Summary
Posttraumatic stress disorder (PTSD) is associated with an increased risk of cardiovascular disease and several other chronic illnesses. Alterations in the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis in PTSD might contribute to these associations but findings regarding SNS and HPA activity in PTSD are heterogeneous. We measured 24-hour urinary catecholamines and cortisol in a large cohort of adult outpatients recruited from 2 Veterans Affairs medical centers. 24-hour urinary norepinephrine, epinephrine, dopamine and cortisol were measured by tandem mass spectrometry. Lifetime and current PTSD were assessed with the Clinician Administered PTSD Scale using DSM-IV-TR criteria. Out of 613 participants, 199 (32.5%) had current PTSD, 100 (16.3%) had lifetime but not current PTSD, and 314 (51.2%) never had PTSD. Patients with current PTSD had significantly higher norepinephrine secretion compared to those without PTSD. Patients in the lifetime PTSD group exhibited lower cortisol values compared to those without PTSD. Participants who never had PTSD showed the lowest norepinephrine and the highest cortisol values. All results remained stable when controlling for potentially confounding variables. This study provides evidence for increased norepinephrine secretion and decreased cortisol in PTSD. Future longitudinal studies are needed to determine whether these changes contribute to adverse health outcomes in patients with PTSD.
Keywords: Posttraumatic stress disorder, catecholamines, norepinephrine, cortisol, cardiovascular disease
1. Introduction
Posttraumatic stress disorder (PTSD) follows exposure to a severe traumatic event. PTSD is characterized by three co-occurring symptom clusters: re-experiencing the trauma, avoidance and hyperarousal.
PTSD is not only of high psychological burden but is also increasingly recognized as an independent risk factor for cardiovascular disease (CVD) (Edmondson, Kronish et al. 2013; Turner, Neylan et al. 2013). Of note, increased catecholamine secretion due to increased sympathetic nervous system (SNS) activity contributes to cardiovascular diseases such as heart failure and essential hypertension (Parati and Esler 2012). Thus, increased catecholamine secretion might partially explain the association between PTSD and CVD and other chronic medical conditions such as type-2 diabetes (Vaccarino, Goldberg et al. 2014).
Indeed, patients with PTSD often show alterations in major neurobiological stress systems: the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis. Several studies have found elevated concentrations of catecholamines in PTSD, including norepinephrine and dopamine (Kosten, Mason et al. 1987; Yehuda, Southwick et al. 1992; Yehuda, Siever et al. 1998; Hawk, Dougall et al. 2000; Young and Breslau 2004; Delahanty, Nugent et al. 2005). However, most of these studies had small sample sizes and differed markedly from each other in the types of trauma, proportion of men and women, and age range of participants studied. The largest study so far included 69 patients with lifetime PTSD. That study found increased norepinephrine in PTSD but only when taking depression into account (Young and Breslau 2004). Of note, some studies did not find elevated catecholamines in patients with PTSD (Pitman and Orr 1990; Inslicht, Marmar et al. 2006). Findings on cortisol secretion in PTSD are even less homogenous (Meewisse, Reitsma et al. 2007). A recent review concluded that PTSD is associated with lower cortisol output, especially in the morning (Morris, Compas et al. 2012). Due to the fact that cortisol is released in pulses and has a clear circadian rhythm, 24-hour urinary cortisol provides an integrated measure that is more reliable than measures from single (or few) time points.
It has been hypothesized that hypocortisolism might contribute to exaggerated catecholamine output in PTSD (Bierer, Tischler et al. 2006; Yehuda and Seckl 2011; Zoladz and Diamond 2013). However, very few studies have measured cortisol and catecholamine levels within the same patients. One study found lower cortisol and higher norepinephrine (but not epinephrine) secretion in women with PTSD compared to those without PTSD (Glover and Poland 2002). Others have found the opposite pattern of hormone release with higher cortisol and unaltered catecholamines (Inslicht, Marmar et al. 2006) or higher catecholamine levels in concert with normal cortisol (Young and Breslau 2004).
Because of these contradictory findings, we aimed to further investigate the association between PTSD and 24-hour urinary catecholamines and cortisol in a large sample of outpatients. Given that the largest PTSD study in the field so far included only 69 patients with (lifetime) PTSD, we enrolled 613 participants, of which 199 (32.5%) had current PTSD, 100 (16.3%) had lifetime but not current PTSD and 314 (51.2%) never had PTSD. Norepinephrine, epinephrine, dopamine and cortisol were measured within the same individuals. We hypothesized patients with PTSD would have higher 24-hour concentrations of catecholamines but lower 24-hour urinary cortisol concentrations.
2. Methods
2.1. Patients
The Mind Your Heart Study is a prospective cohort study designed to examine the association between PTSD and health outcomes. Participants were recruited between February 2008 and June 2010 from outpatient clinics affiliated with two Department of Veterans Affairs (VA) Medical Centers (the San Francisco VA Medical Center and the VA Palo Alto Health Care System, California). Potential participants were excluded if they planned on leaving the area in three years or did not have contact information for follow-up. Potential participants were also excluded if they were unable to walk one block or had a myocardial infarction in the prior six months as a cardiac treadmill test was done for the study. All patients provided written informed consent and appropriate institutional review boards approved the research protocol.
Overall, 1020 patients were assessed for eligibility. One hundred and four patients (10.2%) were found to be ineligible, primarily due to lacking contact information for follow-up (n = 82). Of the remaining 916 eligible patients, 170 (18.6%) declined to participate or did not show up for the baseline interview such that 746 participants were enrolled in the study. Out of these, 621 completed 24-hour urine sampling according to the protocol described below. Four participants were excluded because they provided less than 500ml urine and four participants were excluded because they did not complete full PTSD assessments, resulting in a final sample of 613 participants for this study.
2.2. Clinical Assessment
All participants completed a study visit at the San Francisco VA Medical Center where we administered a self-report questionnaire to determine age, sex, ethnicity, income, education, and medical history. PTSD was evaluated with a clinical interview, the Clinician Administered PTSD Scale (CAPS). The CAPS is a widely structured interview for diagnosing PTSD following the DSM IV criteria with excellent test–retest reliability and internal consistency (Weathers, Keane et al. 2001). We used the standard “1, 2” CAPS scoring rule of at least a score of 1 for frequency and 2 for intensity to establish positivity for a specific symptom. We selected this rule because it has demonstrated high sensitivity and has been widely used in prior studies of PTSD (Weathers, Keane et al. 2001). Current PTSD was assessed based on symptoms within the last month and lifetime PTSD was assessed based on the most symptomatic month since the traumatic event. The PTSD group consisted of subjects with either full or partial PTSD, as partial PTSD is associated with significant impairment in health and functioning (Weathers, Keane et al. 2001). Partial PTSD was defined as meeting diagnostic criteria for the re-experiencing cluster and either avoidance or hyperarousal clusters, in addition to the other CAPS criteria. We also required this group to exhibit symptoms meeting a total CAPS score ≥ 40, as defined by the authors of the CAPS as the lower threshold for PTSD (Weathers, Keane et al. 2001). 199 participants in this study met these criteria for current PTSD (including N=19 with partial PTSD and CAPS score ≥ 40) and 100 met them for lifetime PTSD (including N=24 with partial PTSD and CAPS score ≥ 40). Additionally, the PTSD Check List (PCL) was used, which is a self-report measure and provides a total score and subscores for the PTSD symptom clusters of re-experiencing, avoidance and hyperarousal according to DSM-IV (Blanchard, Jones-Alexander et al. 1996).
To assess comorbid psychiatric diagnosis, we used the depression, anxiety, and mania modules of the World Health Organization CIDI Computer Assisted Personal Interview (Version 21.1.1), which generated diagnoses based on DSM-IV criteria for major depressive disorder, generalized anxiety disorder (GAD), and bipolar disorder. Additionally, we asked whether a doctor or nurse had ever diagnosed the participant with alcoholism or problem drinking or with drug addiction or abuse, respectively.
The Patient Health Questionnaire (PHQ-9) was also used to evaluate depressive symptoms. This self-report instrument measures the frequency of depressive symptoms corresponding to the 9 symptom criteria in the DSM-IV. A standard cut-point of ≥10 is used to define depression and has demonstrated excellent validity when compared with a mental health interview, with a sensitivity of 88% and a specificity of 88% (Kroenke, Spitzer et al. 2001).
2.3. 24-Hour Urinary Cortisol and Catecholamines
We used 24-hour urine sampling as a non-invasive integrated measure of HPA axis and catecholamine activity that could be collected in the subjects’ home environment.
Participants were instructed to collect all urine for 24 hours after the end of their study appointment at the San Francisco VA Medical Center and to keep the urine collection jugs refrigerated at all times. No preservatives were added to the urine jugs.
Research staff arrived at the patients’ homes exactly 24 hours after their study appointments ended to ensure accurately timed specimens and to evaluate compliance with the protocol. If more than 1 hour had passed since the participants’ last void, the subjects were asked to void again to complete the collection. If the sample was reported to be incomplete, or had not been refrigerated, the participants were asked to repeat the collection, and research personnel returned 24 hours later to recollect the urine. If the 3-liter collection jugs were completely full, the subjects were given new jugs and asked to repeat the collection to ensure that no urine had been inadvertently discarded. If participants had urinary incontinence, their samples were deemed inadequate, and no urinary data were recorded for these subjects.
Urinary catecholamine excretion levels (norepinephrine, epinephrine, and dopamine) were measured with high performance liquid chromatography-tandem mass spectrometry at Specialty Laboratories, Incorporated in Valencia, CA. The normal reference range for these assays is 0-500 μg/day for dopamine, 0–20 μg/day for epinephrine, and 0–80 μg/day for norepinephrine. The detection limit was 1.0 μg for epinephrine, 3.0 μg for norepinephrine, and 50 μg for dopamine, and participants with lower values were recoded to these detection limits.
Urinary cortisol was analyzed by high performance liquid chromatography-tandem mass spectrometry at Quest Diagnostics Nichols Institute in San Juan Capistrano, CA. The normal reference range for this assay was 40-50 mcg/day. The detection limit was 1.0 mcg. Cortisol levels for subjects whose cortisol levels were below this detection limit were coded as 1.0 mcg. We also measured urine creatinine over 24 hours and serum creatinine as markers of renal function.
2.4. Statistics
Statistical analyses were performed using SPSS Version 19.0. Demographic data were analyzed using Pearson's Chi2-test for categorical data and ANOVA for continuous data. Cortisol and catecholamines were also analyzed using ANOVA.
In the first step, catecholamine and cortisol values (raw data and log transformed data) were compared between PTSD groups. For analyses of cortisol, participants who reported taking oral steroids (n = 9) were excluded. In a second step, potentially confounding variables were included in the analyses. Stepwise multiple regression analyses were conducted to analyze whether potential confounding variables that differed between PTSD groups influenced catecholamine and cortisol values: age, sex, cigarettes (pack years), working status, angina, hypertension, intake of selective serotonin reuptake inhibitors (SSRI), other antidepressants, antipsychotics, benzodiazepines, and adrenergic antihypertensives, and self-reported depressive symptoms. Associations between cortisol and catecholamines (log transformed data) and PTSD symptom severity (PCL and CAPS scores) were analysed by Pearson's correlation coefficient.
3. Results
3.1. Sample characteristics
Out of the 613 participants, 199 (32.5%) had current PTSD, 100 (16.3%) had lifetime but not current PTSD, and 314 (51.2%) never had PTSD. Sample characteristics are presented in Table 1. There were significant differences between groups in age and sex. Patients with current PTSD were slightly younger compared to those who had never had PTSD, and women were more likely to be in the current PTSD group. There were no differences in level of education and ethnicity. Furthermore, the number of smokers was equally distributed across groups. Patients in the never PTSD group were more likely to be currently employed.
Table 1.
Medical and demographic variables by PTSD status
| never PTSD (N=314) | lifetime PTSD (N=100) | current PTSD (N=199) | statistics | |
|---|---|---|---|---|
| Sample characteristics | ||||
| Age, mean (SD)* | 59.8 (12.1) | 57.4 (10.0) | 57.4 (11.0) | p= .03 |
| Males, N (%)** | 306 (97.5) | 94 (94) | 174 (87.4) | p< .001 |
| High school graduate, N (%) | 312 (99.7) | 100 (100) | 196 (98.5) | p= .18 |
| Whites, N (%) | 130 (41.9) | 41 (41.8) | 82 (41.8) | p= .98 |
| Smokers, N, (%) | 72 (23.2) | 31 (31.0) | 51 (26.0) | p= .28 |
| Pack years, mean (SD)*** | 14.4 (17.8) | 19.4 (18.7) | 18.9 (20.6) | p= .01 |
| Employed, N (%)** | 117 (37.3) | 25 (25.3) | 47 (23.6) | p=.002 |
| Type of trauma (N / %) | ||||
| No traumatic event | 44 (14) | 0 | 0 | |
| Combat | 46 (14.6) | 51 (51) | 121 (60.8) | |
| Other military trauma | 18 (5.7) | 7 (7) | 6 (3.0) | |
| Sexual assault | 13 (4.1) | 11 (11) | 28 (14.1) | |
| Physical assault | 58 (18.5) | 14 (14) | 19 (9.5) | |
| Unexpected death of a loved one | 19 (6.1) | 5 (5) | 5 (2.5) | |
| Accident | 29 (9.2) | 2 (2) | 3 (1.5) | |
| Other | 87 (27.7) | 10 (10) | 17 (8.5) | |
| Childhood trauma (N / %) | ||||
| Trauma before age of 16 | 44 (16.4) | 16 (16) | 25 (12.6) | |
| CAPS score (last month)**** | 3.7 (6.8) | 19.8 (12.6) | 66.1 (19.3) | p<.001 |
| Self-reported depressive symptoms (PHQ-9)**** | 4.0 (4.2) | 7.59 (5.4) | 11.2 (5.6) | p<.001 |
| Psychiatric diagnosis - CIDI (N / %) – last 30 days | ||||
| Depression | 7 (2.2) | 11 (11) | 35 (18.6) | p<.001 |
| Bipolar I | 2 (0.6) | 2 (2) | 9 (4.8) | - |
| Bipolar II | 2 (0.6) | 2 (3) | 7 (3.7) | - |
| GAD | 5 (1.6) | 3 (3) | 16 (8.5) | - |
| Self-reported alcohol and drinking problems (N / %) | ||||
| Alcoholism or problem drinking | 86 (14.2) | 31 (5.1) | 64 (10.6) | p= .53 |
| Drug addiction or abuse | 76 (12.5) | 28 (4.6) | 49 (8.1) | p= .75 |
| Medical conditions N (%) | ||||
| Hypertension | 141 (45.0) | 51 (51.0) | 109 (55.6) | p= .06 |
| Myocardial infarction | 27 (8.6) | 9 (9.0) | 22 (11.2) | p= .61 |
| Angina* | 32 (10.2) | 9 (9.0) | 33 (16.8) | p= .05 |
| Congestive heart failure | 15 (4.8) | 3 (3.0) | 12 (6.1) | p= .50 |
| Stroke | 19 (6.1) | 6 (6.0) | 11 (5.6) | p= .98 |
| Diabetes | 52 (16.6) | 14 (14.0) | 40 (20.4) | p= .34 |
| Medication intake N (%) | ||||
| SSRI**** | 24 (7.6) | 17 (17.0) | 56 (28.1) | p<.001 |
| Tricyclics | 10 (3.2) | 6 (6.0) | 7 (3.5) | p= .43 |
| Other antidepressants**** | 35 (11.1) | 32 (32.0) | 77 (38.7) | p<.001 |
| Antipsychotics*** | 18 (5.7) | 9 (9.0) | 26 (13.1) | p= .02 |
| Benzodiazepines | 11 (3.5) | 10 (10.0) | 18 (9.0) | p=.01 |
| Beta-Blocker | 79 (25.2) | 26 (26.0) | 53 (26.6) | p= .93 |
| ACE inhibitors | 74 (23.6) | 24 (24.0) | 52 (26.1) | p= .80 |
| AT blockers | 13 (4.1) | 6 (6.0) | 16 (8.0) | p= .18 |
| Adrenergic antihypertensives | 30 (9.6) | 8 (8.0) | 30 (15.1) | p= .09 |
| Diuretics | 69 (22.0) | 22 (22.0) | 134 (21.9) | p= .99 |
| Oral steroids | 6 (1.9) | 1 (1.0) | 2 (1.0) | p= .65 |
Bonferroni post-hoc tests: n.s.
Bonferroni post-hoc tests: never PTSD > current PTSD
Bonferroni post-hoc tests: never PTSD < current PTSD
Bonferroni post-hoc tests: never PTSD < lifetime PTSD < current PTSD
Abbreviations: PTSD = posttraumatic stress disorder, PHQ-9 = patient health questionnaire-9=item, SSRI = selective serotonin reuptake inhibitors, ACE = angiotensin-converting-enzyme, AT blockers = angiotensin receptor blockers
Only 7.2% of the sample reported that they had not experienced a traumatic event (all in the “never PTSD” group). The majority of the participants reported combat-related (35.6%) or other military trauma (5.1%) to be the most severe traumatic event. Furthermore, 8.5% of the participants experienced sexual assault and 14.8% physical assault. Additionally, unexpected death of a loved one (4.7%), accident (5.5%) and other traumatic events (18.6%) were reported. Table 1 presents the traumatic events for each group separately. The current PTSD group did not differ from the lifetime PTSD group concerning type of trauma (Chi2 = 6.7, p = .35). Childhood trauma prior to the age of 16 was reported by 85 participants. Presence of childhood trauma was comparable across study groups (Chi2 = 1.4, p = .49, see also Table 1).
As expected, there were significant group differences in CAPS scores (last month). Patients with current PTSD exhibited the highest scores, and the lowest CAPS scores were found in the never PTSD group.
Patients with current PTSD were significantly more likely to have comorbid depression as determined by questionnaire (PHQ-9) and clinical interview (CIDI) measures (p < .001). Only a few participants suffered from comorbid current bipolar disorder or GAD. Due to the small number of cases per group statistical analyses could not be performed for bipolar disorder and GAD. The study groups did not differ concerning alcoholism or problem drinking (Chi2 = 1.3, p = .53) and drug addiction or abuse (Chi2 = 0.6, p = .75). Table 1 also presents self-reported depression measured with the PHQ-9.
Patients with current PTSD were significantly more likely to report angina and there was a trend towards greater report of hypertension (p = .06). Furthermore, patients with PTSD used more psychotropic medication (SSRI, other antidepressants, antipsychotics, and benzodiazepines). Full information on medical conditions and medication intake is given in Table 1.
3.2. 24-Hour Urinary Cortisol and Catecholamines
The groups did not differ in urine creatinine values over 24 hours (p = .65) or serum creatinine (p = .64). Means (SDs) are given in Table 2.
Table 2.
24-Hour Urinary Cortisol and Catecholamines by PTSD status
| 24 hour urine | never PTSD | lifetime PTSD | current PTSD | statistics |
|---|---|---|---|---|
| Cortisol (N:304/97/193) | ||||
| mean(SD) | 30.9 (21.1) | 23.5 (14.6) | 27.2 (20.2) | p = .004* |
| log transformed | 3.2 (0.7) | 3.0 (0.7) | 3.1 (0.7) | p = .002* |
| Epinephrine (N:314/100/199) | ||||
| mean(SD) | 3.9 (3.0) | 3.4 (2.5) | 4.1 (3.5) | p= .18 |
| log transformed | 1.1 (0.8) | 0.9 (0.8) | 1.1 (0.8) | p= .22 |
| Norepinephrine (N:314/100/199) | ||||
| mean(SD) | 50.96 (24.2) | 51.62 (28.3) | 57.17 (27.9) | p= .03** |
| log transformed | 3.80 (0.6) | 3.79 (0.6) | 3.93 (0.5) | p= .02** |
| Dopamine (N:314/100/198) | ||||
| mean(SD) | 187.6 (99.2) | 188.0 (104.1) | 190.6 (95.0) | p= .94 |
| log transformed | 5.1 (0.6) | 5.1 (0.6) | 3.9 (0.6) | p= .78 |
| Urine creatinine over 24h | 1610 (504) | 1566 (559) | 1645 (548) | p= .46 |
| Serum creatinine (mg/dl) | 1.0 (0.3) | 1.0 (0.2) | 1.1 (0.3) | p= .22 |
Bonferroni post-hoc tests: never PTSD > liefetime PTSD
Bonferroni post-hoc tests: never PTSD < current PTSD
Abbreviations: PTSD = posttraumatic stress disorder
Catecholamines
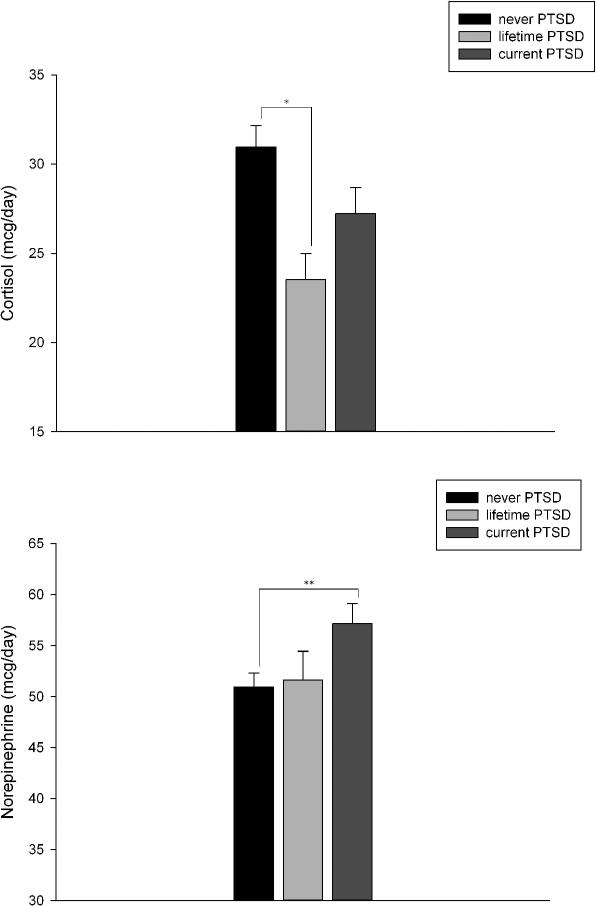
Analyses of norepinephrine revealed a significant group effect (see Table 2). Post-hoc tests demonstrated that patients with current PTSD had higher norepinephrine values compared to patients that never had PTSD (p = .03; Figure 1). There were no significant differences between the current and lifetime PTSD group (p = .12). We reran the analyses excluding the 19 patients in the current PTSD group who had partial PTSD, and this did not change the results (group effect for norepinephrine p = .03).
Figure 1.
24 hour urinary cortisol and norepinephrine in participants by PTSD status
Stepwise regression analyses revealed no significant impact of any entered variable except PTSD status on norepinephrine values (see Table 3). These results emphasize the association between norepinephrine release and PTSD symptomatology.
Table 3.
Stepwise multiple regression analyses: impact of PTSD status and potentially confounding variables on norepinephrine and cortisol
| 24 hour norepinephrine (log)* | β | T | p |
|---|---|---|---|
| Included variable(s) | |||
| PTSD status | .09 | 2.15 | .03 |
| Excluded variables | |||
| Age | .03 | .83 | .41 |
| Sex | .03 | .75 | .46 |
| Cigarettes (pack years) | .06 | 1.36 | .18 |
| Working status | .05 | 1.28 | .20 |
| Angina | −.02 | −.57 | .57 |
| Hypertension | .07 | 1.80 | .07 |
| SSRI | −.05 | −1.24 | .22 |
| other antidepressant | −.02 | −.45 | .65 |
| antipsychotics | .01 | .23 | .77 |
| benzodiazepines | −.03 | −.80 | .42 |
| Adrenergic antihypertensives | .06 | 1.53 | .13 |
| PHQ-9 score | −.005 | −.10 | .92 |
| 24 hour cortisol (log)** | β | T | p |
|---|---|---|---|
| Included variable(s) | |||
| PTSD status | −.08 | −1.99 | .046 |
| Age | −.09 | −2.28 | .02 |
| Sex | .13 | 3.19 | .002 |
| Hypertension | −.22 | −5.28 | <.001 |
| Excluded variables | |||
| Cigarettes pack per year | −.002 | −.04 | .97 |
| Working status | .06 | 1.38 | .17 |
| Angina | −.01 | −.30 | .77 |
| SSRI | −.05 | −1.06 | .29 |
| other antidepressant | −.008 | −.19 | .85 |
| antipsychotics | −.08 | −1.86 | .06 |
| benzodiazepines | .08 | 1.92 | .06 |
| Adrenergic antihypertensives | .03 | .81 | .42 |
| PHQ-9 score | −.03 | −.53 | .59 |
Model estimates: Fdf1,586 = 4.63, p = .032, R2 = .008
Model estimates: Fdf4,568 = 13.67, p <.001, R2 = .09
Abbreviations: log = log transformed values, PTSD = posttraumatic stress disorder, SSRI = selective serotonin reuptake inhibitors, PHQ-9 = patient health questionnaire-9-item
We found no group differences for 24-hour urinary epinephrine and dopamine.
Cortisol
For 24-hour urinary cortisol, ANOVA revealed significant between-group differences. Participants that never had PTSD showed the highest cortisol values (see Table 2). Bonferroni post-hoc tests showed that the never PTSD group differed from both PTSD groups (never PTSD vs. lifetime PTSD p = .004; never PTSD vs. current PTSD p = .059; Figure 1). Patients with lifetime and current PTSD did not differ in their cortisol release. Again, excluding the 19 patients in the current PTSD group who had partial PTSD did not change the results (group effect p = .009).
Stepwise regression analyses revealed the best model fit when PTSD status, age, sex and hypertension were included in the model. PTSD remained a significant predictor of cortisol after controlling for these additional variables. All other predictors were excluded as shown in Table 3.
There was a negative correlation between cortisol and the re-experiencing subscore of the PCL (r = -.09, p = .02). The correlation between the other PCL subscores and cortisol was not significant, however, there was a trend towards significance for the total PCL score (r = -.08, p = .06). PTSD symptom severity as measured with the CAPS did not correlate significantly with cortisol.
Association between Catecholamines and cortisol
For the whole sample there were significant positive associations between cortisol and epinephrine (r = .24, p < .001), norepinephrine (r = .24, p < .001) and dopamine (r = .20, p < .001). Correlation coefficients varied from r = .25 to r = .30 in the current PTSD group and from r =.19 to r = .25 in the never PTSD group (all p < .001). In the lifetime PTSD group, the association between epinephrine and cortisol was not significant, while cortisol and norepinephrine (r = .22, p = .03) as well as dopamine (r = .23, p = .02) were positively correlated.
4. Discussion
This study examined 24-hour urinary cortisol and catecholamine secretion in a large sample of patients with current PTSD, patients with lifetime but not current PTSD, and patients who never had PTSD. Patients with current PTSD had significantly higher norepinephrine secretion compared to those who never had PTSD. Patients with lifetime PTSD exhibited lower cortisol values compared to those that never had PTSD. Participants who never had PTSD showed the lowest norepinephrine and the highest cortisol values. All results remained stable when controlling for potentially confounding variables.
PTSD is associated with a markedly increased risk for cardiovascular disease, and increased sympathetic nervous system activity might be an important underlying mechanism (Edmondson, Kronish et al. 2013; Turner, Neylan et al. 2013; Yufu, Okada et al. 2014). Indeed, in our prior cross-sectional analyses we found that participants with current PTSD were more likely to have ischemia on exercise treadmill testing (Turner, Neylan et al. 2013) and in the current study found they were more likely to report angina pectoris. However, self-report of several other cardiac conditions did not differ by PTSD status. This might be due to the fact that we recruited participants from outpatient VA medical clinics. Thus, healthy participants might have been underrepresented compared to population-based studies. An important goal of this prospective cohort study is to determine whether patients with PTSD have increased CVD events during follow-up and if so, whether catecholamines mediate the association between PTSD and CVD risk. Supporting this hypothesis, several smaller studies have found elevated catecholamines in PTSD (Kosten, Mason et al. 1987; Yehuda, Southwick et al. 1992; Yehuda, Siever et al. 1998; Hawk, Dougall et al. 2000; Young and Breslau 2004; Delahanty, Nugent et al. 2005). Furthermore, PTSD is characterized by a variety of additional SNS alterations such as exaggerated startle response, decreased heart rate variability, baroreflex dysfunction, and increased QT variability on electrocardiograms (Orr, Metzger et al. 2002; Rozanski, Blumenthal et al. 2005). Each of these physiologic measures has been linked to CVD as increases in blood pressure and elevated catecholamines have direct effects on the heart, blood vessels, and platelets (Bedi and Arora 2007; Edmondson and Cohen 2013). Furthermore, exaggerated catecholamine release in response to stressful circumstances (Otte, Neylan et al. 2005) leading to higher concentrations of circulating catecholamines may also contribute to autonomic imbalance (Edmondson and Cohen 2013).
Apart from PTSD, major depression is also an independent risk factor for CVD and mortality (Whooley and Wong 2013). Importantly, major depression is often comorbid with PTSD and has also been associated with altered catecholamine function (Gold and Chrousos 2002; Otte, Neylan et al. 2005; Goddard, Ball et al. 2010). In our study, the association between PTSD and norepinephrine remained stable after controlling for depressive symptoms. Thus, increased norepinephrine seems to be characteristic of PTSD and not merely an artifact of comorbid depression. However, it remains unclear whether elevated norepinephrine is a consequence of PTSD or a pre-existing risk factor. High norepinephrine levels likely contribute to PTSD symptomatology at different stages: at the time of trauma high norepinephrine might facilitate memory consolidation and fear conditioning (van Stegeren 2008), while later high catecholamine activity might be associated with symptoms of hyperarousal and consequent development of CVD.
With regard to cortisol secretion, lower cortisol output has been proposed in patients with PTSD (Yehuda and Seckl 2011; Morris, Compas et al. 2012). However, the literature is far from being consistent (Meewisse, Reitsma et al. 2007). In our sample, we did not find lower cortisol in patients with current PTSD. The picture seems to be more complicated: participants with lifetime PTSD had the lowest cortisol levels, which differed significantly from those who had never PTSD, whereas participants with current PTSD diagnosis had cortisol level between the two other groups. Again, depressive symptoms, which have been found to be associated with higher cortisol levels (Parker, Schatzberg et al. 2003; Pariante and Lightman 2008), did not influence the results. Interestingly, it has been proposed that lower basal cortisol might be a pre-existing risk factor for developing PTSD (Zoladz and Diamond 2013). Indeed, individuals with lower cortisol levels immediately after trauma have been found to have a greater risk for later developing PTSD (Yehuda, McFarlane et al. 1998; Delahanty, Raimonde et al. 2003; Ehring, Ehlers et al. 2008; McFarlane, Barton et al. 2011). The time course of cortisol release during treatment of PTSD has not been investigated extensively. Two case studies suggested that symptom improvement is accompanied by a normalization of HPA axis functioning in PTSD, e.g. basal cortisol levels (Heber, Kellner et al. 2002; Kellner, Yehuda et al. 2002). Furthermore, it has been shown that responders to psychotherapy demonstrate an increase in cortisol levels (Olff, de Vries et al. 2007). Again, only very small samples have been investigated and prior studies have not included control groups without PTSD. Furthermore, it is still unclear whether HPA axis dysregulations are related to PTSD diagnosis or to trauma exposure per se, as suggested by some studies (Seedat, Stein et al. 2003; de Kloet, Vermetten et al. 2007). Our results, though cross-sectional, support the hypothesis that low cortisol levels might be a risk factor for developing PTSD in response to trauma, as both groups of patients, those with current and those with lifetime PTSD, exhibited lower cortisol levels than participants that never had PTSD. However, alternative explanations cannot be ruled out. For example, it is possible that lower cortisol results from chronic enhanced negative feedback and glucocorticoid receptor hypersensitivity that has been shown to develop after childhood trauma and (later) PTSD (Yehuda 2002; McGowan, Sasaki et al. 2009; Wingenfeld and Wolf 2011; van Zuiden, Kavelaars et al. 2013). Furthermore, patients with current PTSD might have experienced more stress during the assessments, which might contribute to the higher cortisol levels in comparison to the lifetime PTSD group.
Our study had two major strengths: the large sample size and measurement of catecholamines and cortisol as an integrated measure over 24 hours within the same participants. For example, a recent meta-analysis of daily cortisol output yielded only 262 traumatized and 320 non-traumatized controls (Morris, Compas et al. 2012). Furthermore, we were able to control for potential confounding variables, including age, sex, medication use, and perhaps most importantly depressive symptoms.
However, several limitations should be considered when interpreting our findings. More than 90% of our participants are men and the mean age was over 50 years. Therefore, it is not known whether our results are applicable to women and younger populations as well. An additional “healthy” control group was not recruited, which might be a limitation. However, it might also be a strength of the study to examine a “real-world” sample of medical outpatients who did not differ markedly in terms of the measured health parameters, which could help isolate the effects of PTSD status. The use of an above-average healthy control sample with no physical or somatic health problems might have lead to an overestimation of assumed PTSD effects. Furthermore, we did not have information about the duration of current PTSD and the time between prior PTSD and remission in the lifetime PTSD group. In addition, though PTSD and several comorbidities were assessed with structured clinical interviews, some important conditions, such as substance abuse, were measured with self-report questionnaires. Finally, it would be informative to investigate not only basal cortisol and catecholamine release but also the acute reactivity of the HPA axis and SNS to trauma- and non-trauma related stressors.
In summary, our results support the hypothesis of a disturbed interplay of HPA axis and SNS activity in PTSD, demonstrated by lower cortisol and exaggerated norepinephrine release. Prospective studies involving measures of HPA axis functionality, SNS activity, and cardiovascular health are needed to establish whether these alterations contribute to the increased risk of cardiovascular disease in patients with PSTD.
Highlights.
Patients with current PTSD had significantly higher 24-hour urine norepinephrine levels than those without PTSD.
Those who never had PTSD showed the lowest norepinephrine and highest cortisol values.
These changes could contribute to adverse health outcomes in patients with PTSD.
Acknowledgements
We gratefully acknowledge the time and efforts of the Mind Your Heart Study participants and staff.
Role of the Funding Source
The Mind Your Heart Study was supported by the National Heart, Lung, and Blood Institute (K23 HL 094765-0), the American Heart Association, the Irene Perstein Foundation, and Departmental funds from the University of California, San Francisco. The funding sources did not have any role in the design of the study, analysis of results, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Wingenfeld conducted the analyses and wrote the first draft of the manuscript. Drs. Otte and Wingenfeld designed the analytic plan. Drs. Cohen and Whooley designed the Mind Your Heart study and supervised study data collection. Dr. Neylan assisted in the PTSD assessment protocol. All authors reviewed and revised the mansucript draft and approved the final manuscript.
Conflict of Interest
Dr. Otte has received honoraria fees for lectures from Lundbeck and Servier and has received compensation as a member of the scientific advisory board of Lundbeck. Dr. Neylan reports receiving study medication from Actelion for a study funded by the Department of Defense and receiving study medication from Glaxo Smith Kline for a study funded by the Department of Veterans Affairs. Drs. Wingenfeld, Whooley, and Cohen report no potential financial conflicts of interest.
References
- Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007;99(6):642–649. [PMC free article] [PubMed] [Google Scholar]
- Bierer LM, Tischler L, et al. Clinical correlates of 24-h cortisol and norepinephrine excretion among subjects seeking treatment following the world trade center attacks on 9/11. Ann N Y Acad Sci. 2006;1071:514–520. doi: 10.1196/annals.1364.055. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, et al. Psychometric properties of the PTSD checklist (PCL). Behavioral Research & Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, et al. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007;32(3):215–226. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, et al. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30(2):121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, et al. Injury severity, prior trauma history, urinary cortisol levels, and acute PTSD in motor vehicle accident victims. J Anxiety Disord. 2003;17(2):149–164. doi: 10.1016/s0887-6185(02)00185-8. [DOI] [PubMed] [Google Scholar]
- Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):548–556. doi: 10.1016/j.pcad.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Kronish IM, et al. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166(5):806–814. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, Ehlers A, et al. Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities of PTSD and depression? Psychiatry Res. 2008;161(1):67–75. doi: 10.1016/j.psychres.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover DA, Poland RE. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27(7):805–819. doi: 10.1016/s0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Ball SG, et al. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27(4):339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Hawk LW, Dougall AL, et al. Urinary catecholamines and cortisol in recent- onset posttraumatic stress disorder after motor vehicle accidents. Psychosom Med. 2000;62(3):423–434. doi: 10.1097/00006842-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Heber R, Kellner M, et al. Salivary cortisol levels and the cortisol response to dexamethasone before and after EMDR: a case report. J Clin Psychol. 2002;58(12):1521–1530. doi: 10.1002/jclp.10102. [DOI] [PubMed] [Google Scholar]
- Inslicht SS, Marmar CR, et al. Increased cortisol in women with intimate partner violence-related posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31(7):825–838. doi: 10.1016/j.psyneuen.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Kellner M, Yehuda R, et al. Longitudinal course of salivary cortisol in post-traumatic stress disorder. Acta Psychiatr Scand. 2002;105(2):153–155. doi: 10.1034/j.1600-0447.2002.01012.x. discussion 155-156. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Mason JW, et al. Sustained urinary norepinephrine and epinephrine elevation in post-traumatic stress disorder. Psychoneuroendocrinology. 1987;12(1):13–20. doi: 10.1016/0306-4530(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, et al. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane AC, Barton CA, et al. Cortisol response to acute trauma and risk of posttraumatic stress disorder. Psychoneuroendocrinology. 2011;36(5):720–727. doi: 10.1016/j.psyneuen.2010.10.007. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, et al. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, et al. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, de Vries GJ, et al. Changes in cortisol and DHEA plasma levels after psychotherapy for PTSD. Psychoneuroendocrinology. 2007;32(6):619–626. doi: 10.1016/j.psyneuen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, et al. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25(2):271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Otte C, Neylan TC, et al. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biol Psychiatry. 2005;57(1):27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33(9):1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Schatzberg AF, et al. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43(1):60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 1990;27(2):245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- Rozanski A, Blumenthal JA, et al. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Seedat S, Stein MB, et al. Plasma cortisol and neuropeptide Y in female victims of intimate partner violence. Psychoneuroendocrinology. 2003;28(6):796–808. doi: 10.1016/s0306-4530(02)00086-0. [DOI] [PubMed] [Google Scholar]
- Turner JH, Neylan TC, et al. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biol Psychiatry. 2013;74(11):861–866. doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, et al. Posttraumatic stress disorder and incidence of type-2 diabetes: A prospective twin study. J Psychiatr Res. 2014 doi: 10.1016/j.jpsychires.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stegeren AH. The role of the noradrenergic system in emotional memory. Acta Psychol (Amst) 2008;127(3):532–541. doi: 10.1016/j.actpsy.2007.10.004. [DOI] [PubMed] [Google Scholar]
- van Zuiden M, Kavelaars A, et al. Predicting PTSD: pre-existing vulnerabilities in glucocorticoid-signaling and implications for preventive interventions. Brain Behav Immun. 2013;30:12–21. doi: 10.1016/j.bbi.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, et al. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Wong JM. Depression and cardiovascular disorders. Annu Rev Clin Psychol. 2013;9:327–354. doi: 10.1146/annurev-clinpsy-050212-185526. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Wolf OT. HPA axis alterations in mental disorders: impact on memory and its relevance for therapeutic interventions. CNS Neurosci Ther. 2011;17(6):714–722. doi: 10.1111/j.1755-5949.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25(2):341–368. vii. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R, McFarlane AC, et al. Predicting the development of posttraumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry. 1998;44(12):1305–1313. doi: 10.1016/s0006-3223(98)00276-5. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Seckl J. Minireview: Stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152(12):4496–4503. doi: 10.1210/en.2011-1218. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Siever LJ, et al. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44(1):56–63. doi: 10.1016/s0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick S, et al. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J Nerv Ment Dis. 1992;180(5):321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder: an epidemiologic community study. Arch Gen Psychiatry. 2004;61(4):394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Yufu K, Okada N, et al. Plasma norepinephrine is an independent predictor of adverse cerebral and cardiovascular events in type 2 diabetic patients without structural heart disease. J Cardiol. 2014 doi: 10.1016/j.jjcc.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Diamond DM. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature. Neurosci Biobehav Rev. 2013;37(5):860–895. doi: 10.1016/j.neubiorev.2013.03.024. [DOI] [PubMed] [Google Scholar]