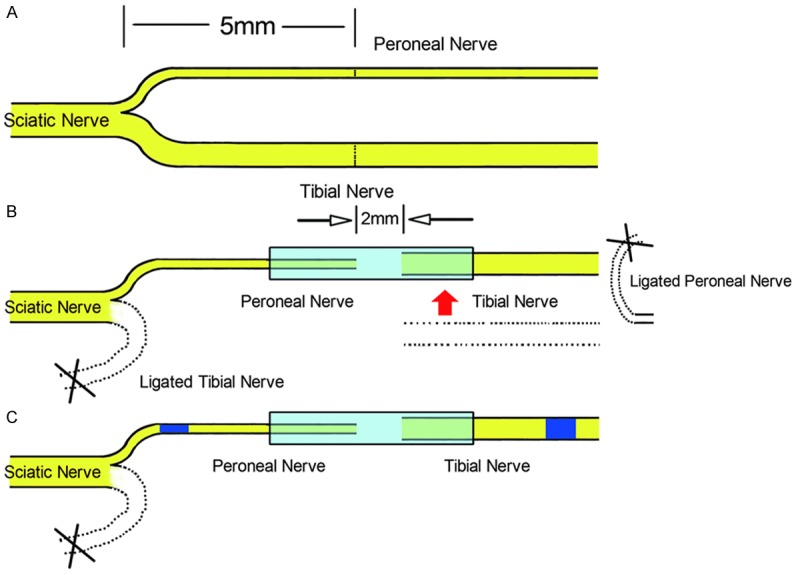
Surgical procedures. A: The right sciatic nerve and its two main branches were exposed, the common peroneal nerve and the tibial nerve were transected at 5 mm distal to the bifurcation. B: The proximal tibial nerve and the distal common peroneal nerve were ligated. C: Then the proximal common peroneal nerve served as a donor nerve and was bridged to the distal tibial nerve via an artificial conduit.
Treatment method
From the next day of the surgery, each rat in the treatment group was treated with 2 ml Lumbricus extract liquid (1 g/ml) by oral gavage once daily, each rat in the normal group and the model group was treated with 2 ml 0.9% NaCl in the same manner at the same time every day. Oral gavage was performed daily until the end of the 12th week.
General observations
Postoperatively, the general condition of the animals was regularly observed, including the activities of the operated limbs, the degree of wound healing, ulcer formation and rotten situation on feet which were caused by self-biting toes.
Walking track analysis
Walking track analysis was done in a confined walkway for the animals in all groups 12 weeks postoperative. The confined walkway had a dark shelter at the end of a corridor (10 × 60 cm). White paper with the appropriate dimensions was placed on the bottom of the track. The rat’s hind limbs were dipped into black ink before the animal was placed at the entrance of the walkway. Foot prints from both sides appeared immediately on the paper after the rat walked down the track. Prints for measurement were chosen at the time of walking, based on clarity and completeness at a point when the rat was walking.
Paired footprint parameters for print length (distance from heel to toe, PL), toe spread (distance from first to fifth toe, TS), and intermediary toe spread (distance from second to fourth toe, IT) were recorded for the left normal control foot (NPL, NTS, NIT) and the corresponding right experimental foot (EPL, ETS, EIT) for each rat (Figure 2).
Figure 2.
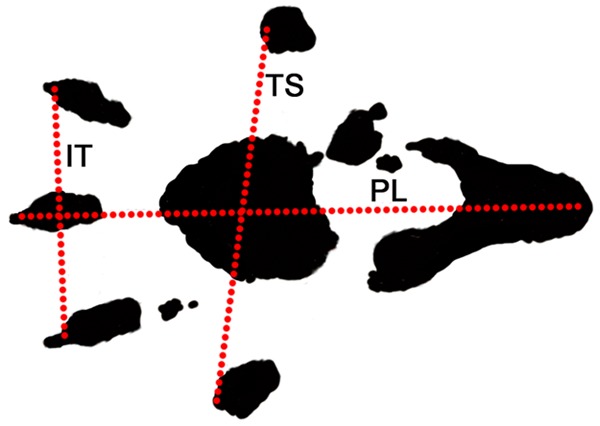
Footprint for walking track analysis. Walking track analysis was done in a confined walkway for the animals in all groups 12 weeks postoperative. White paper was placed on the bottom of the track. Foot prints from both hind limbs appeared immediately on the paper after the rat walked down the track. Paired footprint parameters were recorded. PL: print length; TS: toe spread; IT: intermediary toe spread.
Tibial function index (TFI), being used to assess tibial nerve function, was calculated according to the Bain-Mackinnon-Hunter formula [8]:
TFI = -37.2 ([EPL-NPL]/NPL) + 104.4 ([ETS-NTS]/NTS) + 45.6 ([EIT-NIT]/NIT) -8.8
Electrophysiological assessment
Electrophysiological assessment was conducted 12 weeks post-surgery prior to sacrifice of the animals. Rats were first anesthetized with sodium pentobarbital (30 mg/kg i.p.), then the repaired tibial nerve was exposed, and stimulating bipolar electrodes were placed proximal and distal to the repair site in each group. The recording electrode was placed in the gastrocnemius muscle, while the ground electrode was placed in subcutaneous tissue between the stimulating and recording electrodes. Rectangular pulses (duration 0.1 ms, 0.9 mA, 10 Hz) were used to stimulate the nerves. The Nerve Conduction Velocity (NCV; m/s) was obtained semiautomatically by dividing the distance between the two stimulating sites by the difference in the onset latency.
Histological study
The nerve (including the proximal common peroneal nerve, artificial conduit and distal tibial nerve) was removed from each rat in each group. Tissues were then harvested and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 h at 4°C. The nerves were then rinsed twice in water for 12 h. Two nerve segments were cut, one 5 mm proximal and one 5 mm distal to the chitin conduit (Figure 1C, blue segments). After this step, each sample was stained in 1% osmium tetroxide for 12 h, then dehydrated through a graded series of ethanols and the specimens were then immersed in xylene, embedded in paraffin, then each nerve segment was sliced into 5 μm cross-sections with a Leica tissue microtome. Images from the proximal and distal segments were acquired under a microscope, from which the total number of myelinated axons in each segment was counted by Image J software. After the numbers of proximal and distal myelinated axons were obtained, the amplification ratio was calculated by dividing the number of proximal axons by the number of distal axons, as follows:
Amplification ratio = Number of distal axons/Number of proximal axons
Morphometric measurements were also performed by Image J software. The shortest lengths of the inner and outer margins of the myelin sheath were measured to determine the axon diameter and fiber diameter [9]. After obtaining the axon and fiber diameter, myelin thickness and G-ratio were calculated. Subsequently, a set of five non-overlapping fields was used to evaluate fiber diameter distribution by random sampling from each distal nerve section. The evaluated area covered approximately three-fifth of the total area of one nerve section.
Statistical analysis
The results were expressed as the mean ± SD. The TFI, the NCV, and the morphometric measurements (e.g. the fiber diameter, axon diameter, myelin thickness and G-ratio) in all groups were evaluated with one-way analysis of variance. The amplification ratios in the two surgical groups were evaluated with T-test. A probability of P < 0.05 was considered significant for all statistical comparisons.
Results
General observations
Animals in the two surgical groups were found to have lameness in the operated limbs postoperative, sagging ankles and awkward movements could be seen. Three rats appeared swollen and ulcerated in the operated limbs 1 week after operation, and 3 weeks later, the number of rats with ulcers caused by toe self-biting increased. The motor function of the operated limbs began to recover gradually from the 4th week postoperative, and at the end of the 12th week, most ulcers eventually healed. However, four rats in the surgical groups (two in the model group and two in the treatment group) still had ulcers not healed at the end of the 12th week post-surgery.
Tibial function index (TFI)
The results of Walking Track Analysis demonstrated that TFI in the normal group, model group, and treatment group was -10.01 ± 14.50, -78.15 ± 3.90, and -59.62 ± 24.79, respectively. The TFI in the normal group was significantly higher than that in the model group and the treatment group (P < 0.05). There was no obvious difference of values between the model group and the treatment group (P > 0.05) (Figure 3).
Figure 3.
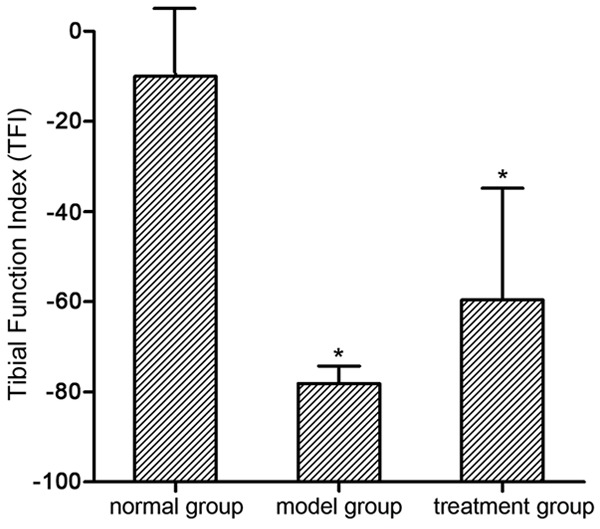
Tibial function index (TFI). Tibial function index (TFI) was measured via walking track analysis in all groups 12 weeks postoperative. Y-axis represents the tibial function index which assessed the tibial nerve function recovery. *P < 0.05 versus normal group; ▲P < 0.05 versus model group.
Nerve conduction velocity (NCV)
According to the electrophysiological assessment conducted 12 weeks post-surgery, NCV in the normal group, model group, and treatment group was 51.38 ± 2.87 m/s, 14.71 ± 3.54 m/s, and 25.93 ± 7.35 m/s, respectively. NCV in the normal group was obviously better than that in the model group and treatment group (P < 0.05). The value in the treatment group was obviously better than that in the model group (P < 0.05) (Figure 4).
Figure 4.
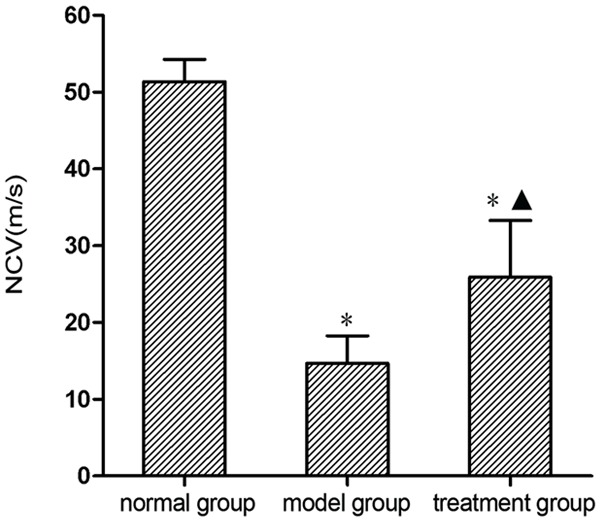
Nerve conduction velocity (NCV). Electrophysiological assessment was conducted to measure nerve conduction velocity in all groups 12 weeks postoperative. *P < 0.05 versus normal group; ▲P < 0.05 versus model group.
Histological study results
From the results of osmium tetroxide staining made at the end of 12th week post-surgery, we could see that: In the proximal and distal nerve segments in the normal group (Figure 5A and 5B), myelin sheathes distributed uniformly. The shape and size of the myelin sheathes looked regular. In the distal segments in the model group and treatment group (Figure 5C and 5D), myelin sheathes distribution looked uneven, with a low density. The shape and size of myelin sheathes looked generally small and irregular.
Figure 5.
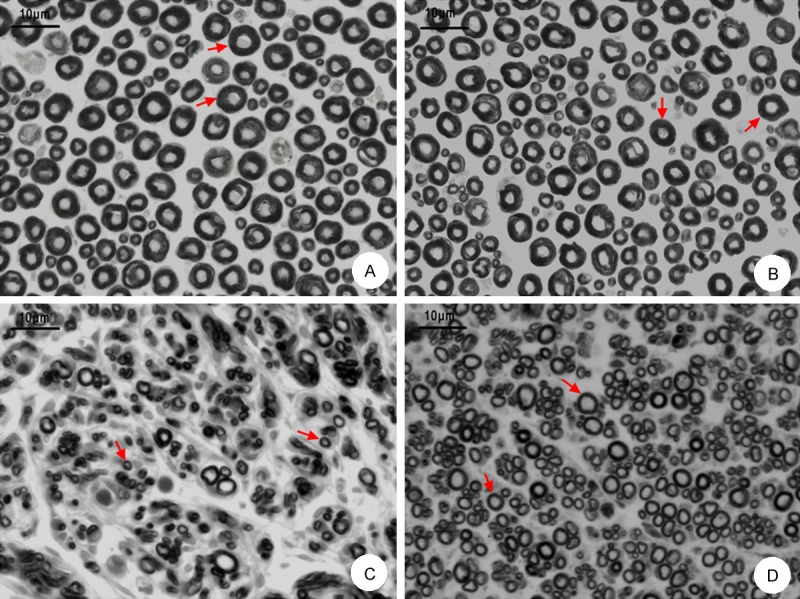
Histological study results in each group. A: Morphological changes in the proximal nerve segment by osmium tetroxide staining (×400); B: Morphological changes in the distal segment in the normal group (×400); C: Morphological changes in the distal segment in the model group (×400); D: Morphological changes in the distal segment in the treatment group (×400). Arrows indicate the myelin sheathes, scale bar indicates 10 μm.
From the site that was 5 mm distal to the artificial conduits, the distal nerve segments in each group were chosen to be measured and analyzed. As shown in Table 1, fiber diameter in the normal group was significantly larger than those in the model group and treatment group (P < 0.05); the value in the treatment group was significantly larger than that in the model group (P < 0.05). Axon diameter in the normal group was significantly larger than those in the model group and treatment group (P < 0.05), and the value in the treatment group was significantly larger than that in the model group (P < 0.05). Myelin thickness in the model group and the treatment group was significantly lower than that in the normal group (P < 0.05); the values in the two surgical groups were not significantly different (P > 0.05).
Table 1.
Morphometric measurements in different groups 12 weeks post-surgery
| Group | |||
|---|---|---|---|
|
| |||
| Normal group | Control group | Treatment group | |
| Number of slices per segment | 5 | 5 | 5 |
| Number of axons per slice | 100 | 100 | 100 |
| Fiber diameter (µm) | 8.15 ± 1.81 | 3.80 ± 0.83* | 5.45 ± 0.89*,▲ |
| Axon diameter (µm) | 3.70 ± 0.80 | 1.74 ± 0.65* | 3.00 ± 0.56*,▲ |
| G-ratio (Axon diameter/Fiber diameter) | 0.46 ± 0.09 | 0.46 ± 0.16 | 0.55 ± 0.06*,▲ |
| Myelin thickness (µm) | 2.25 ± 0.73 | 1.10 ± 0.39* | 1.23 ± 0.30* |
P < 0.05 versus normal group;
P < 0.05 versus model group.
Figure 6 presents the distribution histograms of fiber diameter as measured in the normal group and surgical groups. The histograms compiled from the distal tibial nerves in three groups were generally unimodal. Fiber diameters in normal group, model group and treatment group had main peaks at 9-10 µm, 3-4 µm and 5-6 µm, respectively. The proportion of nerve fibers shifted to larger nerve diameters in normal group and treatment group was larger that in the model group.
Figure 6.
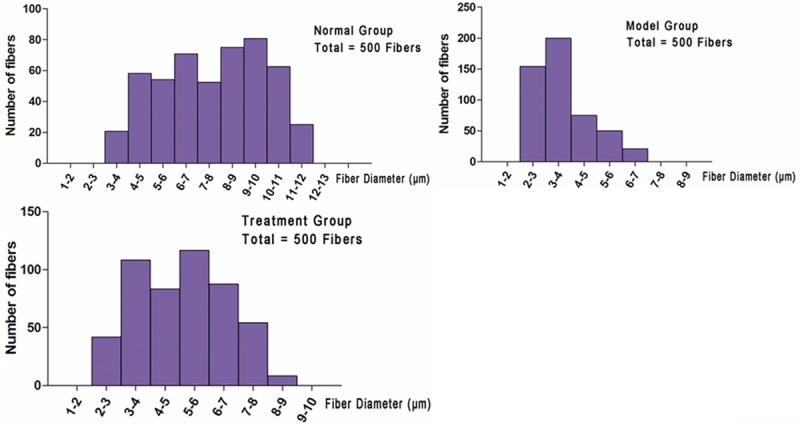
Fiber diameter distribution. A: Histogram of fiber diameter distribution from normal group; B: Histogram of fiber diameter distribution from model group; C: Histogram of fiber diameter distribution from treatment group.
Amplification ratio for nerve regeneration
As shown in Table 2, the amplification ratio for nerve regeneration in the model group was 1.47 ± 0.19, which was obviously less than that in the treatment group (1.96 ± 0.09; P < 0.05).
Table 2.
The number of myelinated fibers and amplification ratio in each group
| Group | Number of slices per segment | Number of proximal fibers | Number of distal fibers | Amplification ratio (distal fibers/proximal fibers) |
|---|---|---|---|---|
| Normal group | 5 | 1972.70 ± 141.66 | 5043.69 ± 291.06 | ------ |
| Control group | 5 | 2052.65 ± 199.60 | 2981.47 ± 367.28 | 1.46 ± 0.17 |
| Treatment group | 5 | 1968.79 ± 126.42 | 3803.21 ± 264.17 | 1.93 ± 0.12* |
P < 0.05 versus model group.
Discussion
Previous studies have demonstrated that there was amplification phenomenon during peripheral nerve regeneration. When a finer nerve was used as a donor to repair the distal damaged nerve, each proximal nerve axon could grow several lateral buds, these lateral buds then grew into the distal myelin sheath tubes and finally reach and dominate the distal target organs [2]. This phenomenon is called amplification phenomenon.
It should be noted that artificial conduit played an important role in amplification effect of peripheral nerve. Artificial conduit not only promotes nerve regeneration and recovery of nerve conduction velocity [10], but also provides a suitable space for nerve amplification effect during regeneration [4]. Artificial conduit can provide a small gap that ensures the generation of the amplification phenomenon, which is superior to suture in situ (e.g. epineurium suture and perineurium suture). The small gap is helpful for the newborn lateral buds to grow toward the distal stump and finally reach the target organ, and the suitable gap between the two nerve segments was 2 mm [11]. Conduit diameter should match with the diameter of repaired nerve, and it can’t be much bigger than that of nerve so as to avoid regenerated fibers grow outside of conduit. In the nerve injury model sutured in situ, there was no gap between the two segments. It is easy to form scar tissue between the two nerve ends, since there is no shielding and protection of conduit. So when the newborn sprouts are blocked by scar tissue, they cannot grow into the distal endoneurial tubes and gradually degenerate. Therefore, when axons establish terminal structures, the number of lateral buds is reduced to the usual single trunk [12], equivalent to the fiber quantity of the original proximal nerve stump. However, when sufficient space between the two nerve segments is supplied by the artificial conduit, simultaneously, the number of distal nerve axons (or endoneurium tubes) is more than that of donor axons, then there was enough space supplied by the distal endoneurium tubes for the newborn buds to grow into, thus the nerve amplification effect arises subsequently. This study verified the nerve amplification effect during peripheral nerve regeneration.
The amplification phenomenon inspired us with a new way to repair nerve defect at a lower cost (using a finer nerve as a donor taken from other parts of the body). On the basis of small gap bridging operation, we use Lumbricus extract as an adjuvant therapy that was expected to be able to promote the amplification effect of peripheral nerve.
Lumbricus contains a variety of active ingredients such as Highly Unsaturated Fatty Acids, Proteins, many Amino Acids, Lipids, Nucleotides, Enzymes, Trace Elements [13]. As multi-component complexes, Lumbricus extract was speculated to be more appropriate than any single factor in the repair of peripheral nerve defect, as it can supply many active ingredients. Furthermore, systemic treatment is consistent with the characteristics of traditional Chinese medicine treating the body as a whole, being more effective than local treatment. Local application of neurotrophins after the peripheral nerve sleeve bridging operations has been reported previous, but the efficacy is limited. Sun H [14] implanted microspheres containing NGF into the neural tube before bridging the transected peripheral nerve, but found that there was no significant difference compared with the control group three months later with respect to nerve conduction velocity, muscle tension and muscle wet weight. Srinivas Madduri [15] advocated the inclusion of neurotrophic factors (NTFs) in the nerve conduit wall, but the effect was not good. Local applications of drugs, not treating the organism as a whole, do not achieve the expected therapeutic effectiveness. Lumbricus extract, as a preparation of traditional Chinese Medicine, may provide a more favorable growth microenvironment for nerve regeneration by inducing various biologically active factors required by nerve regeneration.
Previous studies have shown that Lumbricus extract can improve the total number of regenerated myelinated nerve fibers in adult rats following nerve clamping injury [6], suggesting that Lumbricus extract has potential clinical therapeutic effect during peripheral nerve repair. In this study, the diameter of fiber and axon in the treatment group were larger than that in the model group (P < 0.05). Most importantly, the amplification ratio for nerve regeneration in the treatment group was obviously higher compared with the model group (P < 0.05), indicating that Lumbricus extract promoted recovery of nerve fiber structure, particularly, promoted the amplification effect by inducing each proximal nerve axon to grow more lateral buds. For the NCV, the value in the treatment group was obviously higher than that in the model group (P < 0.05), suggesting that Lumbricus extract promoted recovery of nerve conduction velocity. Regarding the TFI in each group, the values in the two surgical groups were lower than that in the normal group (P < 0.05). Notably, there was no obvious difference between the model group and the treatment group (P > 0.05), suggesting that Lumbricus extract did not promote recovery of neurological function in this experiment. However, due to the number of rats used for walking track analysis to calculate the TFI was very limited (two in the model group and three in the treatment group), excluding the rats with ulcers (couldn’t leave complete footprints), an increased sample size is required for subsequent experiments to evaluate the TFI in the treatment group.
The statistical results confirmed Lumbricus extract can improve the number of peripheral nerve fibers and promote the amplification effect, although it is not certain whether it can promote nerve functional recovery. Lumbricus extract may promote the amplification effect via multifactorial mechanism. The extract has various biological activities. It has mitogenic effect of insulin-like proteins [16], and also exhibit fibrinolytic and anticoagulative activities [17] which was an important effect in many injury conditions. Moreover, Lumbricus contains a kind of proteins with adhesion capabilities (the same to the adhesion of immunoglobulin-like structures) [18]. The immunoglobulin structures in lumbricus extract has been observed, and they can reacted with anti-IgA, anti-IgG and anti-IgM [19]. Furthermore, lumbricus extract can induce synthesis of epidermal growth factor (EGF) and fibroblast growth factor (FGF) during the wound healing on mice skin [20]. Lumbricus extract was speculated to play beneficial effects on peripheral nerve amplification effect via various effects mentioned above. In addition, as a part of Modified Formula Radix Hedysari (MFRH), Lumbricus may play a certain role in peripheral nerve repair mechanism. For example, it may promote nerve regeneration by promoting the secretion of bFGF, NGF and Trk [21], and promotes the proliferation and differentiation of Schwann cells [22]. All of the potential mechanisms mentioned above are required to further explore in our subsequent experiments.
Nerve amplification effect not only occurs in rodents, but also occurs in primates. A previous study on peripheral nerve injury of rhesus monkey, using proximal ulnar nerve as a donor to be simultaneously connected the distal impaired ulnar nerve and musculocutaneous nerve via artificial conduit used to make a mall gap bridging model, has shown that there was compensation amplification phenomenon [2]. Based on this phenomenon and the role of Lumbricus extract in amplification effect, a new idea is generated: regarding the peripheral nerve injury model of rhesus monkey, when Lumbricus extract is used as an adjuvant therapy after surgery, will the amplification effect be better and more significant? This needs to be further explored, expected to provide a more economical method for the clinical repair of peripheral nerve injury at a lower cost.
Conclusions
As a multi-component preparation, Lumbricus extract can promote proximal axons to grow more lateral buds, promote nerve amplification effect and nerve conduction velocity, but whether it can promote nerve function need to be further confirmed.
Acknowledgements
This study was supported by National Natural Science Fund (31171150, 31271284, 81171146, 30971526, 31100860, 31040043, 31371210), Beijing City Science & Technology New Star Classification (A-2008-10), Educational Ministry New Century Excellent Talents Support Project (BMU 20110270), and Chinese National Ministry of Science and Technology 973 Project Planning (No. 2014CB542200).
Disclosure of conflict of interest
None.
References
- 1.Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp Neurol. 1996;137:301–308. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Kou Y, Yin X, Wang Y, Zhang H, Jiang B. The experimental research of nerve fibers compensation amplification innervation of ulnar nerve and musculocutaneous nerve in rhesus monkeys. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:39–43. doi: 10.3109/10731199.2010.494583. [DOI] [PubMed] [Google Scholar]
- 3.Zhao FQ, Zhang PX, Jiang BG. Magnifying effect of conduit bridging in number of nerve fibers of broken peripheral nerves: experiment with rats. Chinese Medical Journal. 2007;87:1043–1047. [PubMed] [Google Scholar]
- 4.Jiang BG, Yin XF, Zhang DY, Fu ZG, Zhang HB. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 2007;58:12–20. doi: 10.1159/000102161. [DOI] [PubMed] [Google Scholar]
- 5.Mu HO, Su XG. Pharmacological studies of Lumbricus. China Pharmaceuticals. 2007;16:61–62. [Google Scholar]
- 6.Wei S, Yin X, Kou Y, Jiang B. Lumbricus extract promotes the regeneration of injured peripheral nerve in rats. J Ethnopharmacol. 2009;123:51–4. doi: 10.1016/j.jep.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 7.An QR. Traditional Decocting Method of Chinese Medicine. China Foreign Medical Treatment. 2009;12:110. [Google Scholar]
- 8.Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods. 2000;96:89–96. doi: 10.1016/s0165-0270(99)00174-0. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda M, Oka Y. The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav. 2012;2:382–390. doi: 10.1002/brb3.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Yin X, Kou Y, Wang Y, Zhang H, Jiang B. The electrophysiology analysis of biological conduit sleeve bridging rhesus monkey median nerve injury with small gap. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:457–463. doi: 10.1080/10731190802375802. [DOI] [PubMed] [Google Scholar]
- 11.Jiang B, Zhang P, Zhang D, Fu Z, Yin X, Zhang H. Study on small gap sleeve bridging peripheral nerve injury. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:55–74. doi: 10.1080/10731190500430149. [DOI] [PubMed] [Google Scholar]
- 12.Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhu WM. 2013, The studies of active ingredient and pharmacological of Lumbricus. Strait Pharmaceutical Journal. 1989;25:25–26. [Google Scholar]
- 14.Sun H, Xu F, Guo D, Yu H. Preparation and evaluation of NGF-microsphere conduits for regeneration of defective nerves. Neurol Res. 2012;34:491–497. doi: 10.1179/1743132812Y.0000000037. [DOI] [PubMed] [Google Scholar]
- 15.Madduri S, Gander B. Growth factor delivery systems and repair strategies for damaged peripheral nerves. J Control Release. 2012;161:274–282. doi: 10.1016/j.jconrel.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Hrzenjak T, Hrzenjak M, Kasuba V, Efenberger-Marinculić P, Levanat S. A new source of biologically active compounds-earthworm tissue (Eisenia foetida, Lumbricus rubellus) Comp Biochem Physiol Comp Physiol. 1992;102:441–447. doi: 10.1016/0300-9629(92)90191-r. [DOI] [PubMed] [Google Scholar]
- 17.Hrzenjak T, Popovic M, Bozic T, Grdisa M, Kobrehel D, Tiska-Rudman L. Fibrinolytic and anticoagulative activities from the earthworm Eisenia foetida. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:825–832. doi: 10.1016/s0305-0491(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 18.Rejnek J, Tuckova L, Zikan J, Tomana M. The interaction of a protein from the coelomic fluid of earthworms with staphylococcal protein A. Dev Comp Immunol. 1991;15:269–277. doi: 10.1016/0145-305x(91)90020-y. [DOI] [PubMed] [Google Scholar]
- 19.Popovic M, Hrzenjak T, Grdisa M, Vukovic S. Adhesins of immunoglobulinelike superfamily from earthworm Eisenia foetida. Gen Pharmacol. 1998;30:795–800. doi: 10.1016/s0306-3623(97)00014-1. [DOI] [PubMed] [Google Scholar]
- 20.Grdisa M, Popovie M, Hrzenjak T. Stimulation of growth factor synthesis in skin wounds using tissue extract (G-90) from the earthworm Eissenia foetida. Cell Biochem Funct. 2004;22:373–378. doi: 10.1002/cbf.1121. [DOI] [PubMed] [Google Scholar]
- 21.Yang DM, A LY, Wei GR, Dong JQ, Jiang BG. The effects of modified formula Radix Hedysari on the expression of bFGF, Trk and NGF in injured sciatic nerve. Clinical Rehabilitation. 2006;22:308–309. [Google Scholar]
- 22.Jiang BG, Jiang Y, Li PJ, Ren XF, Zhang DY, Zhang HB. Extract of the Hedysarum Polybotrys Hand-Ma22 promote Schwann cell differentiation in vitro. China Journal of Microsurgery. 2002;25:38–40. [Google Scholar]


