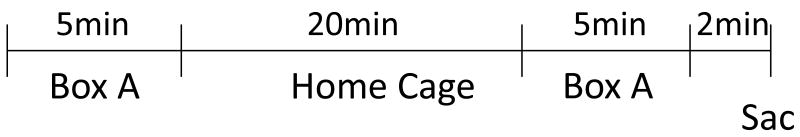1. Introduction
Experiencing emotional trauma, with or without physical trauma, when one perceives great peril to oneself or others can lead to debilitating pathological anxiety and impairment in social and cognitive function. This condition is known as Post-Traumatic Stress Disorder (PTSD) (DSM-IV-TR). Symptoms such as re-experiencing the trauma through intrusive recollections (e.g. nightmares, flash backs), avoidance of trauma-associated cues, and hyperarousal and anxiety (e.g. increased startle reactivity, difficulty sleeping), can lead to impairment in social an occupational functioning that undermine the quality of life of the sufferers and is estimated to cost society over 3 billion dollars annually (VA/DoD, 2010).
Exposure to a traumatic event alone is not sufficient to induce PTSD in all individuals (DiGrande et al., 2011; Kessler et al., 1995), suggesting that there are individual risk factors that confer susceptibility. Some behavioral and environmental risk factors have been documented, such as previous history of PTSD or other anxiety disorders, maternal PTSD, previous trauma, forensic exam following rape, etc. (Boscarino & Adams, 2009; Koenen et al., 2009; Resnick et al., 2007; Yehuda et al., 2008). Biological risk factors are generally unknown or controversial (e.g. levels of glucocorticoid receptors, RGS2), and appear to be significant in particular environmental conditions (Koenen et al., 2009; Norrholm & Ressler, 2009). A possible exception is reduced volume of the right hippocampus (Childress et al., 2013), as suggested by a well-controlled study of twins discordant for combat exposure and PTSD status, which shows that a decreased hippocampal volume, regardless of combat exposure, is a risk factor for PTSD (Gilbertson et al., 2002). However, these findings were not supported by a subsequent study (Kasai et al., 2008), emphasizing the need for studies investigating hippocampal function as a risk factor.
Impaired hippocampal function is well documented in people with established PTSD who experience “recurrent and intrusive distressing recollections of the event, including images, thoughts, or perceptions intrusive recollections of the traumatic event” (DSM-IV-TR). Although this symptomology suggests stronger and longer-lasting encoding of emotion-influenced information (Cahill & McGaugh, 1998; McGaugh, 2004; Roozendaal et al., 2006), recollections of PTSD patients are often fragmented, lacking integrated environmental perceptions (McFarlane, 2000), even to the degree of trauma-related amnesia (Williams, 1994). This apparent discrepancy is resolved by the fact that during a behavioral experience the brain acquires different types of memories (explicit/declarative and implicit) supported by different brain systems (Manns & Eichenbaum, 2006; Packard et al., 2010; Squire et al., 2004). Mounting evidence suggests that PTSD patients have general deficits in declarative and short-term memory (Bremner et al., 1993a; Bremner et al., 1995; Bremner et al., 1993b; Gilbertson et al., 2001; Jenkins et al., 1998; Yehuda et al., 1995). Considering the overwhelming evidence that the hippocampal system (medial temporal lobe system) supports explicit memory (Manns & Eichenbaum, 2006; Packard et al., 1989; Ranganath, 2010; Squire et al., 2004), it is not surprising that PTSD sufferers have impaired hippocampal function during associative tasks (Rauch et al., 2006; Shin & Liberzon, 2010). Whether this impaired hippocampal function is a risk factor for or a sequela of developing PTSD is currently unknown.
We addressed this fundamental question by assessing the integrity of hippocampal function with a cellular imaging method (Arc/Homer1a catFISH) both before (Experiment I) and during (Experiment II) a traumatic event in a rat model of susceptibility for PTSD-like behaviors (Nalloor et al., 2011). The Arc/Homer1a catFISH (cellular compartmental analysis of temporal activity using fluorescence in situ hybridization) method (Vazdarjanova et al., 2002) is a sensitive technique that utilizes the expression of two plasticity-associated immediate-early genes, Arc and Homer1a (H1a), to identify and dissociate large neuronal ensembles activated during either of two events (Nalloor et al., 2012; Ramírez-Amaya et al., 2005; Rosi et al., 2009; Vazdarjanova & Guzowski, 2004; Vazdarjanova et al., 2006). For the purpose of brevity, we are using the term “activated” to describe neurons which have experienced plasticity-inducing events sufficient to initiate transcription of plasticity-related immediate-early genes. Rats pre-classified as susceptible or resistant to developing a PTSD-like phenotype (Nalloor et al., 2012) were allowed to explore a novel place twice (Experiment I) or explore the place once and then receive a footshock during a second exploration (Experiment II). Differences in functional activation of the septal/dorsal and temporal/ventral hippocampus (CA1 and CA3) between the two events were evaluated within the same rats using the Arc/H1a catFISH method and comparisons were made between susceptible and resistant rats.
2. Materials and Methods
2.1 Subjects
Young adult (250-300g) male Sprague-Dawley rats (Charles River Laboratories Inc, Wilmington, MA) were double housed on a 12 hrs light/dark cycle (lights on at 7:00 am) with food and water freely available.
2.2 Behavioral procedures
All behavioral procedures were approved by the Institutional Animal Care and Use Committee (IACUC), Georgia Regents University, Augusta, GA.
All testing was performed between 9:00 am and 5:00 pm by trained observers blinded to the group assignment of the rats and recorded via an overhead camera.
2.3 Classification
Rats were tested for their acoustic startle response and behavior on the elevated plus maze four days after exposure to cat hair (mild stressor). Using a priori set criteria, they were classified as Susceptible (Sus), Resistant (Res), or Intermediate as described in detail in (Nalloor et al., 2011). Only Res and Sus rats were used for Experiments I and II.
2.4 Experiment I (A-A)
The experimental design is summarized in Figure 1. Rats (Res: n = 9; Sus: n= 6) were subjected to two 5-min spatial explorations of a 50cm x 10cm x 19cm box, comprised of an acrylic box with 2 stainless steel plates covering opposing walls and the floor, separated at the floor with a 1 cm gap. The two explorations were separated by 20min, during which time, the rats were returned to their home cages. All rats were decapitated immediately after the second exploration.
Figure 1.
2.5 Experiment II (A-CFC)
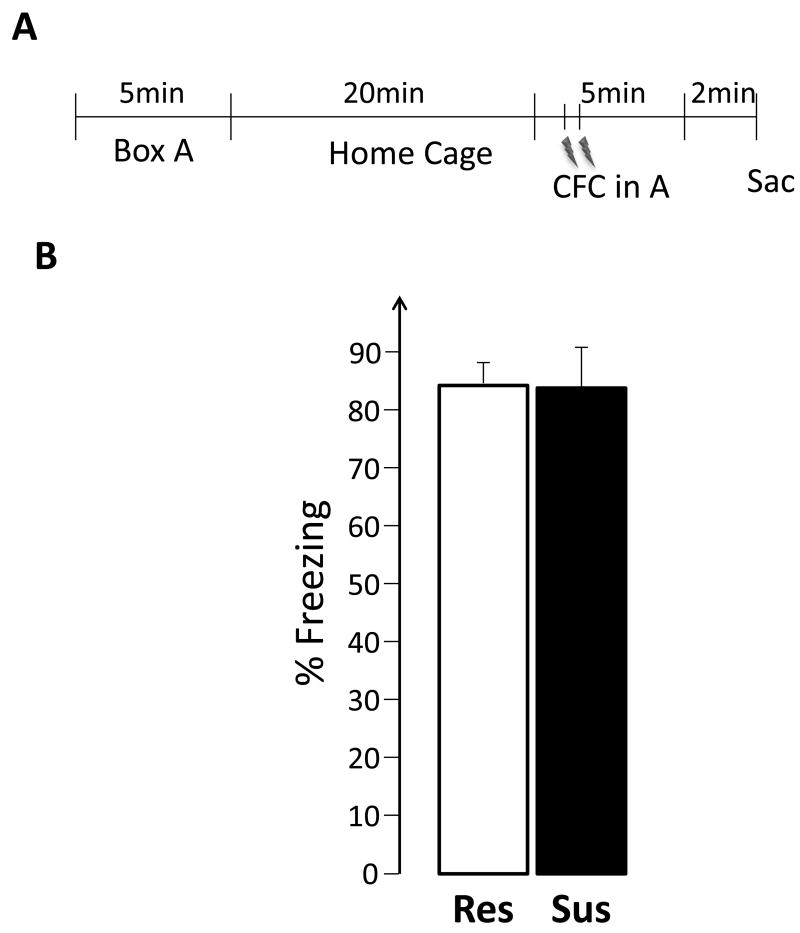
The experimental design is summarized in Figure 5A. Rats (Res: n = 10; Sus: n= 5) were tested as in Experiment I, except that during the second exploration they received 2 foot shocks administered through the stainless steel plates (1mA AC, 1s duration, 30s apart, first shock 30s into the session).
Figure 5.
During both the experiments, fear behavior was measured as time spent freezing, which is defined as lack of movement except that caused by respiration. The first exploration of each experiment is refered to as Event 1 and the second exploration as Event 2.
2.6 Brain harvesting and sectioning
The brains were rapidly harvested and flash-frozen in 2-methyl butane (<2 min) and stored at -80°C. The right hemisphere of brains from Sus and Res rats were blocked together in freezing medium. Twenty micron thick coronal sections were cut on a cryostat and mounted on glass slides. The slides were stored at -20°C till further processing.
2.7 Arc/H1a catFISH
The slides were processed for Arc/H1a in situ hybridization as described by (Vazdarjanova & Guzowski, 2004). Briefly, after fixing and permeabilizing the tissue, a fluorescein-labeled full-length Arc and digoxigenin-labeled H1a antisense riboprobe, targeting the 3′ UTR of H1a mRNA, were applied and hybridized overnight at 56°C. Following a series of stringency washes and a peroxidase quenching step, the digoxigenin tag was detected sequentially with horseradish peroxidase-conjugated anti-digoxigenin antibody (Fab fragments, Roche Diagnostics, Indianapolis, IN) and tyramide amplification reaction with SuperGlo Green (Fluorescent Solutions, Augusta, GA). Following a second peroxidase quenching step, the fluorescein tag was revealed with peroxidase-conjugated anti-fluorescein antibody (Roche) and tyramide amplification reaction with SuperGlo Red (Fluorescent Solutions).
Riboprobes were generated using commercial transcription kits (Ambion MaxiScript, Life Technologies, Grand Island, NY; or AmpliScribe; Epicentre Biotechnologies, Madison, WI) and digoxigenin-UTP or fluorescein-UTP RNA labeling mixes (Roche). Coverslips were mounted and nuclei were counterstained using Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA).
2.8 Image Acquisition and Stereological Analysis
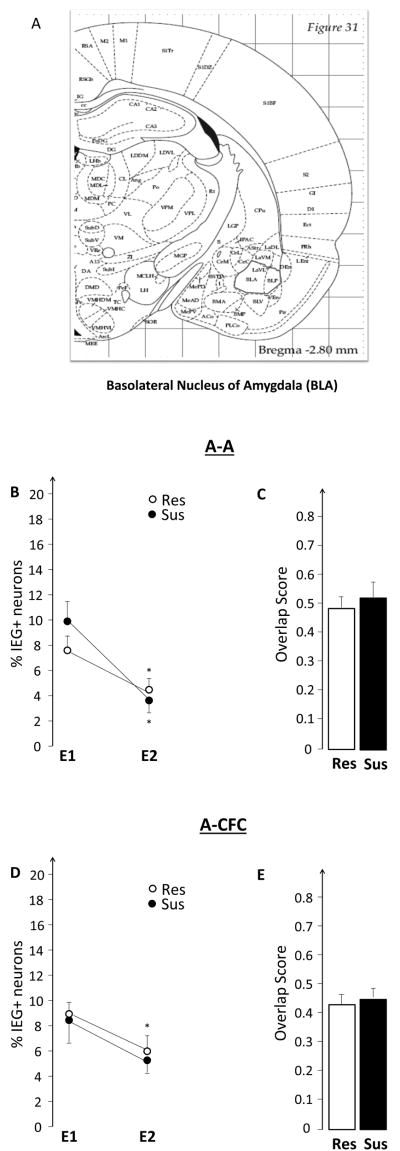
Image stacks from the selected regions (2.8-3.8mm posterior to bregma for dorsal hippocampus and BLA and 4.8- 6.4mm for ventral hippocampus) were collected from at least 3 different slides per animal using a 20× objective on a Zeiss AxioImager/Apotome system. For the dCA3 region, mosaics of the entire region were collected and analyzed. The position of dCA1, vCA1, dCA3, v CA3 and BLA are illustrated in Figures 2A and B, and 8A).
Figure 2.
Figure 8.
Unbiased stereological cell counting and classification were performed as follows: 1) Neuron-like cells in the regions of interest were segmented from the appropriate regions of interest by using an optical dissector method (West, 1999); 2) segmented neurons were classified using Zeiss AxioVision imaging software. Putative glial cells, those with small, intensely, and uniformly stained nuclei, were excluded from the analysis. Cells were classified as Arc+, H1a+ or Arc/H1a+ depending on whether they had foci of transcription for Arc, H1a, or both, respectively. Cells without any foci were classified as Negative. Overlap scores were calculated as: (%Arc/H1a+ - (%E1×%E2)/100)/(%Emin - (%E1×%E2)/100), where %Arc/H1a+ = percentage of total neurons that are Arc/H1a+; %E1 = percentage of cells activated in the first behavioral event = (H1a+ + Arc/H1a+)/total neurons; %E2 = percentage of cells activated in the second behavioral event = (Arc+ + Arc/H1a+)/total neurons; (%E1×%E2)/100 = theoretical probability of cells activated during both events, assuming the two events activated independent groups of neurons; %Emin = lesser of %E1 and %E2, which normalizes the equation to a condition with a perfect overlap. The Overlap Score is 1 for a perfect overlap (%E1 = %E2), and is 0 for two statistically independent %E1 and %E2. For purposes of clarity we refer to all H1a+ cells as an ‘ensemble’ that activated IEG expression during Event 1, and all Arc+ cells as an ensemble that activated IEG expression during Event 2.
2.9 Statistical Analyses
Two-way repeated measures ANOVA with group and event as independent variables was used to compare ensemble size. Statistically significant main effects were followed up by either a one-way RM-ANOVA for within group comparisons or t-tests for between group comparisons. Differences in overlap scores were assessed with one-way ANOVA tests (StatView software, SAS Institute, Cary, NC). Differences were considered statistically significant at p< 0.05.
3. Results
3.1 Experiment I
This experiment tested the hypothesis that the hippocampus of susceptible (Sus), compared to resistant (Res), rats will have suppressed expression of plasticity-related IEGs before experiencing emotional trauma.
3.1.1. Susceptible rats fail to reactivate the dCA1 neuronal ensemble activated during initial exploration of a novel place
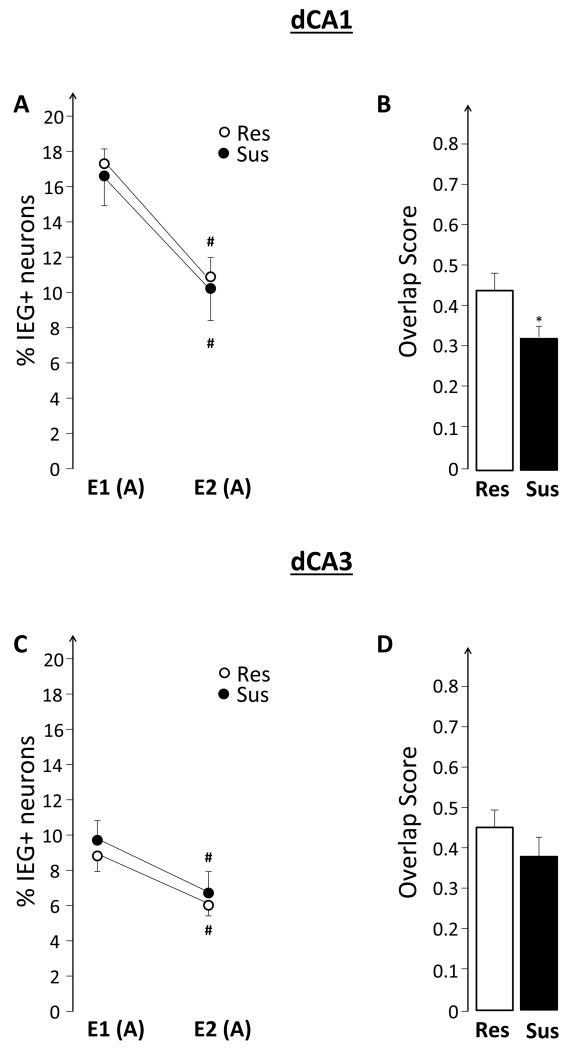
Exploring box A, a novel place, twice lead to a significant decrease in the size of IEG-expressing neuronal ensembles during the second exploration in both Res and Sus rats (event effect F(1,13)= 111.68, p<0.0001, no significant group effect and no significant group x event interaction; Figure 3A). Sus rats, however, activated a qualitatively different neuronal population during the second exploration event compared to that of Res rats, as revealed by the significantly lower overlap score (F(1,13)= 4.84, p<0.05; Figure 3B). None of these changes appear to be related to behavior, as the number of crossings in the experimental box was similar between groups.
Figure 3.
A similar pattern was observed in dCA3 (event effect F(1,13)= 31.43, p<0.0001), except that the overlap was not significantly different between groups.
3.1.2. Susceptible rats show a different pattern of Arc/H1a activation across the ventral hippocampus
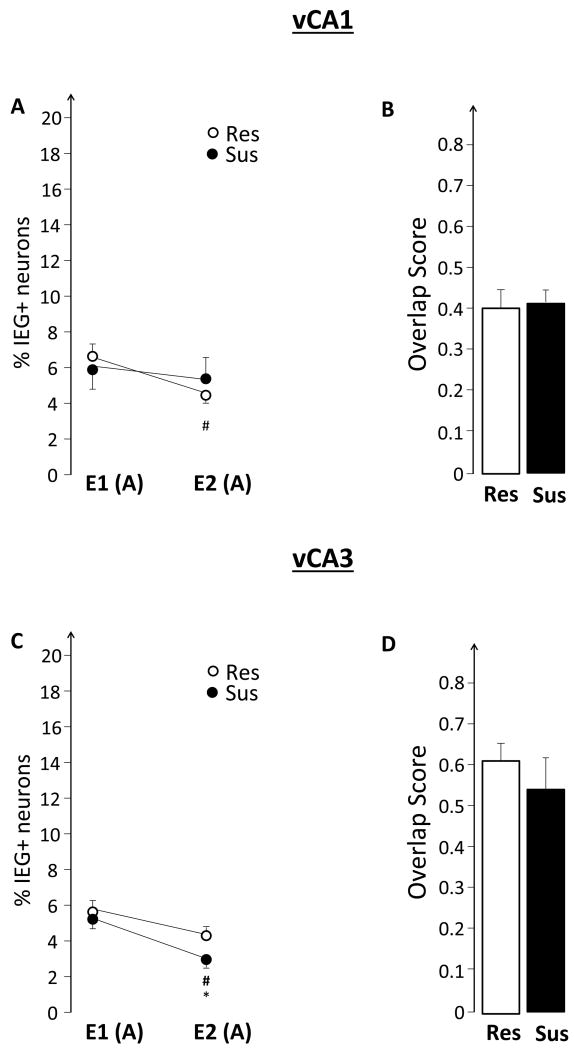
As illustrated in Figure 4A and C, there were no significant differences in the percentage of IEG-expressing neurons during the initial exploration: %E1(Sus) was similar to %E1(Res) in both vCA1 and vCA3. In contrast, during the second exploration Sus rats activated a smaller vCA3 neuronal ensemble: %E2(Sus)< %E2(Res) (F(1,13)= 6.37, p= 0.025). Res rats %E2<%E1 in the vCA1 (F(1,8)=17.11, p<0.01), but in vCA3 %E2 was similar to %E1. Sus rats showed the exact opposite pattern: in vCA1, %E2 was similar to %E1, while in vCA3, %E2< %E1 (F(1,4)=108.30, p<0.001). This pattern of results is consistent with a significant overall event effect (F(1,13)= 14.54, p<0.01) and a trend for a group x event interaction (F(1,13)= 3.74, p=0.07).
Figure 4.
3.1.3. Susceptible and Resistant rats show a similar pattern of activation in the BLA
The BLA has a well-documented role in modulating emotional memories and our vCA3 images were collected from the area of heaviest BLA-ventral hippocampal projections. Therefore, we hypothesized that the smaller %E2(Sus) seen in vCA3 reflected suppressed IEG activation in the BLA of Sus rats during the second exposure. This hypothesis, however, was rejected as Sus and Res rats had similar %E1, %E2, and overlap (Figure 8B and C). For both groups %E2<%E1 (event effect F(1,13)= 18.81, p<0.001; no group effect and no group x event interaction). BLA could have influenced the ventral hippocampus, but these influences did not result from altered IEG expression in the BLA.
3.2 Experiment II
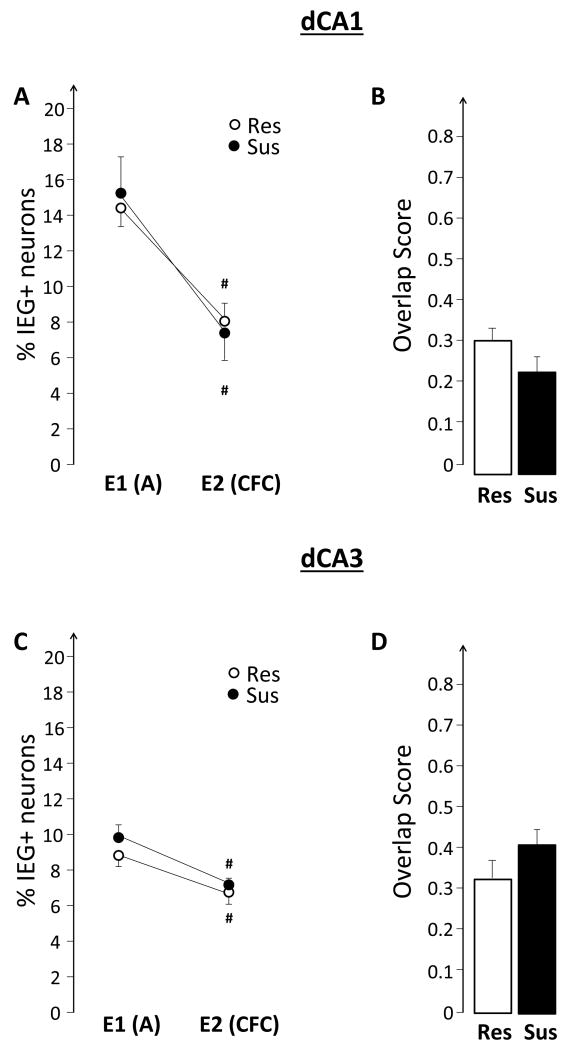
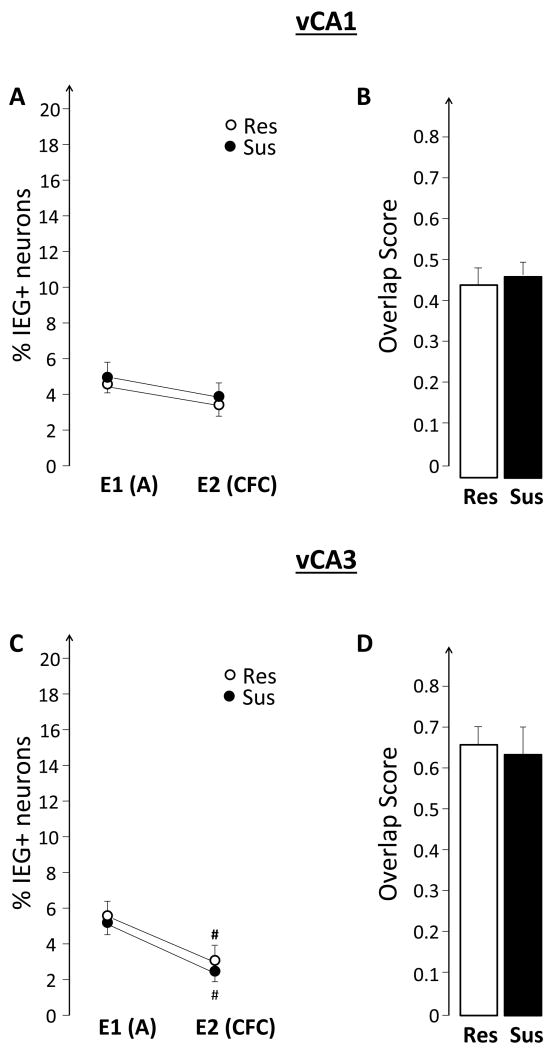
This experiment tested the hypothesis that the hippocampus of susceptible, compared to resistant, rats will show elevated expression of plasticity-related IEGs while experiencing emotional trauma. The results, illustrated in Figures 6 and 7, do not support this hypothesis. There were no differences between Sus and Res rats in either the size of the neuronal ensembles, or in the observed overlap. Similarly, there were no group differences in the BLA (Figure 8D and E).
Figure 6.
Figure 7.
4. Discussion
The results of this set of investigations are consistent with the hypothesis that altered hippocampal function prior to emotional trauma is as a risk factor for a PTSD-like phenotype. We observed impaired hippocampal expression of plasticity-related IEGs (Arc and Homer 1a) and impaired fidelity of network activation during repeated spatial exploration prior to emotional trauma in rats that were pre-classified as susceptible (Sus) to developing a PTSD-like phenotype (Nalloor et al., 2011). Surprisingly, we did not find support for the hypothesis that there is altered hippocampal or basolateral amygdala (BLA) Arc/H1a expression as a result of a traumatic event (Experiment II). Combined, these findings suggest that susceptibility to a PTSD-like phenotype is associated with impaired hippocampal function prior to experiencing emotional trauma in relatively non-stressful conditions.
4.1 Impaired fidelity of hippocampal function prior to emotional trauma
In Experiment I, Sus and Res rats were allowed to learn about a new place by exploring it twice. This experimental design allowed us to compare, between Sus and Res rats, both the size of IEG+ neuronal ensembles activated during the initial and repeated explorations, as well as how similar the IEG+ ensembles activated by both explorations were (overlap scores). Sus rats, compared to Res, showed a decreased overlap of activated ensembles in the dorsal/septal CA1 (dCA1) (Figure 3B), without a difference in the ensemble size (Figure 3A). Combined with previous findings that exploration of the same spatial environment leads to activation of largely overlapping neuronal ensembles as identified both electrophysiologically(Skaggs & McNaughton, 1998) and via the catFISH method (Guzowski et al., 1999; Nalloor et al., 2012; Ramírez-Amaya et al., 2005; Rosi et al., 2009; Vazdarjanova & Guzowski, 2004; Vazdarjanova et al., 2006; Vazdarjanova et al., 2002), the decreased overlap in dCA1 of Sus rats suggests that the fidelity of their hippocampal representations is impaired. The hippocampus in humans is well known to support episodic memory (Manns & Eichenbaum, 2006; Ranganath, 2010; Squire et al., 2004) and there is growing consensus that the rodent hippocampus also encodes the ‘what’, ‘where’ and ‘when’ components of experienced events (Babb & Crystal, 2006; Eichenbaum et al., 2012; Eichenbaum, 2013; Ferbinteanu et al., 2006; Morris, 2001; Nalloor et al., 2012). There is also increasing understanding that while both dCA1 and dCA3 encode the ‘what’ aspects of episodic-like memories, they also contribute differently: the dCA3 supporting spatial encoding, while the dCA1- temporal/sequential encoding (Eichenbaum, 2013; Hunsaker et al., 2008; Hunsaker & Kesner, 2008; Leutgeb, et al., 2004; Nalloor et al., 2012; Vazdarjanova & Guzowski, 2004). In this framework, our findings that Sus rats showed a most pronounced difference from Res rats in the dCA1, but not dCA3, suggest that the impaired fidelity does not concern space, but rather other aspects of the episodic memory for the event. This inference may prove to be essential in informing decisions as to what kind of testing is required to reveal pre-trauma PTSD susceptibility.
4.2 Impaired IEG expression in the ventral hippocampus of Sus rats
The Sus rats had smaller IEG+ neuronal ensembles in the ventral/temporal CA3 (vCA3) region of the hippocampus during the second spatial exploration (Figure 4C). The same rats maintained a similar ensemble size in vCA1, a pattern that was exactly opposite to that displayed by the Res rats (Figure 4A and C). Considering that lesions of the ventral hippocampus affect fear and anxiety behaviors (Blanchard et al., 2005; Kjelstrup et al., 2002), these findings suggest that Sus rats may be more anxious during repeated spatial exploration, or that they have difficulty processing safety. Indeed, the overall pattern of IEG expression in Sus rats is reminiscent to that of naïve or pre-classified rats experiencing fear conditioning (Nalloor et al., 2012)(Figures 3, 4, 6 & 7). We hypothesized that the observed differences may be driven by aberrant input from the BLA, as the images from the vCA1 and vCA3 were collected from the regions with heavy BLA-hippocampal projections (Petrovich et al., 2001; Pikkarainen et al., 1999). Furthermore, we hypothesized that this aberrant input might be expressed as altered IEG expression in the BLA of the same rats. The fact that were no differences between Sus and Res rats in IEG expression in the BLA (Figure 8B and C) rejects the second hypothesis, although it remains possible that the BLA is influencing IEG expression in vCA3, but these influences are based on short-term changes in the BLA that do not lead to altered IEG expression within the BLA itself. Indeed, it should be stressed that Arc expression is indicative of long-term plasticity, as Arc protein is necessary for memory consolidation, but not acquisition (Guzowski et al., 2000; Holloway & McIntyre, 2011; Plath et al., 2006; Ploski et al., 2008; Wang et al., 2006). Future experiments with temporary inactivation of the BLA will clarify the role of the BLA in altered hippocampal representations in Sus rats.
4.3 Hippocampal IEG expression in susceptible rats is similar to that of resistant rats during fear conditioning
The observed differences between Sus and Res rats in the patterns of hippocampal IEG expression before emotional trauma suggested that such altered hippocampal function might be also observed when Sus rats encode an emotionally traumatic event, such as fear conditioning (Experiment II). The findings, however, did not support this hypothesis (Figures 6 and 7). They are, however, consistent with previous findings showing that Sus rats and Res rats have similar levels of fear expression and by inference, similar levels of memory for fear conditioning (Nalloor et al., 2011). Sus rats differed from Res rats in their rate of fear extinction, which may be influenced by the observed impairment of hippocampal function when fear is not externally elicited. Ongoing experiments in our laboratory are designed to test this hypothesis.
4.4 Implications
The current findings reveal a complex pattern of changes in Sus vs. Res rats induced by repeated spatial exploration. They also provide important information that can guide the development of testing tools for revealing susceptibility to a PTSD phenotype. We have a high degree of confidence that the present findings in rats will be applicable to humans, given that the brain systems processing episodic and emotional memories appear to be similar in rats and humans, as illustrated by the successful line of research into emotional memories in humans that was guided by previous findings in rats (McGaugh, 2004). Identifying susceptibility is desirable, as susceptible individuals may choose to minimize behaviors that put them at a higher risk for experiencing traumatic events, or seek early intervention when they experience a traumatic event to minimize the probability of developing PTSD. Importantly, the current findings contribute to understanding the etiology of this condition because they show that altered hippocampal function in susceptible animals exists prior to experiencing emotional trauma.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development; I01BX001978. Further support was provided by R21MH083188 and NSF1138690.
Footnotes
Disclaimer: “The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.”
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, D.C.: 2000. Text Revision. [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Current biology: CB. 2006;16(13):1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neuroscience and biobehavioral reviews. 2005;29(8):1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Adams RE. PTSD onset and course following the World Trade Center disaster: findings and implications for future research. Social psychiatry and psychiatric epidemiology. 2009;44(10):887–898. doi: 10.1007/s00127-009-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Capelli S, Delaney R, McCarthy G, Charney DS. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry research. 1995;59(1-2):97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Charney DS. Deficits in short-term memory in posttraumatic stress disorder. The American journal of psychiatry. 1993a;150(7):1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Steinberg M, Southwick SM, Johnson DR, Charney DS. Use of the Structured Clinical Interview for DSM-IV Dissociative Disorders for systematic assessment of dissociative symptoms in posttraumatic stress disorder. The American journal of psychiatry. 1993b;150(7):1011–1014. doi: 10.1176/ajp.150.7.1011. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in neurosciences. 1998;21(7):294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Childress JE, McDowell EJ, Dalai VVK, Bogale SR, Ramamurthy C, Jawaid A, Schulz PE. Hippocampal volumes in patients with chronic combat-related posttraumatic stress disorder: a systematic review. The Journal of neuropsychiatry and clinical neurosciences. 2013;25(1):12–25. doi: 10.1176/appi.neuropsych.12010003. [DOI] [PubMed] [Google Scholar]
- DiGrande L, Neria Y, Brackbill RM, Pulliam P, Galea S. Long-term posttraumatic stress symptoms among 3,271 civilian survivors of the September 11, 2001, terrorist attacks on the World Trade Center. American journal of epidemiology. 2011;173(3):271–281. doi: 10.1093/aje/kwq372. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Memory on time. Trends in cognitive sciences. 2013;17(2):81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neuroscience and biobehavioral reviews. 2012;36(7):1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, Kennedy PJ, Shapiro ML. Episodic memory--from brain to mind. Hippocampus. 2006;16(9):691–703. doi: 10.1002/hipo.20204. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Gurvits TV, Lasko NB, Orr SP, Pitman RK. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. Journal of traumatic stress. 2001;14(2):413–432. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- Gilbertson Mark W, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature neuroscience. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature neuroscience. 1999;2(12):1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Holloway CM, McIntyre CK. Post-training disruption of Arc protein expression in the anterior cingulate cortex impairs long-term memory for inhibitory avoidance training. Neurobiology of Learning and Memory. 2011;95(4):425–432. doi: 10.1016/j.nlm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Fieldsted PM, Rosenberg JS, Kesner RP. Dissociating the roles of dorsal and ventral CA1 for the temporal processing of spatial locations, visual objects, and odors. Behavioral neuroscience. 2008;122(3):643–650. doi: 10.1037/0735-7044.122.3.643. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiology of learning and memory. 2008;89(1):61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MA, Langlais PJ, Delis D, Cohen R. Learning and memory in rape victims with posttraumatic stress disorder. The American journal of psychiatry. 1998;155(2):278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological psychiatry. 2008;63(6):550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Amstadter AB, Nugent NR. Gene-environment interaction in posttraumatic stress disorder: an update. Journal of traumatic stress. 2009;22(5):416–426. doi: 10.1002/jts.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science (New York, NY) 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- McFarlane AC. Posttraumatic stress disorder: a model of the longitudinal course and the role of risk factors. The Journal of clinical psychiatry. 2000;61(Suppl 5):15–20. discussion 21–23. [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual review of neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Morris RG. Episodic-like memory in animals: psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2001;356(1413):1453–1465. doi: 10.1098/rstb.2001.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalloor R, Bunting KM, Vazdarjanova A. Encoding of emotion-paired spatial stimuli in the rodent hippocampus. Frontiers in behavioral neuroscience. 2012;6:27. doi: 10.3389/fnbeh.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalloor R, Bunting K, Vazdarjanova A. Predicting impaired extinction of traumatic memory and elevated startle. PloS one. 2011;6(5):e19760. doi: 10.1371/journal.pone.0019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164(1):272–287. doi: 10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1989;9(5):1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th. San Diego, CA: Academic Press Inc.; 1998. [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain research. Brain research reviews. 2001;38(1-2):247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. The Journal of comparative neurology. 1999;403(2):229–260. [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(47):12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2005;25(7):1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biological psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Resnick H, Acierno R, Waldrop AE, King L, King D, Danielson C, Kilpatrick D. Randomized controlled evaluation of an early intervention to prevent post-rape psychopathology. Behaviour research and therapy. 2007;45(10):2432–2447. doi: 10.1016/j.brat.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJF, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138(3):901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain: a journal of neurology. 2009;132(Pt 9):2464–2477. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18(20):8455–8466. doi: 10.1523/JNEUROSCI.18-20-08455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual review of neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- VA/DoD. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline For Management of Post-Traumatic Stress. 2010 Retrieved from http://www.healthquality.va.gov/ptsd/cpg_PTSD-FULL-201011612.pdf.
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(23):10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. The Journal of Comparative Neurology. 2006;498(3):317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(29):6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126(2):389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends in Neurosciences. 1999;22(2):51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Williams LM. Recall of childhood trauma: a prospective study of women's memories of child sexual abuse. Journal of consulting and clinical psychology. 1994;62(6):1167–1176. doi: 10.1037//0022-006x.62.6.1167. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ. Learning and memory in combat veterans with posttraumatic stress disorder. The American journal of psychiatry. 1995;152(1):137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bell A, Bierer LM, Schmeidler J. Maternal, not paternal, PTSD is related to increased risk for PTSD in offspring of Holocaust survivors. Journal of Psychiatric Research. 2008;42(13):1104–1111. doi: 10.1016/j.jpsychires.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]