Abstract
Patients with multiple myeloma (MM), who are eligible for autologous stem cell transplantation (ASCT), typically receive a finite period of initial therapy prior to ASCT. It is not clear if patients with suboptimal (less than a partial) response to initial therapy benefit from additional alternative therapy with intent to maximize pre-transplant response. We identified 539 patients with MM who had an ASCT after having achieved less than a partial response (PR) to first line induction chemotherapy between 1995 and 2010. These patients were then divided into two groups: those who received additional salvage chemotherapy prior to ASCT (n=324) and those who had no additional salvage chemotherapy immediately prior to ASCT (n=215). Additional pre-transplant chemotherapy resulted in deepening responses in 68% (complete response in 8% and PR in 60%). On multivariate analysis there was no impact of pre-transplant salvage chemotherapy on treatment related mortality (TRM), risk for relapse, progression free or overall survival. In conclusion, for patients achieving a less than PR to initial induction therapy including with novel agent combinations, additional pre-ASCT salvage chemotherapy improved the depth of response and pre-ASCT disease status but was not associated with survival benefit.
Keywords: Myeloma, Primary Refractory, Autologous Transplant
Introduction
High dose chemotherapy with autologous hematopoietic stem cell transplantation (ASCT) has been shown to improve both overall and disease free survival for patients with multiple myeloma (MM).1-3 Unfortunately, the optimal time to transplant patients after initial therapy to control the disease is not known. In the randomized trials, patients were randomized to ASCT or continuing conventional therapy as long as they did not have evidence of disease progression after a fixed number of cycles of induction chemotherapy. However, there are data to suggest that patients with a lower paraprotein nadir pre-transplant have better outcomes. 4 On the other hand, single center experiences suggest that even patients with disease progression after initial chemotherapy benefit from high dose chemotherapy and ASCT. 5-8The optimal depth of disease response prior to ASCT remains uncertain especially in the context of upfront ASCT for those with a suboptimal response to initial therapy. It is unknown whether such patients should be taken to ASCT immediately or be switched to a salvage regimen to improve the level of response.
In this study we examined the effect of additional salvage chemotherapy on the response rates, progression free survival (PFS) and overall survival (OS) among patients achieving a suboptimal response (defined as less than a partial response (PR)) to initial therapy of newly diagnosed MM.
Patients and Methods
Patients
From a cohort of ASCT recipients for MM between 1995 and 2010 reported to Center for International Blood and Marrow Transplant Research (CIBMTR) within 12 months of the diagnosis, we identified those with suboptimal response to initial therapy. Suboptimal response to first line pre-transplant therapy was defined as a failure to achieve at least a partial response (PR) to first line chemotherapy, Patients who achieved complete response (CR) or PR or were missing information of response to first line chemotherapy were excluded. The study group consisted of patients failing to achieve at least a PR to initial induction therapy and was analyzed in two cohorts: those who received additional salvage chemotherapy after non-response to first line therapy and then proceeded to ASCT (SALVAGE, n=324) and those who had no additional salvage chemotherapy but proceeded to ASCT immediately (NO SALVAGE, n=215). A contemporaneous cohort of those with optimal response to initial therapy consisting of 463 patients with CR and 1626 patients with a PR to first line chemotherapy were included for survival comparisons with the study cohort.
Statistics
Descriptive statistics including demographics variables, disease-related factors and transplant-related factors were tabulated. Characteristics of patients in the two study cohorts were compared using the Mann-Whitney-Wilcoxon test for continuous variables and chi-square test for discrete variables. For discrete variables with small group size, the Fisher's exact test was used for comparison.
Standard International Myeloma Working Group (IMWG) criteria were used for classifying disease responses and defining progression of MM or relapse (REL). 9The probability of PFS and OS were calculated by using the Kaplan-Meier estimator, with the variance estimated by Greenwood's formula. Cumulative incidence curves and probabilities for treatment-related mortality (TRM) were calculated by treating REL as a competing risk. Point wise comparisons were used to analyze outcomes of different interest groups. All tests were two-sided with a significance level of 0.05.
Patients in SALVAGE cohort are likely to wait longer time to receive a transplant than those who receive only one line of treatment. To reduce this potential waiting time bias, a left-truncated version multivariate analysis was performed, where the study clock started at diagnosis with left-truncation time (delay entry time). Multivariate analysis of transplant related mortality (TRM), REL, PFS and OS was performed by using Cox Proportional Hazards Regression models (left truncated to reduce waiting time bias in the SALVAGE group). The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. When the test indicated differential effects over time (non-proportional hazards), models were constructed breaking the post-transplant time course into two periods, using the maximized partial likelihood method to find the most appropriate breakpoint. The proportionality assumptions were further tested. A backward stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect: salvage chemotherapy after first line vs. not. Factors which were significant at a 5% level were kept in the final model. The potential correlation between outcome measures and all significant risk factors was tested. Adjusted probabilities of TRM, relapse, PFS and OS were calculated using the multivariate models. Variables considered in multivariate analysis were: age at transplant, gender, Karnofsky performance score, immunoglobulin sub-type, disease stage, serum creatinine at diagnosis, disease status prior to transplant, conditioning regimens, time from diagnosis to transplant, type of transplant (single/tandem), novel agent use (bortezomib/thalidomide/lenalidomide) and year of transplant.
Results
Patient Characteristics
Among those receiving a first ASCT for MM between 1995 and 2010, 539 patients underwent ASCT within 12 months of diagnosis having failed to achieve a response to initial induction therapy. Of these patients, 215 patients proceeded to ASCT immediately (NO SALVAGE) and 324 patients received additional salvage chemotherapy prior to proceeding to ASCT (SALVAGE). Majority of patients received only one additional line of therapy (76%), with 20% receiving 2 additional lines and 4% receiving more than 2 lines of salvage chemotherapy.
Patients in the SALVAGE and NO SALVAGE cohorts were well matched for age, gender, performance status, immunoglobulin subtype and disease stage (Table 1). A greater proportion of patients in the SALVAGE group had a serum creatinine > 1.5 mg/dL at diagnosis (27% vs 17%). A greater proportion of patients in the NO SALVAGE (60% vs 47% in SALVAGE) group received initial therapy with corticosteroid based regimens (high dose dexamethasone or vincristine/adriamycin/dexamethasone VAD) in 1st line therapy. A greater proportion of patients in the SALVAGE group had ASCT delayed 8-12 months (56% vs 26%) and had their transplants in the later period of analysis between 2005-2010 (55% vs 35%) (Table 1). Within the SALVAGE cohort, institution of salvage chemotherapy resulted in 8% of patients achieving a CR and 60% a PR prior to ASCT. A contemporaneous cohort consisting of 2135 patients (632 patients with CR to 1st line chemotherapy and 1503 patients with a PR) were compared with the non-responding cohort (Figure 1).
Table 1. Baseline Demographics and Characteristics at Transplant.
| Characteristics | NO SALVAGE | SALVAGE | P-value |
|---|---|---|---|
| Number of patients | 215 | 324 | |
| Number of centers | 77 | 85 | |
| Baseline Demographics | |||
| Age at transplant, median (range) | 57 (33-75) | 56 (18-74) | |
| 18-39 | 13 (6) | 23 (7) | 0.872 |
| 40-49 | 40 (19) | 67 (21) | |
| 50-59 | 84 (39) | 113 (35) | |
| 60-69 | 70 (33) | 107 (33) | |
| 70- | 8 (4) | 14 (4) | |
| Male | 131 (61) | 200 (62) | 0.852 |
| KPS at diagnosis ≥80 | 177 (82) | 282 (87) | 0.293 |
| Immunoglobulin Subtype | |||
| IgG | 115 (53) | 175 (54) | 0.289 |
| IgA | 32 (15) | 66 (20) | |
| Light chain | 45 (21) | 59 (19) | |
| Othersa | 17 (8) | 15 (5) | |
| Missing | 6 (3) | 9 (2) | |
| Durie-Salmon Stage | |||
| Stage I | 17 (8) | 15 (5) | 0.093 |
| Stage II | 47 (22) | 61 (19) | |
| Stage III | 104 (48) | 150 (46) | |
| Missing | 47 (22) | 98 (30) | |
| International stage | |||
| Stage I | 49 (23) | 73 (23) | 0.375 |
| Stage II | 51 (24) | 68 (21) | |
| Stage III | 25 (12) | 55 (17) | |
| Missing | 90 (42) | 128 (40) | |
| Serum creatinine at diagnosis >1.5 mg/dL | 36 (17) | 86 (27) | 0.012 |
|
| |||
| No of lines of therapy | |||
| 1 | 215 (100) | 0 (0) | -- |
| 2 | 0 (0) | 245 (76) | |
| 3 | 0 (0) | 65 (20) | |
| ≥4 | 0 (0) | 14 (4) | |
| Sensitivity to second line chemotherapy | 197 (61) | ||
| Sensitivity to third line chemotherapy | 47 (59) | ||
| Sensitivity to fourth line chemotherapy | 2 (14) | ||
| VAD/Corticosteroid based first line therapy | 130 (60) | 152 (47) | 0.002 |
| Bort/Thal/Lena in first line therapy | 69 (32) | 126 (39) | 0.108 |
| Bort/Thal/Lena beyond first line | 186 (57) | ||
|
| |||
| Characteristics at Transplant | |||
| Disease status prior to transplant | |||
| CR | 0 (0) | 25 (8) | <0.001 |
| PR | 0 (0) | 196 (60) | |
| MR/NR/SD | 185 (86) | 88 (27) | |
| PROG/REL | 30 (14) | 15 (5) | |
| Sensitivity for chemotherapy (overall) | |||
| Sensitive | 0 (0) | 221 (68) | <0.001 |
| Resistant | 215 (100) | 103 (32) | |
| Time from diagnosis to HCT, median (range) | 7 (2-12) | 8 (3-12) | |
| <4 months | 76 (35) | 57 (18) | <0.001 |
| 4-8 months | 84 (39) | 85 (26) | |
| 8-12 months | 55 (26) | 182 (56) | |
| Number of transplants | |||
| Single transplant | 149 (69) | 219 (68) | 0.660 |
| Planned tandem transplantc | 38 (18) | 67 (21) | |
| Second salvage transplantd | 28 (13) | 38 (12) | |
| Time between first and second HCT | |||
| <6 months | 38 (58) | 67 (64) | 0.726 |
| 6-12 months | 6 (9) | 8 (8) | |
| 12-24 months | 3 (5) | 8 (8) | |
| 24-36 months | 4 (6) | 4 (4) | |
| >36 months | 15 (23) | 18 (17) | |
| Year of transplant | |||
| 1995-1996 | 18 (8) | 14 (4) | <0.001 |
| 1997-1998 | 41 (19) | 24 (7) | |
| 1999-2000 | 31 (14) | 22 (7) | |
| 2001-2002 | 24 (11) | 38 (12) | |
| 2003-2004 | 26 (12) | 44 (14) | |
| 2005-2006 | 32 (15) | 76 (23) | |
| 2007-2008 | 37 (17) | 85 (26) | |
| 2009-2010 | 6 (3) | 21 (6) | |
| Median follow-up of survivors (range), months | 68 (10-180) | 61 (9-181) | |
Follow-up completeness index as of 12/31/2010: @ 1 year (99%), @ 3 years (95%), @ 5 years (90%)
Other isotype:
line of chemotherapy=1: IgD (n=4), IgM (n=1), non-secretory (n=12)
line of chemotherapy>1: IgD (n=2), IgM (n=1), non-secretory (n=12)
Other conditioning regimsp:
line of chemotherapy=1: cyclophosphamide (n=1)
Planned tandem transplant: Planned and completed tandem transplant within 6 months after first transplant without relapse.
Secondary salvage transplant: Salvage 2nd transplant after relapse followed by 1st transplant.
Figure 1.
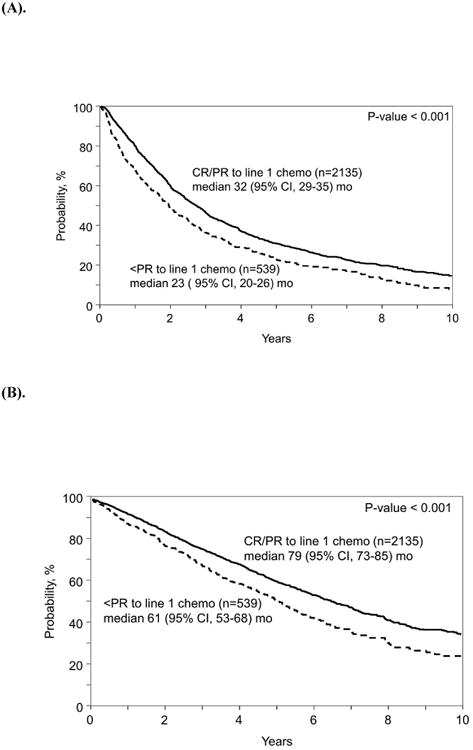
(A). Progression-free survival (PFS) of patients who achieved suboptimal response (<PR) vs. an overall response (CR/PR) to 1st line induction chemotherapy
(B). Overall survival (OS) of patients who achieved suboptimal response (<PR) vs. an overall response (CR/PR) to 1st line induction chemotherapy
Comparison with those with optimal response to initial therapy
Median PFS of patients who achieved a CR or PR to 1st line chemotherapy was superior compared with those that achieved a suboptimal (<PR) response to 1st line chemotherapy (32 mo. vs 23 mo., p< 0.001). (Figure 1a). Median OS of patients who achieved a CR or PR to 1st line chemotherapy was superior to those with suboptimal (<PR) response to 1st line chemotherapy (79 mo. vs 61 mo., p<0.001). (Figure 1b)
Best Responses after ASCT
Best myeloma responses after ASCT in both cohorts are summarized in Table 2. As expected, a higher proportion attained CR in the SALVAGE cohort (19% vs. 9% in the NO SALVAGE). However it is likely that the CR rates for the SALVAGE cohort are an overestimate since centers performing salvage may have been less willing proceed to ASCT in non-responders and those patients were not available for our analysis.
Table 2. Best Response post autologous transplant.
| Best Response post ASCT | Pre-transplant disease status* | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| SALVAGE (N=324) | NO SALVAGE (N=215) | |||||||
|
| ||||||||
| Overall N (%) | CR | PR | MR/NR/SD | PROG/REL | Overall N (%) | MR/NR/SD | PROG/REL | |
| CR | 60 (19) | 17 | 35 | 7 | 1 | 19 (9) | 19 | 0 |
| PR | 128 (40) | 2 | 92 | 30 | 4 | 71 (33) | 60 | 11 |
| MR/NR/SD | 66 (20) | 0 | 32 | 30 | 4 | 68 (32) | 64 | 4 |
| PROG/REL | 50 (15) | 5 | 25 | 15 | 5 | 39 (18) | 28 | 11 |
| Unknown | 20 (6) | 1 | 12 | 6 | 1 | 18 (8) | 14 | 4 |
censored at 2nd transplant if Number of transplants ≥2
Relapse/Progression of MM after ASCT
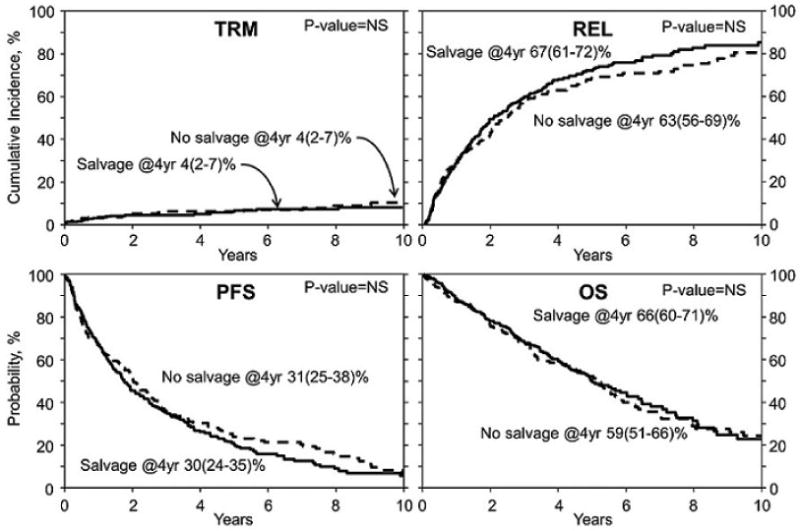
The 4 yr. cumulative incidence of REL in the SALVAGE cohort was 67% (95% C.I 61-72%) and in the NO SALVAGE cohort was 63% (95% C.I 56-69%) (p= 0.44).
Progression Free survival (PFS) and Transplant Related Mortality (TRM)
The 4 yr. PFS in the SALVAGE group was 30% (95% C.I 24-35%) and in the NO SALVAGE group 31% (95% C.I 25-38%) (p= 0.72) (Figure 2) The 4 yr. TRM in the SALVAGE and NO SALVAGE groups was identical at 4% (95% C.I 2-7%) (p= 0.46).
Figure 2. TRM, Relapse/Progression, PFS and OS of SALVAGE vs. NO SALVAGE cohorts.
Overall Survival (OS)
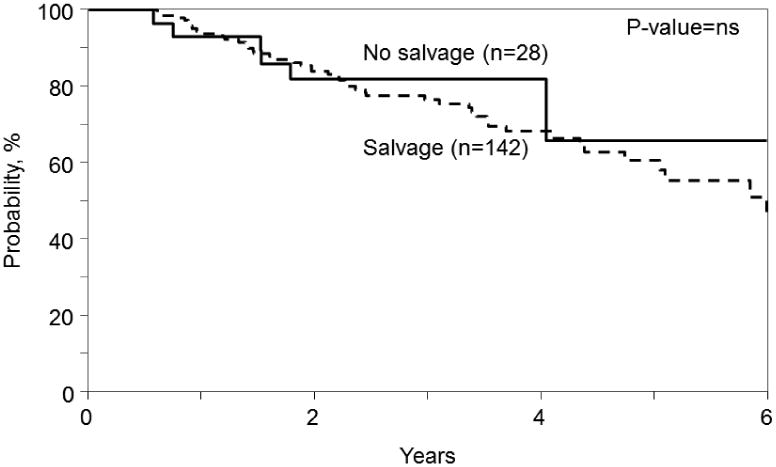
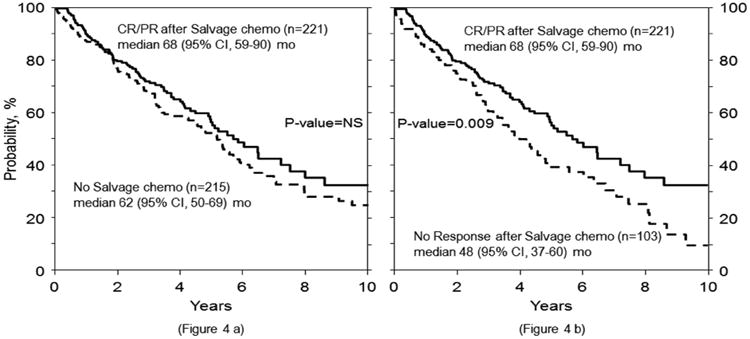
The 4 yr. OS in the SALVAGE group was 66% (95% C.I 60-71%) and in the NO SALVAGE group was 59% (95% C.I 51-66%) (p= 0.14). (Figure 2). Myeloma progression was the major cause of mortality in both the SALVAGE and NO SALVAGE groups. There was no difference in OS in the SALVAGE and NO SALVAGE groups even when the analysis was limited to the subset of patients who received first line therapy with novel agents - bortezomib, thalidomide and lenalidomide (Figure 3). Also, there was no difference in OS in the SALVAGE and NO SALVAGE groups in patients who received bortezomib and/or lenalidomide therapy. (Figure 4) Median OS of patients was 68 (95% CI, 59-90) mo. for those patients who attained CR/PR after SALVAGE. This was superior to the 48 (95% CI 37-60) mo. OS observed in the group that had no response to SALVAGE (p=0.009). However, the median OS of patients for those patients who attained CR/PR after SALVAGE was not better than the 62 (95% CI, 50-69) mo. for those who received NO SALVAGE. (Figure 4).
Figure 3. Effect of salvage on survival in patients with non-response to Bortezomib/Thalidomide/Lenalidomide in 1st line therapy.
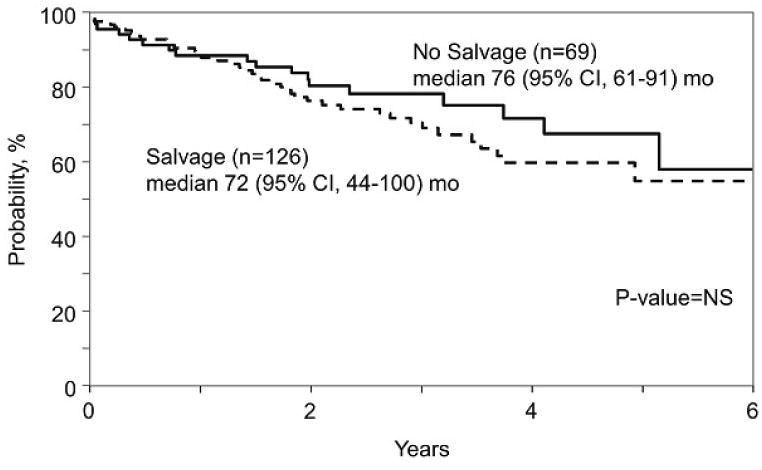
Figure 4. Effect of salvage on survival in patients with receiving Bortezomib and/or Lenalidomide in 1st line therapy.
Multivariate Analysis
On multivariate analysis (Table 3) there was no difference in TRM, REL, PFS and OS between the SALVAGE and NO SALVAGE groups. A baseline creatinine > 1.5mg/dl was predictive of greater TRM (p= 0.008) and also predictive of shorter OS (p= 0.001). We performed additional parallel multivariate analyses for patients receiving transplant within 18 months of diagnosis and within 24 months of diagnosis. These multivariate analyses gave similar results for TRM, REL, PFS and OS.
Table 3. Multivariate analysis with time clock starting at diagnosis.
| TRM | HR | P-value |
|---|---|---|
| Main effect: Salvage vs. No salvage | 0.97 (0.48-1.94) | 0.9260 |
| Creatinine at diagnosis: ≥1.5 vs. <1.5 | 2.7 (1.30-5.71) | 0.0075* |
| Relapse | HR | P-value |
| Main effect: Salvage vs. No salvage | 1.14 (0.92-1.42) | 0.2104 |
| Creatinine at diagnosis: ≥1.5 vs. <1.5 | 1.01 (0.78-1.31) | 0.9168 |
| PFS | HR | P-value |
| Main effect: Salvage vs. No salvage | 1.38 (0.92-1.38) | 0.2407 |
| Creatinine at diagnosis: ≥1.5 vs. <1.5 | 0.81 (0.88-1.41) | 0.3686 |
| OS | HR | P-value |
| Main effect: Salvage vs. No salvage | 0.90 (0.70-1.16) | 0.3965 |
| Creatinine at diagnosis: ≥1.5 vs. <1.5 | 1.60 (1.20-2.12) | 0.0011* |
significant
Discussion
Despite the fact that consolidative ASCT has been adopted as a standard of care for treatment of fit patients with MM for nearly two decades, the appropriate time and duration of pre-transplant therapy for up-front ASCT continues to be debated. There is great heterogeneity in clinical practice with some centers moving patients to ASCT after a predetermined number of cycles of therapy irrespective of depth of response attained, with a few following through with ASCT even in the face of disease progression. Others extend the period of therapy and even consider salvage therapy if a self-determined “desired” level of response is not attained and believe that transplant at the time of minimum disease burden would likely provide the best long-term results. Still others question the value of ASCT in patients already in CR after induction therapy with at least one study suggesting no prolongation in survival for patients in CR after induction therapy.4
Before the advent of proteasome inhibitor and immunomodulatory drugs, regimens like VAD (vincristine, adriamycin and dexamethasone) were frequently used prior to transplantation. Very few patients achieved CR after initial induction therapy and the majority of CRs occurred post-ASCT with CR achieved in 25-35% of patients following a single transplant and 35-50% after tandem transplantation.10. These trials suggested that the deeper responses and especially CR post-transplant is a surrogate for survival in this disease.11-16 However, others challenged the notion of CR as a surrogate to success and have argued that intrinsically aggressive MM, defined by known unfavorable biologic risk factors, overrides the benefit of CR and subgroups of patients with favorable biological risk factors may achieve prolonged survival, often without ever achieving CR.10
The value of depth of response attained pre-transplant has been the subject of analyses by several authors. Modern induction regimens are capable of producing CR in more than 30% of patients prior to ASCT and recent data suggests that CR attained after induction therapy too may be a surrogate for survival.17 Lee at al examined that importance of achieving at least a PR prior to transplant and reported that patients who had novel agent (NA) i.e. bortezomib and/or thalidomide based induction had a significantly shorter OS and PFS when at least a partial response (PR) was not achieved.18 In contrast, in patients who did not receive NAs before ASCT, lack of at least a PR to induction therapy was not associated with a survival disadvantage. Rosinol et al reported that patients with stable disease after induction therapy have an outcome comparable to those with chemosensitive disease.19
Several ongoing large prospective randomized clinical trials are seeking to address the issue of the best modern induction regimen prior to stem cell transplantation. These trials will also hopefully conclusively address the issue of the importance of depth of response attained pre and post ASCT in the era of modern therapies. However, none of these studies are likely to provide answers to the questions of optimal response prior to ASCT and the role of salvage therapy for those achieving “less than a desired level” of response. We therefore chose to query the CIBMTR database in an attempt to address this issue. Our analysis demonstrated that patients achieving a less than partial response (PR) after one line of therapy in initial induction have inferior survival compared with those achieving a PR or CR to initial therapy. Additional lines of salvage therapy prior to ASCT improved the depth of response with a CR in 8% and PR in 60%. Despite the 68% response rate seen in the SALVAGE group this additional salvage therapy prior to ASCT was not associated with an improvement in PFS or OS over those receiving no salvage.
This analysis appears to be of relevance even in the modern era. Though a majority of patients in this experience received VAD as initial therapy, 39% of patients did receive bortezomib and/or an immunomodulatory drug in first line therapy. Salvage therapy did not seem to improve results of ASCT even when the analysis was limited to this sub-group of patients treated with novel agents as part of first line induction therapy
In this study, a greater proportion of patients (82% vs 59%) underwent transplant after 2001 in the SALVAGE vs NO SALVAGE groups. This probably reflects the greater availability of effective therapies for salvage in this era. Also, a greater proportion (56% vs 26%) underwent transplant between 8-12 months post diagnosis in the SALVAGE group. However, a left truncated multivariate analysis was performed to correct for this bias. A subset analysis looking at the median OS of patients who attained CR/PR after SALVAGE was certainly superior to that observed in the group that had no response to SALVAGE (p=0.009). However, the median OS of patients for those patients who attained CR/PR after SALVAGE was not better than those who received NO SALVAGE.
The question of whether patients achieving less than a partial response to induction therapy should undergo salvage therapy remains a relevant one. Although modern three drug induction regimens have been reported to produce a partial response in > 90% of patients, it is still frequent to encounter patients who have less than a partial response with these combination therapies. Also, several patients still receive two drug proteasome inhibitor or immunomodulatory based regimen where rates of PR are lower.
Our analysis has its limitations. Since it was necessarily limited to ASCT recipients, we did not capture patients who received salvage therapy but did not proceed to transplantation. While this is a drawback, it is unlikely to change the conclusion, since such patients who received salvage but not ASCT presumably have a poorer outcome (refractory disease or death during salvage therapy) than the SALVAGE cohort we have assembled. Hence their inclusion will only make the outcomes among the SALVAGE group worse than observed in the current study and will not negate our conclusions. Also important details on cytogenetics that are known to affect outcomes of these patients are lacking. However it can be argued that nonresponse to induction therapy in itself is an adverse risk factor and it is possible that both cohorts are enriched in patients with adverse cytogenetics. Despite these limitations these data indicate that transplant eligible patients who achieve a suboptimal response to initial induction therapy should move on to planned ASCT rather than receiving additional cycles of salvage therapy in a quest to deepen the level of response.
Figure 5. Overall survival (OS) of patients who attained CR/PR after salvage compared with no salvage therapy (4a) and compared with no response to salvage (4b).
Acknowledgments
CIBMTR acknowledgements: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals; Allos Therapeutics, Inc.; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; *Blue Cross and Blue Shield Association; *Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; *Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;*Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children's Foundation; Kiadis Pharma; The Leukemia & Lymphoma Society; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; *Milliman USA, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Perkin Elmer, Inc.; *Remedy Informatics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick's Foundation; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; *Tarix Pharmaceuticals; *TerumoBCT; *Teva Neuroscience, Inc.; *THERAKOS, Inc.; University of Minnesota; University of Utah; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Conflict-of-Interest Statement: The authors have no conflicts-of-interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Lenhoff S, Hjorth M, Holmberg E, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000;95(1):7–11. [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Delasalle K, Feng L, et al. CR represents an early index of potential long survival in multiple myeloma. Bone Marrow Transplant. 2010;45(3):498–504. doi: 10.1038/bmt.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vesole DH, Barlogie B, Jagannath S, et al. High-dose therapy for refractory multiple myeloma: improved prognosis with better supportive care and double transplants. Blood. 1994;84(3):950–956. [PubMed] [Google Scholar]
- 6.Singhal S, Powles R, Sirohi B, et al. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant. 2002;30(10):673–679. doi: 10.1038/sj.bmt.1703717. [DOI] [PubMed] [Google Scholar]
- 7.Alexanian R, Weber D, Delasalle K, et al. Clinical outcomes with intensive therapy for patients with primary resistant multiple myeloma. Bone Marrow Transplant. 2004;34(3):229–234. doi: 10.1038/sj.bmt.1704562. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Lacy MQ, Dispenzieri A, et al. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant. 2004;34(2):161–167. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- 9.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 10.Hari P, Pasquini M, Vesole DH. Cure of multiple myeloma -- more hype, less reality. Bone Marrow Transplant. 2006;37(1):1–18. doi: 10.1038/sj.bmt.1705194. [DOI] [PubMed] [Google Scholar]
- 11.Blade J, Esteve J, Rives S, et al. High-dose therapy autotransplantation/intensification vs continued standard chemotherapy in multiple myeloma in first remission. Results of a non-randomized study from a single institution. Bone Marrow Transplant. 2000;26(8):845–849. doi: 10.1038/sj.bmt.1702622. [DOI] [PubMed] [Google Scholar]
- 12.Alexanian R, Weber D, Giralt S, et al. Impact of complete remission with intensive therapy in patients with responsive multiple myeloma. Bone Marrow Transplant. 2001;27(10):1037–1043. doi: 10.1038/sj.bmt.1703035. [DOI] [PubMed] [Google Scholar]
- 13.Barlogie B, Anaissie E, Haessler J, et al. Complete remission sustained 3 years from treatment initiation is a powerful surrogate for extended survival in multiple myeloma. Cancer. 2008;113(2):355–359. doi: 10.1002/cncr.23546. [DOI] [PubMed] [Google Scholar]
- 14.Harousseau JL, Avet-Loiseau H, Attal M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. J Clin Oncol. 2009;27(34):5720–5726. doi: 10.1200/JCO.2008.21.1060. [DOI] [PubMed] [Google Scholar]
- 15.Hoering A, Crowley J, Shaughnessy JD, Jr, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114(7):1299–1305. doi: 10.1182/blood-2009-03-211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadal E, Gline E, Blade J, et al. High-dose therapy/autologous stem cell transplantation in patients with chemosensitive multiple myeloma: predictors of complete remission. Bone Marrow Transplant. 2004;33(1):61–64. doi: 10.1038/sj.bmt.1704313. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Kim K, Cheong JW, et al. Complete remission status before autologous stem cell transplantation is an important prognostic factor in patients with multiple myeloma undergoing upfront single autologous transplantation. Biol Blood Marrow Transplant. 2009;15(4):463–470. doi: 10.1016/j.bbmt.2008.12.512. [DOI] [PubMed] [Google Scholar]
- 18.Lee SE, Yoon JH, Shin SH, et al. Impact of failed response to novel agent induction in autologous stem cell transplantation for multiple myeloma. Annals of Hematology. 2014;93(4):627–634. doi: 10.1007/s00277-013-1911-1. [DOI] [PubMed] [Google Scholar]
- 19.Rosiñol L, García-Sanz R, Lahuerta JJ, et al. Benefit from autologous stem cell transplantation in primary refractory myeloma? Different outcomes in progressive versus stable disease. Haematologica. 2012 Apr;97(4):616–21. doi: 10.3324/haematol.2011.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]


