Abstract
Background
The delta opioid receptor (DOR) is broadly expressed throughout the nervous system and regulates chronic pain, emotional responses, motivation and memory. Neural circuits underlying DOR activities have been poorly explored by genetic approaches. Here we used conditional mouse mutagenesis to elucidate receptor function in GABAergic neurons of the forebrain.
Methods
We characterized DOR distribution in the brain of Dlx5/6-CreXOprd1fl/fl (Dlx-DOR) mice, and tested main central DOR functions through behavioral testing.
Results
DORs proteins were strongly deleted in olfactory bulb and striatum, and remained intact in cortex and basolateral amygdala. Olfactory perception, circadian activity and despair-like behaviors were unchanged. In contrast, locomotor stimulant effects of SNC80 (DOR agonist) and SKF81297 (D1 agonist) were abolished and increased, respectively. Furthermore, Dlx-DOR mice showed lower levels of anxiety in the elevated plus-maze, opposing the known high anxiety in constitutive DOR knockout animals. Also Dlx-DOR mice reached the food more rapidly in a novelty suppressed feeding (NSF) task, despite their lower motivation for food reward observed in an operant paradigm. Finally, c-fos staining after NSF was strongly reduced in amygdala, concordant with the low anxiety phenotype of Dlx-DOR mice.
Conclusion
Here we demonstrate that DORs expressed in the forebrain mediate the described locomotor effect of SNC80 and inhibit D1-stimulated hyperactivity. Our data also reveal an unanticipated anxiogenic role for this particular DOR subpopulation, with a potential novel adaptive role. DORs therefore exert dual anxiolytic/anxiogenic roles in emotional responses, which may both have implications in the area of anxiety disorders.
Keywords: Delta opioid receptor, Conditional gene knockout, GABAergic forebrain neurons, Locomotion, Motivation, Emotion
Introduction
Mu, delta and kappa opioid receptors are distributed throughout the nervous system and play a central role in pain control, hedonic homeostasis and emotions (1, 2). In the last decade, the delta opioid receptor (DOR) has emerged as an attractive target to reduce chronic pain (3, 4). This receptor is also a key player in several brain processes (5), including the regulation of emotional responses (6), impulsivity (7) or learning and memory (8), and has raised interest in both areas of neurologic and psychiatric disorders. Emotional responses represent a most important aspect of DOR function. Preclinical studies have established a general beneficial role for DOR in reducing levels of anxiety and depressive-like behavior, and delta agonists are in clinical trial for the treatment of mood disorders (3, 9).
DORs are broadly expressed in central and peripheral nervous systems. In the mouse, quantitative autoradiographic binding (10-12) shows particularly abundant protein levels in the olfactory bulb (OB), cortex, striatum and amygdala (Amy). Moderate DOR levels are also found in interpeduncular and pontine nuclei, hippocampus (Hipp), spinal cord (SC) and dorsal root ganglia (DRGs), and low levels in hypothalamus, thalamus, mesencephalon and brain stem (reviewed in (13)). A knock-in mouse line expressing functional fluorescent DORs (14) has allowed anatomical studies of DOR expression with cellular and subcellular details in DRGs (15), enteric neurons (15-18) and the Hipp (16, 17). Refined mapping of DOR expression in the mouse is now possible (19) and provides a basis for understanding DOR activities in the brain and periphery. Analyses of DOR distribution in the human brain shows expression concordant with rodent studies in cortical regions and limbic structures such as Hipp and Amy, as well as basal ganglia and hypothalamus (20-23).
At present, neuron populations and brain circuits where DORs operate in the nervous system have been poorly explored. In pain research, local pharmacology at the level of DRGs and SC has indicated a role for peripheral DORs in pain control (24), and a conditional genetic approach has demonstrated that DORs expressed in small primary nociceptive neurons are essential to reduce persistent pain and mediate delta opioid analgesia (25). In the brain, local pharmacology has provided evidence for an anxiolytic role of DORs at the level of cingulate cortex (Cg Cx) (26), Hipp (27) and Amy (28, 29). However neural populations engaged in DOR-mediated mood control have not been examined by genetic approaches, and DOR-mediated mechanisms underlying motivational and emotional responses, or learning and memory remain unexplored.
In this study we used a Dlx5/6 driver Cre line to genetically inactivate the DOR gene in forebrain GABAergic neurons. We obtained a conditional knockout mouse line that lacks receptors in two main DOR expression sites, i. e. the OB and striatum, including caudate putamen (CPu) and nucleus accumbens (NAc). These mice retain full receptor density in the basolateral amygdala (BLA), which represents a third main site with densest DOR protein levels. We then examined these mice in behaviors known to engage these brain structures and may recruit DOR-mediated controls. Our data reveal an unexpected anxiogenic role for this particular DOR population, which contrasts with the known overall anxiolytic role of the receptor.
Methods and Materials
Animals
The DOR-floxed (Oprd1fl/fl or Ctrl mice) mouse line was described previously (25). Mice were crossed with CMV-Cre mice or Dlx5/6-Cre mice to produce constitutive knockout (CMV-CreXOprd1fl/fl or CMV-DOR) and conditional knockout (Dlx5/6- Oprd1fl/fl or Dlx-DOR) mouse lines, see details in Supplementary. For all behavioral experiment, the Dlx-DOR mice are compared to their control littermates Ctrl mice. In addition, the CMV-DOR mice were also tested in the anxiety-related tests (see Supplementary). Experiments were performed on animals aged between 6 and 18 weeks old, housed 2-4 per cage under standard laboratory conditions (12h dark/light cycle light on at 7am). Food and water were available ad libitum. In the chocolate pellet self-administration experiment, we used males only. For other experiments, both males and females were used, and data were pooled since statistical analysis showed not significant gender effect. All experimental procedures were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the local ethical committee (Comité d'éthique pour l'expérimentation animale IGBMC-ICS).
Quantitative Reverse Transcriptase-PCR
Sampling of brain regions, RNA extraction and quantification were performed according to a previous study (30, 31) and briefly described in Supplementary.
Autoradiographic Binding Assay
Sections were cut from Ctrl, Dlx-DOR and CMV-DOR brains (n = 3) for determination of total DOR binding using [3H] deltorphin-1 as the radiolabeled ligand. On the day of the experiment, sections were thawed and processed according to established protocols (32, 33), with minor modifications. Films exposure, development and analyze were performed as previously described by Kitchen et al. (33). Further details are described in Supplementary.
Agonist-Stimulated [35S]-GTPγS Binding Assays
Membrane preparations and [35S]GTPγS binding assays were performed on brain regions from Ctrl, Dlx-DOR and CMV-DOR mice as described (34) (see Supplementary).
Behavioral Assays
Locomotion, depressive-like behaviors (forced swim and tail suspension tests), anxiety-related behaviors (light/dark box, elevated plus maze and open field tests), novelty-suppressed feeding tests and food self-administration experiments were performed as described in Supplementary.
Drugs
The non-peptidic DOR agonist SNC80 and the dopamine D1 receptor agonist SKF-81297 were used at doses according to previous studies (35, 36). See preparation in Supplementary.
c-Fos immunoreactivity
Measures of c-fos protein expression were performed as reported (37). Further details about sections processing are provided in Supplementary.
Statistical analysis
Statistical differences were determined by analysis of variance (ANOVA) (StatView 5, SAS Institute Inc., Cary, North Carolina) followed by Bonferroni/Dunn post hoc analysis. The F values and experimental degrees of freedom are included in the Results Section. For experiments with two groups, a Student t-test was used. The level of statistical significance was set at p < 0.05. For the behavioral tests during which data were obtained on several periods during the same session (locomotor tests, the Open Field test and despair-like behavior paradigms), the analysis of variance repeated measures was used.
Results
Dlx-DOR mice show DOR deletion mainly in OB and striatum
We used the Cre-LoxP strategy to inactivate the DOR gene (Oprdm1) in forebrain areas. Because DORs are mainly expressed in GABAergic neurons (17, 38, 39), we mated floxed-DOR (Oprd1fl/fl) mice (25) with Dlx-Cre5/6 mice that express Cre recombinase in the forebrain GABAergic neurons (40) to produce conditional (Dlx5/6-Cre X Oprd1fl/fl or Dlx-DOR) mutant mice. We first analyzed DOR transcripts throughout the nervous system using quantitative reverse transcriptase-PCR analysis (Fig. 1A). In mutant mice, DOR mRNA expression was undetectable in OB (OB) and striatum, including CPu and NAc, partially reduced in frontal cortex (FCx) and Amy, and showed normal levels in the spinal cord (SC), periaqueductal gray matter (PAG), brainstem (BS), dorsal raphe nucleus (DRN) and ventral tegmental area (VTA). The genetic deletion, therefore, impacts mainly forebrain areas, consistent with the Dlx5/6-Cre expression pattern ((40) and our unpublished data).
Fig. 1. Neuroanatomical characterization of Dlx-DOR mice.
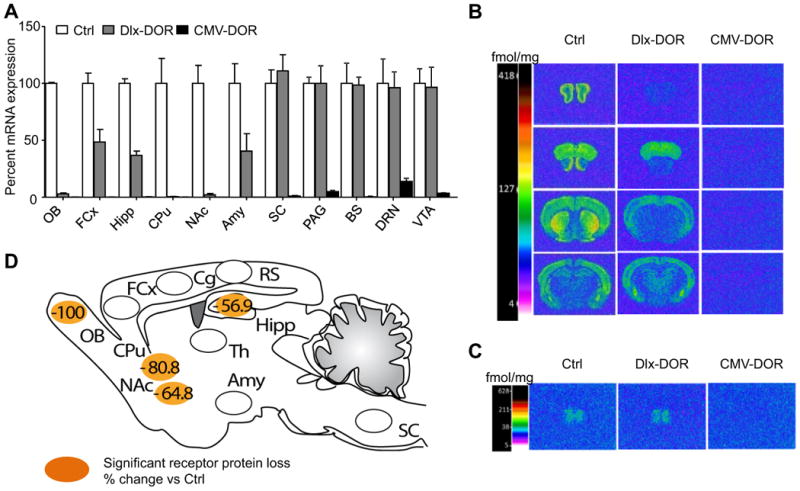
(A) Quantitative RT-PCR. DOR mRNA levels were measured in microdissected samples from Control (Ctrl, white bars), Dlx-DOR (conditional mutant, grey bars) and CMV-DOR (constitutive mutant, black bars) mice (n=3-4/group). Data were normalized using the housekeeping gene 36B4. Expression levels of mutants are expressed as percent change compared to control levels. The DOR transcript was undetectable in OB, CPu and NAc, partially decreased in FCx, Hipp and Amy and unchanged in SC, PAG, BS, DRN and VTA of Dlx-MOR mice, and was undetectable overall in CMV-DOR mice. (B-C) Quantitative DOR ligand binding autoradiography. Brain sections were labeled with [3H] deltorphin-1 and all sections were processed in parallel throughout binding and development of autoradiograms. Representative autoradiograms from brain (B) and SC (C) sections are shown for the three genotypes. The color bar code shows a pseudo-colour interpretation of relative densities from black and white images calibrated in fmol/mg tissue. Non-specific binding was homogenous and at background levels. Values and statistics are shown in Table 1. (D) Summary of DOR protein levels in Dlx-DOR mice compared to control mice, adapted from Table 1. Regions in orange correspond to brain areas showing significant reduction of DOR protein, and numbers represent percent change of DOR protein levels in conditional mutant mice from Table 1. Abbreviations: Amy, amygdala; Cg, cingulated cortex; BS, brainstem; CPu, caudate-putamen nucleus; DRN, dorsal raphe nucleus; FCx, frontal cortex; Hipp, hippocampus; NAc, nucleus accumbens; OB, olfactory bulb; PAG, periaqueducatal gray; RS, retrosplenial cortex; SC, spinal cord; VTA, ventral tegmental area.
We next quantified DOR protein distribution in Dlx-DOR mice, using autoradiographic binding (Table 1 and Fig. 1B-D). There was a strong reduction of [3H] deltorphin-1 binding in external plexiform and internal granular layers of OB, as well as lateral and medial CPu and olfactory tubercles from Dlx-DOR mice. Significant reduction of DOR binding sites was also found in the NAc shell and CA2/3 regions of the Hipp. In contrast there was no significant modification of DOR binding sites throughout cortical areas and BLA subdivisions, and DOR protein levels were also unchanged at the level of SC. Densitometry analysis of CMV-DOR samples confirmed complete DOR deletion in CMV-DOR mice throughout the nervous system.
Table 1. Quantification of specific [3H] deltorphin-1 binding in brain sections from control (Ctrl) and conditional (Dlx-DOR) mutant mice.
Values represent mean ± SEM fmol/mg of tissue equivalent in brain regions of Ctrl and Dlx-DOR mice. Bregma coordinates are taken from the mouse brain atlas of Franklin and Paxinos (63). Specific binding was calculated after the subtraction of non-specific from total [3H] deltorphin-1 binding. Percent change in binding indicates change in Dlx-DOR compared to Ctrl mice. N indicates number of animals per group. No [3H] deltorphin-1 binding could be detected in full knockout brains, data not shown. Two-way ANOVA revealed significant effect of Genotype, Region and Genotype × Region, all p < 0.001. Post-hoc t-test comparisons revealed significant within-region differences compared to WT:
| Region | Bregma | [3H]DELT-1 specific binding (fmol/mg tissue) | % change | ||
|---|---|---|---|---|---|
|
| |||||
| Ctrl (n = 3) | Dlx-DOR (n = 4) | ||||
| Anterior Olfactory Nucleus | 2.46 | ||||
| AON | 42.32 ± 6.34 | 37.60 ± 4.1 | -11.2 | ||
| Motor Cortex | 2.10 | ||||
| Superficial layers | MtCx(Sl) | 105.6 ± 16.4 | 103.1 ± 11.1 | -2.3 | |
| Deep layers | MtCx(Dl) | 92.1 ± 13.3 | 94.6 ± 11.8 | 2.6 | |
| Frontal Cortex | 1.98 | ||||
| Superficial layers | FrCx(Sl) | 112.7 ± 16.5 | 100.2 ± 12.4 | -11.1 | |
| Deep layers | FrCx(Dl) | 83.2 ± 9.8 | 94.6 ± 10.5 | 13.7 | |
| Cingulate Cortex | 1.10 | ||||
| Superficial layers | CgCx(Sl) | 89.5 ± 15.4 | 87.3 ± 14.3 | -2.5 | |
| Deep layers | CgCx(Dl) | 97.4 ± 14.8 | 95.8 ± 15.1 | -1.7 | |
| Frontal-Parietal Cortex | 1.10 | ||||
| Superficial layers | FrPCx(Sl) | 93.3 ± 14.3 | 97.9 ± 13.5 | 4.9 | |
| Deep layers | FrPCx(Dl) | 93.6 ± 14.3 | 93.1 ± 15.8 | -0.5 | |
| Insular Cortex | 1.10 | ||||
| Superficial layers | InCx(Sl) | 120.6 ± 16.0 | 112.4 ± 20.6 | -6.8 | |
| Deep layers | InCx(Dl) | 124.1 ± 19.2 | 115.5 ± 18.0 | -6.9 | |
| Rostral somatosensory Cortex | 1.10 | ||||
| Superficial layers | SsRCx(Sl) | 101.1 ± 8.6 | 84.6 ± 13.9 | -16.3 | |
| Deep layers | SsRCx(Dl) | 81.6 ± 9.7 | 78.9 ± 12.4 | -3.4 | |
| Piriform Cortex | 1.10 | ||||
| Pir | 41.28 ± 6.13 | 40.15 ± 6.42 | -2.7 | ||
| Caudal somatosensory Cortex | -2.06 | ||||
| Superficial layers | SsCCx(Sl) | 118.7 ± 9.5 | 86.9 ± 11.6 | -26.8 | |
| Deep layers | SsCCx(Dl) | 89.9 ± 9.3 | 92.3 ± 15.2 | 2.7 | |
| Perirhinal Cortex | PRhCx | -2.06 | 120.5 ± 19.6 | 106.9 ± 11.1 | -11.3 |
| Auditory Cortex | -2.54 | ||||
| Superficial layers | AuCx(Sl) | 104.9 ± 11.8 | 76.2 ± 10.5 | -27.4 | |
| Deep layers | AuCx(DL) | 96.0 ± 10.7 | 78.6 ± 10.2 | -18.2 | |
| Visual Cortex | -3.52 | ||||
| Superficial layers | ViCx(Sl) | 104.3 ± 9.4 | 89.6 ± 7.5 | -14.1 | |
| Deep layers | ViCx(Dl) | 92.9 ± 11.7 | 71.3 ± 10.2 | -23.2 | |
| Entorhinal Cortex | EntCx | -3.64 | 54.2 ± 8.6 | 52.0 ± 7.4 | -4.1 |
| Olfactory bulb | 3.56 | ||||
| External plexiform Layer | EPl | 200.5 ± 27.1 | 0.0 ± 0.0*** | -100.0 | |
| Internal granular layer | IGl | 84.3 ± 14.0 | 0.0 ± 0.0*** | -100.0 | |
| Nucleus accumbens | 1.18 | ||||
| Core | AcbC | 60.0 ± 11.7 | 24.4 ± 9.2 | -59.3 | |
| Shell | AcbSh | 68.2 ± 16.0 | 20.2 ± 8.3** | -70.3 | |
| Caudate putamen | 1.10 | ||||
| Medial | CPuL | 77.3 ± 21.5 | 22.1 ± 7.0*** | -78.8 | |
| Lateral | CPuM | 128.9 ± 34.6 | 33.2 ± 9.2*** | -82.9 | |
| Tubercle | Tu | 1.10 | 168.4 ± 42.7 | 16.4 ± 6.4*** | -80.3 |
| Septum | 0.74 | ||||
| Medial | MS | 29.7 ± 6.7 | 18.1 ± 7.8 | -39.0 | |
| Lateral | LS | 37.0 ± 8.7 | 22.4 ± 9.2 | -39.4 | |
| Vertical limb of the diagonal band | VDB | 0.74 | 16.9 ± 4.2 | 19.9 ± 6.3 | 17.8 |
| Globus pallidus | GP | -0.22 | 44.8 ± 12.1 | 15.5 ± 6.4 | -65.3 |
| Preoptic area | PoA | -0.22 | 12.6 ± 3.7 | 12.8 ± 5.0 | 1.4 |
| Thalamus | Th | -1.46 | 17.2 ± 2.9 | 21.0 ± 6.3 | 22.4 |
| Amygdala | -1.46 | ||||
| Basolateral | BLA | 77.5 ± 17.4 | 82.6 ± 21.0 | 6.5 | |
| Basomedial | BMA | 76.7 ± 20.8 | 81.2 ± 13.7 | 5.8 | |
| Medial | CeM | 43.2 ± 12.1 | 47.7 ± 15.1 | 10.4 | |
| Hypothalamus | Hypo | -1.46 | 16.3 ± 3.8 | 17.3 ± 5.0 | 6.1 |
| Hippoccampus | |||||
| CA1 | CA1 | -2.06 | 47.6 ± 7.3 | 20.3 ± 2.9 | -57.3 |
| CA2/3 | CA2/3 | -2.06 | 52.1 ± 8.6 | 17.3 ± 3.2* | -66.8 |
| Dentate gyrus | DG | -2.06 | 59.0 ± 8.9 | 28.5 ± 5.6 | -51.7 |
| Dorsal | dHip | -3.80 | 47.4 ± 7.4 | 22.7 ± 4.7 | -52.1 |
| Ventral tegmental Area | VTA | -2.92 | 20.50 ± 3.7 | 20.49 ± 2.79 | -0.05 |
| Presubiculum | Prs | -3.64 | 53.0 ± 13.3 | 31.1 ± 8.9 | -41.4 |
| Periaqueducatal Gray | PAG | -4.04 | 12.09 ± 2.26 | 12.99 ± 0.73 | 7.4 |
| Dorsal Raphe Nucleus | DRN | -4.04 | 13.25 ± 2.48 | 14.17 ± 1.09 | 6.9 |
| Spinal Cord | |||||
| Cervical | C6 | ||||
| Whole section | 16.0 ± 9.5 | 21.1 ± 7.2 | 30.0 | ||
| Superficial layers (lamina I and II) | 24.8 ± 9.3 | 29.4 ± 8.8 | 18.6 | ||
| Laminas III-IV | 17.9 ± 6.9 | 21.6 ± 6.3 | 21.2 | ||
| Lamina X | 16.8 ± 8.5 | 18.9 ± 7.5 | 12.4 | ||
| Ventral horn (laminas VII - IX) | 18.8 ± 6.9 | 22.1 ± 6.7 | 17 | ||
p < 0.05;
p < 0.01;
p < 0.001.
To further confirm lack of protein activity, we measured DOR-mediated G protein activation in brain areas showing reduced receptor binding sites. As expected, agonist-induced [35S]-GTPγS binding (Table S1) was abolished in membrane preparations from OB and CPu, decreased in the hippocampal preparation and comparable to controls in both FCx and SC samples. Receptor signaling therefore fully matches receptor binding in mutant mice.
Dlx-DOR mice show altered locomotor responses to DOR and D1/D3 DAR agonists
We first examined whether DOR loss in CPu and NAc leads to changes in spontaneous locomotor activity and feeding behavior (Table 2). Analysis of total locomotor activity during light (F (2, 20) = 0.31; p>0.05, one-way ANOVA) and dark (F (2, 20) = 0.8; p>0.05, one-way ANOVA) phases revealed no significant difference between Dlx-DOR and control mice. Similarly, no difference in number of distributed food pellets was detected between genotypes (F (2, 20) = 2.05; p>0.05, oneway ANOVA).
Table 2. Basal locomotor activity in control (Ctrl), conditional (Dlx-DOR) and total (CMV-DOR) knockout.
Total locomotor activity was automatically recorded during 20h (from 3 P.M. to 11 A.M.) in actimetry boxes. Values represent the number of infrared beams crossed for the whole session, the light period (8h) or the dark period (12h). There were no statistical differences. CMV-DOR mice showed a trend towards an increased locomotor activity as compared to Ctrl and Dlx-DOR mice. Number of food pellets distributed during the session was measured and no difference was detected. n= 8 per genotype. Statistical analysis was performed using one-way ANOVA.
| Genotype | Total locomotor activity | During light period (8h) | During night period (12h) | Nb of distributed food pellets |
|---|---|---|---|---|
| Ctrl | 4850.43 ± 1282.05 | 2246.29 ± 583.2 | 2604.14 ±707.94 | 288 ± 10.59 |
| Dlx-DOR | 5306.80 ± 851.83 | 2208.6 ± 353.72 | 3098.20 ± 514.76 | 303.3 ± 35.43 |
| CMV-DOR | 6606.33 ± 2013.84 | 2476 ± 703.48 | 4130.33 ± 1320.1 | 209.5 ± 41.96 |
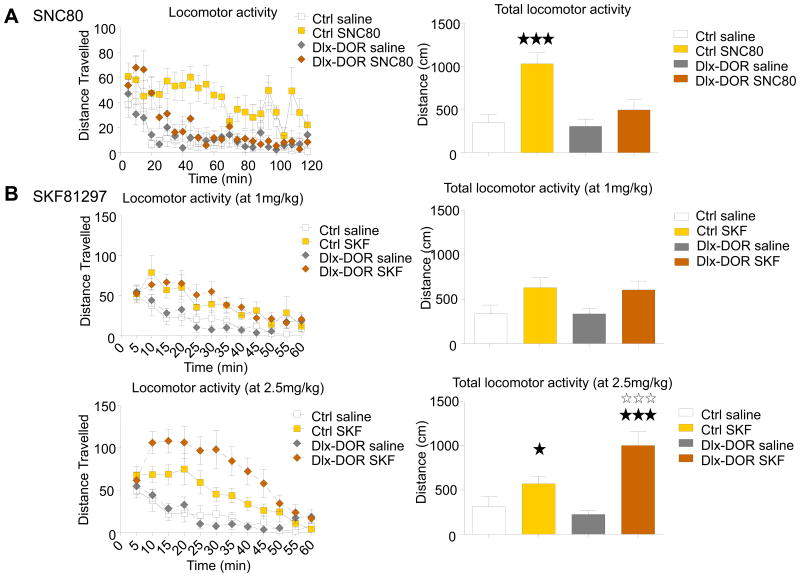
We then examined locomotor stimulant effects of the prototypal DOR agonist, SNC80 (41) in Dlx-DOR mice (Fig 2A) in actimetry cages. Habituation was similar across genotypes (data not shown). SNC80 treatment (10 mg/kg) induced the expected locomotor stimulation in control mice but was inefficient in Dlx-DOR mice. Two-way ANOVA revealed a significant effect of treatment (F (1, 32) = 16.37; p<0.001) and genotype (F (1, 32) = 7.10; p<0.001), and significant treatment × genotype interaction (F (2, 32) = 5.25; p<0.05). Post hoc analysis confirmed that SNC80 treatment significantly enhanced locomotor activity in Ctrl (p<0.001, Bonferroni/Dunn test) but not in Dlx-DOR mice (p>0.05, Bonferroni/Dunn test). Targeted deletion of DORs in forebrain GABAergic neurons therefore abolishes the locomotor stimulant effect of SNC80.
Fig. 2. DOR and D1-mediated locomotor activity.
Dlx-DOR mice and their control littermates (Ctrl) were tested in actimetry boxes for responses to (A) the DOR agonist SNC80 or (B) the dopamine D1 agonist SKF81297. (A) Left panel shows locomotor activity in Ctrl and Dlx-DOR mice treated by intraperitoneal injection of SNC80 (10 mg/kg) or saline over a 2H session, and right panel shows total activity. Activity was significantly increased in SNC80-treated control mice only (n= 8-10 per genotype and treatment). (B) In a second cohort, SKF-81297 was administered subcutaneously (at 1 or 2.5 mg/kg doses). Both Dlx-DOR mice and their control littermates (Ctrl) showed increased locomotor activity compared to vehicle-treated mice, and this effect was significantly stronger in Dlx-DOR mice at the high dose (left, time course; right total activity; n = 9-11 mice per genotype and treatment). Filled and open stars indicate significant treatment or genotype effect, respectively. One star, P<0.05; three stars, P<0.001 (Two-way ANOVA).
To further explore integrity of the basal ganglia circuitry, we examined locomotor stimulant effects of SKF-81297, a D1 dopamine receptor agonist (Fig 2B). At a low dose (1 mg/kg), SKF-81297 induced a locomotor stimulation in both control and Dlx-DOR mice (F(1, 37)=8.63; p<0.01), with no significant difference between genotypes (F (1, 37) = 0.02; p>0.05, Two-way ANOVA). At high dose (2.5 mg/kg), SKF-81297 induced a locomotor stimulation in control animals and this stimulant effect was potentiated in Dlx-DOR mice. Two-way ANOVA performed on total activity scores showed significant effect of treatment (F (1, 37) = 22.23; p<0.0001) and significant genotype × treatment interaction (F (1, 37) = 5.54; p<0.05). Post hoc analysis confirmed that 2.5 mg/kg SKF-81297 stimulates Dlx-DOR mice significantly more than controls (p<0.001, Bonferroni/Dunn test). Thus, selective inactivation of DORs in forebrain GABAergic neurons potentiates D1/D3 dopamine receptor function.
Dlx-DOR mice show reduced levels of anxiety
Our earlier work revealed a depressive-like phenotype in constitutive DOR knockout mice (6). Dlx-DOR mice show major receptor loss in OB and NAc, two areas associated with altered mood (42)((43). We therefore tested Dlx-DOR mice for olfactory discrimination, as well as despairlike behaviors in forced swim and tail suspension tests. Mutant mice behaved similarly to control littermates in all these tasks (Supplementary Results and Figure S1).
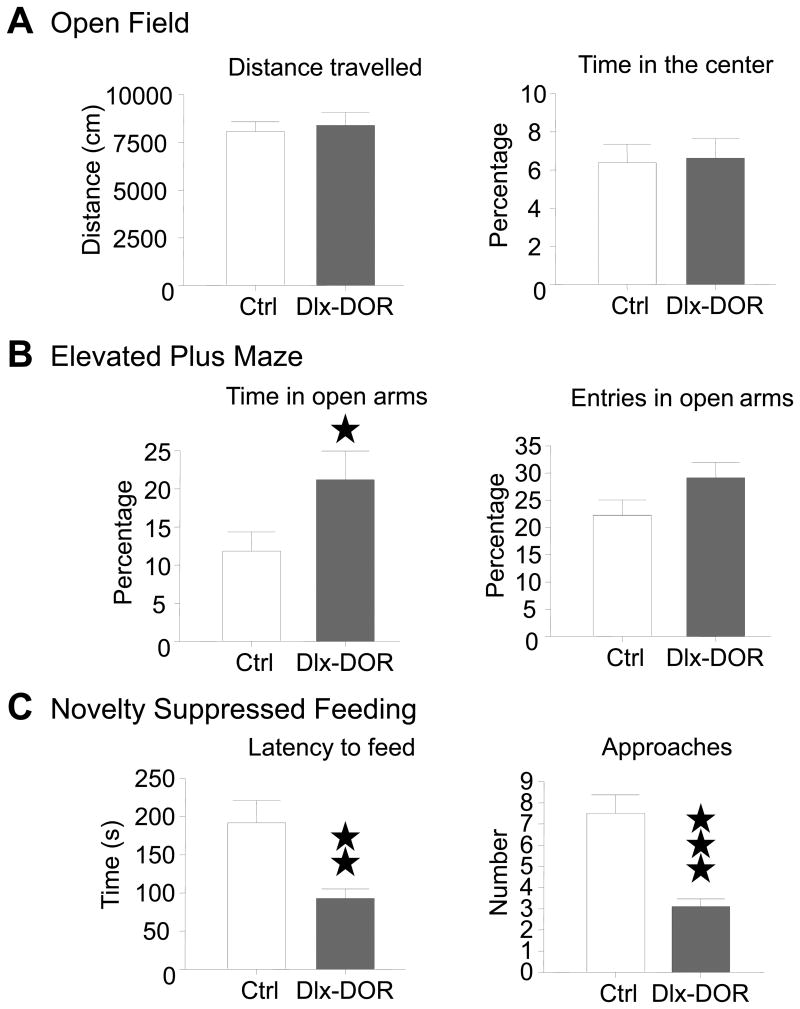
Previous work also indicated that constitutive DOR knockout mice show enhanced anxietylike behavior (6, 44). Many brain structures contribute to anxiety-associated responses, including the Amy (45) and forebrain areas (46) where DORs have either remained intact (Amy) or been deleted (striatum, Hipp) in Dlx-DOR mice. We tested whether the strong DOR depletion in forebrain but not Amy would produce an anxiety-related phenotype (Figure 3). In the open field, Dlx-DOR mice did not differ from controls for both general activity (t (30) = 0.38, p>0.05, Student t-test) and time spent in the arena center (t (30) = 0.17, p>0.05, Student t-test) (Figure 3A). In the elevated plus-maze test, however, a behavioral phenotype was detectable (Figure 3B). Dlx-DOR mice displayed lower anxiety-related behavior compared to controls, as shown by increased time spent in open arms (t (30) = 2.31, p<0.05, Student t-test). Mutant mice also tended to make more entries into open arms, although this effect did not reach statistical significance (t (30) = 1.44, p>0.05, Student t-test). The number of entries in closed arms, an index of locomotor activity, was otherwise unchanged (Ctrl: 11.75 ± 0.72 and Dlx-DOR: 11.19 ±0.79; t (30) = 1.16, p>0.05, Student's t-test). Thus, mice lacking DORs in forebrain GABAergic neurons display lower levels of anxiety, a phenotype that opposes the described increased anxiety-like behaviors in constitutive DOR knockout mice.
Fig. 3. Anxiety-related behaviours.
(A) Open Field. Distance travelled (left) and time spent in center (right) did not differ across genotypes. (B) Elevated plus-maze. Dlx-DOR showed increased time in open arms (left) and a trend to more entries in those arms (right) compared to Ctrl mice. General activity was similar (total visits) between the two groups (data not shown), reflecting no change in spontaneous locomotor activity. (C) Novelty suppressed feeding. Latency to feed was decreased (left), and accordingly number of approaches was decreased also (right) in Dlx-DOR mice compared with Ctrl mice. n=16 per genotype, and filled stars represent significant differences compare to Ctrl mice. One star, P<0.05; two stars, P<0.01; three stars, P<0.001 (Student t-test).
To further examine this unexpected phenotype, we tested Dlx-DOR mice in the novelty suppressed feeding (NSF) task (Figure 3C). In this paradigm, low latency to start eating in a novel environment reflects reduced anxiety-related behavior (47, 48). Dlx-DOR mice showed a shorter latency to feed compared to controls (t (34) = -3.38, p<0.01, Student t-test) and made fewer approaches (t (34) = -5.00, p<0.001, Student t-test). Both parameters, therefore, indicate strong behavioral modifications in mutant mice, consistent with lower anxiety observed in the elevated plus-maze.
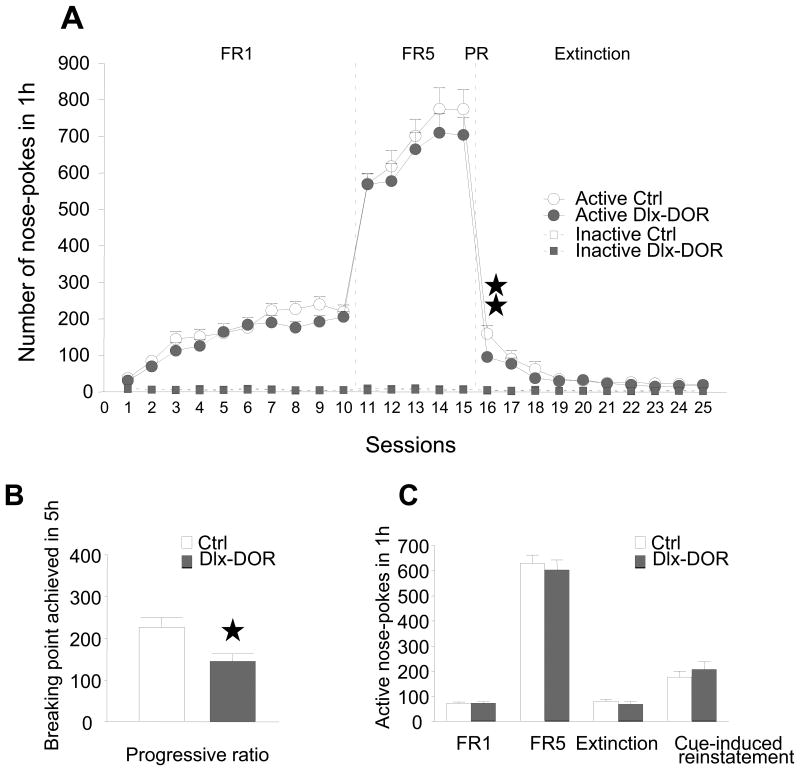
The phenotype observed in NSF may partly result from increased motivation to obtain the food. However, the amount of food pellets consumed was similar between both groups in actimetry cages (Table 2), suggesting that regular food consumption is unchanged in mutant mice. To further test motivation for food in Dlx-DOR mice, we examined motivation to eat palatable chocolate-flavoured pellets in a self-administration (SA) paradigm (Figure 4). Mice from both genotypes discriminated between active and inactive holes during most of the training period, and active nose-poking increased across days (Figure 4A). Control and mutant mice acquired and maintained operant responding similarly in both fixed ratio 1 (FR1) and fixed ratio 5 (FR5) schedules. Number of pellets consumed did not differ between genotypes, as revealed by the two-way ANOVA analysis showing significant effects of day (F(14,518) = 70.37; p<0.001), no main effect of genotype (F(1,37) = 0.29; p>0.05) and no interaction between genotype and day (F(14,518) = 0.76; p>0.05). In addition, both genotypes expressed similar levels of active nose-poking during FR1 (Figure 4C, 72.30 ± 5.45 in Ctrl and 72.38 ± 5.61 in Dlx-DOR mice) and FR5 (629.29 ± 32.68 in Ctrl and 603.21 ± 39.84 in Dlx-DOR mice) reinforced phases (see Table S2 for three-way ANOVA). The targeted DOR deletion in Dlx-DOR mice, therefore, does not modify operant responding to food reward.
Fig. 4. Chocolate flavoured pellets self-administration.
(A) Acquisition and maintenance. Mean number of active and inactive nose-pokes during 10 days of FR1, 5 days of FR5 and 10 days of extinction in 1 h daily sessions. (B) Motivation. Mean breaking point achieved in a session of progressive ratio that was conducted once and lasted 5 h. (C) Cue-induced reinstatement. Mean number of active nose-pokes during the different experimental phases: mean of the 3 days of the acquisition criteria of chocolate self-administration during FR1 and FR5, mean of the 3 days of the acquisition of the extinction criteria and cue-induced reinstatement. Ctrl (n = 23) and Dlx-DOR mice (n = 16). Data are expressed as mean ± SEM. One star, P<0.05; two stars, P<0.01 comparison between genotypes (three-way ANOVA).
In contrast, breaking point values were significantly decreased in Dlx-DOR mice compared to control littermates (F(1,37) = 6.88; p < 0.05) in the progressive ratio (PR) schedule of reinforcement (Figure 4B). Mutant mice, therefore, show reduced motivation for chocolate-flavoured pellets, suggesting that DOR deletion in forebrain GABAergic neurons diminishes motivation for food reward. Altogether, our observations that Dlx-DOR mice show normal food consumption (actimetry boxes), normal acquisition of chocolate pellet self-administration (SA, FR1 and FR5) and reduced motivation for these pellets (SA, PR), strongly suggest that increased motivation for food does not contribute to the low anxiety behavior of Dlx-DOR mice in the NSF task.
Dlx-DOR mice show abnormal neuronal activity in cortex, Amy and NAc following novelty suppressed feeding test
C-fos protein expression is routinely used as a marker of neuronal activity (49). To gain insight into circuit mechanisms underlying the hypoanxiety phenotype of Dlx-DOR mice, we assessed Fos protein expression following animal exposure to the NSF task (Table 3). In a control experiment, c-fos immunoreactivity did not differ across genotypes under basal conditions (Table S3). Also, 24h deprivation alone induced similar c-fos staining in Dlx-DOR and control littermates, except at the level of NAc (t (2) = 8.46, p<0.05, Student t-test) and insular cortex (Ins Cx) (t (2) = 6.4 3, p<0.05, Student t-test) where a food-related response may contribute to distinguish mutant and control mice. After NSF, mutant mice showed a significant decrease of c-fos immunostaining in several brain regions associated to the central integration of emotional components of aversive stimuli, including the Ins Cx (t (14) = 3.04, p<0.01, Student t-test), BLA (t (12) = 3.21, p<0.01, Student t-test) and central nuclei of the amygdala (CeA) (t (12) = 4.56, p<0.001, Student t-test). On the other hand, a significant increase of c-fos protein expression was found in NAc, interfacing emotion, motivation and action. C-Fos expression was otherwise unchanged in all subregions of the CPu, the Cg Cx (Cg Cx), the basomedial nucleus of the amygdala and ventral tegmental area (VTA). Together, the data show that targeted DOR deletion in forebrain GABAergic neurons leads to distinct neuronal activation in mutant and control mice after NSF, which occur mainly in BLA and CeA. Decreased c-fos activation in these two brain regions is consistent with the low anxiety behavior of mutant mice in the task.
Table 3. Quantification of c-fos immunoreactivity in control (Ctrl) and conditional (Dlx-DOR) mice following novelty suppressed feeding.
Ctrl and Dlx-DOR mice were sacrificed 90 min after the NSF test. c-fos protein labeling is obtained by immunocytochemistry on brain sections. The quantification is performed on images acquired on the Hamamatsu scanner and expressed in number of c-fos positive cells per mm2. The level of c-fos positive cells in the Cg Cx, four subregions of the striatum, BMA and VTA is similar in the two groups. However, the quantification reveals a significant reduction of c-fos positive cells in the BLA, CeA and insular cortex on sections from Dlx-DOR in comparison with the Ctrl mice, whereas a significant increase is found in the NAc. n = 6-9 animals per genotype / 4-12 sections per regions / 2 counts per sections.
| Region | Bregma | c-fos positive cells / mm2 | Stats | ||
|---|---|---|---|---|---|
|
| |||||
| Ctrl | Dlx-DOR | ||||
| Nucleus accumbens | NAc | 1.18 | 391.0 ± 38.4 | 497.3 ± 43.2* | p < 0.05 |
| Cingulate cortex | Cg Cx | 1.10 | 986.9 ± 60.3 | 870.2 ± 85.4 | p > 0.05 |
| Insular cortex | Ins Cx | 1.10 | 237.1 ± 22.2 | 165.4 ± 11.9* | p < 0.05 |
| Striatum | 1.10 | ||||
| Dorsomedial | DM | 492.2 ± 95.1 | 448.9 ± 77.5 | p > 0.05 | |
| Dorsolateral | DL | 228.1 ± 50.2 | 253.1 ± 55.3 | p > 0.05 | |
| Ventromedial | VM | 168.0 ± 14.6 | 151.9 ± 9.9 | p > 0.05 | |
| Ventrolateral | VL | 74.6 ± 21.6 | 79.4 ± 19.3 | p > 0.05 | |
| Amygdala | -1.46 | ||||
| Basolateral nucleus | BLA | 228.3 ±26.4 | 138.7 ± 14.1** | p < 0.01 | |
| Basomedial nucleus | BMA | 323.0 ± 65.9 | 232.6 ± 21.4 | p > 0.05 | |
| Central nucleus | CeA | 298.6 ±33.6 | 153.0 ± 12.0*** | p < 0.001 | |
| Ventral Tegmental Area | VTA | -2.54 | 274.17 ± 28.32 | 352.38 ± 33.25 | p > 0.05 |
, P<0.05;
two stars: , P<0.01;
three stars: , P<0.001 (Student t-test).
Discussion
We used Dlx5/6-Cre mice to target the DOR gene in forebrain GABAergic neurons, and obtained conditional knockout mice with a strong deletion of DORs in OB and striatum, while the receptor was preserved in the cortex, BLA, more rostral brain areas and SC. Behavioral analysis of mutant mice provide first genetic evidence that DORs expressed in these brain areas inhibit D1R-mediated locomotor activity and motivation for food reward. Additionally, our study uncovered a novel role for DOR in the regulation of anxiety-related behaviors.
The driver Dlx5/6-Cre mouse line was used previously to delete CB1 receptors from GABAergic neurons of the forebrain (40). Based on the notion that opioid receptors are mostly expressed in GABAergic neurons (16, 17), we anticipated strong decrease of DOR mRNA expression and protein levels throughout the forebrain. Indeed, DORs were almost entirely deleted in OB and striatum. Residual DOR protein in CPu and NAc may arise from DOR protein expression in striatal cholinergic interneurons, or may reflect presynaptic receptors on glutamatergic neurons that mainly project from cortex and Amy to the striatum (50-52). DOR mRNA and protein deletion was otherwise partial in Hipp, and protein levels were fully preserved in cortical areas and Amy. We cannot exclude that, in the latter two areas, limited DOR deletion in discrete neuron populations occurred but was not detected by our methods. In these brain areas also, remaining or intact receptor protein expression could be explained by partial Cre-mediated excision, although in our hands, crossing Dlx5/6-Cre mice with ROSA26 reporter mice showed strong Cre activity at these sites (data not shown). Alternatively, DOR protein expression may occur in non-GABAergic neurons in these brain regions, or could be synthesized and transported from more posterior brain structures. In support of this, Amy showed decreased DOR mRNA, indicating local Cre-mediated DOR gene excision. However, DOR protein levels were maintained, suggesting that the majority of amygdalar receptors are localized presynaptically on afferent terminals. Finally, ectopic Cre expression has been reported in non-GABAergic neurons of hypothalamic areas in Dlx5/6-Cre mice (53), however this did not seem to impact Dlx-DOR mice that show intact DOR protein levels in the hypothalamus (Table 1).
Constitutive DOR knockout mice show enhanced spontaneous locomotor activity (6). We did not observe a similar phenotype in Dlx-DOR mice, suggesting that this particular DOR activity is not regulated at the level of GABAergic forebrain neurons, or simply could not be detected under our experimental conditions. We further observed that the described SNC80-induced hyperlocomotion effect (36, 54) is abolished in Dlx-DOR mice, demonstrating that targeted DORs are essential for the known stimulant effects of the agonist. It is likely that this DOR activity operates at the level of striatum, which plays a prominent role in locomotor activity (55) and shows most effective DOR deletion in conditional mutant mice. Finally, we found potentiated SKF-81297-induced hyperactivity in mutant mice. We have previously reported that constitutive DOR gene knockout and DOR blockage by systemic DOR antagonist treatment, both produce a similar higher sensitivity to SKF-stimulating effects (35). Together with the present study, the data suggest that DORs expressed in striatal GABAergic neurons exert a tonic suppressive effect on striatonigral D1 pathways and the associated locomotor response. Whether DOR/D1R interactions occur directly at the level of D1R-expressing medium spiny neurons or via intrastriatal microcircuitry remains to be determined.
Modified dopaminergic signaling in Dlx-DOR mice may also impact responses to rewarding outcomes. We found that operant responding for palatable food is unchanged in Dlx-DOR mice under FR1 and FR5 schedules. We previously showed that morphine self-administration is preserved in constitutive DOR knockout mice (56), and together the data suggest that DORs do not play a major role in opioid or food reward. However, we found in this study that Dlx-DOR mice show slightly decreased motivation for food reward in a PR schedule. Although DORs overall do not seem to regulate food reward, it is possible that DORs in forebrain GABAergic neurons contribute to some aspects of motivational processes, a hypothesis that deserves further investigations.
DORs were fully removed from the OB in Dlx-DOR mice. We found, however, no main alteration in basic olfactory perception, suggesting that DORs in the OB are not necessary to the detection of olfactory stimuli. Olfactory bulbectomy is a classical model of despair-like behavior (57), and we speculated that Dlx-DOR mice may show a despair-like phenotype, as do constitutive DOR knockout mice (6). Under our experimental conditions however, mutant mice showed no sign of despair behaviour, suggesting that DORs do not tonically regulate emotional circuits associated to OB circuitry and olfaction. Despair-like behaviour in constitutive DOR KO mice therefore, likely results from lack of receptor activity in other brain circuits. It will be interesting to assess Dlx-DOR mice reactivity to stressful odors or in other olfactory tests (Odor-dependent memory and odor detection threshold tasks), in order to determine whether DOR plays any role in olfactory circuitry where the receptor is most densely expressed. In addition, it may be of interest to examine whether the reported SNC80-induced antidepressant effect (58) is altered in the Dlx-DOR mice, to determine whether systemic pharmacological activation recruits forebrain DOR circuits to alleviate depressive-like behaviors.
Dlx-DOR mice show an intriguing low anxiety phenotype. Although no modification of anxiety levels was detected in the open field, mutant mice spent significantly more time in open arms of the elevated plus-maze and showed strongly reduced latency to reach the food in the NSF test, despite their reduced motivation to obtain a food reward in the SA paradigm. The Dlx-DOR mouse phenotype in elevated plus-maze and NSF reflects reduced anxiety-related behavior. The absence of detectable phenotype in the open field may relate to distinct stress levels applied in the different paradigms (e.g. novelty, brightness, openness, privation, elevation (59, 60)). This particular behavior of mutant mice may be more obvious under specific stress conditions, such as food deprivation stress in the case of NSF. Importantly, c-fos analysis immediately after this task further supports the notion of reduced anxiety taking in mutant mice. Thus, neural activation was reduced mainly in lateral and central divisions of the Amy, consistent with a reduced response to anxiogenic stimuli (61, 62). In sum, the data strongly suggest that DORs expressed in GABAergic forebrain neurons normally contribute to increase anxiety. One may speculate that this particular DOR activity, which has not been reported earlier, could contribute to exert an adaptive protective role under threatening situations.
The low anxiety phenotype of Dlx-DOR mice was unexpected, and opposes the established high anxiety-related behavior reported for constitutive DOR KO animals (6, 44). Previous studies, indeed, have shown that total DOR gene knockout and systemic DOR antagonist treatment both increase levels of anxiety (6, 28, 58, 63, 64), while systemic DOR agonists reduce levels of anxiety (58, 64, 65), demonstrating a prevailing anxiolytic DOR activity. Further, local administration of DOR agonists and antagonist in the amygdala support a main contribution of amygdala DORs in this anxiolytic activity (29). A simple interpretation of our data is that DORs exert both anxiolytic and anxiogenic activities, at distinct level of neural circuits possibly involving midbrain (including amygdala) and forebrain structures, respectively. Anxiolytic DOR activity predominates in wild-type animals and is absent in total knockout animals. Anxiogenic DOR activity otherwise has been deleted in Dlx-DOR animals only, which leads to anxiety levels lower than wild-type in these animals, at least in EPM and NSF testing. Future experiments will be required to substantiate this hypothesis.
In conclusion, previous conditional gene knockout studies for cannabinoid CB1 (40) and corticotrophin-releasing hormone receptor 1 (66) have revealed antagonistic receptor activities, which operate in separate neural networks. Using a similar approach, our study reports a novel anxiogenic DOR activity that engages forebrain GABAergic neurons, possibly at the level of corticostriatal networks tightly connecting the Amy (52, 67), a hypothesis that will be tested in future studies. The discovery of dual anxiolytic/anxiogenic roles for DORs, opens novel perspectives in the area of DOR function and anxiety disorders.
Supplementary Material
Acknowledgments
We thank the Mouse clinical Institute, the animal core facility and the imaging platform at the Institut de Génétique et de Biologie Moléculaire et Cellulaire for technical support (Illkirch, France). We are grateful to Elise Le Marchand, Thomas Favier, Gilles Duval and Dzemailj Memedov for the animal care. This work was supported by the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Université de Strasbourg. We would also like to thank the Fondation pour la Recherche Médicale (FRM FDT20120925269), the US National Institutes of Health (National Institute of Drug Addiction, grant #05010 and National Institute on Alcohol Abuse and Alcoholism, grant #16658) for financial support. This work was also supported by the Spanish ‘Instituto de Salud Carlos III’ (RTA, no. RD06/001/001), the Spanish ‘Ministerio de Ciencia e Innovación’ (no. Ministerio de Ciencia e Innovación (SAF2011-29864), the Catalan Government (SGR2009-00131) and ICREA Academia-2008. We are grateful to NeuroPain (Call FP7, EU) for support.
Footnotes
Disclosure/conflict of interest: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sauriyal DS, Jaggi AS, Singh N. Extending pharmacological spectrum of opioids beyond analgesia: multifunctional aspects in different pathophysiological states. Neuropeptides. 2011;45:175–188. doi: 10.1016/j.npep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Lutz PE, Kieffer BL. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–414. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu Sin, Chung P, Kieffer BL. Delta opioid receptors in brain function and diseases. Pharmacol Ther. 2013;140:112–120. doi: 10.1016/j.pharmthera.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 7.Befort K, Mahoney MK, Chow C, Hayton SJ, Kieffer BL, Olmstead MC. Effects of delta opioid receptors activation on a response inhibition task in rats. Psychopharmacology (Berl) 2011;214:967–976. doi: 10.1007/s00213-010-2108-0. [DOI] [PubMed] [Google Scholar]
- 8.Le Merrer J, Faget L, Matifas A, Kieffer BL. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 2012;223:99–106. doi: 10.1007/s00213-012-2693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu Sin, Chung P, Kieffer BL. Delta opioid receptors in brain function and diseases. Pharmacol Ther. 2013 doi: 10.1016/j.pharmthera.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- 11.Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of mu-,delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- 12.Goody RJ, Oakley SM, Filliol D, Kieffer BL, Kitchen I. Quantitative autoradiographic mapping of opioid receptors in the brain of delta-opioid receptor gene knockout mice. Brain Res. 2002;945:9–19. doi: 10.1016/s0006-8993(02)02452-6. [DOI] [PubMed] [Google Scholar]
- 13.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbs E, Faget L, Scherrer G, Kessler P, Hentsch D, Vonesch JL, et al. Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience. 2012;221:203–213. doi: 10.1016/j.neuroscience.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rezai X, Faget L, Bednarek E, Schwab Y, Kieffer BL, Massotte D. Mouse delta opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells. Cell Mol Neurobiol. 2012;32:509–516. doi: 10.1007/s10571-011-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole DP, Pelayo JC, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW. Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology. 2011;141:982–991. e981–988. doi: 10.1053/j.gastro.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Becker J, et al. A mu-delta opioid receptor brain atlas reveals co-occurrence in neurons essential for survival. Brain Structure and Function. 2014 doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonin F, Befort K, Gaveriaux-Ruff C, Matthes H, Nappey V, Lannes B, et al. The human delta opioid receptor: genomic organization, cDNA cloning, functional expression and distribution in human brain. Molecular Pharmacology. 1994;46:1015–1021. [PubMed] [Google Scholar]
- 21.Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Zubieta JK, Price JC, Flesher JE, Madar I, Lever JR, et al. Quantification of delta-opioid receptors in human brain with N1′-([11C]methyl) naltrindole and positron emission tomography. J Cereb Blood Flow Metab. 1999;19:956–966. doi: 10.1097/00004647-199909000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012;124:223–228. doi: 10.1016/j.drugalcdep.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaveriaux-Ruff C. Opiate-Induced Analgesia: Contributions from Mu, Delta and Kappa Opioid Receptors Mouse Mutants. Curr Pharm Des. 2013 doi: 10.2174/138161281942140105163727. [DOI] [PubMed] [Google Scholar]
- 25.Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Narita M, Kuzumaki N, Kaneko C, Hareyama N, Miyatake M, Shindo K, et al. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem. 2006;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- 27.Solati J, Zarrindast MR, Salari AA. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci. 2010;64:634–641. doi: 10.1111/j.1440-1819.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- 28.Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 29.Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, et al. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–2984. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Kitchen I, Leslie FM, Kelly M, Barnes R, Crook TJ, Hill RG, et al. Development of delta-opioid receptor subtypes and the regulatory role of weaning: radioligand binding, autoradiography and in situ hybridization studies. J Pharmacol Exp Ther. 1995;275:1597–1607. [PubMed] [Google Scholar]
- 33.Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Research. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- 34.Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL. Impaired Hippocampus-Dependent and Facilitated Striatum-Dependent Behaviors in Mice Lacking the Delta Opioid Receptor. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nozaki C, Le Bourdonnec B, Reiss D, Windh RT, Little PJ, Dolle RE, et al. delta-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther. 2012;342:799–807. doi: 10.1124/jpet.111.188987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Merrer J, Gavello-Baudy S, Galey D, Cazala P. Morphine self-administration into the lateral septum depends on dopaminergic mechanisms: Evidence from pharmacology and Fos neuroimaging. Behav Brain Res. 2007;180:203–217. doi: 10.1016/j.bbr.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stumm RK, Zhou C, Schulz S, Hollt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 40.Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. Differential behavioral tolerance to the delta-opioid agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-me thoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts AJ, Gold LH, Polis I, McDonald JS, Filliol D, Kieffer BL, et al. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol Clin Exp Res. 2001;25:1249–1256. [PubMed] [Google Scholar]
- 45.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 46.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 47.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 48.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- 50.Buot A, Yelnik J. Functional anatomy of the basal ganglia: limbic aspects. Rev Neurol (Paris) 2012;168:569–575. doi: 10.1016/j.neurol.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- 52.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. J Comp Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, et al. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–279. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 55.Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J. 2012;31:640–653. doi: 10.1038/emboj.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Merrer J, Plaza-Zabala A, Boca CD, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta Opioid Receptor Gene Impairs Place Conditioning But Preserves Morphine Reinforcement. Biol Psychiatry. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Kelly JP, Wrynn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 58.Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- 59.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 60.File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Curr Protoc Neurosci. 2004;Chapter 8(Unit 8):3. doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- 61.Adamec R, Toth M, Haller J, Halasz J, Blundell J. A comparison of activation patterns of cells in selected prefrontal cortical and amygdala areas of rats which are more or less anxious in response to predator exposure or submersion stress. Physiol Behav. 2012;105:628–638. doi: 10.1016/j.physbeh.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- 63.Saitoh A, Yoshikawa Y, Onodera K, Kamei J. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- 64.Perrine SA, Hoshaw BA, Unterwald EM. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vergura R, Balboni G, Spagnolo B, Gavioli E, Lambert DG, McDonald J, et al. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides. 2008;29:93–103. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 66.Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM, et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science. 2011;333:1903–1907. doi: 10.1126/science.1202107. [DOI] [PubMed] [Google Scholar]
- 67.Callaway CW, Hakan RL, Henriksen SJ. Distribution of amygdala input to the nucleus accumbens septi: an electrophysiological investigation. J Neural Transm Gen Sect. 1991;83:215–225. doi: 10.1007/BF01253391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.