Abstract
Aim
Remote follow-up (FU) of implantable cardiac defibrillators (ICDs) allows for fewer in-office visits in combination with earlier detection of relevant findings. Its implementation requires investment and reorganization of care. Providers (physicians or hospitals) are unsure about the financial impact. The primary end-point of this randomized prospective multicentre health economic trial was the total FU-related cost for providers, comparing Home Monitoring facilitated FU (HM ON) to regular in-office FU (HM OFF) during the first 2 years after ICD implantation. Also the net financial impact on providers (taking national reimbursement into account) and costs from a healthcare payer perspective were evaluated.
Methods and results
A total of 312 patients with VVI- or DDD-ICD implants from 17 centres in six EU countries were randomised to HM ON or OFF, of which 303 were eligible for data analysis. For all contacts (in-office, calendar- or alert-triggered web-based review, discussions, calls) time-expenditure was tracked. Country-specific cost parameters were used to convert resource use into monetary values. Remote FU equipment itself was not included in the cost calculations. Given only two patients from Finland (one in each group) a monetary valuation analysis was not performed for Finland. Average age was 62.4 ± 13.1 years, 81% were male, 39% received a DDD system, and 51% had a prophylactic ICD. Resource use with HM ON was clearly different: less FU visits (3.79 ± 1.67 vs. 5.53 ± 2.32; P < 0.001) despite a small increase of unscheduled visits (0.95 ± 1.50 vs. 0.62 ± 1.25; P < 0.005), more non-office-based contacts (1.95 ± 3.29 vs. 1.01 ± 2.64; P < 0.001), more Internet sessions (11.02 ± 15.28 vs. 0.06 ± 0.31; P < 0.001) and more in-clinic discussions (1.84 ± 4.20 vs. 1.28 ± 2.92; P < 0.03), but with numerically fewer hospitalizations (0.67 ± 1.18 vs. 0.85 ± 1.43, P = 0.23) and shorter length-of-stay (6.31 ± 15.5 vs. 8.26 ± 18.6; P = 0.27), although not significant. For the whole study population, the total FU cost for providers was not different for HM ON vs. OFF [mean (95% CI): €204 (169–238) vs. €213 (182–243); range for difference (€−36 to 54), NS]. From a payer perspective, FU-related costs were similar while the total cost per patient (including other physician visits, examinations, and hospitalizations) was numerically (but not significantly) lower. There was no difference in the net financial impact on providers [profit of €408 (327–489) vs. €400 (345–455); range for difference (€−104 to 88), NS], but there was heterogeneity among countries, with less profit for providers in the absence of specific remote FU reimbursement (Belgium, Spain, and the Netherlands) and maintained or increased profit in cases where such reimbursement exists (Germany and UK). Quality of life (SF-36) was not different.
Conclusion
For all the patients as a whole, FU-related costs for providers are not different for remote FU vs. purely in-office FU, despite reorganized care. However, disparity in the impact on provider budget among different countries illustrates the need for proper reimbursement to ensure effective remote FU implementation.
Keywords: Implantable cardioverter defibrillator, Remote monitoring, Devices, Follow-up, Health economics
See page 143 for the editorial comment on this article (doi:10.1093/eurheartj/ehu372)
Introduction
Over the last years remote monitoring of implanted devices like implantable defibrillators (ICD) has been introduced. All stakeholders see potential benefits: physicians and hospitals (the ‘providers’) may optimize performance by reducing the number of in-hospital visits, while specialist nurses and/or technicians can filter alerts from the remote system, hence possibly saving physician time.1 For patients, the technology provides a more continuous follow-up (FU) and saves time for visits to outpatient clinics that often do not result in specific actions.2,3 Many studies have shown that remote technology results in patient care that is non-inferior to classical FU with in-office visits.1,4–8 Especially daily remote monitoring results in earlier detection of device and patient-related problems which translate into earlier clinical decision-making, less inappropriate shocks and improved device longevity.7–9 The CONNECT trial also showed a significant reduction in the length of cardiovascular hospitalizations9 and the IN-TIME trial even showed a mortality benefit in ICD patients with heart failure.10 Some trials have looked as secondary end-points at the cost impact from the perspective of the healthcare payer.6,11 There is data indicating that healthcare payers may have a dominant strategy option with remote ICD monitoring, since costs may be reduced and outcomes improved with remote FU.
While investigators have evaluated the time needed from physicians and nurses/technicians for remote monitoring,12–14 a formal cost analysis from the provider viewpoint has never been performed. Nevertheless, the net financial impact on providers will influence their willingness to change care models from reimbursed in-hospital visits to remote evaluation, especially when the latter is not reimbursed and given the investments needed to train nurses and employ those for the FU activities.15 Industry has to provide additional hardware (transmitters), set up infrastructure (servers) and take care of communication costs, which are not covered separately from the device price. If all stakeholders want to stimulate a reorganization of FU for ICD patients towards remote monitoring, they need to know its cost structure to ensure proper financing for the future. Therefore, the primary objective of this EuroEco trial was to evaluate the impact on the cost for providers when relying on Home Monitoring (HM)-based FU compared with classical FU with only in-office visits. Since reimbursement systems are very heterogeneous in different European countries, secondary objectives were to study the impact on the providers' net clinical income for FU, and the costs for ICD FU with or without remote monitoring from a healthcare payer perspective. Further secondary end-points were the rate of in-office FU visits with relevant findings and patient-reported quality-of-life assessed by the SF-36 questionnaire.
Methods
Study design
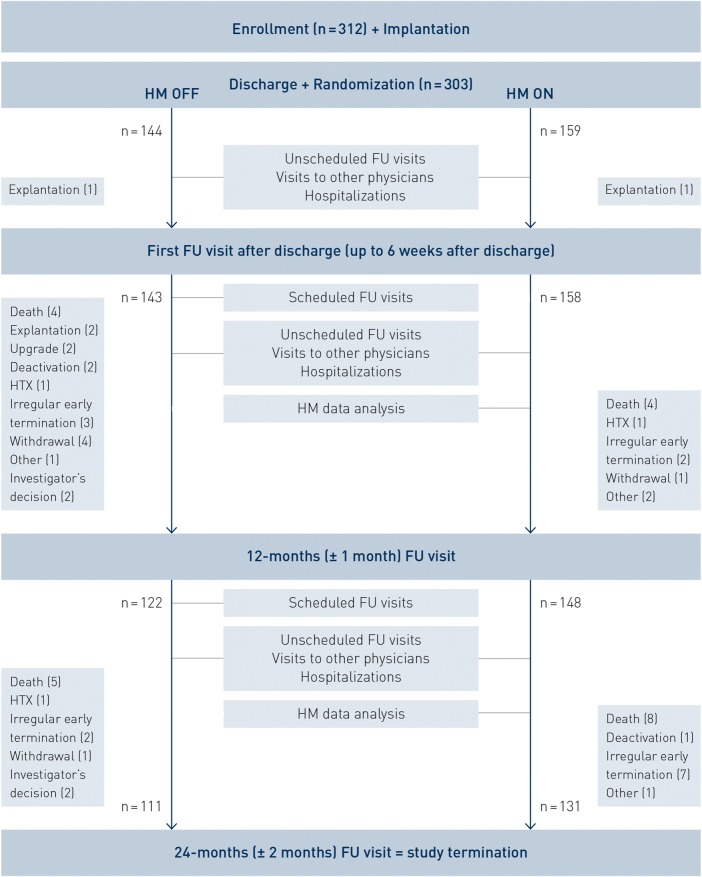
The European Health Economic Trial on HM in ICD Therapy was a randomized, non-blinded, parallel-design trial in which 17 centres from six European countries (Belgium 3 centres, Finland 1, Germany 4, UK 3, Spain 4, The Netherlands 1) participated. To be enrolled, patients had to meet the standard indication for a new or replacement VVI- or DDD-ICD enabled with HM technology capable of electrogram transmission (Biotronik ICDs models Lumos, n = 3, or Lumax, all others). There were no further clinical in- or exclusion criteria except age ≥18 years. A CRT-cohort was started as an extension and is still enrolling. Patients were enrolled prior to ICD implantation, after which they were randomized 1 : 1 to receive regular in-office FU visits (HM OFF group) or a HM facilitated FU regime (HM ON group) for the next 2 years. Three in-office FUs were mandatory for all the patients (within 6 weeks after discharge, 12 ± 1 and 24 ± 2 months after discharge) (see Figure 1). Further in-office FU visits were scheduled according to each centre's individual routine for the HM OFF patients only. Unscheduled in-office FU visits initiated by the physician or the patient could occur in both patient groups at any time during the study. Patients randomized to HM ON were under continuous, automatic remote monitoring during the entire study. The frequency of HM data analysis and the response to HM alerts was left to the investigators' discretion.
Figure 1.
Flow of patients between enrolment and end of follow-up. See text for further details. Patients with ‘irregular early termination’ did not terminate the study with a pre-specified final 24-month follow-up. However, for one patient in the HM OFF group and three patients in the HM ON group, data were available beyond 22 months after implant discharge. HM, Home Monitoring; FU, follow-up; HTX, heart transplantation; ‘upgrade’, implantable cardiac defibrillator upgrade to a cardiac resynchronization device.
The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Ethics Committees of all participating institutions and the trial was registered with clinicaltrials.gov under identifier NCT00776087. All the patients gave their written informed consent to participate in the trial.
Medical and resource use data collection
All medical contacts of the patients were recorded over the course of the trial from paper or electronic charts, including contacts with the provider (in-office, planned or web-based data review following alerts, internal discussions, phone calls) as well as visits to other physicians, additional medical examinations, and hospital admissions. All actions taken were evaluated for clinical relevance by an investigator blinded to group assignment and based on pre-specified criteria. A second blinded scientist double-checked the decisions. Patients self-reported their quality-of-life using SF-36 at randomization, and after 1 and 2 years of FU.
For all provider contacts (including internal discussions between staff members, phone calls, and unscheduled visits), staff time consumption (type of staff and duration) was tracked, often using purpose-built electronic documentation tools (web-based time-tracking software that was activated at the opening and closure of any contact; dedicated chronometers; automatic logging of calls on mobile phones of nurses and/or physicians). All study personnel was trained to log time on contacts for EuroEco patients. Data were later entered in paper-based case report forms. The hospital charts of the patients were clearly labelled to remind study personnel about the patient's inclusion in the trial and the need for time logging.
Monetary valuation: healthcare costs
Cost analysis was conducted from the provider perspective and from the healthcare payer perspective. Provider net income was calculated as the difference between payer reimbursement for FU services and provider cost related to FU. Country-specific tariffs and staff costs were used to convert measured resource use into monetary values and calculate the costs per patient. Non-medical costs (overhead) were derived from calculated staff time and estimated office space, based on nationally accepted percentages. Given only two patients from Finland (one in each group) cost calculations were not performed for this country. Remote monitoring equipment itself was not included in the cost calculations because of its arbitrary values; we rather opted for an analysis that shows payers possible margins for reimbursement of the technology based on their overall budget impact.
Payer costs were based on diagnosis-related groups tariffs, national or regional fee-for-service tariffs, public general hospital tariffs, or publications at the end of 2013. If geographic differences were noted within a single country, a mid-point estimate of the various observed tariffs was taken. All costs were expressed in Euro (€). If not available, 2013 costs were estimated by applying an appropriate inflation factor to previous year costs using the country-specific consumer price index.
A stratified exploratory analysis comparing countries with different reimbursement systems was pre-specified as a secondary end-point. The challenges of an appropriate method for analysing healthcare data from different jurisdictions have been discussed by others.16–18 A recommended method to calculate the costs for a given country is to consider the resource use of all patients in the sample and then apply to these resources cost data for that country. However, this method can only be applied if resource use is homogeneous within the sample. Key resource utilization data from the different countries (number of FU visits, time spent by healthcare workers, hospitalization data) were therefore analysed using generalized linear modelling to verify whether they were sufficiently homogeneous to be a valid basis for calculating country-specific costs. Overall heterogeneity in resource use was detected. Two sets of countries with homogeneous utilization (mainly based on the number of FU visits to the provider centre) were identified, comprising Belgium, The Netherlands, and the UK in one group and Finland, Germany, and Spain in another.
Statistical methods
The sample size estimate was based on an anticipated reduction of total time needed for FU by 14.5 min over 2 years, derived from early non-randomized trials and investigator databases. For a two-sided significance level of 5% to detect this difference and a power of 80%, 312 patients were needed. Ahead of database closure, statistical analysis plans were developed for the clinical and the health economic evaluation, respectively, defining all analytical details. The SAS software (SAS Institute, Inc., USA) was used to evaluate utilization data and to undertake cost calculations. Categorical variables were described by number and frequency of the non-missing data and compared between arms using the Fisher̀s exact test. Continuous variables such as number of FU visits were not normally distributed and were therefore compared between arms using the non-parametric Mann–Whitney U test. All tests were interpreted two-sided, at a 5% level of significance.
For the analysis of the SF-36v2 questionnaires, Health Outcomes Scoring Software 4.5 (QualityMetric Incorporated, Lincoln, RI, USA) was used. Thereby, raw data of the SF36-items were transformed into eight subscores by using the QualityMetric's Missing Score Estimator. These variables were transformed in subscores based on 2009 US norms, from which the mental health sum-score MCS and physical health sum-score (PCS) were calculated. In addition to both sum-scores, subscores before norm-basing were analysed with respect to any difference between HM ON vs. HM OFF to rule out any bias caused by using the standard US norm for European patients. For the purpose of comparative analysis, the non-parametric Mann–Whitney U test was applied here as well.
Cost per patient was described by mean, standard deviation, median, minimum, maximum, and 95% confidence intervals (CI). Confidence intervals were generated using the non-parametric bootstrapping method with 1000 replicates. If the 95% CI for difference excluded zero, the cost differences was considered statistically significant at the P = 0.05 level. The data were bootstrapped by study arm to ensure the treatment balance remained constant. Costs were calculated for the whole mix of patients as enrolled in the study, and also by country based on its corresponding grouping of resource utilization data (see above).
Pre-specified subgroups were evaluated in a multivariate analysis for their impact on provider and payer costs. These subgroups were single- vs. dual-chamber ICD, primary vs. secondary prevention indication, NYHA class ≤II and ≥III, and age below vs. above median, apart from HM ON vs. OFF and country.
Results
Patient characteristics and study execution
Of 312 patients enrolled between July 2008 and July 2011, 303 were eligible for FU data analysis and constituted the actual sample size (159 randomized to HM ON, 144 randomized to HM OFF) since nine patients terminated the study prematurely without providing any data after randomization (BE n = 87, FI n = 2, DE n = 88, UK n = 47, ES n = 54, NL n = 25). The baseline characteristics of the two study groups were similar (Table 1) except for the rate of patients with a primary prophylactic indication, which was higher in the HM ON group.
Table 1.
Patient characteristics and study execution
| Variable | All patients (n = 303) | HM OFF (n = 144) | HM ON (n = 159) |
|---|---|---|---|
| Mean age ± SD, years | 62.4 ± 13.1 | 62.9 ± 12.3 | 62.0 ± 13.9 |
| Male gender, % | 80.5 | 83.3 | 78.0 |
| Mean LVEF ± SD, % | 39.4 ± 15.1 | 39.5 ± 15.6 | 39.2 ± 14.8 |
| Mean QRS duration ± SD, ms | 112 ± 27 | 112 ± 28 | 111 ± 26 |
| Primary/secondary prophylactic indication, %* | 50.8/49.2 | 44.1/55.9 | 57.0/43.0 |
| Single-/dual-chamber ICD, % | 60.7/39.3 | 61.1/38.9 | 60.4/39.6 |
| Replacement, n (%) | 37 (12.2) | 16 (10.4) | 21 (13.2) |
| Medication, % | |||
| Beta-blocker | 81.8 | 83.3 | 80.5 |
| ACE-inhibitor or ARB | 65.7 | 71.5 | 60.4 |
| Diuretic | 53.1 | 56.9 | 49.7 |
| Anti-arrhythmic | 23.1 | 25.7 | 20.8 |
| Mean FU time, months ± SD | 21.8 ± 6.3 | 21.2 ± 7.0 | 22.4 ± 5.5 |
| Termination before 24 months visit, n (%) | 61 (20.1) | 33 (22.9) | 28 (17.6) |
| Irregular early termination (see note)a, n (%) | 14 (4.6) | 5 (3.5) | 9 (5.7) |
| Withdrawal of consent (%) | 6 (2.0) | 5 (3.5) | 1 (0.6) |
| Death, n (%) | 21 (6.9) | 9 (6.3) | 12 (7.5) |
| Investigators decision, n (%) | 4 (1.3) | 4 (2.8) | 0 |
| Device switched off/explanted/upgraded, n (%) | 9 (3.0) | 7 (4.9) | 2 (1.3) |
| Heart transplantation, n (%) | 3 (1.0) | 2 (1.4) | 1 (0.6) |
| Other, n (%) | 4 (1.3) | 1 (0.7) | 3 (1.9) |
| FU for early termination patients, months ± SD | 11.9 (8.3) | 10.2 (7.5) | 13.8 (9.0) |
*P = 0.029 (Fisher’s exact test) for difference in rate of primary prophylactic indication between HM ON and HM OFF. Further differences between groups were statistically insignificant. ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction.
aPatients with ‘Irregular early termination’ did not terminate the study regularly with a final 24-month FU. However, for one patient in the HM OFF group and three patients in the HM ON group, data (e.g. HM information, AE-reports, etc.) was available beyond 22 months after implant discharge.
Six patients crossed over from HM OFF to ON for medical reasons. In four cases, the investigator stopped study participation for these patients, while the other two were analysed according to the intention-to-treat principle. A total of 61 patients (20.1%) terminated the study before the pre-specified 24-month FU visit, mainly due to death or other clinical end-points (Figure 1). The remaining 242 patients terminated the study as planned at the 24-month FU. The median FU duration after randomization was 24.0 months (IQR: 23.1–24.5) in those patients and 11.9 months (IQR: 5.1–19.4) in patients with early termination, without differences between study groups.
Resource utilization during follow-up
During the whole study period, HM ON patients had significantly less scheduled in-office FU visits than HM OFF patients (Table 2). Despite a significantly higher number of unscheduled in-office FU visits in the HM ON group, the total number of FU visits was still significantly lower with HM ON (3.79 ± 1.67 vs. 5.53 ± 2.32, P < 0.001). On the other hand, the HM ON group had significantly more FU contacts outside of FU visits (i.e. phone calls) and internal discussions among staff (Table 2). There were no significant changes in the utilization of other healthcare services, although there were numerically fewer cardiovascular hospitalizations and shorter length-of-stay in the HM ON patients (Table 2).
Table 2.
Resource use during 2 years of implantable cardiac defibrillator follow-up, and average time needed for each of the follow-up activities
| Activity | Average number of services Mean (SD) Median (IQR) |
Average time needed (min) Mean (SD) Median (IQR) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HM OFF (n = 144) | HM ON (n = 159) | P-value | HM OFF (n = 144) |
HM ON (n = 159) |
P-value | |||||
| Physician | Nurse | Technician | Physician | Nurse | Technician | |||||
| Total FU visits | 5.53 (2.32)5.00 (2) | 3.79 (1.67)3.00 (1) | <0.001 | |||||||
| Scheduled visits | 4.92 (1.89)5.00 (2) | 2.84 (0.81)3.00 (0) | <0.001 | 13.49 (8.84)13.80 (12.0) | 10.35 (8.98)9.11 (12.1) | 6.65 (10.40)0.00 (13.8) | 14.19 (9.28)12.88 (12.0) | 11.08 (9.51)9.42 (12.9) | 6.40 (12.46)0.00 (10.3) | NS |
| Unscheduled | 0.62 (1.25)0.00 (1) | 0.95 (1.50)0.00 (1) | =0.005 | 13.43 (12.33)12.50 (17.0) | 8.82 (10.81)5.00 (12.5) | 9.59 (13.27)0.00 (19.5) | 16.32 (11.9)13.33 (14.0) | 9.59 (11.13)7.00 (15.0) | 6.67 (12.25)0.00 (10.0) | NS |
| Other FU contacts | 1.01 (2.64)0.00 (0) | 1.95 (3.29)1.00 (2) | <0.001 | |||||||
| Physician | 0.06 (0.38)0.00 (0) | 0.22 (0.64)0.00 (0) | =0.003 | 8.93 (4.96)10.00 (5.0) | 5.44 (3.95)4.00 (2.2) | NS | ||||
| Nurse | 0.89 (2.48)0.00 (0) | 1.53 (2.97)0.00 (2) | 0.002 | 5.31 (3.33)4.40 (3.3) | 5.91 (5.72)4.20 (3.9) | NS | ||||
| Technician | 0.08 (0.31)0.00 (0) | 0.23 (0.89)0.00 (0) | NS | 16.28 (11.9)13.00 (11.5) | 12.04 (11.93)10.75 (11.8) | NS | ||||
| Remote monitoring services | 0.06 (0.31)0.00 (0) | 11.02 (15.28)6.00 (15) | <0.001 | |||||||
| Physician | 0.01 (0.12)*0.00 (0) | 1.86 (4.54)0.00 (1) | <0.001 | 00 (0) | 2.87 (3.21)2.00 (17) | |||||
| Nurse | 0.04 (0.29)*0.00 (0) | 7.74 (12.82)2.00 (12) | <0.001 | 02.13 (0) | 3.40 (2.30)2.85 (12) | |||||
| Technician | 0.01 (0.08)*0.00 (0) | 1.47 (6.78)0.00 (0) | <0.001 | 00 (0) | 2.86 (3.49)1 (16) | |||||
| Internal discussions | 1.28 (2.92)0.00 (2) | 1.84 (4.20)1.00 (2) | 0.03 | |||||||
| Physician | 4.38 (3.84)3.00 (4.7) | 5.31 (4.57)4.03 (3.8) | NS | |||||||
| Nurse | 4.80 (4.26)3.38 (4.1) | 4.14 (3.28)3.12 (3.3) | NS | |||||||
| Technician | 4.34 (4.11)3.04 (5.1) | 4.96 (5.55)3.00 (4.9) | NS | |||||||
| Other physician visits | 4.64 (8.31)2.00 (5) | 5.91 (8.73)2.00 (7) | 0.214 | |||||||
| Outpatient examinations initiated during FU | 0.69 (1.59)0.00 (1) | 0.58 (1.25)0.00 (1) | 0.852 | |||||||
| CV hospitalizations | 0.85 (1.43)0.00 (1) | 0.67 (1.18)0.00 (1) | 0.233 | |||||||
| Length of hospital stay (days) | 8.26 (18.6)0.00 (7) | 6.31 (15.5)0.00 (5) | 0.266 | |||||||
CV, cardiovascular; FU, follow-up; IQR, inter-quartile range; NS, not significant; SD, standard deviation.
aSince some patients crossed-over from the OFF to the ON group, their remote FU contacts were counted in the HM OFF group (ITT).
The average time spent on each of the above-mentioned contacts was not significantly different for HM ON vs. OFF patients, neither for physicians, nurses nor technicians (Table 2). Figure 2 shows the cumulative time spent on every patient for all contacts combined: HM did not change the total time needed for follow-up, nor the cumulative time for all types of personnel combined: over the study period, 176 min of staff time per patient were required for HM ON vs. 178 min in HM OFF patients (NS). Only physicians spent significantly less time for patients in HM ON, but the difference was small (64 vs. 73 min, P = 0.028).
Figure 2.
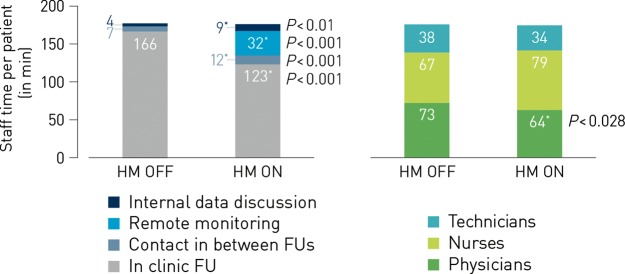
Staff time spent during 2 years of implantable cardiac defibrillator follow-up. The time needed to follow-up the average patient for 2 years did not change with HM. Over the study period, 178 min of staff time per patient were required for HM OFF vs. 176 min in HM ON patients. Significant changes are indicated by an asterisk, and the P-value is mentioned. Left: the time saved by the reduction of in-clinic follow-ups is re-directed to performing remote monitoring, call patients more frequently and discuss patient data more often. Right: only physician time was significantly lower with HM ON, albeit to a small extent.
Costs for follow-up
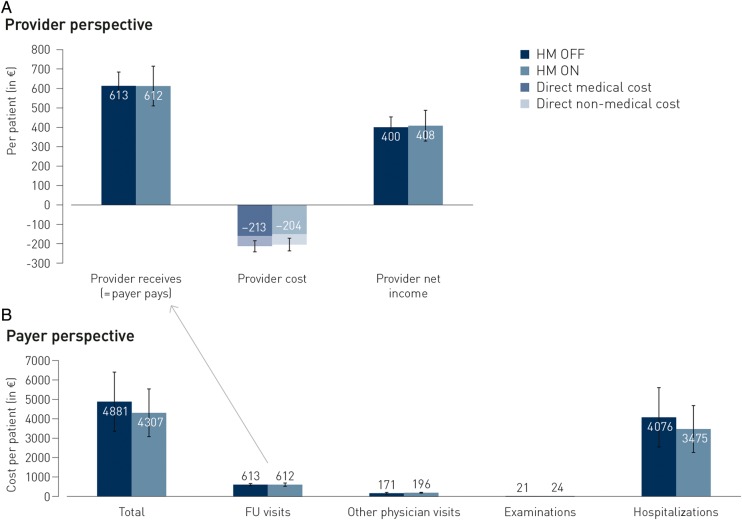
Figure 3A shows the monetary valuation of the time needed by different provider personnel during FU and the non-medical costs (mainly overhead): from a provider perspective, the average cost for FU during 2 years was not different for the HM ON and OFF groups [mean (95% CI): €204 (169–238) vs. €213 (182–243); range for difference (€−36 to 54), NS], without differences in either direct medical or non-medical costs. For the average trial patient, also the FU-related reimbursement was identical, leading to an unaffected net clinical income for the provider [profit of €408 (327–489) vs. €400 (345–455); range for difference (€−104 to 88), NS].
Figure 3.
Primary and main secondary end-points: average costs per patient over two years of follow-up. None of these trial average values for all patients combined were significantly different.
From a payer perspective (Figure 3 B), there was a small albeit not statistically significant decrease in total costs over a 2-year period (excluding the cost of the device and of any remote monitoring equipment or communication costs). The main cost driver for payers is the number of hospitalization days for patients: in line with numerically fewer hospitalizations and a shorter length-of-hospital stay, there was a numerical (non-significant) reduction in hospitalization costs. There was no difference in the costs related to non-provider physician visits or examinations.
Multivariable testing showed that the cost for providers was only significantly dependent on the country (P < 0.001), while for total payer cost only the type of ICD (VVI vs. DDD) had a significant impact (P < 0.001). Costs were not dependent on primary or secondary indication for implant (Table 3).
Table 3.
Multivariable regression of provider and total payer costs vs. different independent variables
| Dependent variable | Independent variable | Coefficient (SE) | P-value |
|---|---|---|---|
| Provider cost | HM ON vs. OFF | −0.119 (0.079) | 0.13 |
| Age ≤median vs. >median | −0.041 (0.080) | 0.61 | |
| NYHA class ≤II vs. ≥III | 0.039 (0.116) | 0.74 | |
| Single vs. dual chamber ICD | −0.142 (0.087) | 0.10 | |
| Primary vs. secondary ICD | −0.012 (0.084) | 0.89 | |
| Country | –a | <0.001 | |
| Total payer cost | HM ON vs. OFF | −0.226 (0.326) | 0.17 |
| Age ≤median vs. >median | −0.212 (0.157) | 0.18 | |
| NYHA class ≤II vs. ≥III | −0.366 (0.237) | 0.12 | |
| Single vs. dual chamber ICD | −0.585 (0.176) | <0.001 | |
| Primary vs. secondary ICD | 0.217 (0.183) | 0.24 | |
| Country | –a | 0.011 |
Based on a generalized linear model with a gamma distribution and a log link.
aThe variable ‘country’ was analysed for whether there was any relevant differences in the results for the different countries. Subsequent pairwise comparisons were undertaken, showing that the provider cost per patient for all countries differ significantly from each other.
SE, standard error.
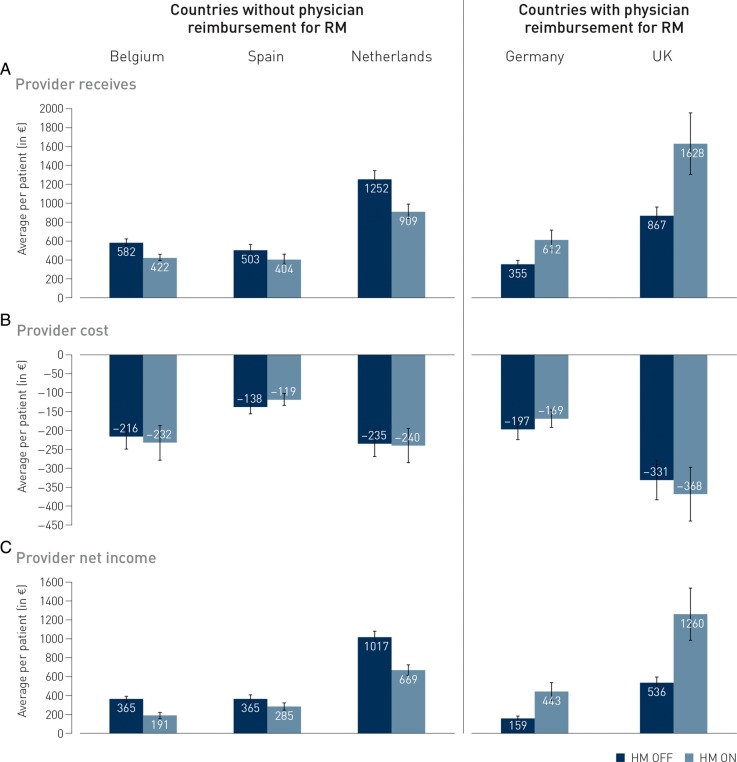
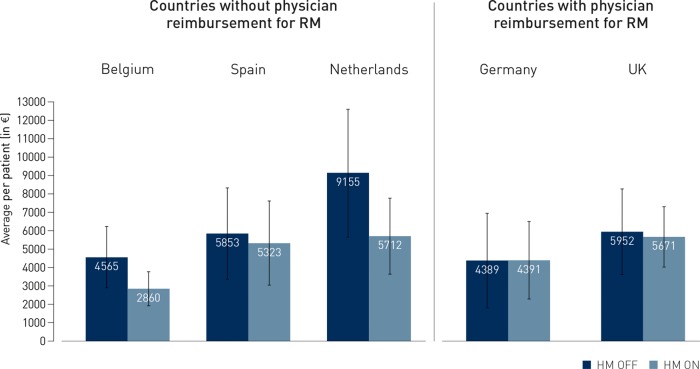
Figure 4 illustrates the heterogeneity among countries, with less profit for providers in the absence of specific remote FU reimbursement (left side: Belgium, Netherlands, and Spain) vs. maintained or increased profit in case such reimbursement exists (right side: Germany and UK). Nevertheless, the overall costs from the payer perspective were numerically lower in countries without reimbursement, and were similar in those with reimbursement (Figure 5).
Figure 4.
Country-dependent variations in provider costs and income. Although provider costs for follow-up are similar for HM ON or OFF patients in all countries, the net income impact of either follow-up strategy is dependent on the existing reimbursement provision for remote monitoring-related activities by the provider (physicians, nurses, and technicians): in countries without such provisions (and usually only a fee-for-service for in-person visits), net income of the providers decreases, while it increases if remote monitoring reimbursement is available (UK and Germany).
Figure 5.
Country-dependent total healthcare payer costs during 2 years of follow-up. Even in countries where remote monitoring reimbursement is available (UK and Germany), total costs for healthcare payers over 2 years of follow-up do not increase.
Other secondary end-points
There were no differences between HM ON and OFF in any of the SF-36 patient-reported quality of life measures, not at baseline, and not at 1 year or 2 years of FU, and there were no changes during the course of the study in any of the measures: PCS at enrolment was 44.7 ± 9.3 for HM ON vs. 45.2 ± 9.7 for HM OFF, with respective values at 1 year 43.9 ± 11.1 vs. 45.1 ± 10.2, and at 2 years 44.4 ± 11.0 vs. 45.4 ± 10.6; MCS at enrolment 48.8 ± 10.6 for HM ON vs. 46.8 ± 11.3 for HM OFF, and at 1 and 2 years 49.3 ± 10.2 vs. 48.9 ± 10.2 and 48.0 ± 11.5 vs. 47.8 ± 10.8, respectively (all inter- and intragroup comparisons non-significant). In the HM ON group, 32.0% of in-office FU visits (193/603) resulted in a clinically relevant finding/action (‘actionable visits’) compared with 26.8% (213/795) in the HM OFF group (P < 0.05).
Discussion
Although remote monitoring technology of cardiac implantable devices entered the clinical field ∼10 years ago, EuroEco is the first trial estimating the cost for providers in setting up an organization based on such technology. Prior trials have shown that such FU is non-inferior clinically from a major events perspective, and even has clinical advantages like earlier actionability on clinical- or device-related findings, fewer and shorter hospitalizations, fewer inappropriate shocks, longer battery longevity, and even lower mortality.1,6,7,9,10,14 Taken together with data on the existing reimbursement situation (defining provider income) and on the costs from a payer perspective, EuroEco also allowed to estimate the impact on the net income of physicians and hospitals. The financial impact is an important determinant for physicians and hospitals when considering adoption of a remote monitoring-based FU. The findings in the overall EuroEco population showed no change in provider cost of HM based vs. classical FU, nor on their net income. The HM ON arm included significantly more patients with a primary prophylaxis indication (who could be expected to require less intensive FU or have less complications), but multivariate analysis did not show a significant impact of this variable on provider costs. Although the sample size calculation was based on time investment findings, cost valuation was not used as basis for the power calculations. The aspect of heterogeneity in cost between the countries was underestimated. This may have decreased the initially calculated power. Nevertheless, the very close values for both arms for total time investment and for the primary end-point make it very unlikely that a higher sample size might have found different overall results.
In contrast to the overall trial population result, the patterns of provider net income in individual countries are of most relevance when interpreting the study data. In countries that have already implemented reimbursement for remote monitoring (like the UK and Germany), provider net income will increase, thus leading to an incentive to use remote monitoring. It is very plausible that in other countries (like Belgium, The Netherlands, or Spain in our study), there may be more incentives to maintain classic in-office FUs, since that is the only way to get reimbursement for FU activities, costs being similar.
An interesting observation is that the total cost for the insurance payer decreases, albeit not significantly, and does not even increase in countries that have already implemented remote FU reimbursement, showing that there is room for such proper compensation of providers. EuroEco observed numerically fewer and shorter hospitalizations, findings that corroborate with similar results from other ICD trials such as ECOST (with non-significant lower hospital costs) and CONNECT (showing a significantly shorter length of stay)9,11 as well as from pacemaker trials such as COMPAS and Oedipe.7,19 EuroEco did not make any cost-effectiveness calculations, since the payer perspective was not the primary one, and since the trial was not powered to detect significant differences in clinical outcome parameters. As the SF-36 measurements indicated, we could also not detect a significant difference in health-related quality-of-life, although prior trials have shown a high level of patient acceptance and satisfaction.3
In our cost analysis, we deliberately did not take into account any remote monitoring equipment itself, nor did we include communication costs, because such costs could be criticized as being arbitrary since mainly based on unilateral estimates by the manufacturer. Nevertheless, given the overall lower costs for payers with remote monitoring, EuroEco will allow informed discussions between all parties (payers, providers, and manufacturers) to come to balanced reimbursement scenarios for all. Scientific organizations and industry have already developed frameworks on how to provide appropriate reimbursement for device telemonitoring, with general principles and country-specific measures.20,21 The EuroEco data may certainly serve to specify these recommendations further.
While we are confident about the direction of the findings on income impact of remote monitoring in different countries, we want to raise caution about the absolute values of the calculated costs and reimbursements which should not be taken for granted. First, there was no statistical significance in any of the cost comparisons HM ON vs. OFF in the individual country scenarios, which may be related to the smaller groups as result of the splitting of the whole population due to heterogeneity of utilization data. Second, cost data were derived from country-based national databases and tariffs, which may not always be fully representative of a specific hospital's situation. Third, only a few centres per country participated and these centres do not necessarily represent the average practice in each country. Also, there may have some underreporting of the utilization data in the trial. In contrast to other clinical trials, in which a pre-specified regimen is guiding data gathering, investigators and nurses in EuroEco had to develop a reflex of documenting all contacts and their timing when dealing with study ICD patients, often unexpectedly (alerts, phone calls, …). Although we developed specific tools to facilitate this work, some contacts may have gone undocumented. Nevertheless, our time measurements found higher time consumption for FU in EuroEco compared with some previous trials. For example, the total nurse + technician time for remote FU (i.e. excluding the in-office visits) documented in EuroEco was 59 min per patient over 2 years, which would equal to ∼246 min per month for 100 patients followed. Earlier trials estimated this cumulative time ∼43, 59,14,22 and 660 min,13 a difference which may be related to the different tasks included in the analysis.
The saving in physician time was only modest in EuroEco (73 vs. 64 min over 2 years; P = 0.028) and total physician time was higher than in other trials,13,22 despite a similar significant reduction of in-office visits. This may be due to a learning curve, where physicians tended to do more of the HM work in the early phase of adoption, while relaying more to trained nurses in a later phase. EuroEco was started in 2008, i.e. short after HM technology was adopted in the clinical field. Also, our trial did a much more detailed and diverse time tracking of physician commitments during FU than other studies, including internal discussions, phone calls and unscheduled visits. This can also explain why other trials also reported a much lower physician- than nurse-time (10–25%) while both were comparable over the full trial period in our study.13,14 Nevertheless, our data indicate that HM allows reducing physician time and shifting it to other competent staff members, which is one of the prime drivers of healthcare reform all over the world. The disparity between nurse and technician time, as seen in EuroEco, may reflect country-based differences that in essence might not be fundamental: both nurses and technicians were specifically qualified personnel for device FU in all centres. Hence, the activities performed by a study nurse in one centre could be regarded interchangeable with the responsibilities of technicians in other practices/countries. Also the EHRA report on resource use during device FU revealed these regional differences.23
This EuroEco report only deals with VVI- and DDD-ICD patients. The study has been extended by also including heart failure patients with a cardiac resynchronization ICD (CRT-D), for whom a more complex and time-intensive clinical and device FU can be anticipated. It needs to be seen in how far the health-economic results reported here can be extended to the CRT-D population.
Conclusion
For all the patients as a whole in the EuroEco trial, FU-related costs for providers were not different for FU based on remote monitoring vs. purely in-office FU of VVI- or DDD-ICD patients, despite reorganized care with clearly different utilization patterns. The costs for the payers of healthcare were also statistically similar, although with numerically decreased costs if HM ON, related to numerically fewer and shorter hospitalizations. Disparity in the impact on provider budget among different countries illustrates the need for proper reimbursement to ensure effective implementation of remote FU.
Funding
This work was supported by an unrestricted grant from BIOTRONIK SE & Co KG, Berlin, Germany. Funding to pay the Open Access publication charges for this article was provided by Biotronik.
Disclosures
L.A. reports grants from Biotronik, during the conduct of the study. P.B. reports grants from Biotronik, during the conduct of the study and received grants from St Jude and also grants from Boston Scientific, outside the submitted work. I.F.-L. reports personal fees from BOSTON SC, St Jude Medical, and Biotronik, during the conduct of the study. H.H. reports other from Biotronik, grants and personal fees from Biotronik, grants from Boston-Scientific, grants from St. Jude Medical, during the conduct of the study and received personal fees from Pfizer/BMS, Daiicchi-Sankyo, Bayer, Boehringer-Ingelheim, Merck, outside the submitted work. G.H. reports grants and other from Biosense Webster, grants and other from St Jude Medical, grants and other from Biotronik, other from Medtronic, grants and other from Stereotaxis, grants and other from Boston Scientific, during the conduct of the study, grants and other from Biosense Webster, grants and other from St Jude Medical, grants and other from Biotronik, other from Medtronik, grants from Stereotaxis, other from Boston Scientific, outside the submitted work. S.L. reports grants from BIOTRONIK, during the conduct of the study. A.M. reports grants from Biotronik, during the conduct of the study. A.S. is an employee of Biotronik SE & Co KG, Berlin, Germany.
Conflict of interest: none declared.
Acknowledgements
The authors wish to acknowledge the support of Cristina Ivanescu, Konstantina Skaltsa, Xandra Lie, and Anke van Engen, all of Quintiles Consulting, a subsidiary of Quintiles Ltd, UK, who contributed with their statistical and health economic expertise to this comprehensive data evaluation. Many thanks also to Beate Wenzel, Sascha Mrosk, and Thuan Tran, all of the Center for Clinical Research at BIOTRONIK SE & Co KG, Berlin, Germany, for their contribution to the conduct of this study and the data evaluation.
References
- 1.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 2.Heidbuchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, Ector H. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10:351–357. doi: 10.1093/europace/eun010. [DOI] [PubMed] [Google Scholar]
- 3.Ricci RP, Morichelli L, Quarta L, Sassi A, Porfili A, Laudadio MT, Gargaro A, Santini M. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace. 2010;12:674–679. doi: 10.1093/europace/euq046. [DOI] [PubMed] [Google Scholar]
- 4.Spencker S, Coban N, Koch L, Schirdewan A, Muller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483–488. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 5.Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through Home Monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54–61. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Piccini JP, Knight D, Stewart M, Clapp-Channing N, Sanders GD. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. 2010;21:545–550. doi: 10.1111/j.1540-8167.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 7.Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, Binet D, Daubert JC, Investigators CT. A randomized trial of long-term remote monitoring of pacemaker recipients (the COMPAS trial) Eur Heart J. 2012;33:1105–1111. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida JS, Aliot E, Boursier M, Bizeau O, Kacet S Investigators Et. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J. 2013;34:605–614. doi: 10.1093/eurheartj/ehs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH, Investigators C. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Sogaard P Group ftI-TS. Benefit of implant-based multiparameter telemonitoring in patients with heart failure: results of the randomized IN-TIME trial. Lancet. 2014;384:583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 11.Guedon-Moreau L, Lacroix D, Sadoul N, Clementy J, Kouakam C, Hermida JS, Aliot E, Kacet S on behalf of the EtI. Costs of remote monitoring vs. ambulatory follow-ups of implanted cardioverter defibrillators in the randomized ECOST study. Europace. 2014;16:1181–1188. doi: 10.1093/europace/euu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164–170. doi: 10.1093/europace/eum289. [DOI] [PubMed] [Google Scholar]
- 13.Vogtmann T, Stiller S, Marek A, Kespohl S, Gomer M, Kuhlkamp V, Zach G, Loscher S, Baumann G. Workload and usefulness of daily, centralized home monitoring for patients treated with CIEDs: results of the MoniC (Model Project Monitor Centre) prospective multicentre study. Europace. 2013;15:219–226. doi: 10.1093/europace/eus252. [DOI] [PubMed] [Google Scholar]
- 14.Ricci RP, Morichelli L, D'Onofrio A, Calo L, Vaccari D, Zanotto G, Curnis A, Buja G, Rovai N, Gargaro A. Effectiveness of remote monitoring of CIEDs in detection and treatment of clinical and device-related cardiovascular events in daily practice: the HomeGuide Registry. Europace. 2013;15:970–977. doi: 10.1093/europace/eus440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heidbuchel H. Telemonitoring of implantable cardiac devices: hurdles towards personalised medicine. Heart. 2011;97:931–939. doi: 10.1136/hrt.2009.188789. [DOI] [PubMed] [Google Scholar]
- 16.Vemer P, Rutten-van Molken MP. The road not taken: transferability issues in multinational trials. Pharmacoeconomics. 2013;31:863–876. doi: 10.1007/s40273-013-0084-z. [DOI] [PubMed] [Google Scholar]
- 17.Willke RJ, Glick HA, Polsky D, Schulman K. Estimating country-specific cost-effectiveness from multinational clinical trials. Health Econ. 1998;7:481–493. doi: 10.1002/(sici)1099-1050(199809)7:6<481::aid-hec353>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, Reed SD, Rutten F, Sculpher M, Severens J. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12:409–418. doi: 10.1111/j.1524-4733.2008.00489.x. [DOI] [PubMed] [Google Scholar]
- 19.Halimi F, Clementy J, Attuel P, Dessenne X, Amara W Investigators Ot. Optimized post-operative surveillance of permanent pacemakers by home monitoring: the OEDIPE trial. Europace. 2008;10:1392–1399. doi: 10.1093/europace/eun250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubner S, Auricchio A, Steinberg JS, Vardas P, Stone P, Brugada J, Piotrowicz R, Hayes DL, Kirchhof P, Breithardt G, Zareba W, Schuger C, Aktas MK, Chudzik M, Mittal S, Varma N. ISHNE/EHRA expert consensus on remote monitoring of cardiovascular implantable electronic devices (CIEDs) Europace. 2012;14:278–293. doi: 10.1093/europace/eur303. [DOI] [PubMed] [Google Scholar]
- 21.PricewaterhouseCoopers. Moving towards good practice in the reimbursement of CIED telemonitoring. 2012. A study conducted in five European countries: Germany, Italy, Spain, the Netherlands and the UK http://www.eucomed.org/uploads/Modules/Publications/whitepaper_reimbursementciedtelemonitoring.pdf .
- 22.Ricci RP, Morichelli L, D'Onofrio A, Calo L, Vaccari D, Zanotto G, Curnis A, Buja G, Rovai N, Gargaro A. Manpower and outpatient clinic workload for remote monitoring of patients with cardiac implantable electronic devices: Data from the HomeGuide Registry. J Cardiovasc Electrophysiol. 2014 doi: 10.1111/jce.12482. doi:10.1111/jce.12482. [DOI] [PubMed] [Google Scholar]
- 23.Boriani G, Auricchio A, Klersy C, Kirchhof P, Brugada J, Morgan J, Vardas P European Heart Rhythm A, Eucomed. Healthcare personnel resource burden related to in-clinic follow-up of cardiovascular implantable electronic devices: a European Heart Rhythm Association and Eucomed Joint Survey. Europace. 2011;13:1166–1173. doi: 10.1093/europace/eur026. [DOI] [PubMed] [Google Scholar]