Abstract
Bevacizumab (BEV) is widely used for treatment of patients with recurrent glioblastoma. It is not known if there are differences in outcome between early versus delayed BEV treatment of recurrent glioblastoma. We examined the relationship between the time of starting BEV treatment and outcomes in patients with recurrent glioblastoma. In this retrospective chart review, we identified patients with recurrent glioblastoma diagnosed between 2005 and 2011 who were treated with BEV alone or BEV-containing regimens. Data was analyzed to determine overall survival (OS) from time of diagnosis and progression free survival (PFS) from time of starting BEV. A total of 298 patients were identified, 112 patients received early BEV, 133 patients received delayed BEV, and 53 patients were excluded because they either progressed within 3 months of radiation or received BEV at the time of diagnosis. There was no significant difference in PFS between patients that received early BEV and those that received delayed BEV (5.2 vs. 4.3 months, p = 0.2). Patients treated with delayed BEV had longer OS when compared to those treated with early BEV (25.9 vs. 20.8 months, p = 0.005). In patients with recurrent glioblastoma, there was no significant difference in PFS from the time of starting BEV between early and delayed BEV. Although patients treated with delayed BEV seemed to have longer OS, a conclusion regarding OS outcome requires further prospective trials. These results may indicate that delaying treatment with BEV is not detrimental for survival of patients with recurrent glioblastoma.
Keywords: Delayed bevacizumab, Early bevacizumab, Recurrent glioblastoma, Survival outcome
Introduction
Glioblastoma is the most common malignant primary brain tumor in adults [1]. Despite using an aggressive approach with multimodality therapy, survival is poor with a median survival of only 15 months [2]. Unfortunately, treatment options have remain limited. Bevacizumab (BEV), a humanized monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), received accelerated approval in 2009 for treatment of recurrent glioblastoma by Food and Drug Administration [3]. Following its approval in recurrent glioblastoma many clinicians have sought to determine the optimal timing for using BEV but this question remains unanswered [4].
Previous studies have shown that recurrent glioblastoma after BEV failure are aggressive and resistant to subsequent salvage therapies [5]. Upon cessation of BEV, rapid clinical deterioration has been observed seemingly as a result of this aggressive phenotype and increased vasogenic edema [6]. This has led to the concern that treating recurrent glioblastoma with BEV early in the disease might lead to a more aggressive phenotype on recurrence and may reduce any expected survival benefit. In this retrospective study, we examined the survival outcome of patients with recurrent glioblastoma treated with early BEV versus those treated with delayed BEV.
Methods and materials
Patients
This retrospective chart review study was approved by the institutional review board (IRB) of the University of Texas MD Anderson Cancer Center. Data on a total of 298 patients diagnosed with recurrent glioblastoma and gliosarcoma, who were treated with BEV alone or BEV-containing regimens between July 2005 and June 20 [1] 1 were collected from our database. All the patients had a pathologically-confirmed diagnosis of glioblastoma and gliosarcoma. Collected data included age at diagnosis, sex, date of diagnosis, extent of resection, date of first recurrence, date of start of BEV, number of recurrences before start of BEV, Karnofsky performance score (KPS) at the time of start of BEV, date of recurrence after start of BEV, and date of death or last follow-up.
Treatment
Treatment characteristics recorded included extent of resection (biopsy only, subtotal or gross total resection) determined by reviewing reports of post-operative imaging (MRI or CT), or operative reports (if imaging was not available). Treatment after surgery with radiation, radiation with chemotherapy or chemotherapy was recorded. Early BEV treatment was defined as treatment with BEV at the first disease progression, and delayed BEV treatment was defined as treatment with BEV at the second progression or later.
Statistical analysis
Patients who were treated with BEV at diagnosis were excluded. Patients with radiographic disease progression within 3 months of completion of radiation treatment were excluded from the survival analysis because of their radiographic progression may be a pseudo-progression secondary to radiation treatment, unless progression was pathologically-confirmed. Progression-free survival (PFS) was defined as duration of time between the dates of first BEV treatment to disease progression, death or last follows up. OS was defined as duration of time from date of surgery to death or last follow up. PFS and OS were estimated using Kaplan–Meier methods, and log-rank tests were performed to compare survival curves between patients who received early BEV and those who received delayed BEV. Cox regression models were built to evaluate the association of clinical factors with survival. Differences between groups were calculated using the χ2 test, the t test or the Mann–Whitney test. Data were analyzed using Graphpad prism 5 (Graphpad software, Inc., La Jolla, CA) and SPSS 21 (IBM, Chicago, IL).
Results
Patient characteristics
A total of 298 patients with recurrent glioblastoma who received BEV were identified. Histology was confirmed as glioblastoma at MD Anderson for all the patients, of whom 12 (4.2 %) patients progressed from lower grade tumors. Fifteen patients were excluded because they received BEV at the time of diagnosis, and 38 patients were excluded because they could have had pseudo-progression. Among 245 patients included in the analysis, 142 (58 %) were men and 103 (42 %) were women, with a median age of 51.9 years. The KPS at the time of diagnosis was available in 224 (91.4 %), and KPS at diagnosis was ≥70 in 208 (92.9 %) patients and <70 in 16 (7.1 %) patients. Solitary tumors were found in 247 (87.3 %) patients and multiple tumors were found in 36 (12.7 %) patients. Median time from diagnosis to first progression was not significantly different in early BEV patients when compared to delayed BEV patients (8.1 vs. 7.6 months, p = 0.1) (Table 1).
Table 1.
Patient characteristics
| Characteristic | Early BEV (N = 112) | Delayed BEV (N = 133) | P |
|---|---|---|---|
| Age (median) | 52.1 | 51.7 | NS |
| Sex n (%) | |||
| Male | 67(59.8 %) | 75 (56.4 %) | NS |
| Female | 45 (40.2 %) | 58 (43.6 %) | |
| KPS (mean) | 83.7 | 80.4 | 0.045* |
| Extent of surgery n (%) | |||
| Biopsy | 14 (13.1 %) | 11 (8.5 %) | NS |
| Subtotal | 30 (28 %) | 41 (31.8 %) | NS |
| Gross total | 63 (58.9 %) | 77 (59.7 %) | NS |
| Chemoradiation | |||
| Yes | 105 (93.8 %) | 117 (88 %) | NS |
| No | 7 (6.2 %) | 16 (12 %) | |
| Number of lesions n (%) | |||
| Single | 97 (86.6 %) | 119 (89.5 %) | NS |
| Multiple | 15 (13.4 %) | 14 (10.5 %) | |
| Median time to start of BEV (months) | 9.8 | 16 | <0.0001* |
| Median time to first recurrence (months) | 8.1 | 7.6 | NS |
KPS Karnofsky performance score, NS no statistical significance
statistically significant
Treatment
All 245 patients underwent surgery, and we were able to determine the extent of resection in 236 patients, with gross total resection in 140 (59.3 %) patients, subtotal resection in 71 (30.1 %) patients and biopsy in 25 (10.6 %) patients. Following surgery, 222 (90.6 %) patients underwent radiation with concurrent chemotherapy. BEV or BEV-containing regimen (Table 2) was started after the first progression (early BEV) in 112 (45.7 %) patients and after the second progression or later in 133 (54.3 %) patients, with a median time from diagnosis to start of BEV of 9.8 months and 16 months, respectively (Table 1). The dose of BEV was 10 mg/kg every two weeks in 93 (83 %) early BEV patients and in 105 (78.9 %) delayed BEV patients. In the remaining patients BEV was given at a dose of 5 mg/kg every 2 weeks in 10 (8.9 %) early BEV patients and 15 (12.3 %) in delayed BEV patients, and at a dose of 7.5 mg/kg every 2 weeks in 9 (8.1 %) early BEV patients and in 13 (9.8 %) delayed BEV patients. There was no significant difference in the dose of BEV between the two groups (p = 0.5).
Table 2.
Treatment regimens of early and delayed BEV groups
| Regimen | Early BEV (N) | Delayed BEV (N) |
|---|---|---|
| BEV | 23 | 32 |
| BEV/irinotecan | 58 | 77 |
| BEV/temozolomide | 13 | 7 |
| BEV/carboplatin | 6 | 5 |
| BEV/carmustine | 1 | 1 |
| BEV/lomustine | 1 | 0 |
| BEV/cis-retinoic acid | 4 | 1 |
| BEV/capecitabine | 1 | 1 |
| BEV/erlotinib | 2 | 0 |
| BEV/gemcitabine | 0 | 1 |
| BEV/etoposide | 0 | 1 |
| BEV/temsirolimus | 0 | 1 |
| BEV/tamoxifen | 0 | 1 |
| BEV/temozolomide/6-thioguanine | 0 | 1 |
| BEV/carboplatin/etoposide | 1 | 0 |
| BEV/irinotecan/cis-retinoic acid | 2 | 0 |
| BEV/imatinib/hydroxyurea | 0 | 1 |
| BEV/procarbazine/lomustine/6-Thioguanine/hydroxyurea | 0 | 3 |
| Total | 112 | 133 |
Survival and progression
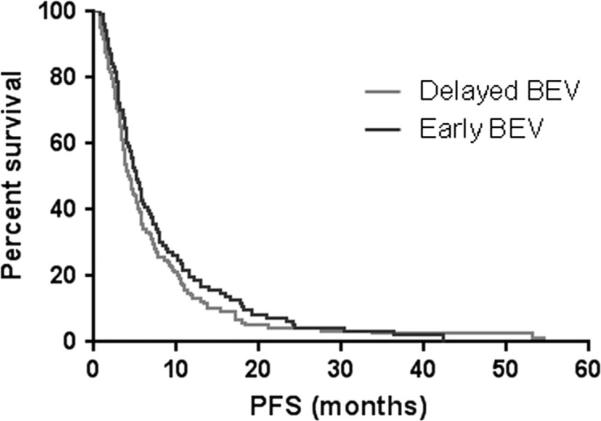
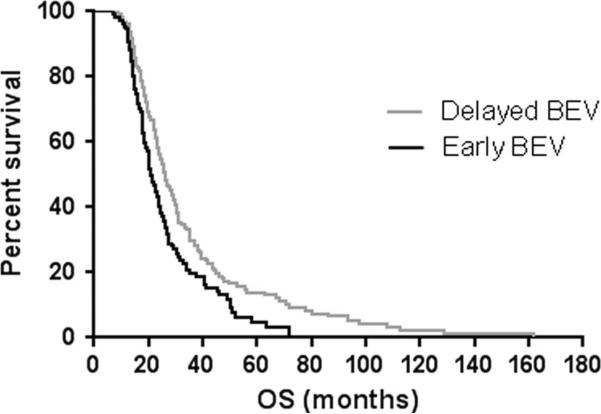
Median PFS was not significantly different between patients treated with early BEV and those treated with delayed BEV (5.2 vs. 4.3 months, p = 0.2) (Fig. 1). However, median OS was significantly higher in patients treated with delayed BEV when compared to those treated with early BEV (25.9 vs. 20.8 months, p = 0.005) (Fig. 2).
Fig. 1.
Kaplan–Meier estimates of progression-free survival (PFS) by early bevacizumab (early BEV) and delayed bevacizumab (delayed BEV) (p = 0.2)
Fig. 2.
Kaplan–Meier estimates of overall survival (OS) by early bevacizumab (early BEV) and delayed bevacizumab (delayed BEV) (p = 0.005)
Univariate analysis demonstrated that KPS, extent of resection (biopsy vs. gross total resection), number of recurrences before start of BEV (1 vs. ≥2), and time from diagnosis to start of BEV were the strongest predictors of OS. Neither age, number of lesions, chemoradiation, or time from diagnosis to start of BEV affected survival (Table 3). Multivariate analysis (including KPS and extent of resection) demonstrated that time from diagnosis to start of BEV and the number of recurrences before BEV were significantly associated with survival (Table 4), indicating an association between delayed BEV treatment and a small but significant favorable overall survival.
Table 3.
Univariate Cox model analysis for overall survival
| Covariate | Estimate | Standard error | Hazard ratio | 95 % CI | P |
|---|---|---|---|---|---|
| Age | 0.012 | 0.006 | 1.012 | 1–1.022 | 0.05 |
| KPS | –0.011 | 0.005 | 0.989 | 0.979–0.999 | 0.04* |
| Number of lesions | |||||
| Multiple vs. single | 0.005 | 0.136 | 1.005 | 0.769–1.312 | 0.97 |
| Extent of resection | |||||
| GTR vs. Biopsy | –0.465 | 0.232 | 0.628 | 0.398–0.990 | 0.045* |
| STR vs. Biopsy | –0.534 | 0.246 | 0.586 | 0.362–0.949 | 0.03* |
| Chemoradiation | |||||
| Yes vs. No | 0.325 | 0.228 | 1.383 | 0.885–2.161 | 0.15 |
| Time to 1st recurrence | –0.001 | 0.001 | 0.999 | 0.997–1.001 | 0.3 |
| Number of recurrences | |||||
| Before BEV: 1 vs. ≥2 | 0.377 | 0.138 | 1.459 | 1.114–1.910 | 0.006* |
| Time from diagnosis to start of BEV | –0.041 | 0.005 | 0.960 | 0.950–0.970 | 0.0001* |
KPS Karnofsky performance score, GTR gross total resection, STR subtotal resection, NS no statistical significance
statistically significant
Table 4.
Multivariate Cox model for overall survival
| Covariate | Estimate | Standard error | Hazard ratio | 95 % CI of HR | P |
|---|---|---|---|---|---|
| Number of recurrences | |||||
| Before BEV: 1 vs. ≥2 | 0.380 | 0.154 | 1.462 | 1.082–1.976 | 0.01* |
| Time to start of BEV | –0.043 | 0.006 | 0.958 | 0.947–0.969 | 0.0001* |
statistically significant
Discussion
BEV is the most recent FDA approved agent for recurrent glioblastoma based on improvements in radiographic response and 6-month progression free survival rates. [7–10] In previous trials, although the population treated with BEV included patients with initial recurrence in addition to patients with two or more relapses, there was no published results on a difference in survival outcome between these groups. Thus, the issue of early versus delayed treatment with BEV for recurrent glioblastoma has become particularly relevant after studies have shown that after BEV failure tumors may be more aggressive and resistant to subsequent treatments [11, 12]. Our study demonstrated that for patients with recurrent glioblastoma delaying treatment with BEV was not detrimental to the favorable effect of BEV on progression-free survival. Although there was a statistically significant difference in overall survival from time of diagnosis, it is difficult to make a conclusion regarding the overall survival outcome in this retrospective analysis.
The results of this study provide evidence that patients with recurrent glioblastoma might still benefit from BEV when the treatment is delayed. The strategy of delaying BEV treatment relies on the observation that there is no effective treatment for BEV failure, which leads to the hypothesis that treatment with BEV results in a more aggressive tumor after BEV failure. The mechanisms underlying this phenomenon are not fully understood but may include triggering alternate pro-angiogenic pathways are following sustained anti-VEGF therapy involving other endothelial growth factors including fibroblast growth factor and platelet-derived growth factor [13, 14], hypoxia-induced recruitment of numerous bone marrow-derived cells that have the ability to promote new blood vessels [11], stimulation of endothelial cells to recruit pericytes to generate tumor endothelial cells more resistant to future anti-angiogenic treatments. [15] Furthermore, glioblastoma cells have shown the capability to alter to a more aggressive, mesenchymal phenotype by invading into normal brain via vessel co-option [16], where tumor cells exploit normal blood vessels to spread throughout the brain when tumor angiogenesis is hindered [17].
On the other hand, there is evidence that BEV may not be associated with an increased distant or diffuse recurrence of malignant gliomas at the time of failure when compared to patients treated with BEV-free regimens [18], which may contradict the hypothesis that BEV treatment may result in more aggressive tumors after failure.
There are several limitations to this study. First, it is a retrospective analysis of data that doesn't control all of the potential confounding factors affecting survival outcome. Although we have attempted to control for known clinical factors associated with improved survival, other prognostic factors including RPA class and molecular markers (e.g. IDH1 mutation and MGMT promoter methylation status) that we do not have available for review were not included in the univariate or multivariate analyses. Another possible confounding factor is that patients with more aggressive disease may be more likely to be treated with BEV earlier than those with less aggressive disease. We used the time from diagnosis to first relapse as a surrogate marker for the aggressiveness of tumors. There was no significant difference between the time to first relapse between patients who were treated with early BEV versus those patients treated with delayed BEV. The study did not include data about the molecular profile of the tumors which might have had a prognostic significance or the salvage treatment after BEV failure.
The question of the impact of the timing of starting BEV on survival outcome was—at least partially—evaluated in prospective phase III clinical trials (RTOG 0825 and AVAglio trials) randomizing patients to either receive Bev or placebo with standard radiation with concurrent temozolomide, and no overall survival benefit was found in both studies [19, 20]. In addition, in our study we exclusively investigated the timing of BEV in the setting of recurrent disease and not in newly diagnosed glioblastoma or in those with pseudo-progression.
A recent retrospective study evaluated the impact of deferred use of BEV on survival outcome in glioblastoma patients [21]. The conclusion was that delaying the use of BEV did not diminish its efficacy on survival, which supports the findings in our study. This study included patients treated with upfront BEV and stratified patients into groups according to the recurrence at which BEV was initiated (upfront, 1st, 2nd or 3rd+ recurrences). In addition, this study extended the analysis to study the difference in the outcome between single-agent BEV and BEV with added chemotherapy, survival after BEV failure and characteristics of patients unlikely to continue any treatment after their first failure. Unlike our current study, their study did not exclude those patients who had, or could have had, pseudo-progression and did not address the time from diagnosis to BEV initiation as a variable that could potentially affect survival.
In conclusion, our retrospective study showed that delaying the use of BEV and seeking alternate treatment for recurrent glioblastoma is not associated with a worse survival outcome. This retrospective study has limitations but provide reasonable evidence that there may not be detrimental effects associated with delayed use of BEV in patients with recurrent glioblastoma. However, further prospective investigation is recommended.
Acknowledgments
Dr. Gilbert has a consultant or advisory relationship with and received honoraria from Gnenetech, Novartis, Merck and EMD-Serono. He also received research funding from Merck, Galaxo Smith Kline and Genentech; Dr. Yung has a consultant or advisory relationship with Novartis, Merck and Actelion; and received honoraria from Merck and Novartis. He also received research funding from Daiichi Sankyo; Dr. Puduvalli has a consultant or advisory relationship with Novartis, and received honoraria from Novartis and Merck. He also received research funding from Celgene, Genentech, Pfizer and Merck; Dr. De Groot has a consultant or advisory relationship with Genentech and VBL therapeutics, and received honoraria from Merck. He also received research funding from Sanofi-Aventis, AstraZeneca, and EMDSerono.
Footnotes
Conflict of interest Mohamed A. Hamza, Jacob J. Mandel, Charles A. Conrad: nothing to disclose
Contributor Information
Mohamed A. Hamza, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA OhioHealth, 500 Thomas Lane, Suite 2E, Columbus, OH 43214, USA.
Jacob J. Mandel, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
Charles A. Conrad, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
Mark R. Gilbert, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
W. K. Alfred Yung, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA.
Vinay K. Puduvalli, Division of Neuro-oncology, The Ohio State University Wexner Medical Center, Arthur G. James Cancer Hospital, 320 W 10th Avenue, Starling Loving Hall Suite M410, Columbus, OH 43210, USA
John F. DeGroot, Department of Neuro-Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030, USA
References
- 1.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. doi:10.1634/theoncologist.2009-0121.Epub. [DOI] [PubMed] [Google Scholar]
- 4.Argirios M, Teri NK. New treatment options in the management of glioblastoma multiforme: a focus on bevacizumab. Onco Targets Ther. 2010;3:27–38. doi: 10.2147/ott.s5307. Published online 2010 June 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 6.Ananthnarayan S, Bahng J, Roring J, et al. Time course of imaging changes of glioblastoma during extended bevacizumab treatment. J Neurooncol. 2008;88:339–347. doi: 10.1007/s11060-008-9573-x. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19. 8721. Epub 2009 Aug 31. [DOI] [PubMed] [Google Scholar]
- 8.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 10.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. doi:10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. doi:10.1212/WNL.0b013e 3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-oncology. 2010;12(3):233–242. doi: 10.1093/neuonc/nop027. doi:10.1093/neuonc/nop027. Epub 2010 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong J, Grunstein J, Tejada M, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23(14):2800–2810. doi: 10.1038/sj.emboj.7600289. Epub 2004 Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. doi:10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piao Y, Liang J, Holmes L, et al. Glioblastoma resistance to anti-VEGF therapy is associated with myeloid cell infiltration, stem cell accumulation and mesenchymal phenotype. Neuro-Oncology. 2012;14(11):1379–1392. doi: 10.1093/neuonc/nos158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wick A, Dorner N, Schafer N, et al. Bevacizumab does not increase the risk of remote relapse in malignant glioma. Ann Neurol. 2011;69(3):568–592. doi: 10.1002/ana.22336. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert MR, Dignam J, Won M, et al. RTOG 0825: phase III double-blind placebo-controlled trial evaluating bevacizumab (Bev) in patients (Pts) with newly diagnosed glioblastoma (GBM). J Clin Oncol. 2013;31(suppl) abstr 1. [Google Scholar]
- 20.Henriksson R, Bottomley A, Mason W, et al. Progression-free survival (PFS) and health-related quality of life (HRQoL) in AVAglio: a phase III study of bevacizumab (Bv), temozolomide (T), and radiotherapy (RT) in newly diagnosed glioblastoma (GBM). J Clin Oncol. 2013;31(suppl) doi: 10.1200/JCO.2014.60.3217. abstr 2005. [DOI] [PubMed] [Google Scholar]
- 21.Piccioni DE, Selfridge J, Mody RR, et al. Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro-oncology. 2014 doi: 10.1093/neuonc/nou028. doi:10.1093/neuonc/nou028. [DOI] [PMC free article] [PubMed] [Google Scholar]