Abstract
Multidrug resistance-associated protein 1 (MRP1/ABCC1) is the first identified member of ABCC subfamily which belongs to ATP-binding cassette (ABC) transporter superfamily. It is ubiquitously expressed in almost all human tissues and transports a wide spectrum of substrates including drugs, heavy metal anions, toxicants, and conjugates of glutathione, glucuronide and sulfate. With the advance of sequence technology, many MRP1/ABCC1 polymorphisms have been identified. Accumulating evidences show that some polymorphisms are significantly associated with drug resistance and disease susceptibility. In vitro reconstitution studies have also unveiled the mechanism for some polymorphisms. In this review, we present recent advances in understanding the role and mechanism of MRP1/ABCC1 polymorphisms in drug resistance, toxicity, disease susceptibility and severity, prognosis prediction, and methods to select and predict functional polymorphisms.
Keywords: multidrug resistance-associated protein 1, ABCC1, single nucleotide polymorphism, drug resistance, prognosis, disease susceptibility
Multidrug resistance-associated protein 1 (MRP1/ABCC1) is a member of the ATP-binding cassette (ABC) transporter superfamily which contains 49 members in human that are divided into 7 subfamilies, named from ABCA to ABCG (http://nutrigene.4t.com/humanabc.htm)[1-2]. MRP1/ABCC1 is the first identified gene in the ABCC subfamily and was cloned from a multidrug resistant small cell lung cancer cell line H69AR[3]. Subsequent studies revealed the important role of MRP1/ABCC1 as an exporter of drugs and metabolites in many physiological, pathological and pharmacological processes. Thus, polymorphism is likely an important feature of MRP1/ABCC1 in disease susceptibility, drug response, and treatment outcomes[4]. In this review, we will evaluate recent advances in discovery of MRP1/ABCC1 polymorphisms and understanding their potential clinical applications.
1 STRUCTURE AND TISSUE DISTRIBUTION
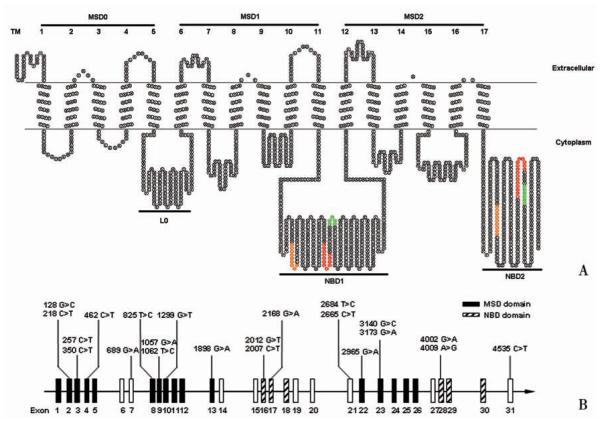
The MRP1/ABCC1 gene is located in chromosome 16p13. 1 and spans approximately 200 kb. It contains 31 exons and encodes a protein of 1 531 amino acid residues with an apparent molecular weight of 180-190 kD[3-5]. MRP1/ABCC1 is an atypical ABC transporter with three membrane-spanning domains (MSD) and two cytosolic nucleotide binding domains (NBD) [6]. While MSD1 and MSD2 each consists of 6 transmembrane ( TM) segments, MSD0 has 5 TM segments with a predicted extracellular amino terminus (Fig. 1A). However, recent studies showed that the amino terminus of human MRP1/ABCC1 may have an unusual U-shaped structure which possibly serves as a gate for MRP1/ABCC1 function[7-9].
Fig. 1.
A:Schematic representation of the topological structure of MRP1/ABCC1 protein predicted using TOPO2 program with modification (http://www.sacs.ucsf.edu/TOPO-run/wtopo.pl). The consensus sequences of Walker A and B are highlighted in orange and green, respectively. The ABC-signature motif is highlighted in red. TM, transmembrane; MSD, membrane spanning domain; NBD, nucleotide-binding domain. B: Distribution of clinically relevant MRP1/ABCC1 exon polymorphisms.
The sequence of MSD is highly divergent among different members of ABC transporter family, consistent with MSD’s possible function in determining substrate specificity[10]. Thus, polymorphisms in this domain may affect the substrate spectrum of MRP1/ABCC1. While a typical ABC transporter has two MSDs, the additional MSD0 of human MRP1/ABCC1 is peculiar and its function is not yet fully elucidated. However, our recent studies showed that MSD0 contributes to MRP1/ABCC1 homo-dimerization[11-12].
In contrast to MSD, NBD is highly conserved among different ABC transporters. It is responsible for binding and hydrolysis of ATP to provide energy for substrate transport[10]. Similar to other ABC transporters, the NBD of MRP1/ABCC1 has two consensus motifs designated as “Walker A” and “Walker B”[13] and a third consensus motif designated as ABC-signature motif of approximate 13 amino acids between Walker A and Walker B[10]. These highly conserved motifs are critical for MRP1/ABCC1 function and a single mutation may abolish the activity of the whole protein[14-15]. Thus, polymorphisms in NBD may produce inactive MRP1/ABCC1.
MRP1/ABCC1 appears to be ubiquitously expressed in almost all human tissues[16-18]. Its expression level is high in lung, spleen, testis, kidney, placenta, thyroid, bladder and adrenal gland, but low or no expression in some cells of circulatory system, such as eosinophils, helper T-cells and erythrocytes[19]. MRP1/ABCC1 is also expressed in blood-brain, blood-testis and blood-cerebrospinal fluid (CSF) barriers, which was thought to contribute to protection of these organs by keeping out toxic substances[20-21]. Indeed, it has been shown that accumulation of etoposide in CSF increased 10-fold in MRP1/ABCC1 knockout mice[20]. At the cellular level, in contrast to the apical membrane location of other ABC transporters, MRP1/ABCC1 is predominantly located in the basolateral membrane of polarized cells[22-23]. Thus, MRP1/ABCC1 likely pumps its substrate into the interstitial space of body, rather than excreting them into bile, urine or gut.
2 SUBSTRATES
MRP1/ABCC1 can transport a wide spectrum of substrates ranging from anticancer drugs to fluorescent dye (Tab. 1). A wide variety of anticancer drugs including anthracyclines, epipodophyllotoxins, vinca alkaloids, camptothecins, methotrexate and mitoxantrone are known substrates of MRP1/ABCC1 and, thus, MRP1/ABCC1 over-expression leads to multidrug resistance in cancer chemotherapy. In addition to anticancer drugs, MRP1/ABCC1 also transports many other types of drugs, such as anti-HIV drugs. Therefore, MRP1/ABCC1 gene polymorphisms may affect patient response to chemotherapy of these diseases. Previously, we have shown that G2168A polymorphism significantly reduced MRP1/ABCC1 activity in resistance to anthracyclines, vinca alkaloids and etoposide[24].
Tab. 1.
Clinically relevant substrates of MRP1/ABCC1 *
| Type of substrates | Examples |
|---|---|
| Drugs | Anticancer drugs |
| Vinca alkaloids: vinblastine and vincristine | |
| Epipodophyllotoxins: etoposide (VP-16) and teniposide | |
| Camptothecins: topotecan, irinotecan and SN-38 | |
| Methotrexate and mitoxantrone | |
| Other drugs | |
| Anti HIV drugs; ritonavir and saquinavir | |
| Antibiotics: difloxacin and grepafloxicin | |
| Tyrosine kinase inhibitors: imatinib mesylate and gefitinib | |
| Heavy metal anions | Arsenite |
| Arsenate | |
| Trivalent and pentavalent antimonials | |
| Glutathione conjugates(-GS) | Dinitrophenyl-GS |
| Etacrynic acid-GS | |
| Doxorubicin-GS | |
| Cyclophosphamide-GS | |
| Melphalan-GS | |
| Aflatoxin B1-epoxide-GS | |
| Hydroxynonenal-GS | |
| Prostaglandin A2-GS | |
| Glutathione (GSH, GSSG) | |
| Glucuronide conjugates (-G) | Bilirubin-G |
| Estradiol 17βD-G | |
| Hyodeoxycholate-G | |
| Etoposide (VP-16)-G | |
| NS-38-G | |
| Sulfate conjugates (-S) | Estrone-3-S |
| Taurocholate-3-S | |
| Dehydroepiandrosterone-3-S | |
| Sulfatolithocholyl taurine | |
| Folates | Folic acid |
| L-leucovorin | |
| Toxicants | Aflatoxin B1 |
| Methoxychlor | |
| Fenitrothion | |
| Others | Leukotrienes C4, D4 and E4 |
| Curcuminoids | |
| Calcein |
Another important group of substrates of MRP1/ABCC1 is organic anion conjugates including glutathione, glucuronides and sulfate conjugates. Transporting these conjugates helps cells to remove toxins and protect tissues from damage[25-26]. LTC4, a very important mediator of inflammatory response which controls vascular permeability and smooth muscle contraction, is another high affinity substrate of MRP1/ABCC1[19,27]. Thus, MRP1/ABCC1 polymorphisms may affect therapeutic efficiency of some LTC4 targeting drugs, such as montelukast and zileuton [28-29].
3 POLYMORPHISMS
A large number of naturally occurrmg MRP1/ABCC1 polymorphisms have been identified with most studies in Asian and Caucasian populations[30-37]. A comprehensive list of naturally occurring MRP1/ABCC1 polymorphisms in different populations can be found in several publicly accessible databases [Pharmacogenetics Research Network: http://www.pharmgkb.org; National Central for Biotechnology Information (NCBI): http://www.ncbi.nlm.nih.gov/snp; Japanese Single Nucleotide Polymorphisms (JSNP) database: http://snp.ims.u-tokyo.ac.jp/; International HapMap Project: www.hapmap.org/].
Most identified MRP1/ABCC1 polymorphisms are single nucleotide polymorphisms (SNPs), although repeats, insertions and deletions are also found. There are vast ethnical differences in MRP1/ABCC1 polymorphism distribution and frequency, especially between Asian and Caucasian. For example, G2168A is a common SNP in the Asian population, but it has not been found in Caucasian[24]. On the contrary, G2012T polymorphism is common in Caucasian, but not found in Asian populations [31]. Most MRP1/ABCC1 polymorphisms have a very low frequency (< 5%), which indicating that MRP1/ABCC1 is a highly conserved gene. The majority of identified polymorphisms are located in the untranslational region (UTR) and introns and few polymorphisms are located in the coding region. Polymorphisms in the coding region are more likely to be functional and can be divided into three types: synonymous (no change in amino acid sequence resulting in a wild-type protein), non-synonymous (change in amino acid sequence resulting in a mutant protein), and nonsense (change to a stop codon resulting in a truncated protein). Up to date, only 14 non-synonymous polymorphisms have been identified with very low frequencies and no nonsense polymorphism has been found (Fig. 1). These non-synonymous polymorphisms were intensively studied both in vitro and in vivo since they could be easily recreated using site-directed mutagenesis and they might affect the expression and function of MRP1/ABCC1[24-39]. Although the polymorphisms in the non-coding region do not affect the sequence of the protein, they are also important and can be used as genetic markers[40-41].
4 ASSOCIATION OF MRP1/ABCC1 POLYMORPHISMS WITH THERAPEUTIC RESPONSE
As discussed above, many therapeutic drugs are substrates of MRP1/ABCC1. Thus, it is conceivable that some MRP1/ABCC1 polymorphisms may affect treatment responses and toxicities. Tab. 2 lists MRP1/ABCC1 polymorphisms that have been studied for their association with therapeutic responses. One of these polymorphisms, G2012T which was first identified by Conrad et al.[34] in Caucasian population, has been extensively studied. It causes mutation of a highly conserved Gly671 to Val. Investigation of its potential relationship with response to atorvastatin in treatment of hypercholesterolemia, telatinib in treatment of solid tumors, and induction therapy of leukemia, however, showed no significant correlattion with treatment responses[42-44]. Consistent with these clinical observations, in vitro studies also showed that the mutant MRP1/ABCC1 carrying this mutation had no detectable difference in drug transport activity from the wild type MRP1/ABCC1 [34], Thus, the G2012T polymorphism may not have functional impact on chemotherapy.
Tab. 2.
Association of MRP1/ABCC1 polymorphisms with therapeutic response
| Polymorphisms | rs number | Amino acid exchange | Location | Drugs | Disease/Observation | References |
|---|---|---|---|---|---|---|
| G2012T | rs45511401 | Gly671Val | Exon 16 | Atorvastatin | Hypercholesterolemia/No correlation | [42] |
| Telatinib | Solid tumor/No correlation | [44] | ||||
| Induction Therapy | Leukemia/No correlation | [43] | ||||
| G4002A | rs2239330 | No change | Exon 28 | Gemcitabine, cisplatin, taxane, methotrexate | Pancreatic cancer/No correlation | [45-47] |
| Citalopram | Major depressive disorder/Strong correlation | [48] | ||||
| G2168A | rs4148356 | Arg723Gln | Exon 17 | Platinum | Ovarian cancer/Correlation | [50] |
| Taxane | Ovarian cancer/Correlation | [50] | ||||
| A4009G | rs28364006 | Alal337Thr | Exon 28 | Methotrexate | Psoriasis/Correlation | [49] |
| IVS23 G-1960A | rs2238476 | No change | Intron 23 | Methotrexate | Psoriasis/Correlation | [49] |
| IVS9 T-176C | rs35592 | No change | Intron 9 | Methotrexate | Psoriasis/Correlation | [49] |
| T2684C | No change | Exon 21 | Leukemic/No correlation | [43] | ||
| C2007T | rs2301666 | No change | Exon 16 | Leukemic/No correlation | [43] | |
| G2012T | rs45511401 | Gly671Val | Exon 16 | Leukemic/No correlation | [43] | |
| C2665T | No change | Exon 21 | Leukemic/No correlation | [43] | ||
| IVS1 C-14840T | rsll9774 | No change | Intron 1 | Montelukast | Asthma/Correlation | [28] |
| Zileuton | Asthma/Correlation | [29] | ||||
| GCC repeat | No change | 5′UTR | Azithromycin | Cystic fibrosis/No correlation | [72] | |
| IVS18 C -30G | rs2074087 | No change | Intron 18 | Taxanes | Ovarian cancer/No correlation | [47] |
Another extensively studied polymorphism is G4002A, a synonymous SNP located in exon 28. Several studies exploring the correlation of G4002A polymorphism and responses to anticancer drugs gemcitabine, cisplatin, taxanes and methotrexate showed no significant association in pancreatic cancer patients [45-47]. However, Lee et al. [48] found that this polymorphism was strongly associated with the response of patients with major depressive disorder to antidepressant citalopram. Although patients with the G4002A polymorphism had a 4. 7-fold increase in citalopram response, there is no evidence that G4002A polymorphism of MRP1/ABCC1 in the blood-brain barrier affects citalopram uptake and if citalopram is a substrate of MRP1/ABCC1. Another non-synonymous polymorphism located in exon 28, A4009G, was found to correlate with methotrexate therapeutic efficacy in a study of 374 chronic plaque psoriasis patients who received methotrexate monotherapy[49]. It was found that the heterozygous A4009G in the responders is significantly higher than that in non-responders, suggesting that the A4009G polymorphism may increase methotrexate responses. However, it has not yet been determined if the A4009G polymorphism affects MRP1/ABCC1 expression, trafficking, or function. Future studies on the possible effects of the A4009G polymorphism on these aspects of MRP1/ABCC1 are needed.
A well studied polymorphism that has been shown to significantly reduce drug transport activity of MRP1/ABCC1 is G2168A[24]. It has also been shown to increase chemotherapy response in advanced ovarian cancer patients[50]. In the study of advanced ovarian cancer patients, several other polymorphisms of MRP1/ABCC1 (T825C, T1062C, T1684C, C2007T and G4002A) were also investigated. However, none of these polymorphisms were found to significantly associate with chemotherapy responses. Thus, the G2168A polymorphism may be an indicator of chemotherapy response of advanced ovarian cancers. However, whether this polymorphism also affects chemotherapy responses of other human cancers need to be investigated.
In addition to the polymorphisms in the coding region, some polymorphisms in the non-coding region of MRP1/ABCC1 are also found to associate with drug responses. Two such polymorphisms in the non-coding region are IVS23 G-1960A and IVS9 T-176C located in intron 23 and 9, respectively. They both have been shown to significantly associate with methotrexate response in psoriasis patients and patients carrying these polymorphisms appear to have worse response to methotrexate treatment[49]. Another example of polymorphisms in the non-coding region is IVSI C-14840T which is located in intron 1 and has been found to correlate with significantly higher response to both montelukast and zileuton in asthma patients than wild-type homozygotes [28-29]. Thus, polymorphisms in MRP1/ABCC1 may affect montelukast and zileuton response and lung function. Interestingly, in another study of two independent cohorts, polymorphisms of MRP1/ABCC1 in the 3'-UTR (G3361A and A2615G) and IVS14 C-1575T also significantly correlate with lung function[51]. While 3'-UTR G3361A correlates with higher forced expiratory volume at one second (FEV1), 3'-UTR A2615G correlates with lower FEVI. Another polymorphism, IVS14 C-1575T in the intron 14 of MRP1/ABCC1, correlates with highly excessive FEVI decline. However, how these polymorphisms in the non-coding region possibly affect MRP1/ABCC1 is not yet known. It is also unknown if MRP1/ABCC1 plays any role in lung function. While the polymorphisms in the UTR may affect the translation and expression of MRP1/ABCC1, the polymorphisms in the intron may affect RNA processing. Clearly, these hypothetical mechanisms of action and the role of MRP1/ABCC1 in lung function needs to be investigated in the near future.
5 ASSOCIATION OF MRP1/ABCC1 POLYMORPHISMS WITH PROGNOSIS PREDICTION
Based on the above discussion of association of MRP1/ABCC1 polymorphisms with therapeutic response, it is tempting to speculate that polymorphisms of MRP1/ABCC1 may be used as markers to predict prognosis. Indeed, two polymorphisms have been shown to associate with prognosis (Tab. 3). In a study of possible contribution of four non-synonymous polymorphisms of MRP1/ABCC1 to neuroblastoma outcome in a cohort of 195 Caucasian patients, it was found that the presence of the G2010T polymorphism has significant improvement in outcome[52]. It was also found that the G2010T polymorphism reduces the stability and expression level of MRP1/ABCC1 mRNA. Hence, it is possible that patients with the G2010T polymorphism may have reduced level of MRP1/ABCC1, which would enhance drug response and increase chemotherapy efficacy. In another study of correlating 5'-UTR G-1666A polymorphism with hepatocellular carcinoma (HCC) outcome in 162 Chinese patients, it was found that the mutant genotype carriers had better prognosis with increased 4-year disease free survival[53]. Using in vitro electrophoretic mobility shift assay (EMSA), these authors also found that the mutant allele had much less binding affinity to nuclear proteins, suggesting that this promoter polymorphism may cause decreased transcription of MRP1/ABCC1. However, whether this promoter polymorphism inhibits MRP1/ABCC1 transcription has not yet been demonstrated. It is also unknown if the nuclear proteins that bind to this region are involved in the transcription of MRP1/ABCC1. Never-theless, these polymorphisms may be used as makers predicting prognosis and survival in neuroblastoma and HCC.
Tab. 3.
Association of MRP1/ABCC1 polymorphisms with prognosis prediction
6 ASSOCIATION OF MRP1/ABCC1 POLYMORPHISMS WITH DRUG TOXICITY
Since some toxicants and drug metabolites are also substrates of MRP1/ABCC1, possible association of MRP1/ABCC1 polymorphisms and drug toxicity is also of importance and interest to investigate. In this regard, correlation of MRP1/ABCC1 polymorphisms and drug-induced neuropathy is mostly studied (Tab. 4). In a recent study correlating polymorphisms of MRP1/ABCC1 (IVS9A8G, IVS11C-48T, T1684C, IVS18C-30G, G4002A and IVS30A18G) with irinotecan-induced neutropenia in cancer patients, it was found that the TT genotype carriers of IVSI1 C-48T had significant lower neutrophil count (ANC) in patients receiving irinotecan monotherapy[54]. Irinotecan-induced neutropenia is thought to be due to production of the cytotoxic irinotecan metabolite, SN-38, which is a substrate of MRP1/ABCC1. Consistent with this study, MRP1/ABCC1 polymorphism has also been found to correlate with peripheral neuropathy induced by vincristine[55]. In this study of 833 myeloma patients, it was found that the carriers of MRP1/ABCC1 polymorphism IVS16 A1695T were more likely to develop vincristine-induced peripheral neuropathy than the wild type carriers. Similar to SN-38, it is also speculated that this polymorphism may decrease MRP1/ABCC1-mediated transport of vincristine and, thus, increases vincrinstine-induced peripheral neuropathy. However, the molecular mechanisms need further investigation.
Tab. 4.
Association of MRP1/ABCCl polymorphisms with drug toxicity
| Polymorphisms | rs number | Amino acid exchange | Location | Drugs | Drug toxicity/Disease/Observation | References |
|---|---|---|---|---|---|---|
| G4002A | rs2239330 | No change | Exon 28 | Irinotecan | Neutropenia/Solid tumor/Correlation | [54] |
| Methotrexate | Overall MTX toxicity/Rheumatoid arthritis/No correlation | [46] | ||||
| IVS11 -48C > T | rs3765129 | No change | Intron 11 | Irinotecan | Neutropenia/Solid tumor/Correlation | [54] |
| IVS9 A8G | rs35588 | No change | Intron 9 | Irinotecan | Neutropenia/Solid tumor/No correlation | [54] |
| T1684C | rs35605 | No change | Exon 13 | Irinotecan | Neutropenia/Solid tumor/No correlation | [54] |
| IVS30 A18G | rs212088 | No change | Intron 30 | Irinotecan | Neutropenia/Solid tumor/No correlation | [54] |
| IVS3 G-3198A | rsll075291 | No change | Intron 3 | Methotrexate | Hepatic and gastrointestinal toxicity/Psoriasis/Correlation | [49] |
| IVS4 G409A | rsl967120 | No change | Intron 4 | Methotrexate | Hepatic and gastrointestinal toxicity/Psoriasis/Correlation | [49] |
| IVS5 G413A | rs3784862 | No change | Intron 5 | Methotrexate | Hepatic and gastrointestinal toxicity/Psoriasis/Correlation | [49] |
| IVS5 A-7942G | rs246240 | No change | Intron 5 | Methotrexate | Hepatic and gastrointestinal toxicity/Psoriasis/Correlation | [49] |
| IVS5 G-1641A | rs3784864 | No change | Intron 5 | Methotrexate | Hepatic and gastrointestinal toxicity/Psoriasis/Correlation | [49] |
| IVS23 G-1960A | rs2238476 | No change | Intron 23 | Methotrexate | Hepatic and gastrointestinal toxicity/Psoriasis/Correlation | [49] |
| IVS14 C115T | No change | Intron 14 | Methotrexate | Overall MTX toxicity/Rheumatoid arthritis/No correlation | [46] | |
| IVS18 C-30G | rs2074087 | No change | Intron 18 | Methotrexate | Overall MTX toxicity/Rheumatoid arthritis/No correlation | [46] |
| G2012T | rs45511401 | Gly671Val | Exon 16 | Doxorubicin | Cardiotoxicity/NHL/Correlation | [56] |
| IVS16 A1695T | rs3887412 | No change | Intron 16 | Vincristine | Peripheral neuropathy/Multiple myeloma/Correlation | [55] |
One interesting polymorphism is G2012T, which shows correlation with doxorubicin toxicity in non-Hodgkin lymphoma patients[56]. The patients with this polymorphism have more anthracycline-induced cardiotoxicity than the wild-type patients. It was thought that the special subcellular localization of MRP1/ABCC1 in cardiomyocytes, in both plasma and lysosome membranes, permits sequestration of doxorubicin in lysosomes and prevent doxorubicin cardiotoxicity[17-57]. However, it has been demonstrated previously that the G2012T polymorphism of MRP1/ABCC1 has no effect on its function and substrate transport activity[34]. Thus, it is not clear how this polymorphism affects anthrocycline-induced cardiotoxicity. Furthermore, since multidrug chemotherapy was used for these cohorts of patients, interpretation of these observations should be cautious. Doxorubicin mono-therapy and further investigation of G2012T mutation on MRP1/ABCC1 activity in transporting doxorubicin would help clarify this issue.
Several other polymorphisms of MRP1/ABCC1 (IVS3 G-3198A, IVS4 G409A, IVS5G413A, IVS5 A-7942G, IVS5G-1641A and IVS23 G-1960A) have been found to significantly correlate with methotrexate toxicity in liver and GI tract of psoriasis patients[49]. All these polymorphisms are located in introns and form a haplotype although it is not yet known if they affect the expression of MRP1/ABCC1 individually or as a haplotype. Based on the above discussion, MRP1/ABCC1 polymorphisms are likely important genetic indicators in drug toxicity during chemotherapy.
7 ASSOCIATION OF MRP1/ABCC1 POLYMORPHISMS WITH DISEASE SUSCEPTIBILITY AND SEVERITY
Association of MRP1/ABCC1 with disease susceptibility has also been identified (Tab. 5). In a case control study of 500 lung cancer patients and 517 cancer free control subjects in Chinese population, Wang et al. [58] detected the association of three polymorphisms in the 3'-UTR of MRP1/ABCC1 (C543T, T866A and T1512C) with lung cancer susceptibility. They found that subjects carrying mutant allele of 3'-UTR T866A had an increased risk of lung cancer. However, the other two polymorphisms had no significant correlation with lung cancer susceptibility. Further investigation showed that these three polymorphisms form a haplotype and the GTA haplotype was associated with increased risk of lung cancer compared with the most prevailing AAA haplotype. Therefore, this polymorphism haplotype may increase lung cancer predisposition in Chinese population. We recently identified association of another non-synonymous polymorphism G2168A with lung cancer susceptibility[59]. In our study of 77 lung cancer patients and 71 control individuals in Chinese population, we showed that the subjects carrying the G2168A allele had 3. 5 fold increased risk (adjusted OR = 3. 42; 95% CI, 1. 29 - 9. 06; P =0. 013) of lung cancer compared with wild-type carriers. Further stratified analysis showed that the elderly people (> 50 years) carrying mutant allele of this polymorphisms were more likely to develop lung cancer (adjusted OR, 4. 10; 95% CI, 1. 25-13. 48; P =0. 020) than younger ones. Taken together, it is possible that MRP1/ABCC1 polymorphisms may play important roles in lung cancer susceptibility. Although the mechanism of MRP1/ABCC1 action in lung cancer susceptibility is unknown, it is tempting to speculate that MRP1/ABCC1 may protect lung tissues against carcinogens by preventing them from entering bronchial epithelial cells. Carriers of these MRP1/ABCC1 polymorphisms are likely more susceptible to carcinogenesis due to reduced protection by MRP1/ABCC1. This possibility is consistent with our observation that the G2168A polymorphism decreases MRP1/ABCC1 function in drug transport activity (unpublished observations). However, further clinical studies are needed to test this possibility.
Tab. 5.
Association of MRP1/ABCC1 polymorpliisms witli disease susceptibility and severity
| Polymorphisms | rs number | Amino acid exchange | Location | Diseases | Phenotype/Observation | References |
|---|---|---|---|---|---|---|
| 3′-UTRT866A | rs212090 | No change | 3′-UTR | Lung cancer | Susceptibility/Correlation | [58] |
| COPD | Severity/Correlation | [61] | ||||
| 3′-UTR C543T | rs3743527 | No change | 3′-UTR | Lung cancer | Susceptibility/No correlation | [58] |
| 3′-UTRT1512C | rs212091 | No change | 3′-UTR | Lung cancer | Susceptibility/No correlation | [58] |
| T825C | rs246221 | No change | Exon 8 | Autism | Susceptibility/No correlation | [73] |
| G2168A | r9tt48356 | Arg723Gln | Exon 17 | Lung cancer | Susceptibility/Correlation | [59] |
| 5′-UTR G -260C | rs504348 | No change | Promoter | Cystic fibrosis | Severity/Correlation | [60] |
| 3′-UTR G3361A | rs4148382 | No change | 3′-UTR | COPD | Severity/Correlation | [61] |
| Lung function | Severity/Correlation | [51] | ||||
| 5′-UTR C435G | rs504348 | No change | 5′-UTR | COPD | Severity/No correlation | [61] |
| IVS1 T5977G | rs4781699 | No change | Intron 1 | COPD | Severity/No correlation | [61] |
| IVS14 C-1575T | rs35621 | No change | Intron 14 | COPD | Severity/No correlation | [61] |
| Lung function | Severity/Correlation | [51] | ||||
| 3′-UTR A2615G | rs212093 | No change | 3′-UTR | Lung function | Severity/Correlation | [51] |
Possible impact of MRP1/ABCC1 polymorphisms on disease severity has also been reported in other studies (Tab. 5). In a study of 203 cystic fibrosis (CF) patients, it was found that the G-260C polymorphism in the 5'-UTR of MRP1/ABCC1 significantly increased CF severity[60]. Patients with CC genotype had earlier onset of chronic colonization by Pseudomonas aeruginosa (PA). Although in vitro study showed no impact of this polymorphism on promoter transcriptional activity, mRNA levels, basal and cAMP-induced anion transport, the possibility that this polymorphism affects translation/synthesis of MRP1/ABCC1 and, thus, its expression level cannot be ruled out.
In another study of five MRP1/ABCC1 polymorphisms (3'-UTR T866A, 3'-UTR G3361A, 5'-UTR C-435G, IVSl T5977G and IVS14 C-1575T) and their possible effect on chronic obstructive pulmonary disease (COPD) severity, it was found that the 3'-UTR T866A was associated with higher FEV1 level and less airway wall inflammation while the 3'-UTR G3361A was associated with lower FEV1 level and higher inflammation. However, the other three polymorphisms have no significant association with COPD severity[61]. The mechanism of the 3'-UTR T866A in affecting COPD severity remains unknown. However, it is speculated that 3'-UTR T866A may affect MRP1/ABCC1 mRNA stability together with another 3'-UTR polymorphism 801 C > GR[51-61] They were found to be in complete linkage disequilibrium[40]. Clearly, MRP1/ABCC1 polymorphisms are likely associated with lung cancer susceptibility and with COPD and CF disease severity. However, whether and how each polymorphism possibly affects disease susceptibility and severity need to be investigated in the future.
8 CONCLUSIONS SPECTIVES
Since the discovery of MRP1/ABCC1 in 1992, many MRP1/ABCC1 polymorphisms have been identified. Most of the identified polymorphisms are synonymous and have low frequency, indicating that MRP1/ABCC1 is a highly conserved gene. Some of the MRP1/ABCC1 polymorphisms have been found to associate with drug response, prognosis, toxicity, disease susceptibility and severity. Some of these polymorphisms have also been shown to affect MRP1/ABCC1 expression or function which may indicate the underling mechanism of association with the observed phenotype. With the advances of next generation sequencing, International HapMap Project and 1 000 Genomes Project[62-63], more MRP1/ABCC1 polymorphisms are likely to be identified. However, identifying functional MRP1/ABCC1 polymorphisms and their mechanisms of action will not be easy. Thus, both opportunities and challenges exist.
Because not every polymorphism is functional, selecting potentially functional polymorphisms for further clinical relevance study is important considering the large number of polymorphisms is to be identified. Use of in silico and bioinformatics tools such as SIFT, PANTHER and Polyphen algorithms to detect sequence conservation can help identify the likely functional polymorphism since sequences that are highly conserved across different species tend to be functionally important[64-66]. However, this strategy should be used with caution due to both false positive and negative predictions. For example, G689A, G1057A and G3173A polymorphisms of MRP1/ABCC1 are predicted as deleterious polymorphisms using SIFT. However, none of these polymorphisms adversely affects MRP1/ABCC1 function[24-39].
Examination of polymorphism databases shows that most polymorphisms are located in introns and UTRs. In addition, some polymorphisms located in the exons are synonymous polymorphisms. Thus, study of sequence conservation will unlikely be able to predict if these polymorphisms are functional. For these polymorphisms, a genome-wide approach to identify polymorphisms of positive and negative selection is helpful[41-67]. Positive selection is an evolutionary process and the positively selected polymorphisms contribute to the favorable phenotype of species and, thus, these polymorphisms may be of higher frequency in the population and important for the gene function[66-68]. Opposite to positive selection, negative selection is the decline of disadvantage phenotype and harmful and, thus, the negatively selected polymorphisms usually have very low frequency (minor allele frequency < 0. 05) in the population although they may be important for the function and rare drug adverse effects[66]. Both strategies have been used to identify functional MRP1/ABCC1 polymorphisms[38,41,56,67]. However, it is noteworthy that combination of sequence conservation and evolutionary features may be more powerful than any approach alone to predict and identify functional polymorphisms.
Another challenge is to understand how each polymorphism affects gene function. While it is easy to study the effect of the non-synonymous polymorphisms on the structure and function of MRP1/ABCC1 by re-creating the mutant protein and analyzing the protein in cell lines[24-39], it is challenging to investigate the synonymous or non-coding region polymorphisms due to complexity of their functional gene effect by different mechanisms such as transcription, splicing, RNA stability, and combined haplotype [40, 49, 53, 69-70].
Acknowledgments
Foundation items This work was support ed by a grant from NIH (ROI CA120221) and China Scholarship Council and Scholarship Award for Excellent Doctoral Student granted by Ministry of Education of China.
Biography
YIN Jiye , Ph . D. , mainly engaged in the research of pharmacogenetics.
REFERENCES
- [1].Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters[J] Int J Toxicol. 2006;25(4):231–259. doi: 10.1080/10915810600746023. [DOI] [PubMed] [Google Scholar]
- [2].Mo W, Zhang JT. Oligomerization of human ATP-binding cassette transporters and its potential significance in human disease [J] Expert Opin Drug Metab Toxicol. 2009;5(9):1049–1063. doi: 10.1517/17425250903124371. [DOI] [PubMed] [Google Scholar]
- [3].Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line[J] Science. 1992;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- [4].Conseil G, Deeley RG, Cole SP. Polymorphisms of MRP1(ABCC1) and related ATP-dependent drug transporters [J] Pharmacogenet Genomics. 2005;15(8):523–533. doi: 10.1097/01.fpc.0000167333.38528.ec. [DOI] [PubMed] [Google Scholar]
- [5].Grant CE, Kurz EU, Cole SP, et al. Analysis of the intron-exon organization of the human multidrug-resistance protein gene (MRP) and alternative splicing of its mRNA [J] Genomics. 1997;45(2):368–378. doi: 10.1006/geno.1997.4950. [DOI] [PubMed] [Google Scholar]
- [6].Mo W, Liu JY, Zhang JT. Biochemistry and pharmacology of human ABCC1/MRP1 and its role in detoxification and in multidrug resistance of cancer chemotherapy in recent advances on cancer research and therapy[M] In: Pestka S, Shi Y, Liu XY, editors. Recent advances on cancer research and therapy. Elsevier; 2011. in press. [Google Scholar]
- [7].Yang Y, Chen Q, Zhang JT. Structural and functional consequences of mutating cysteine residues in the amino terminus of human multidrug resistance-associated protein 1 [J] J Biol Chem. 2002;277(46):44268–44277. doi: 10.1074/jbc.M207003200. [DOI] [PubMed] [Google Scholar]
- [8].Chen Q, Yang Y, Li L, et al. The amino terminus of the human multidrug resistance transporter ABCC1 has a U-shaped folding with a gating function [J] J Biol Chem. 2006;281(41):31152–31163. doi: 10.1074/jbc.M603529200. [DOI] [PubMed] [Google Scholar]
- [9].Chen Q, Yang Y, Liu Y, et al. Cytoplasmic retraction of the amino terminus of human multidrug resistance protein 1 [J] Biochemistry. 2002;41(29):9052–9062. doi: 10.1021/bi025634s. [DOI] [PubMed] [Google Scholar]
- [10].Hipfner DR, Deeley RG, Cole SP, et al. Structural, mechanistic and clinical aspects of MRP1 [J] Biochim biophys Acta. 1999;1461(2):359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- [11].Yang Y, Mo W, Zhang JT. Role of transmembrane segment 5 and extracellular loop 3 in the homodimerization of human AB-CC1[J] Biochemistry. 2010;49(51):10854–10861. doi: 10.1021/bi101350x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang Y, Liu Y, Dong Z, et al. Regulation of function by dimerization through the amino-terminal membrane-spanning domain of human ABCC1/MRP1 [J] J Biol Chem. 2007;282(12):8821–8830. doi: 10.1074/jbc.M700152200. [DOI] [PubMed] [Google Scholar]
- [13].Walker JE, Saraste M, Runswick MJ, et al. Distantly related sequences in the alpha-and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold[J] EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Szentpétery Z, Sarkadi B, Bakos E, et al. Functional studies on the MRP1 multidrug transporter: characterization of ABC-signature mutant variants[J] Anticancer Res. 2004;24(2A):449–455. [PubMed] [Google Scholar]
- [15].Szentpétery Z, Kern A, Liliom K, et al. The role of the conserved glycines of ATP-binding cassette signature motifs of MRP1 in the communication between the substrate-binding site and the catalytic centers[J] J Biol Chem. 2004;279(40):41670–41678. doi: 10.1074/jbc.M406484200. [DOI] [PubMed] [Google Scholar]
- [16].Zaman GJ, Versantvoort CH, Smit JJ, et al. Analysis of the expression of MRP, the gene for a new putative transmembrane drug transporter, in human multidrug resistant lung cancer cell lines[J] Cancer Res. 1993;53(8):1747–1750. [PubMed] [Google Scholar]
- [17].Flens MJ, Zaman GJ, Van der Valk P, et al. Tissue distribution of the multidrug resistance protein [J] Am J Pathol. 1996;148(4):1237–1247. [PMC free article] [PubMed] [Google Scholar]
- [18].St-Pierre MV, Serrano MA, Macias RI, et al. Expression of members of the multidrug resistance protein family in human term placenta [J] Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- [19].Chang XB. A molecular understanding of ATP-dependent solute transport by multidrug resistance-associated protein MRP1 [J] Cancer Metastasis Rev. 2007;26(1):15–37. doi: 10.1007/s10555-007-9041-7. [DOI] [PubMed] [Google Scholar]
- [20].Wijnholds J, deLange EC, Scheffer GL, et al. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier [J] J Clin Invest. 2000;105(3):279–285. doi: 10.1172/JCI8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mercier C, Masseguin C, Roux F, et al. Expression of P-glycoprotein(ABCB1) and Mrp1(ABCC1) in adult rat brain: focus on astrocytes[J] Brain Res. 2004;1021(1):32–40. doi: 10.1016/j.brainres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- [22].Evers R, Zaman GJ, van Deemter L, et al. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells[J] J Clin Invest. 1996;97(5):1211–1218. doi: 10.1172/JCI118535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roelofsen H, Vos TA, Schippers IJ, et al. Increased levels of the multidrug resistance protein in lateral membranes of proliferating hepatocyte-derived cells [J] Gastroenterology. 1997;112(2):511–521. doi: 10.1053/gast.1997.v112.pm9024305. [DOI] [PubMed] [Google Scholar]
- [24].Yin JY, Huang Q, Yang Y, et al. Characterization and analyses of multidrug resistance-associated protein 1 (MRP1/ABCC1) polymorphisms in Chinese population[J] Pharmacogenet Genomics. 2009;19(3):206–216. doi: 10.1097/FPC.0b013e328323f680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Leslie EM, Deeley RG, Cole CP. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters[J] Toxicology. 2001;167(1):3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- [26].Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cmoat/MRP2 [J] Semin Liver Dis. 1998;18(4):359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- [27].Leier I, Jedlitschky G, Buchholz U, et al. Characterization of the ATP-dependent leukotriene C4 export carrier in mastocytoma cells[J] Eur J Biochem. 1994;220(2):599–606. doi: 10.1111/j.1432-1033.1994.tb18661.x. [DOI] [PubMed] [Google Scholar]
- [28].Lima JJ, Zhang S, Grant A, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma [J] Am J Respir Crit Care Med. 2006;173(4):379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tantisira KG, Lima J, Sylvia J, et al. 5-lipoxygenase pharmacogenetics in asthma: overlap with cys-leukotriene receptor antagonist loci [J] Pharmacogenet Genomics. 2009;19(3):244–247. doi: 10.1097/FPC.0b013e328326e0b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Saito S, Iida A, Sekine A, et al. Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR) [J] J Hum Genet. 2002;47(4):147–171. doi: 10.1007/s100380200018. [DOI] [PubMed] [Google Scholar]
- [31].Moriya Y, Nakamura T, Horinouchi M, et al. Effects of polymorphisms of MDR1, MRP1, and MRP2 genes on their mRNA expression levels in duodenal enterocytes of healthy japanese subjects[J] Biol Pharm Bull. 2002;25(10):1356–1359. doi: 10.1248/bpb.25.1356. [DOI] [PubMed] [Google Scholar]
- [32].Oselin K, Mrozikiewicz PM, Gaikovitch E, et al. Frequency of MRP1 genetic polymorphisms and their functional significance in caucasians: detection of a novel mutation G816a in the human MRP1 gene[J] Eur J Clin Pharmacol. 2003;59(4):347–350. doi: 10.1007/s00228-003-0625-z. [DOI] [PubMed] [Google Scholar]
- [33].Fukushima-Uesaka H, Saito Y, Tohkin M, et al. Genetic variations and haplotype structures of the ABC transporter gene AB-CC1 in a japanese population[J] Drug Metab Pharmacokinet. 2007;22(1):48–60. doi: 10.2133/dmpk.22.48. [DOI] [PubMed] [Google Scholar]
- [34].Conrad S, Kauffmann HM, Ito K, et al. Identification of human multidrug resistance protein 1(MRP1) mutations and characterization of a G671V substitution[J] J Hum Genet. 2001;46(11):656–663. doi: 10.1007/s100380170017. [DOI] [PubMed] [Google Scholar]
- [35].Perdu J, Germain DP. Identification of novel polymorphisms in the pM5 and MRP1(ABCC1) genes at locus 16p13. 1 and exclusion of both genes as responsible for pseudoxanthoma elasticum[J]. Hum Mutat. 2001;17(1):74–75. doi: 10.1002/1098-1004(2001)17:1<74::AID-HUMU14>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [36].Wang H, Hao B, Zhou K, et al. Linkage disequilibrium and haplotype architecture for two ABC transporter genes (ABCC1 and ABCG2) in Chinese population: implications for pharmacogenomic association studies[J] Ann Hum Genet. 2004;68:563–573. doi: 10.1046/j.1529-8817.2003.00124.x. Pt 6. [DOI] [PubMed] [Google Scholar]
- [37].Ito S, Ieiri I, Tanabe M, et al. Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cmoat, in healthy japanese subjects[J] Pharmacogenetics. 2001;11(2):175–184. doi: 10.1097/00008571-200103000-00008. [DOI] [PubMed] [Google Scholar]
- [38].Wang Z, Sew PH, Ambrose H, et al. Nucleotide sequence analyses of the MRP1 gene in four populations suggest negative selection on its coding region [J] BMC Genomics. 2006;7:111. doi: 10.1186/1471-2164-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Létourneau IJ, Deeley RG, Cole SP. Functional characterization of non-synonymous single nucleotide polymorphisms in the gene encoding human multidrug resistance protein 1 (MRP1/ABCC1) [J] Pharmacogenet Genomics. 2005;15(9):647–657. doi: 10.1097/01.fpc.0000173484.51807.48. [DOI] [PubMed] [Google Scholar]
- [40].Leschziner G, Zabaneh D, Pirmohamed M, et al. Exon sequencing and high resolution haplotype analysis of ABC transporter genes implicated in drug resistance[J] Pharmacogenet Genomics. 2006;16(6):439–450. doi: 10.1097/01.fpc.0000197467.21964.67. [DOI] [PubMed] [Google Scholar]
- [41].Wang Z, Wang B, Tang K, et al. A functional polymorphism within the MRP1 gene locus identified through its genomic signature of positive selection [J] Hum Mol Genet. 2005;14(14):2075–2087. doi: 10.1093/hmg/ddi212. [DOI] [PubMed] [Google Scholar]
- [42].Moretti IR, Cristina AR, Sorkin SA, et al. ABCB1 and ABCC1 expression in peripheral mononuclear cells is influenced by gene polymorphisms and atorvastatin treatment [J] Biochem Pharmacol. 2009;77(1):66–75. doi: 10.1016/j.bcp.2008.09.019. [DOI] [PubMed] [Google Scholar]
- [43].Mahjoubi F, Akbari S, Montazeri M, et al. MRP1 polymorphisms(T2684C, C2007T, C2012T, and C2665T) are not associated with multidrug resistance in leukemic patients[J] Genet Mol Res. 2008;7(4):1369–1374. doi: 10.4238/vol7-4gmr482. [DOI] [PubMed] [Google Scholar]
- [44].Steeghs N, Gelderblom H, Wessels J, et al. Pharmacogenetics of telatinib, a VEGFR-2 and VEGFR-3 tyrosine kinase inhibitor, used in patients with solid tumors[J] Invest New Drugs. 2011;29(1):137–143. doi: 10.1007/s10637-009-9347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tanaka M, Okazaki T, Suzuki H, et al. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome[J] Cancer. 2011;117(4):744–751. doi: 10.1002/cncr.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ranganathan P, Culverhouse R, Marsh S, et al. Methotrexate (MTX) pathway gene polymorphisms and their effects on MTX toxicity in caucasian and african american patients with rheumatoid arthritis[J] J Rheumatol. 2008;35(4):572–579. [PubMed] [Google Scholar]
- [47].Marsh S, Paul J, King CR, et al. Pharmacogenetic assessment of toxicity and outcome after platinum plus taxane chemotherapy in ovarian cancer: the scottish randomised trial in ovarian cancer [J] J Clin Oncol. 2007;25(29):4528–4535. doi: 10.1200/JCO.2006.10.4752. [DOI] [PubMed] [Google Scholar]
- [48].Lee SH, Lee MS, Lee JH, et al. MRP1 polymorphisms associated with citalopram response in patients with major depression[J] J Clin Psychopharmacol. 2010;30(2):116–125. doi: 10.1097/JCP.0b013e3181d2ef42. [DOI] [PubMed] [Google Scholar]
- [49].Warren RB, Smith RL, Campalani E, et al. Genetic variation in efflux transporters influences outcome to methotrexate therapy in patients with psoriasis [J] J Invest Dermatol. 2008;128(8):1925–1929. doi: 10.1038/jid.2008.16. [DOI] [PubMed] [Google Scholar]
- [50].Obata H, Yahata T, Quan J, et al. Association between single nucleotide polymorphisms of drug resistance-associated genes and response to chemotherapy in advanced ovarian cancer[J] Anticancer Res. 2006;26(3B):2227–2232. [PubMed] [Google Scholar]
- [51].Siedlinski M, Boezen HM, Boer JM, et al. ABCC1 polymorphisms contribute to level and decline of lung function in two population-based cohorts [J] Pharmacogenet Genomics. 2009;19(9):675–684. doi: 10.1097/FPC.0b013e32832f5eff. [DOI] [PubMed] [Google Scholar]
- [52].Pajic M, Murray J, Marshall GM, et al. ABCC1 G2012T single nucleotide polymorphism is associated with patient outcome in primary neuroblastoma and altered stability of the ABCC1 gene transcript[J] Pharmacogenet Genomics. 2011;21(5):270–279. doi: 10.1097/FPC.0b013e328343dd5f. [DOI] [PubMed] [Google Scholar]
- [53].Zhao J, Yu BY, Wang DY, et al. Promoter polymorphism of MRP1 associated with reduced survival in hepatocellular carcinoma[J] World J Gastroenterol. 2010;16(48):6104–6110. doi: 10.3748/wjg.v16.i48.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Innocenti F, Kroetz DL, Schuetz E, et al. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics[J] J Clin Oncol. 2009;27(16):2604–2614. doi: 10.1200/JCO.2008.20.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Annemiek B, Corthals SL, Jongen LJ, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the hovon-65/GMMG-HD4 trial [J] Lancet Oncol. 2010;11(11):1057–1065. doi: 10.1016/S1470-2045(10)70206-0. [DOI] [PubMed] [Google Scholar]
- [56].Wojnowski L, Kulle B, Schirmer M, et al. NAD(P) H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity [J] Circulation. 2005;112(24):3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- [57].Rajagopal A, Simon SM. Subcellular localization and activity of multidrug resistance proteins[J] Mol Biol Cell. 2003;14(8):3389–3399. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wang H, Jin G, Wang H, et al. Genetic susceptibility of lung cancer associated with common variants in the 3'untranslated regions of the adenosine triphosphate-binding cassette B1 (ABCB1) and ABCC1 candidate transporter genes for carcinogen export[J] Cancer. 2009;115(3):595–607. doi: 10.1002/cncr.24042. [DOI] [PubMed] [Google Scholar]
- [59].Yin JY, Han LF, Huang Q, et al. ABCC1 polymorphism Arg723Gln (2168G > A) is associated with lung cancer susceptibility in a Chinese population [J] Clin Exp Pharmacol Physiol. 2011;38(9):632–637. doi: 10.1111/j.1440-1681.2011.05571.x. [DOI] [PubMed] [Google Scholar]
- [60].Mafficini A, Ortombina M, Sermet-Gaudelius I, et al. Impact of polymorphism of multidrug resistance-associated protein 1 ABCC1 gene on the severity of cystic fibrosis [J] J Cyst Fibros. 2011;10(4):228–233. doi: 10.1016/j.jcf.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [61].Budulac SE, Postma DS, Hiemstra PS, et al. Multidrug resistance-associated protein-1 (MRP1) genetic variants, MRP1 protein levels and severity of COPD[J] Respir Res. 2010;11:60. doi: 10.1186/1465-9921-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].International HapMap Consortium The international hapmap project[J] Nature. 2003;426(6968):789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- [63].Pennisi E. Genomics. 1000 genomes project gives new map of genetic diversity[J] Science. 2010;330(6004):574–575. doi: 10.1126/science.330.6004.574. [DOI] [PubMed] [Google Scholar]
- [64].Thomas PD, Campbell MJ, Kejariwal A, et al. PANTHER: a library of protein families and subfamilies indexed by function [J] Genome Res. 2003;13(9):2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ng PC, Henikoff S. Predicting deleterious amino acid substitutions[J] Genome Res. 2001;11(5):863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pang GS, Wang J, Wang Z, et al. Predicting potentially functional SNPs in drug-response genes [J] Pharmacogenomics. 2009;10(4):639–653. doi: 10.2217/pgs.09.12. [DOI] [PubMed] [Google Scholar]
- [67].Wang Z, Wang J, Tantoso E, et al. Signatures of recent positive selection at the ATP-binding cassette drug transporter superfamily gene loci [J] Hum Mol Genet. 2007;16(11):1367–1380. doi: 10.1093/hmg/ddm087. [DOI] [PubMed] [Google Scholar]
- [68].Bamshad M, Wooding SP. Signatures of natural selection in the human genome [J] Nat Rev Genet. 2003;4(2):99–111. doi: 10.1038/nrg999. [DOI] [PubMed] [Google Scholar]
- [69].Mishra PJ, Humeniuk R, Longo-sorbello GS, et al. A miR-24 microrna binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance [J] Proc Natl Acad Sci USA. 2007;104(33):13513–13518. doi: 10.1073/pnas.0706217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hofmann MH, Blievernicht JK, Klein K, et al. Aberrant splicing caused by single nucleotide polymorphism C516G > T [Q172H], a marker of CYP2B6 * 6, is responsible for decreased expression and activity of CYP2B6 in liver[J] J Pharmacol Exp Ther. 2008;325(1):284–292. doi: 10.1124/jpet.107.133306. [DOI] [PubMed] [Google Scholar]
- [71].Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance[J] Pharmacogenomics. 2008;9(1):105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- [72].Nicolis E, Pasetto M, Cigana C, et al. The GCC repeat length in the 5′UTR of MRP1 gene is polymorphic: a functional characterization of its relevance for cystic fibrosis [J] BMC Med Genet. 2006;7:7. doi: 10.1186/1471-2350-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Serajee FJ, Nabi R, Zhong H, et al. Polymorphisms in xeno-biotic metabolism genes and autism[J] J Child Neurol. 2004;19(6):413–417. doi: 10.1177/088307380401900603. [DOI] [PubMed] [Google Scholar]