Abstract
PURPOSE
WHO grade II low-grade gliomas (LGGs) with high risk factors for recurrence are mostly lethal despite current treatments. We conducted a phase I study to evaluate the safety and immunogenicity of subcutaneous vaccinations with synthetic peptides for glioma-associated antigen (GAA) epitopes in HLA-A2+ adults with high-risk LGGs in the following three cohorts: 1) patients without prior progression, chemotherapy or radiation therapy (RT); 2) patients without prior progression or chemotherapy but with prior RT, and 3) recurrent patients.
METHODS
GAAs were IL-13Rα2, EphA2, WT1, and Survivin. Synthetic peptides were emulsified in Montanide-ISA-51 and given every 3 weeks for 8 courses with intramuscular injections of poly-ICLC, followed by q12week booster vaccines.
RESULTS
Cohorts 1, 2, and 3 enrolled 12, 1, and 10 patients, respectively. No regimen-limiting toxicity was encountered except for one case with Grade 3 fever, fatigue and mood disturbance (Cohort 1). ELISPOT assays demonstrated robust IFN-γ responses against at least 3 of the 4 GAA epitopes in 10 and 4 cases of Cohorts 1 and 3, respectively. Cohort 1 patients demonstrated significantly higher IFN-γ responses than Cohort 3 patients. Median progression-free survival (PFS) periods since the 1st vaccine are 17 months in Cohort 1 (range 10–47+) and 12 months in Cohort 3 (range 3–41+). The only patient with large astrocytoma in Cohort 2 has been progression-free for over 67 months since diagnosis.
CONCLUSION
The current regimen is well tolerated and induces robust GAA-specific responses in WHO grade II glioma patients. These results warrant further evaluations of this approach.
INTRODUCTION
WHO grade II LGGs are slow-growing primary brain tumors with an extremely high risk for undergoing transformation into more aggressive and lethal WHO grade III or IV high-grade gliomas (HGGs) (1). Even with the combination of available therapeutic modalities [i.e., surgery, radiation therapy (RT), chemotherapy], the invasive growth and resistance to therapy exhibited by these tumors results in recurrence (a majority of cases as HGGs) and death in most patients (1–3).
Immunotherapeutic modalities, such as vaccines, may offer safe and effective treatment options for these patients. The slower growth rate of LGGs (in contrast to HGGs) should allow sufficient time for multiple immunizations and hence high levels of anti-glioma immunity. Because patients with LGGs are likely not as immuno-compromised as patients with HGG, they may exhibit greater immunological response to and benefit from the vaccines. Further, the generally mild toxicity of vaccines may help maintain a higher quality of life than is experienced with current cancer therapy.
Based on encouraging data from a phase I vaccine trial targeting multiple human leukocyte antigen (HLA)-A2 restricted GAA cytotoxic T-cell (CTL) epitopes in patients with recurrent HGGs (4), we conducted a pilot study of subcutaneous vaccinations with synthetic peptides for GAA epitopes emulsified in Montanide-ISA-51 every 3 weeks for 8 courses as well as intramuscular administration of poly-ICLC (5, 6) in WHO grade II gliomas with high risk for recurrence. GAAs for these peptides are IL-13Rα2 (7, 8), EphA2 (9), Wilms’ tumor gene product 1 (WT1) (10), and Survivin (11), all of which contain HLA-A2 restricted CTL epitopes (7–11). While IL-13Rα2 (12) and EphA2 (13) are typically expressed in HGGs, Survivin (14) and WT1 (15) are frequently expressed at high levels in grade II, III and IV astrocytomas (14, 15). Using immunohistochemistry, Uematsu et al. have shown 100% of glioma specimens (n=29; grades II–IV), but not normal brain tissues, contain Survivin-positive cells (14). Interestingly, high level expression of Survivin was associated with poor prognosis in patients with grade II or III astrocytomas (14). Oji et al. have shown expression of WT1 protein in 5 of 6 LGG, and in 18 of 18 HGG cases, with a trend of higher expression levels in HGGs (15). WT1 protein was not detected in the normal glial cells contained in the tumor specimens (15). A pan-HLA-DR tetanus toxoid peptide (TetA830) was included to enhance general helper CD4+ T-cell response.
Our rationale is to offer both immunotherapeutic and immuno-prophylactic potential to reduce the risk of tumor recurrence, which could translate into improved survival. Therapeutically, this approach could suppress the expansion of indolently growing neoplastic LGG cells. Prophylactically, it could prevent the growth of glioma cells that undergo anaplastic transformation. The primary objectives were to assess tolerability of this novel regimen, and its potential for inducing GAA-targeted immune responses.
PATIENTS AND METHODS
Patients
HLA-A2+ adults (≥ 18 years of age) with WHO grade II LGG who met the following criteria were enrolled with informed consent and approvals by the institutional review board (IRB) and US Food and Drug Administration (FDA) (BB-IND#13624). Enrollment criteria included: Cohort 1 (with no prior RT) and Cohort 2 (with prior RT) (both cohorts in UPCI 07-057; ClinicalTrials.gov Identifier: NCT00795457): Histologically diagnosed WHO grade II astrocytoma or oligoastrocytoma that had not progressed since the initial surgery/biopsy, but with at least one of the three following high-risk factors: 1) age ≥ 40 years old; 2) incomplete resection (post-op MRI showing >1cm residual disease, based on the maximum dimension of residual T2 or fluid-attenuated inversion-recovery [FLAIR] abnormality from the edge of the surgical cavity either laterally, antero-posteriorly, or supero-inferiorly) or 3) the pre-resection tumor size is ≥ 4 cm (the maximum preoperative tumor diameter, based on the axial and/or coronal T2 or FLAIR MR images) as each of these conditions represents an independent risk factor for WHO grade II LGG patients (16, 17). Cohort 3 (UPCI 08-135; NCT00874861): Histologically diagnosed WHO grade II glioma with recurrence. Patients were required to have a Karnofsky performance status of > 60, adequate liver and renal function, and off corticosteroids for at least 4 weeks prior to study enrollment.
Study Design
Patients received subcutaneous injections of GAA-derived HLA-A*0201-restricted peptides (300 µg/peptide/dose) and a pan-HLA-DR-binding tetanus toxoid peptide (TetA830–845; 200 µg/dose) emulsified in Montanide ISA-51 (Seppic) and concurrent intramuscular injections of poly-ICLC (20 µg/kg, Hiltonol, Oncovir, Inc), every 3 weeks for 8 vaccines. Participants were evaluated for adverse events (AEs), regimen-limiting toxicities (RLTs), and treatment response by clinic visits, laboratory testing, and MR imaging. At 15, 18, 21 and 24 weeks after starting vaccination, immune response was assessed by ELISPOT assay on peripheral blood mononuclear cells (PBMCs). Patients demonstrating no clinical or radiological progression (per RECIST criteria) without RLT had the option of continuing to receive vaccination at 12-week intervals for up to 2 years after initial vaccination. For such patients, additional immunological and MRI evaluations were obtained at 12-week intervals.
Toxicity Assessment and Stopping Rules
Each trial was monitored for treatment-related AEs using NCI CTC3.0. The following were considered to be RLTs: ≥Grade 2 hypersensitivity or allergic reaction; ≥Grade 3 non-hematologic toxicity; ≥Grade 3 hematologic toxicity that recurred despite 50% poly-ICLC dose reduction or did not resolve to ≤grade 1 by the time the next dose was due. Stopping rules were implemented such that the treatment was considered excessively toxic, warranting accrual be halted, if at any time the observed rate of RLT was ≥33% and at least 2 RLTs had been observed.
Peptides
HLA-A2–restricted peptides used in this study were: ALPFGFILV (IL-13Rα2345–353:1A9V) (7); TLADFDPRV (EphA2883–891) (9); LMLGEFLKL (Survivin96–104:M2) (11); YMFPNAPYL (WT1 126–134:Y1) (10) admixed with AQYIKANSKFIGITEL (TetA830–845) (18). The peptides were produced using automated solid-phase synthesis by NeoMPS (PolyPeptide Group, San Diego, CA). Peptides were tested in multiple quality-assurance assays including purity, sterility, identity, potency, pyrogenicity and stability.
ELISPOT Assays
Enzyme-linked immunosorbent spot (ELISPOT) assays were performed on PBMCs obtained and cryopreserved before vaccination (Week 0), at Weeks 15, 18, 21, 24 and q12 weeks as described previously (4, 19, 20) with minor modifications. Batched Ficoll-isolated PBMC samples from each patient were evaluated simultaneously following in vitro stimulation with irradiated autologous dendritic cells loaded with wild-type IL-13Rα345–353, EphA2883–891, Survivin96–104, WT1 126–134 and TetA830–845. A positive ELISPOT response was defined as a ≥ 2-fold increase in spot-forming T-cells (CD8+ cells for GAAs, CD4+ cells for TetA830–845) over the pre-vaccine level and ≥50 spots/100,000 cells at any of two consecutive post-vaccine time points against the same antigen[s] (Weeks 12, 15, 18, 21 and 24). Also, the number of post-vaccine spots was required to be at least double that at baseline, and at least three times the standard-deviation of the pre-vaccine value.
Radiological Response Monitoring
Tumor size was assessed before vaccination (Week 0) and at weeks 12 and 24, and q12 weeks thereafter using MRI scans. Response was evaluated according to RECIST criteria using T2-weighted FLAIR images.
Statistical Methods
This pilot study was designed to assess safety and immunologic efficacy in patients who met eligibility criteria described in the patients section. Each cohort was intended to enroll 9 patients, and was to be analyzed separately to provide a point estimate of immune response as assessed by the ELISPOT assay. A cohort was considered worthy of further investigation if there were at least 4 ELISPOT responses among 9 patients. Patients who received fewer than 4 vaccines were replaced by other patients for primary endpoint analyses. Each statistical analysis is discussed in the result section. Two-sided P values ≤ 0.05 were considered statistically significant.
RESULTS
Demographics and Clinical Characteristics
Between February 2009 and December 2011, 12, 1 and 10 eligible patients were enrolled in cohorts 1, 2 and 3, respectively (Table 1). Twenty one of 23 patients completed the scheduled initial 8 immunizations; two patients (patients 1 in Cohort 1 and 2 in Cohort 3) were withdrawn from the protocol due to early tumor progression (Table 2). Four patients completed six additional booster vaccinations. Immunologic and safety data are presented on patients who had at least four vaccinations (21 patients; Table 2), and at least one vaccination (23 patients; Table 3), respectively.
Table 1.
Summary for Demographics and clinical Characteristics of Participating Patients
| Cohort | 1 | 2 | 3 |
|---|---|---|---|
| No. of Patients | 12 | 1 | 10 |
| No. who completed 8 vacs. | 11 | 1 | 9 |
| Male/Female | 8/4 | 0/1 | 4/6 |
| Median Age (Years) | 40.2 | 26.0 | 39.3 |
| Range (Years) | 29–57 | 26 | 26–49 |
| Tumor Histology (A/OA/O) | 8/4/0 | 1/0/0 | 2/5/3 |
| 1p/19q loss (deletion detected/not deleted/not examined) | 2/5/5 | 0/0/1 | 1/4/5 |
| IDH1/2 mutations (mutation detected/not detected/ not examined) | 5/0/7 | 0/0/1 | 2/0/8 |
Table 2.
Demographics, and Clinical and Immunological Responses for Each Patient
| Cohort | ID | Gender | Age | Tumor Type |
Tumor Size |
Previous Tx |
# of Vac |
IFN-γ ELISPOT | PFS | Dx to 1st V |
OS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL13Rα2 | EphA2 | WT1 | Sur | Tet | |||||||||||
| 1 | |||||||||||||||
| 1 | M | 42 | OA | 774 | None | 3 | NA | NA | NA | NA | NA | 3 | 7 | 14 | |
| 2 | F | 29 | A | 1960 | None | 12 | 40 | 54 | 39 | 2 | NA | 17 | 6 | 57 | |
| 3 | M | 47 | A | 4085 | None | 10 | 82 | 322 | 419 | 350 | 186 | 14 | 3 | 25 | |
| 4 | F | 34 | A | 3361 | None | 8 | 288 | 359 | 262 | 196 | 0 | 10 | 2 | >50 | |
| 5 | M | 31 | A | 121 | None | 7 | 180 | 119 | 271 | 49 | 474 | >47 | 11 | >58 | |
| 6 | M | 57 | A | 5780 | None | 11 | 145 | 236 | 144 | 112 | 77 | 17 | 2 | >48 | |
| 7 | M | 35 | A | 1972 | None | 9 | 0 | 533 | 377 | 69 | 90 | 14 | 4 | 33 | |
| 8 | M | 49 | A | 241 | None | 12 | 20 | 5 | 4 | 7 | 267 | 19 | 10 | >48 | |
| 9 | F | 38 | A | 496 | None | 14 | 189 | 118 | 120 | 132 | 461 | >42 | 4 | >46 | |
| 10 | M | 51 | OA | 1136 | None | 14 | 41 | 342 | 700 | 125 | 151 | >37 | 10 | >47 | |
| 11 | M | 39 | OA | 1836 | None | 12 | 304 | 193 | 285 | 116 | 69 | 19 | 5 | 43 | |
| 12 | F | 30 | OA | 2520 | None | 8 | 51 | 514 | 253 | 81 | 56 | 11 | 2 | >29 | |
| 2 | 1 | F | 26 | A | 1782 | RT | 14 | 21 | 128.5 | 40 | 5 | NA | >45 | 22 | >67 |
| 3 | |||||||||||||||
| 1 | F | 49 | A | 5344 | None | 10 | 6 | 0 | 8 | 3 | 0 | 11 | 26 | >88 | |
| 2 | F | 44 | A | 1512 | RT | 3 | NA | NA | NA | NA | NA | 2 | 44 | 57 | |
| 3 | M | 36 | OA | 1236 | BCNU & TMZ | 10 | 82 | 210 | 47 | 450 | 337 | 12 | 66 | 96 | |
| 4 | F | 28 | OA | 3522 | None | 11 | 38 | 30 | 19 | 21 | 31 | 13 | 65 | >110 | |
| 5 | M | 35 | OA | 1154 | TMZ | 8 | 6 | 32 | 18 | 14 | 23 | 6 | 36 | 74 | |
| 6 | F | 49 | OA | 442 | None | 8 | 97 | 707 | 109 | 50 | 41 | 6 | 52 | >96 | |
| 7 | F | 38 | O | 1591 | None | 10 | 24 | 14 | 43 | 354 | 31 | 12 | 17 | >60 | |
| 8 | M | 26 | O | 1591 | None | 8 | 0 | 27 | 9 | 0 | 5 | >41 | 11 | >52 | |
| 9 | F | 39 | O | 4489 | None | 11 | 13 | 97 | 203 | 134 | 0 | 16 | 57 | >93 | |
| 10 | M | 49 | OA | 226 | TMZ & RAD001 | 14 | 10 | 230 | 9 | 0 | 28 | 29 | 132 | >164 | |
Table 3.
Adverse Events (N=23)
| Grade 1 | Grade 2 | Grade 3 | ||||
|---|---|---|---|---|---|---|
| Adverse Event | No. | % | No. | % | No. | % |
| Blood/Bone Marrow | ||||||
| Leukocytopenia | 3 | 13 | ||||
| Injection site reactions | ||||||
| Redness, induration, pruritis, pain | 15 | 65 | 8 | 35 | ||
| Constitutional symptoms | ||||||
| Fatigue (lethargy, malaise, asthenia) | 8 | 35 | 12 | 52 | 1 | 4 |
| Fever | 11 | 48 | 5 | 22 | 1 | 4 |
| Myalgia | 11 | 48 | 3 | 13 | ||
| Body ache | 10 | 44 | 6 | 26 | ||
| Headache | 8 | 35 | 9 | 39 | ||
| Insomnia | 2 | 9 | 2 | 9 | ||
| Light headed/dizziness | 4 | 17 | 1 | 4 | ||
| Arthralgia | 1 | 4 | 1 | 4 | ||
| Weight Loss | 2 | 9 | ||||
| Diaphoresis | 1 | 4 | ||||
| Gastrointestinal | ||||||
| Diarrhea | 1 | 4 | ||||
| Vomiting | 3 | 13 | 1 | 4 | ||
| Nausea | 5 | 22 | 1 | 4 | ||
| Anorexia | 3 | 13 | ||||
| Abdominal Pain | 1 | 4 | ||||
| Dermatological | ||||||
| Skin rash | 2 | 9 | ||||
| Dry skin | 1 | 4 | ||||
| Pulmonary/Upper Respiratory | ||||||
| Rhinitis/Runny nose | 2 | 9 | 2 | 9 | ||
| Pharyngitis | 2 | 9 | 1 | 4 | ||
| Cough | 3 | 13 | ||||
| Neurological | ||||||
| Seizure | 5 | 22 | ||||
| Visual Disturbances | 3 | 13 | ||||
| Other (Neuropathy, ataxia) | 3 | 13 | 4 | 17 | ||
| Metabolic | ||||||
| Hypoglycemia, Hyponatremia, Hypercholestermia, etc | 6 | 26 | ||||
| Microbiological | ||||||
| Infection | 3 | 13 | ||||
Summary of Systemic Toxicities
The primary objective of this study was to assess safety, given that this was the first such trial in patients with WHO grade II glioma (Table 3). Principal toxicities included grade I and II injection site reactions (100%) and flu-like symptoms (fatigue, myalgias, fever, headache), which were usually limited to 48 hours after each vaccine and were controlled with acetaminophen or ibuprofen. Grade 1 leukopenia developed in 3 patients in Cohort 3. No instances of autoimmunity were encountered. No RLT has been encountered except for one case in Cohort 1 who presented with Grade 3 fever and fatigue following the 7th vaccine. The symptoms subsided by the use of over-the-counter non-steroidal anti-inflammatory drug by the next day.
Induction of Epitope-Specific Immune Responses against GAAs
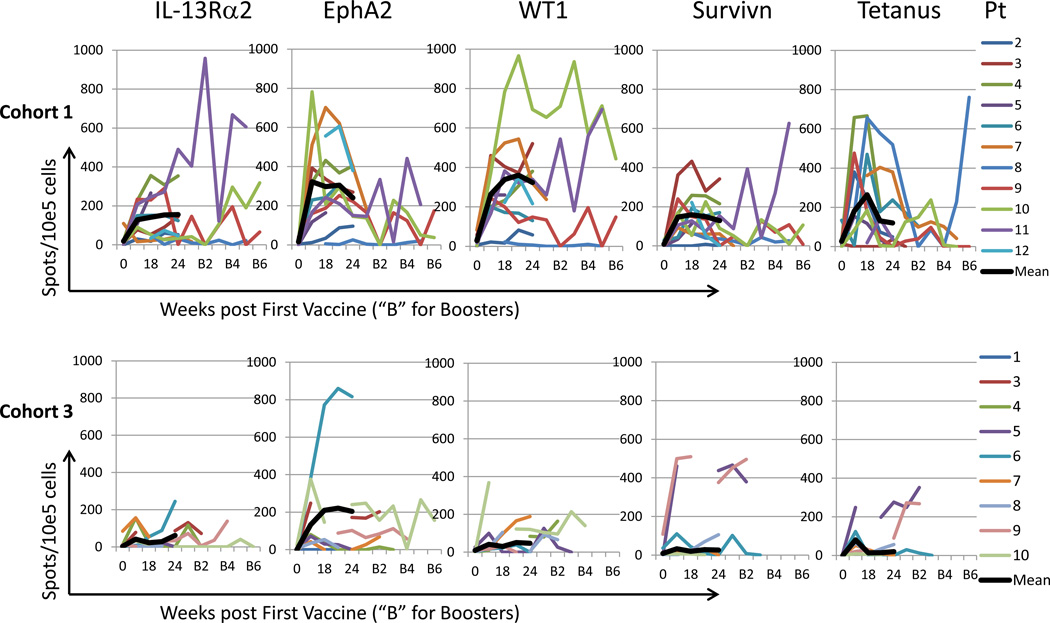
All but two patients (one in Cohort 1 and one in Cohort 3), who had disease progression before the first post-vaccine PBMC sampling on Week 15, had PBMCs available for immunological analysis. In 10 of 11, 1 of 1, and 5 of 9 evaluable patients in Cohorts 1, 2 and 3, respectively, vaccination induced immune reactivity to at least one of the vaccine-targeted GAAs by IFN-γ ELISPOT assays (Table 2). Positive IFN-γ responses against at least 3 of the 4 GAA epitopes were observed in 9 of 11, and 3 of 9 cases in Cohorts 1 and 3, respectively. Nine of 10 in Cohort 1 but only 1 of 9 in Cohort 3 responded to the Tet peptide. The time course and magnitude of the IFN-γ ELISPOT responses in these immunologically evaluable patients in Cohorts 1 and 3 are summarized in Figure 1.
Figure 1. IFN-γ ELISPOT assays on each of vaccine-targeted antigens in Cohorts 1 and 3.
Time course of glioma-associated antigen epitope-specific T-cell responses evaluated by IFN-γ enzyme-linked immunosorbent spot (ELISPOT) analyses in 11 and 9 patients in Cohorts 1 (upper panels) and 3 (lower panels), respectively, who received at least 5 vaccinations. The Week 0 spot numbers are included and post-vaccine spot numbers are not subtracted by Week 0 spot numbers.
When magnitude of IFN-γ ELISPOT responses was compared against each of the 4 GAAs between Cohorts 1 and 3 (Table 4), Cohort 1 patients demonstrated a significantly higher magnitude of IFN-γ response than Cohort 3 patients for IL-13Rα2 (p=0.030), WT1 (p=0.0098) and Tetanus (p=0.021) epitopes as well all 4 GAA epitopes combined (p=0.031). The EphA2 epitope also demonstrated the same trend but without statistical significance (p=0.095). Interleukin (IL)-5 ELISPOT assays were performed to assess type-2 adaptive immune responses against the vaccine-targeted GAAs in 6 (Patients 2–7), one, and 6 (Patients 1,3,4,6–8) in Cohort 1, 2 and 3, respectively (Table 4). In corresponding cases, IFN-γ responses were significantly higher than IL-5 responses in each of IL13Rα2, EphA2 and WT1 epitopes (p=0.0020, 0.0059, 0.014). The survivin (p=0.067), but not the Tetanus (p=0.32) epitope showed a similar trend.
Table 4.
Summary of Statistical Analyses
| Comparison | P-Value | Groups | Median | IQR | Method | |
| IFN-γ ELISPOT in Cohorts 1 and 3 |
IL-13Rα2 | 0.030 | Cohort 1 | 81.5 | 40.2,185 | Wilcoxon Test (median values are spots/10e5 cells) |
| Cohort 3 | 13.3 | 6.00,37,5 | ||||
| EphA2 | 0.095 | Cohort 1 | 236 | 119,350 | ||
| Cohort 3 | 32.0 | 27.3,210 | ||||
| WT1 | 0.0098 | Cohort 1 | 262 | 132,331 | ||
| Cohort 3 | 18.5 | 8.67,47.0 | ||||
| Survivin | 0.45 | Cohort 1 | 112 | 59.2,128 | ||
| Cohort 3 | 20.5 | 3.00,134 | ||||
| All 4 GAAs | 0.031 | Cohort 1 | 224 | 147,260 | ||
| Cohort 3 | 20.5 | 3.00,134 | ||||
| Tetanus | 0.021 | Cohort 1 | 139 | 21.0,318 | ||
| Cohort 3 | 27.0 | 19.0,41.4 | ||||
| IFN-γ and IL-5 ELISPOT (All Cohorts Combined) |
IL-13Rα2 | 0.0020 | IFN-γ | 81.5 | 30.8,120 | |
| IL-5 | 1.50 | 0.00,13.0 | ||||
| EphA2 | 0.0059 | IFN-γ | 210 | 41.5,341 | ||
| IL-5 | 3.33 | 1.25,5.75 | ||||
| WT1 | 0.014 | IFN-γ | 109 | 40.9,266 | ||
| IL-5 | 23.0 | 8.67,67.6 | ||||
| Survivin | 0.067 | IFN-γ | 69.0 | 34.9,273 | ||
| IL-5 | 23.0 | 2.75,43.5 | ||||
| Tetanus | 0.32 | IFN-γ | 36.8 | 11.8,110 | ||
| IL-5 | 68.8 | 23.2,276 | ||||
| Comparison | P-Value | CV for Cox or rho for Spearman | Method | |||
| PFS and IFN-γ ELISPOT |
Cohort 1 | 0.95 | 0.0000662 | Cox Proportional Hazards Model; Likelihood Ratio Test |
||
| Cohort 3 | 0.095 | 0.00261 | ||||
| PFS for Cohorts 1 and 3 | 0.26 | 0.612 | ||||
| Baseline Tumor Size and PFS |
0.24 | 0.00018 | ||||
| Age and IFN-γ ELISPOT | 0.46 | 0.17 | Spearman Test | |||
| Baseline Tumor Size and Overall IFN-γ ELISPOT Response |
0.21 | 0.20 | ||||
We also evaluated possible associations between baseline IFN-γ ELISPOT values and PFS or subsequent vaccine responses (i.e., whether pre-existing baseline responses contribute to vaccine effects) as shown in Supplementary Table 1. There was a positive association between the baseline response against EphA2 and PFS (p=0.046), but not with any other vaccine-targeted antigen. There were no associations between baseline responses and subsequent responses in any of the vaccine-targeted antigens.
Clinical Outcomes
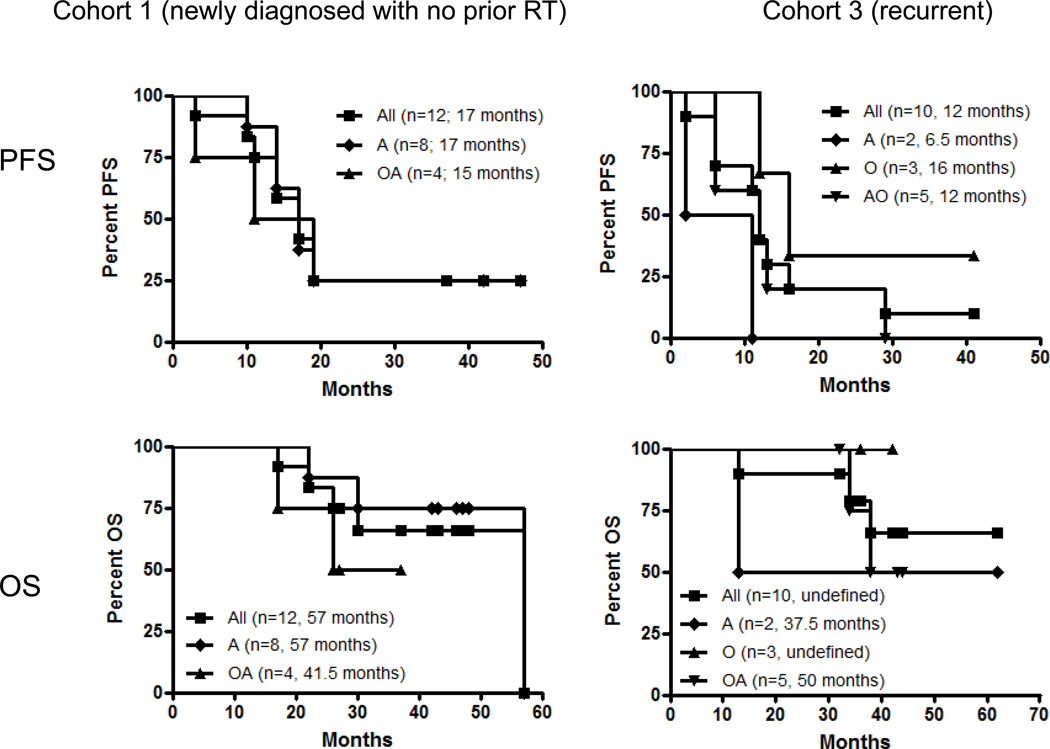
Although the primary goal of this study was to provide an analysis of safety and immuno-reactivity, preliminary outcome data were obtained (Table 2 and Figure 2). Median PFS periods since the 1st vaccine are 17 months (Cohort 1; range 3–42+) and 12 months (Cohort 3; range 3–37+) (Figure 2). Because patients in Cohorts 1 and 2 were allowed to enter the study at any time following diagnosis as long as they do not have recurrence, there was a considerable variability in the time period between diagnosis and the 1st vaccine (Table 2). The median PFS since diagnosis is 21 months for Cohort 1. In Cohort 1, 3 patients still remain progression-free (37, 42 and 47 months to date; Table 2). The only patient with large astrocytoma in Cohort 2 has been progression-free for over 45 months since the 1st vaccine (Supplementary Figure 1). In Cohort 3, there is one patient who is progression-free to date at 41 months since the first vaccine. Among patients who completed at least 8 vaccines, 7 of 10, 1 of 1, and 7 of 9 in Cohorts 1–3, respectively, are alive to date (29–58, 67 and 52–164 months since diagnosis for Cohorts 1–3, respectively; Table 2 and Figure 2). There was one patient in each of Cohorts 1 and 3 who had to be taken off the study after 4 vaccines due to rapid tumor progression. Both cases were found to have recurred with glioblastoma upon resection of the recurrent tumor. No patients had a partial or complete response. We also evaluated median PFS and OS in each of the pathological diagnoses (Figure 2), but observed no significant differences between the pathological types with small sample numbers.
Figure 2. PFS and OS since the 1st vaccine.
In parentheses, months indicate median PFS or OS for the group. OA, oligoastrocytoma; A, astrocytoma; O, oligodendrioglioma. P>0.2 for all inter-pathological type comparisons (Log-rank test).
In regard to biological and clinical correlates, although no statistically significant association between IFN-γ ELISPOT response and PFS was observed, given the modest numbers of patients on this trial, a trend was observed in Cohort 3 (p=0.095; Table 4). Although we had hypothesized that baseline tumor size might be negatively associated with IFN-γ ELISPOT response and PFS, no trends were observed to support this (Table 4). No statistically significant association was observed between IFN-γ ELISPOT and prior use of TMZ (Cohort 3), age (Table 4) or lymphopenia (not shown). In regard to associations between immune responses and genetic markers, such as chromosome 1p/19q deletion, p53 and Isocitrate dehydrogenase (IDH) mutations, our attempts were challenged because 13 of the total 23 cases were biopsied or resected for pathological diagnosis before 2010, prior to implementation of IDH mutation analyses even in major medical centers, and 16 of the total 23 cases were referred from institutions from distant areas (Table 1).
DISCUSSION
This is, to our knowledge, the first clinical study of peptide-based vaccination using novel GAA-derived epitopes and adjuvant poly-ICLC in “high-risk” WHO grade II LGGs. Our findings demonstrate tolerability and immunological activity of this approach.
In our IFN-γ ELISPOT analyses, Cohort 1 patients demonstrated significantly higher magnitudes of responses than Cohort 3 patients against IL-13Rα2-, WT1-, Tetanus-epitopes and overall 4 GAA epitopes combined (Figure 1 and Table 4). These data strongly suggest that WHO grade II astrocytoma or oligoastrocytoma patients without prior treatment other than surgery may be a particularly suitable group of patients for vaccine treatments. Furthermore, because our recent pilot study of GAA-peptide vaccines in combination with poly-ICLC in newly diagnosed pediatric glioma patients (20) utilized the same vaccine schedule (i.e., every 3 weeks for 8 vaccines) and methods for IFN-γ ELISPOT assays, we made a preliminary comparison of IFN-γ ELISPOT data between Cohort 1 patients in the current study and those in the pediatric study (Supplementary Table 2). Cohort 1 patients demonstrated significantly higher magnitudes of response against EphA2 (p=0.00095) and survivin (p=0.0031), although data could be confounded by other factors, such as the use of RT in the pediatric patients. The current study enrolled only one patient in Cohort 2 due to paucity of patients who were interested in the study. This was somewhat surprising but may suggest that those patients who receive upfront RT may tend to receive chemotherapy as well. Nonetheless, the current study supports further development of vaccine approaches in WHO grade II adult LGG patients.
We observed considerable levels of inter-patient variability in IFN-γ ELISPOT data (Figure 1). This is likely, at least partially, due to different frequencies of antigen-reactive precursor CD8+ T-cells among patients. We also noted some fluctuations of responses along the time course in individual patients as also seen in our previously published studies (4, 20). This could be possibly due to one or both of the following events: 1) migration of GAA-reactive T-cells in systemic circulation (i.e., detectable in PBMC) to the tumor tissue and/or lymph nodes, as possible memory T-cell development; and 2) an induction of tolerance and/or exhaustion of GAA-reactive T-cells. Nonetheless, our positive response criteria (Materials and Methods) require elevated spot numbers must be seen at least two consecutive post-vaccine time points against the same antigen[s], assuring consistency of immune response for determining immunological responders using PBMC-based immune assays.
In the current study, we evaluated relative magnitudes of IFN-γ and IL-5 ELISPOT responses as readouts of type-1 and type-2 adaptive immune responses (21). These are appropriate to compare, as the ELISPOT measures the frequency of cytokine-producing cells per number of cells plated, but we also recognize that these measures are only examples of one type 1 cytokine and one type 2 cytokine. To capture more comprehensive picture of type 1 vs. type 2 immuno-skewing, in our future analyses, a broader cytokine analysis would need to be performed to measure the total type 1 and type 2 cytokines (and other types), perhaps by a multiplexed Luminex assay for antigen-stimulated lymphocyte supernatants.
We have previously demonstrated that tumor-specific type-1 T cells, which predominantly secrete IFN-γ (22), but not type-2 T-cells, can efficiently traffic into brain tumor sites and mediate effective therapeutic efficacy (23) via type-1 chemokine CXCL10 (23–26) and an integrin receptor VLA-4 (6, 27–30). However, cancers, including gliomas, secrete numerous type-2 cytokines (31–33) that promote tumor proliferation (34, 35) and immune escape (36). Our preclinical studies (5, 6) and prior phase I/II clinical study in recurrent WHO grade III/IV HGG patients (4) have indicated that poly-ICLC promotes type-1 polarization of T-cell responses against vaccine-targeted GAAs. Although limited numbers of cases were evaluated for IL-5 ELISPOT, our data further support the ability of our vaccine regimen for promoting type-1 (i.e., IFN-γ-driven) GAA-specific T-cell responses. While our strategy emphasizes promotion of type-1 responses, it has also been reported that coordinated T-cell and humoral responses against NY-ESO-1 antigen contribute to superior outcome patients (37), but the observation could also relate to the high immunogenicity of NY-ESO-1. Expanding assessments of serological responses in future trials would help identify the role for humoral responses in a peptide/T cell-driven study.
Post-vaccine tissues were available for assessing antigen expression in 3 cases (Patients 3, 6 and 8 in Cohort 1). All cases showed positive immunoreactivity at least for IL-13Rα2 and survivin (Supplementary Figure 2). Among these cases, both pre-and post-vaccine tissues were available from only one case (Patient 3 in Cohort 1). Pre-vaccine tumor sections showed diffuse immunoreactivity for IL-13Rα2 and EphA2, heterogeneous expression of survivin, but absent expression of WT1. In contrast, post-vaccine tumor sections obtained at the time of recurrence as WHO grade III anaplastic astrocytoma demonstrated diffuse and high-level expression of all 4 antigens. These findings contrast from previous observations in patients receiving peptide-vaccines targeting epidermal growth factor receptor (EGFR) viii (38), in which recurrent tumors showed absence of EGFR viii expression. Infiltration of CD8+ T-cells was evaluated in Patients 6 and 8 and found to be sparse (Supplementary Figure 2). Two of the 3 patients (Patients #3 and #6) showed positive IFN-γ ELISPOT responses against all 4 GAAs, suggesting that the systemically induced GAA-specific CD8+ effector T-cells in these 2 patients may have failed to: 1) sufficiently traffic to the tumor site (39) and/or 2) mediate cytotoxic effects against GAA-expressing glioma cells for a variety of reasons, including the lack of antigen-processing components (40) and local immunosuppression in the tumor environment (41). While we recognize the importance of overcoming these issues, we also think that these observations from 2 cases do not necessarily provide us with any conclusion about the vaccine efficacy. This is because, in these cases, the recurrent tumor has already acquired resistance and/or escaped from the vaccine response. On the other hand, the tumor from patients who display sustained positive clinical response or stable disease will never be evaluated unless we implement prospective studies to evaluate the tumors following the study interventions.
In our previous vaccine clinical studies using poly-ICLC (4, 20, 42), a number of cases demonstrated initial imaging changes that can suggest immunotherapy failure, which was followed by improvement by observations alone or dexamethasone treatment. In the current study, however, despite the robust induction of IFN-γ response in PBMC, we did not observe any apparent case of such “tumor pseudoprogression”. Nevertheless, this does not preclude a possibility that some patients may have been prematurely withdrawn from the study based on MRI findings suggesting progressive disease. Indeed, Patient 6 in Cohort 3 has been radiologically and clinically stable without any active anti-tumor therapy for longer than 33 months since this patient was withdrawn from our study due to radiological progression after the initial 8 vaccinations. Novel imaging technologies as well as more appropriate response criteria for brain tumor immunotherapy need to be developed (42).
In regard to common genetic mutations and novel immunotherapy targets in LGG, mutations of the isocitrate dehydrogenase (IDH) metabolic enzymes IDH1 and IDH2 have been found to be frequent and early genetic alterations in astrocytomas and oligodendrogliomas (43). Mutation of IDH1 occurs early in glioma progression, with somatic mutations of the R132 residue of IDH1 identified in the majority (>70%) of grades II and III astrocytomas and oligodendrogliomas, as well as in secondary GBMs that develop from these LGG (44, 45). It has been recently reported that the IDH1(R132H) mutation contains an immunogenic epitope suitable for mutation-specific vaccination in the context of major histocompatibility complexes (MHC) class II (46). Further refinement of vaccine-targeted antigens and identification of novel antigens for LGGs are warranted for development of more effective vaccine strategies for LGGs, such as personalized vaccines based on biopsy-based antigen-characterization in each patient.
In summary, the current study demonstrated promising immunoreactivity in high-risk groups of WHO grade II LGG patients. These data support larger studies of GAA peptide-based vaccination in patients with LGG, in which clinical efficacy will be assessed as the primary endpoint. However, preliminary data with recurrent tumors suggest that the vaccine regimen may not eliminate tumor cells expressing vaccine-targeted antigens. Further studies are warranted to understand mechanisms limiting the efficacy of the current approach.
Supplementary Material
Statement of Translational Relevance.
World Health Organization (WHO) grade II low-grade gliomas (LGGs) are slow-growing primary brain tumors with very high risk of progression following conventional therapies. More than 50% of these patients eventually recur with aggressive high-grade gliomas (HGG), and most patients eventually die of the disease. Development of immunotherapeutic approaches, such as vaccines, may be particularly appropriate because patients with LGG are likely not to be as immunocompromised as patients with HGG, and the slower growth rate of LGG (in contrast with HGG) should allow sufficient time to administer multiple immunizations, which may induce high levels of anti-glioma immunity. We evaluated synthetic peptides for human leukocyte antigen (HLA)-A2-restricted cytotoxic T-cell (CTL) epitopes derived from glioma-associated antigens (GAAs) in patients with high-risk low-grade gliomas. Our results with safety and robust inductions of GAA-specific CD8+ T-cell responses support further development of this approach.
Acknowledgments
Shari Reynolds and Rebecca Bishop for regulatory management; Physicians who referred their patients; Jennifer Mabold, C.R.N.P. and Clinical Translational Science Institute for patient care; patients and their families. Research Supports from National Institutes of Health (NIH) Grants No. 1R21CA133859 (HO) and P01 CA132714 (HO) and Musella Foundation for the overall conduct of the study (HO). This project used UPCI shared resources (Clinical Research Services, Immunological Monitoring and Cellular Products Laboratory, Biostatistics Facility) that are supported in part by NIH P30CA047904.
Footnotes
Dr. Potter contributed to the design of the study when he was an active faculty at University of Pittsburgh, but performed the analyses as an independent consultant after he retired from University of Pittsburgh.
Disclosures: Hideho Okada is an inventor in the U.S. Patent Application No. 60,611, 797 (Utility Patent Application) “Identification of An IL-13 Receptor Alpha2 Peptide Analogue Capable of Enhancing Stimulation of Glioma-Specific CTL Response”. An exclusive licensing agreement has been completed on this application between University of Pittsburgh and Stemline, Inc. Due to the potential conflicts of interest (COI), Hideho Okada complied with COI management policies of University of Pittsburgh, and did not solely interpret any data in the current study.
References
- 1.Brown PD, Shaw EG, Gunderson LL, Tepper JE. Clinical Radiation Oncology. Philadelphia, PA: Churchill-Livingstone; 2006. Low-Grade Gliomas; pp. 493–514. [Google Scholar]
- 2.Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. Journal of Neurosurgery. 2011;115:948–965. doi: 10.3171/2011.7.JNS101238. [DOI] [PubMed] [Google Scholar]
- 3.van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJB, Jaeckle K, Junck L, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. The Lancet Oncology. 2011;12:583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 4.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-Cell Responses Against Novel Glioma-Associated Antigen Peptides and Clinical Activity by Vaccinations With {alpha}-Type 1 Polarized Dendritic Cells and Polyinosinic-Polycytidylic Acid Stabilized by Lysine and Carboxymethylcellulose in Patients With Recurrent Malignant Glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu X, Fallert-Junecko B, Fujita M, Ueda R, Kohanbash G, Kastenhuber E, et al. Poly-ICLC promotes the infiltration of effector T cells into intracranial gliomas via induction of CXCL10 in IFN-α and IFN-γ dependent manners. Cancer Immunology, Immunotherapy. 2010;59:1401–1409. doi: 10.1007/s00262-010-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Nishimura F, Sasaki K, Fujita M, Dusak JE, Eguchi J, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eguchi J, Hatano M, Nishimura F, Zhu X, Dusak JE, Sato H, et al. Identification of interleukin-13 receptor alpha2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 8.Okano F, Storkus WJ, Chambers WH, Pollack IF, Okada H. Identification of a novel HLA-A*0201 restricted cytotoxic T lymphocyte epitope in a human glioma associated antigen, interleukin-13 receptor 2 chain. Clin Cancer Res. 2002;8:2851–2855. [PubMed] [Google Scholar]
- 9.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, et al. EphA2 as a Glioma-Associated Antigen: A Novel Target for Glioma Vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinilla-Ibarz J, May RJ, Korontsvit T, Gomez M, Kappel B, Zakhaleva V, et al. Improved human T-cell responses against synthetic HLA-0201 analog peptides derived from the WT1 oncoprotein. Leukemia. 2006;20:2025–2033. doi: 10.1038/sj.leu.2404380. [DOI] [PubMed] [Google Scholar]
- 11.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 12.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 13.Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 14.Uematsu M, Ohsawa I, Aokage T, Nishimaki K, Matsumoto K, Takahashi H, et al. Prognostic significance of the immunohistochemical index of survivin in glioma: a comparative study with the MIB-1 index. J Neurooncol. 2005;72:231–238. doi: 10.1007/s11060-004-2353-3. [DOI] [PubMed] [Google Scholar]
- 15.Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S, Jomgeow T, et al. Overexpression of the Wilms' tumor gene W T1 in primary astrocytic tumors. Cancer Sci. 2004;95:822–827. doi: 10.1111/j.1349-7006.2004.tb02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, et al. Recurrence following neurosurgeon-determined gross total resection of adult supratentorial low-grade glioma: Results of a prospective clinical trial. J Neurosurg. 2008;109:835–841. doi: 10.3171/JNS/2008/109/11/0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw E, Arusell R, Scheithauer B, O'Fallon J, O'Neill B, Dinapoli R, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 18.Slingluff CL, Jr, Yamshchikov G, Neese P, Galavotti H, Eastham S, Engelhard VH, et al. Phase I trial of a melanoma vaccine with gp100 (280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin Cancer Res. 2001;7:3012–3024. [PubMed] [Google Scholar]
- 19.Okada H, Lieberman FS, Walter KA, Lunsford LD, Kondziolka DS, Bejjani GK, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Potter DM, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. J Clin Oncol. 2014;32:2050–2058. doi: 10.1200/JCO.2013.54.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatsumi T, Kierstead LS, Ranieri E, Gesualdo L, Schena FP, Finke JH, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura F, Dusak JE, Eguchi J, Zhu X, Gambotto A, Storkus WJ, et al. Adoptive transfer of Type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–4487. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 24.Fujita M, Zhu X, Sasaki K, Ueda R, Low KL, Pollack IF, et al. Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol. 2008;180:2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- 25.Fujita M, Zhu X, Ueda R, Sasaki K, Kohanbash G, Kastenhuber ER, et al. Effective Immunotherapy against Murine Gliomas Using Type 1 Polarizing Dendritic Cells--Significant Roles of CXCL10. Cancer Res. 2009;69:1587–1595. doi: 10.1158/0008-5472.CAN-08-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki K, Zhao X, Pardee AD, Ueda R, Fujita M, Sehra S, et al. Stat6 signaling suppresses VLA-4 expression by CD8+ T cells and limits their ability to infiltrate tumor lesions in vivo. The Journal of Immunology. 2008;181:104–108. doi: 10.4049/jimmunol.181.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki K, Pardee AD, Okada H, Storkus WJ. IL-4 inhibits VLA-4 expression on Tc1 cells resulting in poor tumor infiltration and reduced therapy benefit. Eur J Immunol. 2008;38:2865–2873. doi: 10.1002/eji.200838334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki K, Zhu X, Vasquez C, Nishimura F, Dusak JE, Huang J, et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 2007;67:6451–6458. doi: 10.1158/0008-5472.CAN-06-3280. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki K, Pardee AD, Qu Y, Zhao X, Ueda R, Kohanbash G, et al. IL-4 Suppresses Very Late Antigen-4 Expression Which is Required for Therapeutic Th1 T-cell Trafficking Into Tumors. Journal of Immunotherapy. 2009;32:793–802. doi: 10.1097/CJI.0b013e3181acec1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol. 1996;105:344–352. doi: 10.1046/j.1365-2249.1996.d01-753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weller M, Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res. 1995;21:128–151. doi: 10.1016/0165-0173(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 33.Nitta T, Hishii M, Sato K, Okumura K. Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Res. 1994;649:122–128. doi: 10.1016/0006-8993(94)91055-3. [DOI] [PubMed] [Google Scholar]
- 34.Jarnicki AG, Lysaght J, Todryk S, Mills KHG. Suppression of Antitumor Immunity by IL-10 and TGF-beta-Producing T Cells Infiltrating the Growing Tumor: Influence of Tumor Environment on the Induction of CD4+ and CD8+ Regulatory T Cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 35.Prokopchuk O, Liu Y, Henne-Bruns D, Kornmann M. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer. 2005;92:921–928. doi: 10.1038/sj.bjc.6602416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seo N, Hayakawa S, Takigawa M, Tokura Y. Interleukin-10 expressed at early tumour sites induces subsequent generation of CD4<sup>+</sup>T-regulatory cells and systemic collapse of antitumour immunity. Immunology. 2001;103:449–457. doi: 10.1046/j.1365-2567.2001.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108:16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS, et al. Immunologic Escape After Prolonged Progression-Free Survival With Epidermal Growth Factor Receptor Variant III Peptide Vaccination in Patients With Newly Diagnosed Glioblastoma. Journal of Clinical Oncology. 2010;28:4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 40.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, et al. LOH in the HLA Class I region at 6p21 is Associated with Shorter Survival in Newly Diagnosed Adult Glioblastoma. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-2861. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn BJ, Pollack IF, Okada H. Immune-checkpoint blockade and active immunotherapy for glioma. Cancers (Basel) 2013;5:1379–1412. doi: 10.3390/cancers5041379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada H, Pollack IF. Do we need novel radiologic response criteria for brain tumor immunotherapy? Expert Review of Neurotherapeutics. 2011;11:619–622. doi: 10.1586/ern.11.49. [DOI] [PubMed] [Google Scholar]
- 43.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C, Pirozzi CJ, Lopez GY, Yan H. Isocitrate dehydrogenase mutations in gliomas: mechanisms, biomarkers and therapeutic target. Curr Opin Neurol. 2011;24:648–652. doi: 10.1097/WCO.0b013e32834cd415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichimura K. Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol. 2012;29:131–139. doi: 10.1007/s10014-012-0090-4. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature. 2014 doi: 10.1038/nature13387. advance online publication. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.