Abstract
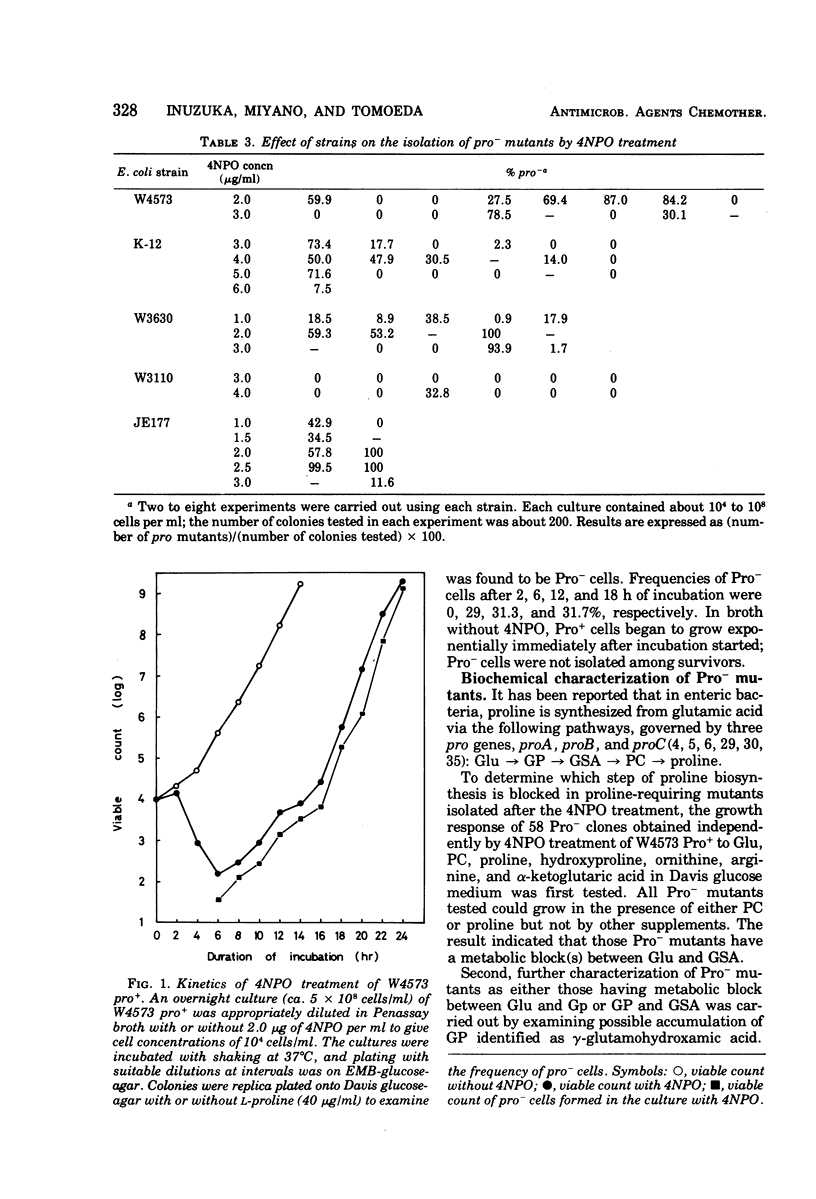
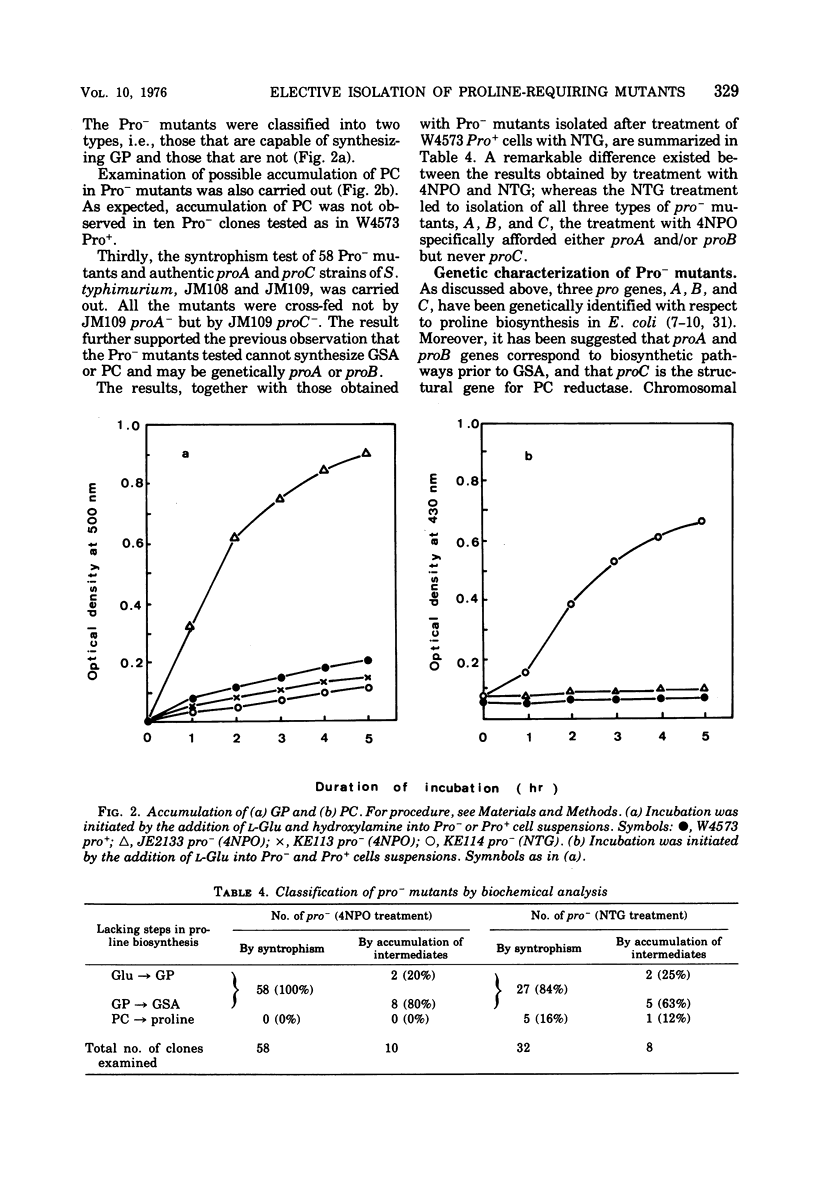
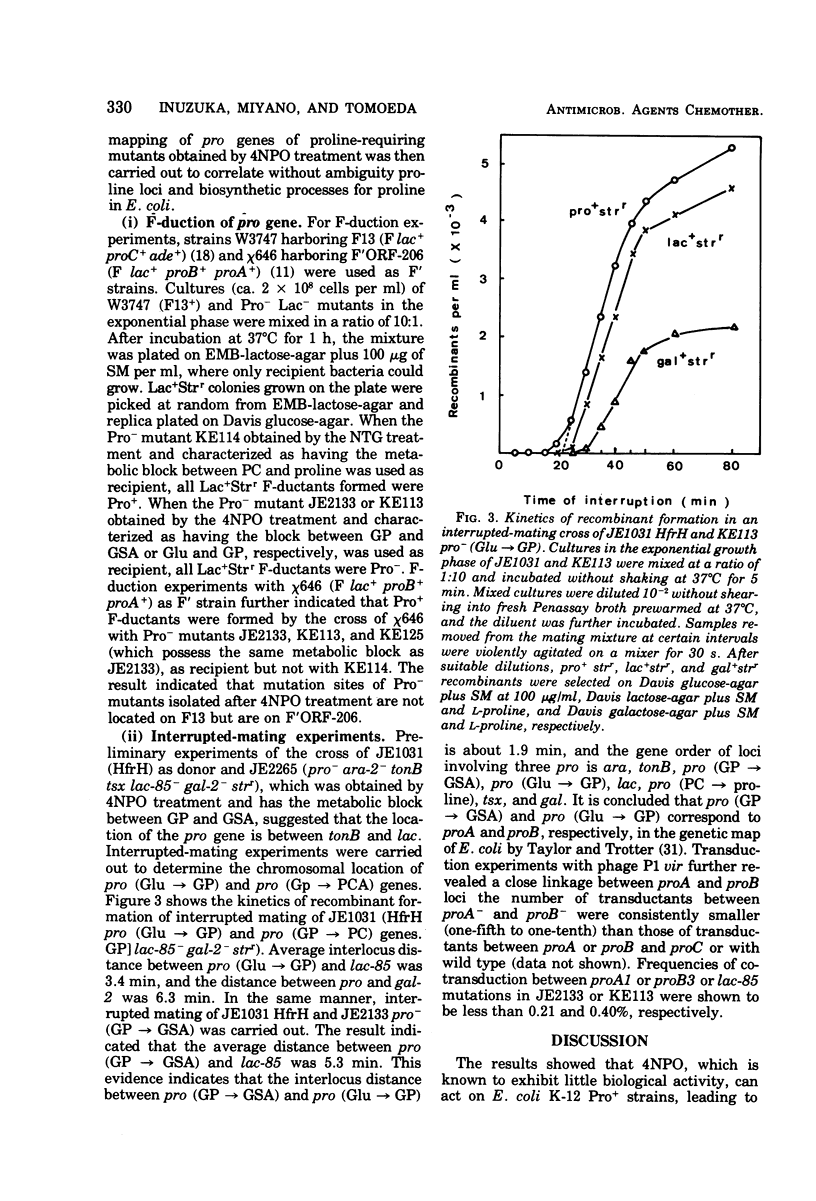
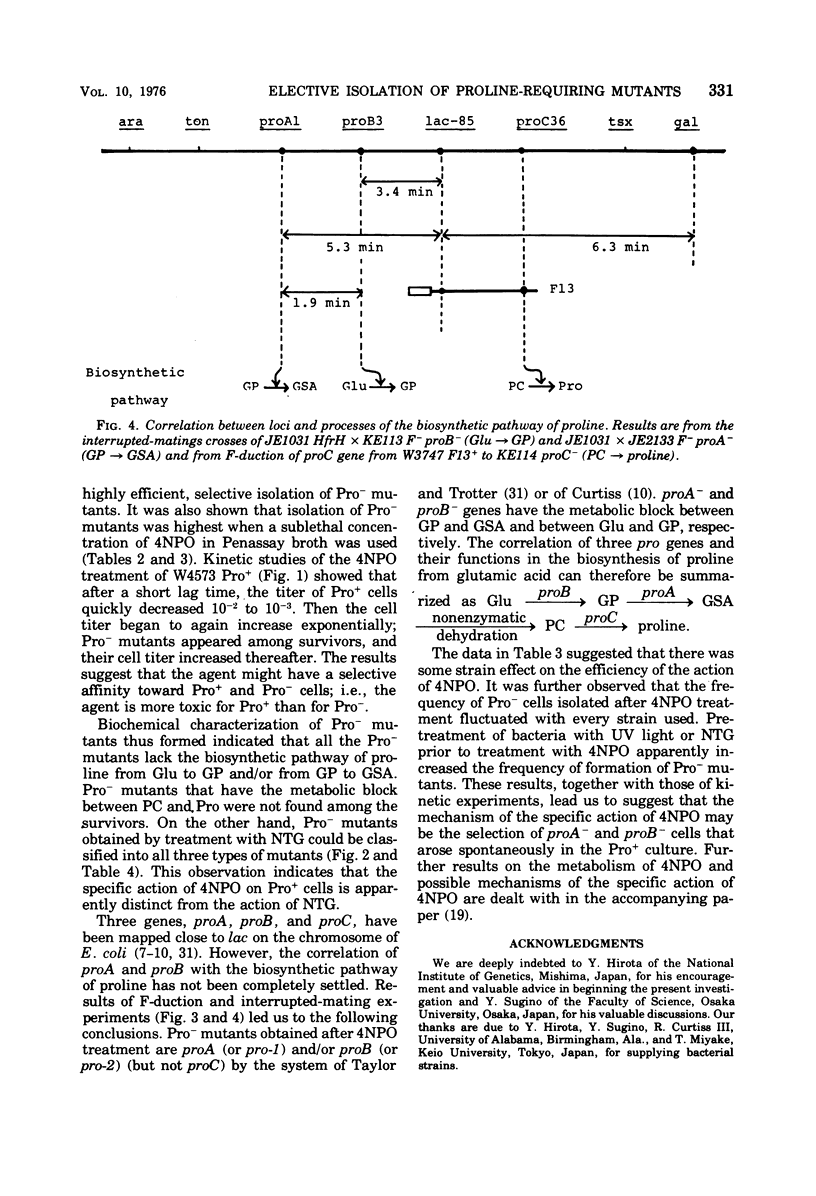
A specific action of 4-nitropyridine 1-oxide on Escherichia coli K-12 Pro+ strains leading to highly efficient, selective isolation of Pro− mutants is described. Incubation of Pro+ cells with a sublethal concentration of 4-nitropyridine 1-oxide in Penassay broth gave Pro− mutants, which lacked either the biosynthetic pathway of proline from glutamic acid to glutamyl γ-phosphate (proB−) or the pathway from glutamyl γ-phosphate to glutamic γ-semialdehyde (proA−) or both. Pro− mutants, which have the metabolic block between Δ1 pyrroline-5-carboxylate (the cyclized dehydration product of glutamic γ-semialdehyde) and proline (proC−) were not found among survivors. Treatment of Pro+ cells with N-methyl-N′-nitro-N-nitrosoguanidine led to isolation of all three types of Pro− mutants, suggesting that the action of 4-nitropyridine 1-oxide on Pro+ cells is apparently distinct from the action of N-methyl-N′-nitro-N-nitrosoguanidine. F-duction and interrupted mating experiments led to determination of the correlation between proline loci and the biosynthetic pathway of proline from glutamic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki M., Koga C., Kawazoe Y. Carcinogenicity of 4-nitroquinoline 1-oxide analogs: pyridine series. Gan. 1971 Aug;62(4):325–327. [PubMed] [Google Scholar]
- Baich A., Pierson D. J. Control of proline synthesis in Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):397–404. doi: 10.1016/0304-4165(65)90345-4. [DOI] [PubMed] [Google Scholar]
- Baich A. Proline synthesis in Escherichia coli. A proline-inhibitable glutamic acid kinase. Biochim Biophys Acta. 1969 Dec 30;192(3):462–467. doi: 10.1016/0304-4165(69)90395-x. [DOI] [PubMed] [Google Scholar]
- Baich A. The biosynthesis of proline in Escherichia coli: phosphate-dependent glutamate -semialdehyde dehydrogenase (NADP), the second enzyme in the pathway. Biochim Biophys Acta. 1971 Jul 20;244(1):129–134. doi: 10.1016/0304-4165(71)90129-2. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Rossi J. J. Proline excretion and indirect suppression in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1974 Jun;118(3):928–934. doi: 10.1128/jb.118.3.928-934.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broda P., Collins J. F. Gross map distances and Hfr transfer times in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):747–752. doi: 10.1128/jb.117.2.747-752.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condamine H. Mutants des voies de biosynthèse et de dégradation de la proline chez E. coli K 12. Ann Inst Pasteur (Paris) 1971 Jan;120(1):9–22. [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSOWICZ N., WAINFAN E., BOREK E., WAELSCH H. The enzymatic formation of hydroxamic acids from glutamine and asparagine. J Biol Chem. 1950 Nov;187(1):111–125. [PubMed] [Google Scholar]
- Hirota Y., Inuzuka M., Tomoeda M. Elective selection of proline-requiring mutants. J Bacteriol. 1966 Jun;91(6):2392–2392. doi: 10.1128/jb.91.6.2392-.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka M., Toyama H., Miyano H., Tomoeda M. Specific action of 4-nitropyridine 1-oxide on Escherichia coli K-12 Pro+ strains leading to the isolation of proline-requiring mutants: mechanism of action of 4-nitropyridine 1-oxide. Antimicrob Agents Chemother. 1976 Aug;10(2):333–343. doi: 10.1128/aac.10.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Miyake T, Demerec M. Proline Mutants of Salmonella Typhimurium. Genetics. 1960 Jun;45(6):755–762. doi: 10.1093/genetics/45.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAHARA W., FUKUOKA F., SUGIMURA T. Carcinogenic action of 4-nitroquinoline-N-oxide. Gan. 1957 Jun;48(2):129–137. [PubMed] [Google Scholar]
- Okabayashi T., Ide M., Yoshimoto A., Otsubo M. Mutagenic activity of 4-nitroquinoline 1-oxide and 4-hydroxyaminoquinoline 1-oxide on bacteria. Chem Pharm Bull (Tokyo) 1965 May;13(5):610–611. doi: 10.1248/cpb.13.610. [DOI] [PubMed] [Google Scholar]
- PEISACH J., STRECKER H. J. The interconversion of glutamic acid and proline. V. The reduction of delta 1-pyrroline-5-carboxylic acid to proline. J Biol Chem. 1962 Jul;237:2255–2260. [PubMed] [Google Scholar]
- STRECKER H. J. The interconversion of glutamic acid and proline. I. The formation of delta1-pyrroline-5-carboxylic acid from glutamic acid in Escherichia coli. J Biol Chem. 1957 Apr;225(2):825–834. [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoeda M., Inuzuka M., Kubo N., Nakamura S. Effective elimination of drug resistance and sex factors in Escherichia coli by sodium dodecyl sulfate. J Bacteriol. 1968 Mar;95(3):1078–1089. doi: 10.1128/jb.95.3.1078-1089.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristram H., Thurston C. F. Control of proline biosynthesis by proline and proline analogues. Nature. 1966 Oct 1;212(5057):74–75. doi: 10.1038/212074a0. [DOI] [PubMed] [Google Scholar]
- Yoshinaga F., Takeda Y., Okumura S. Glutamate kinase activity in Brevibacterium flavum: relationship between L-proline and L-glutamine biosynthesis. Biochem Biophys Res Commun. 1967 Apr 20;27(2):143–149. doi: 10.1016/s0006-291x(67)80053-6. [DOI] [PubMed] [Google Scholar]


