Abstract
In the mammalian white matter, glycogen-derived lactate from astrocytes plays a critical role in supporting axon function using the astrocyte-neuron lactate transfer shuttle (ANLTS) system with specialized monocarboxylate transporters (MCTs). A rapid breakdown of glycogen to lactate during increased neuronal activity or low glucose conditions becomes essential to maintain axon function. Therefore astrocytes actively regulate their glycogen stores with respect to ambient glucose levels such that high ambient glucose upregulates glycogen and low levels of glucose depletes glycogen stores. Although lactate fully supports axon function in the absence of glucose and becomes a preferred energy metabolite when axons discharge at high frequency, it fails to benefit axon function during an ischemic episode in white matter. Emerging evidence implies a similar lactate transport system between oligodendrocytes and the axons they myelinate, suggesting another metabolic coupling pathway in white matter. Therefore the conditions that activate this lactate shuttle system and the signaling mechanisms that mediate activation of this system are of great interest. Future studies are expected to unravel the details of oligodendrocyte-axon lactate metabolic coupling to establish how white matter components metabolically cooperate and that lactate may be the universal metabolite to sustain CNS function.
Keywords: D-lactate, white matter, axon, oligodendrocytes, end-feet
INTRODUCTION
Energy homeostasis in the brain is maintained predominantly by oxidative metabolism of glucose, primarily to fulfill the energy demand associated with ionic gradients in neurons and astrocytes. The mobilization of glycogen in astrocytes serves to enhance the availability of glucose for neuronal glycolytic and oxidative metabolism at the onset of stimulation. The evolutionary selection process for glycogen metabolism reflects the requirements of rendering large amounts of glucose into an osmotically stable but readily soluble substrate for rapid mobilization. Therefore glycogen serves as a glucose reserve and substrate buffer for local energy demand. The regulation of glycogen is under the control of enzymatic machinery that is exquisitely sensitive to cell energy status. Mobilization of glycogen in the brain is under allosteric control of adenylates (ATP, ADP and AMP) via AK- and CK-mediated reactions as well as AMP-activated and/or AMP-dependent protein kinases.
At the cellular level, the sodium-potassium pump (Na+/K+ pump) plays a major role in neuron-astrocyte metabolic interactions that form the basis for coupling between neuronal activity and energetics (Munzer et al., 1994; Hertz et al., 2007). The astrocytic Na+/K+ pump is exquisitely sensitive to increases in extracellular K+ (Henn et al., 1972; Hajek et al., 1996; Honegger and Pardo, 1999), which a) allocates astrocytes primarily to clear the extracellular K+ that accumulates upon neuronal activity, and b) makes astrocytes high energy-demanding cells (Attwell and Laughlin, 2001). As a result, astrocytes act as sensors to neuronal activity levels and the energy demand of astrocytes during neuronal activity is substantially high, in particular when other ATP-dependent reactions occur such as glutamate-glutamine pathway cycles. Because 1) glycogen and glycogen phosphorylase activity are confined to astrocytes (Phelps, 1972; Pfeiffer-Guglielmi et al., 2003), 2) astrocytes are strategically positioned to uptake glucose via glucose transporter 1 (GLUT 1) from the blood circulation (Maher et al., 1994; Simpson et al., 1994; Vannucci et al., 1997), and 3) astrocytic end-feet store glucose as glycogen (Chih et al., 2001), astrocytes are the principal cells uniquely equipped to maintain energy homeostasis (Magistretti and Pellerin, 1996).
Establishing the physiological role of brain glycogen raised challenging and at times conflicting questions. Historically, Kuffler (Kuffler and Nicholis, 1964; Kuffler and Potter, 1964; Kuffler and Nicholls, 1966; Kuffler et al., 1966) proposed the idea that glial cells contribute to neuronal energy metabolism, but it was Tsacopoulus (Tsacopoulos et al., 1994) who established metabolite transfer from glial cells to neuronal cells, albeit in the non-mammalian honeybee retina model system. Early versions of the astrocyte-neuron lactate shuttle hypothesis (ANLSH) were eventually refined to assert that lactate produced by active neurons and astrocytes is released into a lactate pool that is eventually used by neurons at rest or during activity. The revised hypothesis included that activation of glycolysis and production of lactate can take place in active neurons in addition to astrocytes. Since then, accumulating evidence has shown that there is a functional astrocyte-neuron lactate transfer shuttle (ANLTS) in multiple CNS regions (Magistretti et al., 1994; Wender et al., 2000; Pellerin et al., 2002). In the mammalian CNS, white matter tracts comprise long-range axons with varying degrees of myelination and their associated glial cells. Due to the challenge of meeting the metabolic needs of transport and signal conduction of these long-range axons, lactate is a critical energy substrate. This review focuses on glia-derived lactate transfer to axons in optic nerve, a pure white matter tract with fully myelinated axons, and astrocyte glycogen and oligodendrocyte glycolysis as potential sources of lactate to support axon function.
ASTROCYTE-NEURON LACTATE TRANSFER SHUTTLE (ANLTS) IN WHITE MATTER
Mammalian white matter tracts are metabolically challenged, even under physiological conditions, due to extensive axonal energy demand and significant glial cell maintenance (Ransom and Orkand, 1996; Harris and Attwell, 2012). Despite this, they have a relatively restricted substrate supply due to a unique vascular network that is much less dense compared to gray matter (Moody et al., 1990). White matter architecture further complicates energy metabolism because oligodendrocytes wrap myelin around axons to facilitate axon transport, but this inevitably forms a barrier around the axons that restricts extracellular metabolites from gaining access to axons (Nave, 2010a, b). There is morphological evidence that astrocytes contact axons at nodes of Ranvier (Black and Waxman, 1988), presumably to uptake energy metabolites via their end-feet (Butt et al., 1994) and deliver energy substrate to axons via ANLTS (Pellerin et al., 1998). This shuttle system in principle states that capillary glucose taken up by astrocyte end-feet is stored as glycogen in astrocytes, to be delivered to axons as lactate when needed (Dringen et al., 1993). While astrocytes do not release glucose, they are capable of releasing lactate via specialized monocarboxylate transporters (MCTs). The cellular expression pattern of MCTs is heterogeneous and directs one-way traffic of lactate from astrocytes to axons. Among several isoforms of MCTs, MCT1 is expressed by tissues that export lactate and MCT2 is expressed by tissues that consume lactate, so MCT1 is ideally expressed by astrocytes while axons express MCT2 (Poole et al., 1996; Broer et al., 1997). The architectural organization of ANLTS in white matter tracts includes: 1) the cell-specific distribution of lactate dehydrogenase (LDH) isozymes to convert pyruvate to lactate (LDH5) in astrocytes and to convert lactate to pyruvate (LDH1) in neuronal elements (Bittar et al., 1996); 2) cell-specific locations of MCTs; and 3) the strategic location of astrocytes at neurovascular units to store glycogen. This anatomical organization supports an astrocyte-derived lactate shuttle in white matter while raising the question of whether this shuttle is functional and whether lactate is a preferred or secondary energy substrate coupled to white matter metabolism.
IS LACTATE THE SUBSTRATE OF CHOICE FOR AXONAL ENERGY?
A direct correlation between astrocyte glycogen content and axon function (Brown et al., 2003) established a link between astrocyte-derived L-lactate and axonal metabolic coupling (Tekkok et al., 2005) in mouse optic nerve (MON). Astrocytes and myelinated axons maintain 3-dimensional cell-to-cell interactions in MONs, providing an in vitro preparation where extracellular glucose levels and glycogen content can be modified to correlate with axon function and architectural structure to study the interactions between these parameters. Astrocytes possess the enzymes glycogen synthase and glycogen phosphorylase (Pellegri et al., 1996; Pfeiffer-Guglielmi et al., 2003), so glycogen synthesis and breakdown are maintained in a steady state but can also be precisely adjusted to meet changing energy demands of axons. Consistent with this, glycogen levels in MONs are sustained over time (7 pmol/μg protein) when extracellular glucose concentration is kept at 10 mM. Removal of glucose (0 mM Glucose for 60 min) causes a gradual reduction in glycogen content and after 20 min of aglycemia, glycogen content rapidly declines, reaching its lowest value at around 30 min (Brown et al 2003). Axon function remains constant during the first 20 min of aglycemia, but starts to decline and eventually decreases to zero following a time course parallel to glycogen levels. Using a MON preparation, the dependence of axon function on glycogen content can be tested extensively. For instance, incubating MONs in low glucose concentrations (2 mM, 120 min) before aglycemia induction depletes glycogen stores, leading to rapid loss of axon function upon induction of aglycemia (Brown et al 2003). Expectedly, increasing glycogen content by incubating MONs in high glucose concentrations (30 mM for 120 min) sustains axon function for a longer duration upon removal of glucose. Glycogen content quantification confirms that incubating MONs in low or high glucose for 120 min regulates glycogen stores, hence axon function becomes more vulnerable when glycogen stores are depleted (Brown et al 2003). On the other hand increasing only ambient glucose in the extracellular environment for shorter periods of time (30 min) does not modify levels of glycogen stores in astrocytes and fails to support axon function and recovery following aglycemia. These studies provide proof-of-principle that the lactate shuttle is optimally functional in white matter and that in the absence of glucose, glycogen is broken down to provide lactate to support axon function (Brown et al., 2003).
When axons are stimulated at low frequencies, they are able to use either glucose or lactate, raising the question of whether there is a preferential metabolite following higher frequency activity. Indeed, high frequency stimulation drastically decreases MON glycogen content under normoglycemic conditions. Most evidence to date supports that in white matter, lactate arising from glycogen mobilization in astrocytes is shuttled to axons using distinct MCT isoforms found on astrocytes and axons to sustain axon function when glucose is low or enhanced firing is required (Brown et al 2003). These data emerged from experiments that used D-lactate, a metabolically inert but transportable competitive inhibitor of L-lactate. MCTs are stereo-specific with a preference for L isomers but are still significantly permeable to D isomers, so D-Lactate acts as a competitive inhibitor. Replacing glucose in the extracellular environment with L-lactate (20 mM for carbon equivalent of 10 mM glucose) supports axon function such that under normoglycemic conditions, axon function remains constant or similar numbers of axons recover following an episode of aglycemia. Conversely, D-Lactate (20 mM) accelerates axon function loss by competitively blocking astrocyte-derived L-lactate use by axons. In conditions where astrocyte glycogen content is increased, axon function is preserved during aglycemia and recovery is significantly improved. However, addition of D-lactate during aglycemia expedites axon function loss and dramatically reduces axonal recovery. Similarly when glycogen content was reduced by incubating MONs in low glucose (2 mM for 120 min), axon function remained stable during steady-state activity, but the addition of D-lactate sharply depressed conduction, leading to complete loss of axon function and verifying that D-lactate blocks glycogen-derived lactate and hampers axonal conduction. Interestingly, a train of high frequency stimulation causes a prominent drop in glycogen content and a slight but transient depression in axon function, suggesting that lactate is the primary energy metabolite during increased physiological activity. Addition of D-lactate during high frequency stimulation leads to a prominent decline in axon function, confirming that lactate is the preferred substrate to support axon function (Tekkok et al., 2005).
These results collectively support a well-defined lactate shuttle system between astrocytes and neurons in a fully myelinated pure white matter tract. Localization of MCT1 on astrocytes and MCT2 on axons (Brown et al., 2003) substantiates the idea that astrocytic glycogen fuels neighboring axons when glucose is low or when there is increased activity. It is also plausible that axons may subsist primarily on L-lactate, even in the presence of glucose. It is critical to maintain axon function to properly relay information in the CNS, therefore the implication of glycogen-derived lactate use under control conditions or during recovery from an injury, as well as the ability to recruit synapses upon high frequency stimulation of axons during learning and memory, expand the importance of this shuttle. Of particular interest is whether this support system is impaired during aging, contributing to age-dependent neurodegenerative diseases. These questions currently remain under investigation.
CAN LACTATE BE USED AS A SUBSTRATE DURING ISCHEMIA?
In a series of experiments, localized application of glutamate caused cortical injury, which was attenuated by the presence of L-lactate. Likewise, infusion of D-lactate aggravated the glutamate-induced cortical injury size, implying that lactate is neuroprotective and neurons prefer lactate to derive energy during excitotoxicity. Consistent with this, amino acid release increases during the reperfusion period following an ischemic attack, which is attenuated in the presence of L-lactate (Alessandri et al., 1996; Ros et al., 2001).
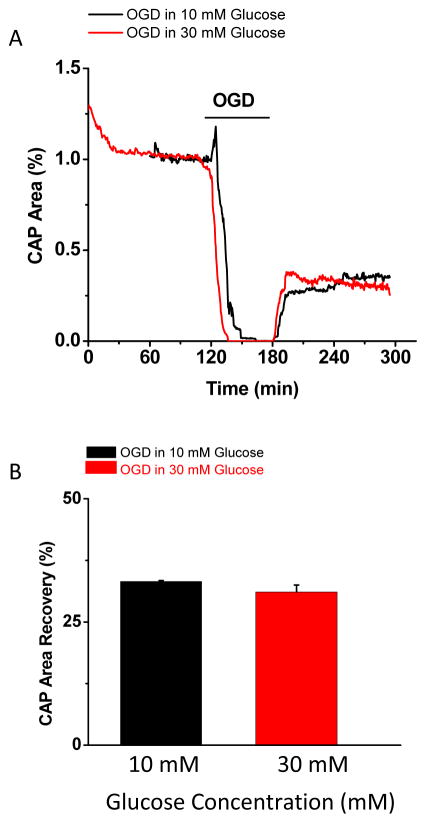
Do these principles apply to ANLTS in white matter? The presence of glycogen-derived lactate under ischemic conditions failed to support axon function in white matter (Figure 1). After incubating MONs in high glucose (30 mM for 120 min) to upregulate glycogen stores, oxygen and glucose depletion (OGD for 60 min) caused a more rapid drop in axon function following high glucose incubation (Figure 1A), but there was comparable recovery (Figure 1B) to levels observed under normoglycemic conditions (10 mM glucose). Furthermore, increasing ambient glucose levels (30 mM glucose for 30 min) did not impact axon recovery in MONs. Ischemia, which is produced experimentally by removing glucose and oxygen from the tissue and mimics the effects of stroke where a thrombus interrupts blood supply to parts of the brain, results in decreased glycogen levels. Unlike aglycemia, where glucose is absent but other available energy reserves can be metabolized aerobically, during ischemia oxidative phosphorylation is inhibited. While it is accepted that the integrated function of the mammalian brain requires oxygen, white matter has been shown to be relatively resistant to anoxia (Tekkok et al., 2003). This is because the energy yield from glucose is sufficient to sustain a subgroup of axons during anoxia in rodent optic nerve. However, glycogen-derived lactate in the presence of combined OGD is unable to provide fuel and is not neuroprotective. Note that glycogen is metabolized to yield glycolytic energy during ischemia and may be the preferential substrate for neuronal elements in the penumbra region as opposed to the core stroke area. Therefore it is plausible that white matter adjacent to the core stroke area may benefit from lactate availability (Alessandri et al., 1996; Ros et al., 2001).
Figure 1.
Lactate cannot be used as a substrate during ischemia. (A) Time course of compound action potential (CAP) area shows that following oxygen glucose deprivation (OGD), axon function recorded in MONs incubated in high (30 mM, red) glucose drops faster than in MONs incubated in normal (10 mM, black) glucose, but both groups recover to similar levels following 60 minutes of OGD. (B) Histograms show that CAP area recovery is similar between the two groups and is independent of lactate availability during OGD (60 min).
ARE OLIGODENDROCYTES A CONSUMER OR A SOURCE OF LACTATE?
More recent evidence supports that oligodendrocytes display metabolic diversity similar to neurons and can utilize both glucose and lactate to extract energy and to synthesize lipids to deposit as myelin along axons (Sanchez-Abarca et al., 2001). Oligodendrocytes contact multiple axons and oligodendroglial cytoplasm extends into multiple myelinating shafts that face the periaxonal space, establishing a network of access to axons. During the process of myelination, each oligodendrocyte produces large amounts of myelin beyond its own mass, making them metabolically demanding during development. Once myelination is complete, maintaining the complex architecture of myelin continues to be a metabolic challenge due to the slow and difficult process of myelin delivery. Recent evidence suggests that this morphological organization is the basis of a metabolic interaction between oligodendrocytes and axons beyond current concepts (Funfschilling et al., 2012). Oligodendrocytes are charged with adjusting the efficiency of signal propagation through precise myelination of axons as the activity and metabolic needs of the CNS change. It is estimated that glucose delivery at the nodes of Ranvier may be sufficient to maintain mitochondrial ATP production under steady state conditions without any glial cell support. On the other hand, with increased activity in longer and larger fibers, both oligodendrocytes and astrocytes supply axons with pyruvate/lactate as the need arises (Nave, 2010a, b; Funfschilling et al., 2012; Lee et al., 2012). This supports the suggestion that the lactate shuttle is not restricted to astrocytes and axons, but rather that another specialized lactate shuttle exists between oligodendrocytes and their myelinated axons (Amaral et al., 2013). Recent genetic studies propose that oligodendrocytes sustain axonal energy metabolism by performing aerobic glycolysis and delivering lactate to the myelinated compartments via monocarboxylate transporters (MCTs) (Funfschilling et al 2012). This metabolic support depends on non-compacted regions of myelin that connect oligodendrocyte cell bodies to the periaxonal cytosolic space under the myelin sheath. These tube-like cytosolic compartments called myelinic channels run parallel to axons facing the axonal internode and facilitate metabolic exchange via MCTs. Abnormal metabolic support by oligodendrocytes is now accepted to underlie various diseases, ranging from amyotrophic lateral sclerosis to psychiatric diseases (Nave and Ehrenreich, 2014).
CONCLUSIONS
In white matter, astrocytes store glycogen and glycogen breakdown occurs when glucose concentration is low or most importantly during increased energy demand under normoglycemic conditions, demonstrating that CNS white matter glycogen plays a crucial role as an energy substrate during physiological conditions to maintain function (Brown et al., 2003). Thus a role for glycogen as an energy buffer, rather than an energy reserve, is now well-established in white matter (Brown et al., 2003). In this role brain glycogen provides an energy substrate in the form of glycogen-derived lactate, even in the presence of normoglycemic concentrations of glucose, to support function when ambient glucose alone is insufficient to meet immediate tissue energy demands. Providing tissue with an unlimited energy substrate allows indefinite maintenance of function during high intensity stimuli, indicating that the ability to support function during increased tissue energy demand is dependent on the availability of a suitable energy substrate. Therefore astrocyte glycogen content determines neuronal and axonal survival (Swanson and Choi, 1993) and consequently once glycogen content is depleted during constant hypoglycemia, brain function fails and may suffer irreversible injury (Auer, 1986; Frier and Fisher, 1999). Up-regulation or down-regulation of glycogen content modifies latency to axon failure (Wender et al., 2000; Brown et al., 2003). Similarly, blocking lactate uptake into axons by application of D-lactate, a competitive blocker of L-lactate uptake, accelerates axon function failure during aglycemia. Taken together these data indicate that glycogen content present in the tissue at the onset of aglycemia determines the duration of axon function (Brown et al., 2003), a relationship that may have profound implications for patients that suffer from type-1 diabetes mellitus and experience frequent silent white matter infarcts leading to declining cognitive function. Similarly, axon function as the presynaptic input dictates synaptic transmission and synaptic plasticity underlying learning and memory-related events that decline with age and in neurodegenerative diseases. Therefore all evidence points toward astrocyte-derived lactate as a crucial substrate and this metabolic coupling is validated in both gray and white matter portions of the brain. Interestingly a recent concept questions whether oligodendrocytes support axon function by delivering lactate, proposing another metabolic coupling in white matter. The conditions that activate this oligodendrocyte-axon lactate shuttle system and its signaling mechanisms are of great interest, particularly because oligodendrocytes are in a position to influence the susceptibility of injured axons to undergo degeneration or to become resistant. Therefore, impaired oligodendrocye-axon lactate shuttle is proposed to contribute to chronic demyelinating and psychiatric diseases (Nave and Ehrenreich 2014). Future studies are expected to unravel the details of oligodendrocyte-axon lactate metabolic coupling to establish that all white matter components metabolically cooperate and that lactate may be the universal metabolite to sustain CNS function.
Acknowledgments
This work was supported by grants from the American Stroke Association and the National Institute of Aging (NIA) to SB, as well as a gift from Rose Mary Kubik. Selva Baltan has previously published as Selva Tekkök.
References
- Alessandri B, Landolt H, Langemann H, Gregorin J, Hall J, Gratzl O. Application of glutamate in the cortex of rats: a microdialysis study. Acta Neurochir Suppl. 1996;67:6–12. doi: 10.1007/978-3-7091-6894-3_2. [DOI] [PubMed] [Google Scholar]
- Amaral AI, Meisingset TW, Kotter MR, Sonnewald U. Metabolic aspects of neuron-oligodendrocyte-astrocyte interactions. Front Endocrinol (Lausanne) 2013;4:54. doi: 10.3389/fendo.2013.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Auer RN. Progress review: hypoglycemic brain damage. Stroke. 1986;17:699–708. doi: 10.1161/01.str.17.4.699. [DOI] [PubMed] [Google Scholar]
- Bittar PG, Charnay Y, Pellerin L, Bouras C, Magistretti PJ. Selective distribution of lactate dehydrogenase isoenzymes in neurons and astrocytes of human brain. J Cereb Blood Flow Metab. 1996;16:1079–1089. doi: 10.1097/00004647-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. The perinodal astrocyte. Glia. 1988;1:169–183. doi: 10.1002/glia.440010302. [DOI] [PubMed] [Google Scholar]
- Broer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkök S, Ransom BR. Glycogen regulation and functional role in mouse white matter. J of Physiol. 2003;549(2):501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Berry M. Astrocyte associations with nodes of Ranvier: ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J Neurocytol. 1994;23:486–499. doi: 10.1007/BF01184072. [DOI] [PubMed] [Google Scholar]
- Chih C, Lipton P, Roberts EL. Do active cerebral neurons really use lactate rather than glucose? TiNS. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Frier BM, Fisher BM. Hypoglycemia in Clinical Diabetes. New York: John Wiley and Sons, Ltd; 1999. [Google Scholar]
- Funfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Mobius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek I, Subbarao KV, Hertz L. Acute and chronic effects of potassium and noradrenaline on Na+, K+-ATPase activity in cultured mouse neurons and astrocytes. Neurochem Int. 1996;28:335–342. doi: 10.1016/0197-0186(95)00081-x. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn FA, Haljamae H, Hamberger A. Glial cell function: active control of extracellular K + concentration. Brain Res. 1972;43:437–443. doi: 10.1016/0006-8993(72)90399-x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. Journal of Cerebral Blood Flow & Metabolism. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Honegger P, Pardo B. Separate neuronal and glial Na+,K+-ATPase isoforms regulate glucose utilization in response to membrane depolarization and elevated extracellular potassium. Journal of Cerebral Blood Flow & Metabolism. 1999;19:1051–1059. doi: 10.1097/00004647-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholis JG. Glial Cells in the Central Nervous System of the Leech; Their Membrane Potential and Potassium Content. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964;248:216–222. doi: 10.1007/BF00348592. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Potter DD. Glia in the Leech Central Nervous System: Physiological Properties and Neuron-Glia Relationship. J Neurophysiol. 1964;27:290–320. doi: 10.1152/jn.1964.27.2.290. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nature Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–387. doi: 10.1111/j.1749-6632.1996.tb34449.x. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Naichen Y, Pellerin L, De Rham S, Martin JL. Regulation of astrocyte energy metabolism by neurotransmitters. Ren Physiol Biochem. 1994;17:168–171. doi: 10.1159/000173810. [DOI] [PubMed] [Google Scholar]
- Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. American J of Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- Munzer JS, Daly SE, Jewell-Motz EA, Lingrel JB, Blostein R. Tissue- and isoform-specific kinetic behavior of the Na,K-ATPase. Journal of Biological Chemistry. 1994;269:16668–16676. [PubMed] [Google Scholar]
- Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010a;468:244–252. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nature Reviews Neuroscience. 2010b;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Nave KA, Ehrenreich H. Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry. 2014;71:582–584. doi: 10.1001/jamapsychiatry.2014.189. [DOI] [PubMed] [Google Scholar]
- Pellegri G, Rossier C, Magistretti PJ, Martin JL. Cloning, localization and induction of mouse brain glycogen synthase. Brain Res Mol Brain Res. 1996;38:191–199. doi: 10.1016/0169-328x(95)00305-c. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bonvento G, Chatton JY, Pierre K, Magistretti PJ. Role of neuron-glia interaction in the regulation of brain glucose utilization. Diabetes Nutr Metab. 2002;15:268–273. discussion 273. [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte- neuron lactate shuttle. Dev Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. Journal of Neurochemistry. 2003;85:73–81. doi: 10.1046/j.1471-4159.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Phelps CH. Barbiturate-induced glycogen accumulation in brain. An electron microscopic study. Brain Res. 1972;39:225–234. doi: 10.1016/0006-8993(72)90797-4. [DOI] [PubMed] [Google Scholar]
- Poole RC, Sansom CE, Halestrap AP. Studies of the membrane topology of the rat erythrocyte H+/lactate cotransporter (MCT1) Biochem J. 1996;320:817–824. doi: 10.1042/bj3200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom BR, Orkand RK. Glial-neuronal interactions in non-synaptic areas of the brain: Studies in the optic nerve. TiNS. 1996;19:352–358. doi: 10.1016/0166-2236(96)10045-x. [DOI] [PubMed] [Google Scholar]
- Ros J, Pecinska N, Alessandri B, Landolt H, Fillenz M. Lactate reduces glutamate-induced neurotoxicity in rat cortex. J Neurosci Res. 2001;66:790–794. doi: 10.1002/jnr.10043. [DOI] [PubMed] [Google Scholar]
- Sanchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 2001;36:321–329. doi: 10.1002/glia.1119. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Vannucci SJ, Maher F. Glucose transporters in mammalian brain. Biochem Soc Trans. 1994;22:671–675. doi: 10.1042/bst0220671. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Choi DW. Glial glycogen stores affect neuronal survival during glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13:162–169. doi: 10.1038/jcbfm.1993.19. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Ransom BR. Axon function persists during anoxia in mammalian white matter. J of Cereb Blood Flow & Metab. 2003;23:1340–1347. doi: 10.1097/01.WCB.0000091763.61714.B7. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J of Neurosci Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Veuthey AL, Saravelos SG, Perrottet P, Tsoupras G. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J Neurosci. 1994;14:1339–1351. doi: 10.1523/JNEUROSCI.14-03-01339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]