Abstract
Rationale
Granulocyte macrophage colony stimulating factor (GM-CSF, Csf2) is a growth factor for myeloid-lineage cells that has been implicated in the pathogenesis of atherosclerosis and other chronic inflammatory diseases. However, the role of GM-CSF in advanced atherosclerotic plaque progression—the process that gives rise to clinically dangerous plaques—is unknown.
Objective
To understand the role of GM-CSF in advanced atherosclerotic plaque progression.
Methods and Results
Ldlr−/− mice and Csf2−/−Ldlr−/− mice were fed a Western-type diet for 12 wks, and then parameters of advanced plaque progression in the aortic root were quantified. Lesions from the GM-CSF-deficient mice showed a substantial decrease in two key hallmarks of advanced atherosclerosis, lesional macrophage apoptosis and plaque necrosis, which indicates that GM-CSF promotes plaque progression. Based on a combination of in vitro and in vivo studies, we show that the mechanism involves GM-CSF-mediated production of IL-23, which increases apoptosis susceptibility in macrophages by promoting proteasomal degradation of the cell-survival protein Bcl-2 and by increasing oxidative stress.
Conclusion
In LDL-driven atherosclerosis in mice, GM-CSF promotes advanced plaque progression by increasing macrophage apoptosis susceptibility. This action of GM-CSF is mediated by its IL-23-inducing activity rather than its role as a growth factor.
Keywords: Atherosclerosis, GM-CSF, IL-23, apoptosis, plaque necrosis, growth factors, cytokines, vulnerable plaque
INTRODUCTION
GM-CSF is generally considered a hematopoietic growth factor with particular roles in myeloid cell development, and mice lacking GM-CSF or its receptor have deficits in specific populations of non-lymphoid tissue resident dendritic cells under homeostatic conditions1. However, these mice have normal levels of myeloid immune cell populations in the peripheral circulation and lymphoid organs1. Thus, it is important to consider other roles for GM-CSF in physiologic and pathophysiologic settings, such as its ability to promote cytokine production. For example, GM-CSF primes macrophages for the production of pro-inflammatory cytokines following exposure to LPS or TNF-α2 and induces IL-23 production in dendritic cells (DCs) and macrophages3, 4.
Understanding the role of GM-CSF in atherosclerosis, particularly its effect on the types of necrotic plaques that give rise to acute atherothrombotic disease in humans, is important for a number of reasons. First, atherosclerosis is driven by a variety of lesional myeloid cell processes5, suggesting a potentially important role for this myeloid cell-relevant protein. Second, GM-CSF production by cultured macrophages is induced by incubation with atherogenic lipoproteins6, and GM-CSF is expressed in murine and human atherosclerotic lesions7, 8. Third, in a small study in which GM-CSF was administered to patients with stable coronary artery disease to improve collateral artery formation, several of the subjects suffered acute coronary events9. In this context, in a pre-clinical study of GM-CSF therapy for atherosclerosis in rabbits, there were features suggesting accelerated advanced plaque progression despite a decrease in overall intimal area10. Fourth, GM-CSF is administered to cancer patients following chemotherapy to mobilize stem cells11, while anti-GM-CSF therapy is under trial for treatment of rheumatoid arthritis and multiple sclerosis12. Because these treatments are offered to patients who may have sub-clinical coronary artery disease, it is important to understand the role of GM-CSF in advanced plaque progression.
In theory, both growth factor and non-growth factor roles of GM-CSF could be important in atherosclerosis. In animal models of atherosclerosis, the effects of GM-CSF deficiency or exogenous GM-CSF administration on atherosclerosis have been variable and dependent upon the specific animal model tested7, 10, 13, 14. However, most of these studies used models and reported endpoints most relevant to early atherogenesis, such as lesion size and cellularity, not advanced plaque progression. In this regard, most clinically relevant plaques in humans are distinguished not by their large size and cellularity but rather by features of plaque instability, notably plaque necrosis15. A major cause of advanced plaque necrosis is accelerated lesional macrophage apoptosis coupled with defective efferocytic clearance of the dead cells, leading to post-apoptotic necrosis and necrotic core formation16. Advanced plaques are also characterized by excessive oxidative stress, which promotes macrophage apoptosis17, 18.
To address this gap, we conducted a study in Csf2−/−Ldlr−/− mice subjected to prolonged Western diet feeding and focused on lesional cell apoptosis and necrotic core formation. We observed that the aortic root lesions of these GM-CSF-deficient mice had a substantial decrease in apoptotic cells, plaque necrosis, and oxidative stress compared with lesions of control Ldlr−/− mice. The mechanism involves GM-CSF-mediated induction of IL-23 in myeloid cells, which then sensitizes macrophages to apoptosis via proteasomal degradation of Bcl-2. The decrease in Bcl-2 increases caspase-9 activation and promotes pro-apoptotic oxidative stress. Thus, a non-growth factor function of GM-CSF promotes advanced plaque progression through an IL-23-mediated signaling pathway in macrophages that increases their susceptibility to apoptosis. These findings reveal a new pathway that contributes to advanced lesional macrophage apoptosis, which may be relevant to contemplated or actual situations where GM-CSF or IL-23 are used as a treatment modality in humans.
METHODS
Animals and animal maintenance
Csf2−/− mice on a C57BL/6J background were generously provided by Dr. Bruce Trapnell (University of Cincinnati College of Medicine). Csf2−/− mice were bred with C57BL/6J Ldlr−/− mice (Jackson labs) to generate Csf2−/−Ldlr−/− mice. 6-wk-old Ldlr−/− or Csf2−/−Ldlr−/− mice were fed a Western-type diet (Harlan Teklad, TD88137) ad libitum for 12 wks to generate advanced atherosclerotic lesions. All protocols were approved by the Columbia University Institutional Animal Care and Use Committee (IACUC).
Atherosclerotic lesion analysis and metabolic profiling
Animals were euthanized at the end of the WD feeding period using isoflurane inhalation, and blood was withdrawn by cardiac puncture. The heart with the aortic root attached was harvested, embedded in OCT, and frozen on dry ice. Aortic root sections were prepared using a cryomicrotome and then stained with hematoxylin and eosin. Six sections per mouse were quantified for total lesion area and necrotic area as described previously19. Briefly, the intimal region containing lesions are demarcated and quantified using ImagePro Plus by a person blinded to the experimental groups. Similarly, the necrotic area is marked and quantified as an area of the lesion that is devoid of cellular nuclei. Plasma cholesterol and triglycerides were measured using the Cholesterol E kit and Triglyceride M Color B kit from Wako. Fasting blood glucose was measured using glucose test strips and a glucometer. Plasma insulin was analyzed using an insulin ELISA kit (Crystal Chem).
Apoptosis and in situ efferocytosis assays
Apoptosis in cultured macrophages was assayed using Alexa fluor-conjugated annexin-V labeling (Life Technologies), followed by fluorescence microscopy. A total of 600 cells per group were analyzed to quantify the percentage of cells that were annexin-V positive. Apoptosis in atherosclerotic lesions was detected by TUNEL staining using the TMR red in situ cell death detection kit (Roche) following the manufacturer’s protocol. The TUNEL-stained sections were analyzed by microscopy and quantification was conducted using ImageJ. Lesional apoptosis was also assayed using activated-caspase-3 immunofluorescence microscopy20. In situ efferocytosis quantification was carried out as described previously21, 22. Briefly, aortic root sections were stained with TUNEL followed by anti-F4/80 immunohistochemistry to label lesional macrophages. Efferocytosis efficiency was quantified by counting the number of apoptotic cells that were co-localized or juxtaposed to F4/80-labeled macrophages (“associated”) vs. those that were not associated with macrophages (“free”).
Statistics
The data are displayed as mean ± SEM. The “n” numbers for each group are indicated in the Figure legends. All data presented in this study fit into a normal distribution and hence a Student’s two-tailed t-test was used for determining statistical significance between two groups, whereas, a one-way ANOVA with Bonferroni’s correction was applied while evaluating statistical significance between multiple groups. The difference between the means were considered significant when the p-value was less than 0.05.
Detailed Methods are provided in the Online Data Supplement.
RESULTS
Aortic root lesions of western diet-fed Csf2−/−Ldlr−/− mice show decreases in lesional cell apoptosis and plaque necrosis
To understand the role of GM-CSF in advanced atherosclerosis, GM-CSF-deficient mice in an atherosclerosis-prone LDLR knockout background (Csf2−/−Ldlr−/−) and control Ldlr−/− mice were fed a Western-type diet (WD) for 12 weeks. We first confirmed that GM-CSF was absent in the atherosclerotic lesions of Csf2−/−Ldlr−/− mice (Online Figure I). Further, we observed no significant differences between the two groups of mice in terms of body weight, total cholesterol, plasma triglycerides, fasting blood glucose, or plasma insulin (Online Table I). When the endpoint of total aortic root lesional area was assessed, we found that the two cohorts were remarkably similar (Figure 1A-B), which is largely consistent with a previous study13. Also consistent with previous studies7, 8, we observed that lesional macrophages, DCs, and SMCs were the major producers of GM-CSF in lesions of Ldlr−/− mice (Online Figure II). Because GM-CSF is an important hematopoietic growth factor, we next analyzed the immune cell distribution in the lesions. Overall plaque cellularity was comparable between the two groups of mice (Online Figure IIIA). The number of CD11clowF4/80+ cells (macrophages) was not altered by GM-CSF deficiency (Online Figure IIIB). However, as reported in a previous study13, we found a modest (~20%) but statistically significant decrease in the CD11chiMHCIIhi cell population, presumably dendritic cells (DCs), in the double knockout plaques (Online Figure IIIB). There was also a decrease in T cells in the Csf2−/−Ldlr−/− lesions (Online Figure IIIB). Note that GM-CSF deficiency was not associated with significant changes in the peripheral blood monocyte or neutrophil count (Online Figure IIIC).
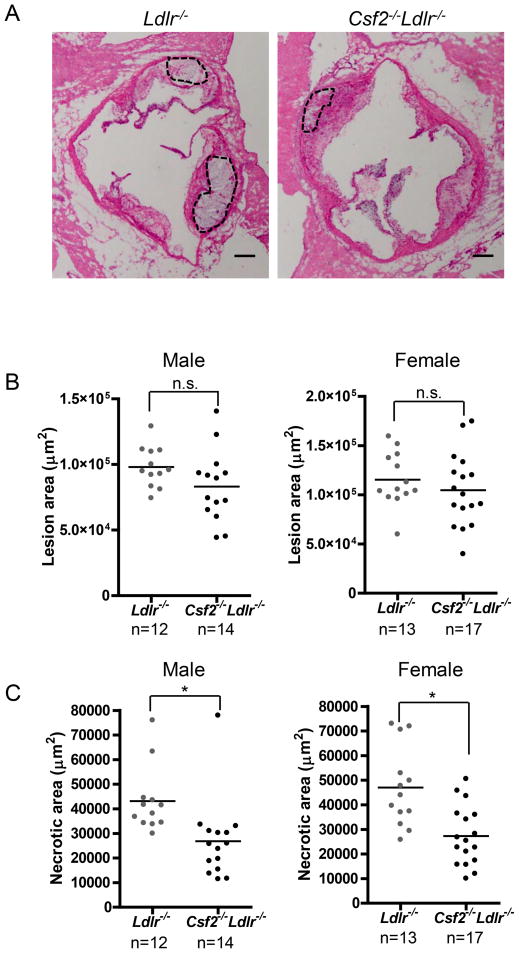
Figure 1. Necrotic area is decreased in the aortic root lesions of GM-CSF-deficient Ldlr−/− mice.
A, Representative images of H&E-stained aortic root sections of 12-wk WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice. The necrotic regions are indicated by the broken lines. Bar, 50 μm. B–C, Measurement of total atherosclerotic lesion area and necrotic area. *, p<0.05; n.s., no significant difference.
We next examined necrotic area in the lesions, because this endpoint represents a critical advanced lesional characteristic that determines plaque vulnerability in human atherosclerotic lesions15. There was an approximately 50% decrease in the necrotic area in the GM-CSF-deficient mice (Figure 1A and C). Atherosclerotic plaque necrosis is mediated in large part by the combination of lesional cell apoptosis and defective apoptotic cell clearance (efferocytosis)23. To measure apoptosis, we used the TUNEL staining method and found that the absolute number and percentage of TUNEL-positive lesional cells was significantly lower in the GM-CSF-deficient mice (Figure 2A–C). The percentage of lesional cells that was positive for cleaved caspase-3, another marker of apoptosis, was also significantly lower in the GM-CSF deficient mice (Figure 2D and Online Figure IV). This decrease in lesional cell apoptosis in GM-CSF-deficient mice was due to lower numbers and percentages of apoptotic macrophages and DCs, while the extent of smooth muscle cell apoptosis was similar between the two groups of mice (Online Figure V). Moreover, the ratio of apoptotic cells associated with macrophage phagocytes vs. those that were free of phagocytes was similar between the two groups of mice (Figure 2E), which indicates that efferocytosis was not affected by GM-CSF deficiency. Two other features of advanced atherosclerosis thinning of the fibrous cap and decreased intimal elastin content, was not affected by GM-CSF deficiency (Online Figure VI, A and B). Thus, GM-CSF deficiency specifically decreases lesional macrophage and DC apoptosis and plaque necrosis in advance aortic root lesions of WD-fed Ldlr−/− mice, which suggested to us a specific mechanism of action.
Figure 2. Lesional apoptotic cells are lower in GM-CSF-deficient Ldlr−/− mice.
A, Representative images of TUNEL-stained aortic root sections of 12-wk WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice (red), with DAPI-nuclear counterstain (blue), and their corresponding H&E-stained images. Bar, 25 μm. B–D, Quantification of lesional TUNEL and activated caspase-3 data. E, In situ efferocytosis was assayed by labeling apoptotic cells with TUNEL (red) and macrophages with anti-F4/80 (green), followed by quantification of the ratio of apoptotic cells that are associated with macrophages vs. those that are not (“free”). The red arrow depicts apoptotic cells that are associated with macrophages. Nuclei were stained with Hoechst dye (blue). Bar, 5 μm. For B–D, n=10 mice per group; *, p<0.05; n.s., no significant difference.
GM-CSF deficient mice have decreased lesional cytokines, including IL-23
To understand the mechanism of decreased apoptosis in the lesions of GM-CSF-deficient mice, we tested several possibilities. If CD11chi cells were intrinsically more susceptible to apoptosis than CD11cloF4/80+ cells, then Csf2−/−Ldlr−/− lesions, which have a decrease in CD11chi cells (above), might simply be populated with a higher percentage of cells that are relatively resistant to apoptosis. However, as shown above, these two subpopulations of cells showed similar decreases in apoptosis in the Csf2−/−Ldlr−/− lesions (Online Figure V). Additionally, cultured DCs and macrophages exposed to atherosclerosis-relevant pro-apoptotic factors such as 7-ketocholesterol (7KC) and oxidized-LDL showed similar susceptibility to apoptosis (data not shown). A decrease in apoptosis-susceptible neutrophils in the double knockout lesions could also provide an explanation, but the lesions from the two groups of mice had similarly low numbers of neutrophils (Online Figure IIIB). Thus, the decrease in lesional apoptosis in Csf2−/−Ldlr−/− lesions cannot be explained by an increase in the ratio of apoptosis-resistant:susceptible cell types.
We next examined whether the lesions of Csf2−/−Ldlr−/− mice had an alteration in cytokines that may lead to a decrease in apoptosis. The mRNA levels of pro- and anti-inflammatory cytokines in the lesions of the two groups of mice were quantified by RT-qPCR of lesional RNA obtained by laser capture microdissection (LCM). We found a significant decrease in the expression of IFN-γ and IL-2 in the GM-CSF-deficient lesions (Figure 3A), consistent with a decrease in lesional T cells (above). Further analysis of T cell subset mRNA expression indicated a significant decrease in lesional Th1 and Th17 profiles, while Th2 and Tregs were unaffected (Figure 3B). The decrease in lesional Th1 cells is consistent with the known role of GM-CSF in skewing T cell differentiation toward a Th1 phenotype. A similar decrease in Th1 cell profile was observed in the spleens of GM-CSF-deficient mice (Online Figure VIIA). However, there were no significant differences between the two groups of mice in the numbers of total T cells, CD4+ T cells, CD8+ T cells, or regulatory T cells in the spleen or peripheral blood (Online Figure VIIB-E). Consistent with a decrease in Th17 cells in the lesions of Csf2−/−Ldlr−/− mice, expression of the mRNA for IL-17A, the major cytokine produced by Th17 cells, was also decreased in the lesions of this cohort (Figure 3A). Previous studies have shown that IL-23, a cytokine induced by GM-CSF, is critical for Th17 cell differentiation and survival3, 24. In agreement with these reports, we found decreased levels of IL-23 in the double knockout lesions (Figure 3A and 3C), while serum IL-23 levels were unchanged between the two groups of mice (Online Figure VIII). Macrophages and DCs are the major producers of IL-23 in atherosclerotic lesions (Online Figure IX), and their production of IL-23 was significantly decreased in the GM-CSFdeficient mice (Online Figure X). Finally, consistent with the lack of changes in the numbers of lesional Tregs and macrophages, lesional Il10 and Tgfb mRNA were similar in Ldlr−/− mice and Csf2−/−Ldlr−/− mice (Figure 3A). In summary, the lesions of WD-fed Csf2−/−Ldlr−/− mice are characterized by decreases in the mRNAs for specific T cell cytokines, particularly Il17, and a decrease in Il23.
Figure 3. Aortic root lesions of GM-CSF-deficient Ldlr−/− mice have lower levels of IL-23.
A–B, RNA was isolated by LCM of lesional cells in 12 wk WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice, and mRNA of the indicated genes was quantified by RT-qPCR. All data are normalized to Actb mRNA. n=5 mice per group; *, p<0.05; n.s., no significant difference. C, Atherosclerotic lesional extracts obtained from 12 wk WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice were assayed for IL-23 by ELISA. n=10 mice per group; *, p<0.05; n.s., no significant difference.
IL-23 increases apoptosis susceptibility in cultured macrophages, and restoration of IL-23 in Csf2−/−Ldlr−/− mice increases lesional apoptosis
IL-17 plays a pro-apoptotic role in vascular endothelial cells25 and in cardiomyocytes post ischemia-reperfusion injury26, while IL-23 has been reported to play a role in apoptosis of self-reactive thymocytes during T cell selection27 and of leukemic cells in B-acute lymphoblastic leukemia28. We therefore tested whether IL-17 or IL-23 could induce apoptosis in cultured macrophages under basal conditions or when exposed to 7-ketocholesterol (7KC), a pro-apoptotic oxysterol present in human atherosclerotic lesions29, 30. Apoptosis was assessed by annexin-V staining, which labels externalized phosphatidylserine on the plasma membrane of apoptotic cells. Treatment of macrophages with IL-17 or IL-23 alone did not lead to a significant increase in the number of annexin-V+ cells (Figure 4A–B). Similarly, treatment of macrophages with IL-17 did not result in enhancement of 7KC-induced apoptosis (Figure 4A). However, IL-23 treatment led to a significant, dose-dependent increase in 7KC-induced macrophage apoptosis (Figure 4B and Online Figure XI), and this effect was abrogated by co-incubation with a neutralizing antibody against the IL-23 receptor (IL-23R) (Figure 4C). The neutralizing effect of the IL-23R antibody was validated by demonstrating blockage of IL-23-induced STAT3 phosphorylation in cultured macrophages (data not shown). IL-12 and IL-23 share a common subunit and certain common functions31, but IL-12 did not enhance macrophage apoptosis (Figure 4C). The effect of IL-23 in sensitizing macrophages to apoptosis was not specific to 7-KC: both oxidized LDL32 and the combination of an ER stressor and oxidized phospholipid (thapsigargin and KOdiA-PC)33 gave similar results (Online Figure XII). In contrast, TNF-α, IL-2, IFN-γ, and IL-6, which are higher in the lesions of Ldlr−/− vs. Csf2−/−Ldlr−/− mice, did not increase basal or 7KC-induced apoptosis susceptibility in cultured macrophages (Online figure XIII). Finally, consistent with our in vivo data that GM-CSF-deficient mice have decreased apoptosis of lesional DCs as well as macrophages, we found that cultured bone marrow-dervied DCs demonstrated enhanced susceptibility to 7KC-induced apoptosis in the presence of IL-23 (Online Figure XIV). These combined data demonstrate that IL-23 enhances the susceptibility of macrophages and DCs to apoptosis induced by certain athero-relevant apoptotic factors in an IL-23R-dependent manner.
Figure 4. IL-23 increases the susceptibility of cultured macrophages to 7KC-induced apoptosis.
A, Bone marrow-derived macrophages were treated with the indicated concentration of IL-17, 35 μM 7KC, or a combination of IL-17 and 7KC for 18 h. Apoptosis was quantified by counting annexin V-labeled and total cells in fluorescence microscopic images. B, Similar to A, but the macrophages were treated with IL-23 instead of IL-17. C, Similar to B, but some of the groups received either control IgG or neutralizing antibody against the IL-23 receptor (anti-IL23R, 1 μg/ml) or rIL-12 (100 ng/ml). *, p<0.05 vs. control; #, p<0.05 vs. the 7KC-treated group; ¶, p<0.05 vs. the 7KC+IL-23-treated group; n.s., no significant difference. The data are representative of 3 independent experiments.
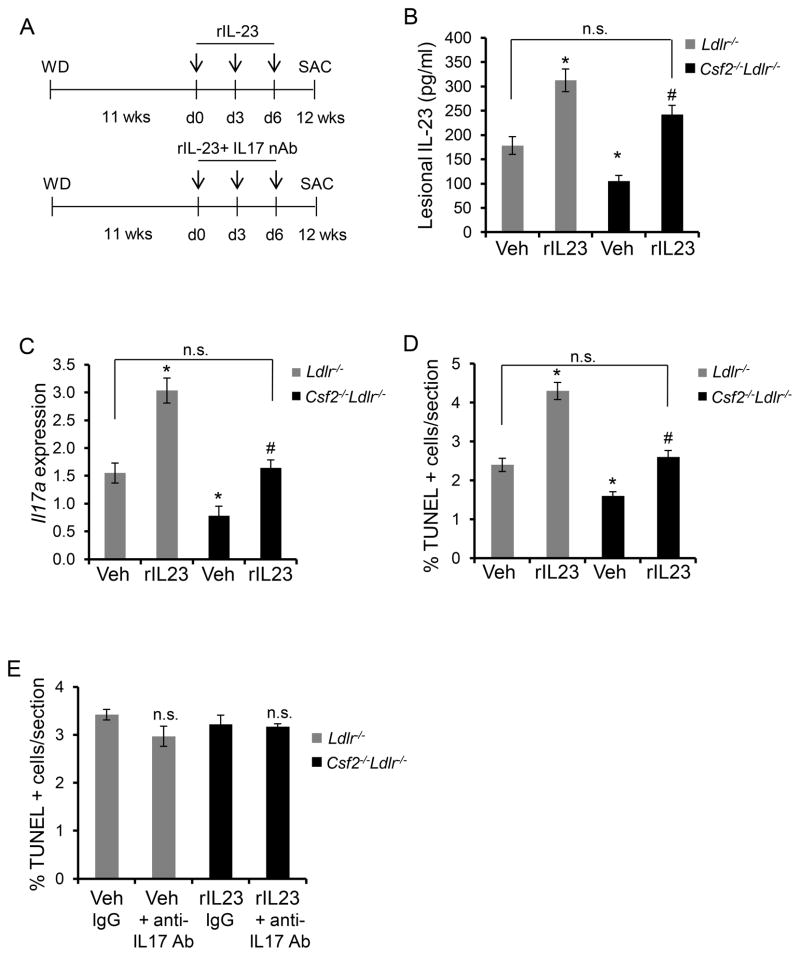
To test the role of IL-23 in lesional macrophage apoptosis in vivo, we administered recombinant mouse IL-23 (rIL-23) to Ldlr−/− or Csf2−/−Ldlr−/− mice as per the scheme illustrated in Figure 5A. The primary aim was to restore lesional IL-23 levels in the GM-CSF-deficient mice and to evaluate the effect of this restoration on lesional cell apoptosis. Using an IL-23 ELISA assay of lesional extracts and a pilot IL-23 dosing experiment, we found a dose of rIL-23 that restored the level of lesional IL-23 in GM-CSF-deficient mice close to the level of lesional IL-23 in control (Veh) Ldlr−/− mice (Figure 5B; compare 1st and 4th bars). Because ELISA is a measure of immunogenic rather than bio-active IL-23, we analyzed the functional activity of IL-23 by measuring the mRNA level of one of its target genes, Il17a. Consistent with the ELISA data, Il17a mRNA in the lesions of IL-23-treated Csf2−/−Ldlr−/− mice was restored close to the level in control Ldlr−/− mice (Figure 5C; compare 1st and 4th bars). However, restoration of IL-23 levels did not affect the expression levels of other cytokine genes such as Tnfa, Ifng, and Il2, which remained lower in the GM-CSF-deficient mice (Online Figure XV). Using this dose of IL-23, we found that lesional apoptosis in IL-23-restored Csf2−/−Ldlr−/− mice was increased to the level of that in control Ldlr−/− mice (Figure 5D; compare 1st and 4th bars). Moreover, consistent with the lack of an effect of IL-17 on apoptosis susceptibility in cultured macrophages (above), neutralization of IL-17 activity by administration of anti-IL-17 antibody34 did not affect lesional cell apoptosis in the IL-23-restored mice or any of the other groups of mice (Figure 5E). As a positive control for the IL-17 antibody, we demonstrated that the level of the IL-17 target mRNA, Il6, was decreased in the lesions of anti-IL-17-treated mice (Online Figure XVI). These data, combined with our data with cultured macrophages (above), support the hypothesis that the decrease in lesional IL-23 in Csf2−/−Ldlr−/− mice plays an important role in the decrease of lesional cell apoptosis in these mice.
Figure 5. Restoration of IL-23 in Csf2−/−Ldlr−/− Mice Increases Lesional Apoptosis.
A, Schematic showing the time of i.v. administration of rIL-23 (5 μg/kg) or a combination of rIL-23 and anti-IL17 (100 μg/mouse) neutralizing antibody in WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice. Control mice were treated with vehicle (Veh). B, Extracts of aortic root sections obtained from the indicated groups of mice were assayed for IL-23 by ELISA. C, LCM-RT-qPCR quantification of Il17a mRNA, normalized to Actb, in the lesions of the indicated groups of mice. D–E, Percent TUNEL-positive cells in the atherosclerotic lesions of the indicated groups of mice. For A–D, n=3 mice for the control group and n=6 mice for the rIL-23-treated group, and for E, n=5 mice per group; *, p<0.05 vs. vehicle-treated Ldlr−/− mice; #, p<0.05 vs. vehicle-treated Csf2−/−Ldlr−/− mice; n.s., no significant difference.
IL-23 promotes ubiquitin-mediated degradation of the cell survival protein Bcl-2
7KC induces apoptosis in macrophages via activation of the mitochondrial-caspase-9 pathway of apoptosis35. We therefore investigated whether this pathway might also be required in IL-23-mediated enhancement of 7KC-induced macrophage apoptosis. Caspase-9 is activated by proteolytic cleavage of the inactive, full-length protein (pro-caspase-9) into a shorter length active protease36. Because activated caspase-9 protein is very short-lived in the 7KC-macrophage model, caspase-9 activation is measured by quantifying the disappearance of pro-caspase 9. We found that IL-23 treatment enhanced 7KC-mediated loss of pro-caspase-9 (Online Figure XVIIA), indicating enhanced caspase-9 activation. Most importantly, knockdown of caspase-9 blocked apoptosis in 7KC-treated cells and prevented the IL-23 increment in apoptosis (Online Figure XVIIB).
Although the 7KC + IL-23 result does not necessarily prove a direct role for caspase-9 in IL-23 enhancement of apoptosis, because this enhancement requires 7KC-induced apoptosis in the first place, these findings led us to explore further a protein that is known to affect the mitochondrial pathway of apoptosis, Bcl-237. Bcl-2 was of additional interest because of a report showing that it can protect leukemia cells from IL-23-induced apoptosis28. In this context, we found that treatment of macrophages and DCs with IL-23, but not 7KC, led to a significant down-regulation of Bcl-2 protein expression (Figure 6A and Online Figure XVIIIA). IL-23 did not decease Bcl2 mRNA (Online Figure XVIIIB), indicating that the observed decrease in Bcl-2 protein is not due to transcriptional inhibition or decrease in mRNA stability. We next determined if the decrease in Bcl-2 was regulated by proteasome-mediated degradation, which has been demonstrated in other settings in which Bcl-2 levels are regulated38. Consistent with this mechanism, MG-132, a proteasome inhibitor, abrogated the IL-23-mediated decrease in Bcl-2 (Figure 6B). One of the mechanisms by which Bcl-2 is targeted for proteasomal degradation is via dephosphorylation of Ser87, which serves as a signal for poly-ubiquitination by ubiquitin ligases38. Because ubiquitination of endogenous proteins is difficult to detect, we overexpressed full-length mouse Bcl-2 in control and IL-23-treated macrophages and then conducted an immunoprecipitation-immunoblot experiment. The data show a significant decrease in phospho-Ser-Bcl-2 in IL-23-treated macrophages compared with control cells (Figure 6C, middle blot). Moreover, when the same lysates were immunoblotted for ubiquitin, we found that there was an increase in high-molecular weight bands between 50–150 kDa in the extracts from IL-23-treated macrophages, indicating that IL-23 promotes polyubiquitination of Bcl-2 (Figure 6C, lower blot). Thus, the ability of IL-23 to promote Bcl-2 dephosphorylation and subsequent ubiquitination is a plausible mechanism for IL-23-mediated Bcl-2 down-regulation.
Figure 6. IL-23 induces ubiquitin-mediated degradation of Bcl-2.
A–B, Immunoblot of Bcl-2 in macrophages treated with IL-23 (10 ng/ml) or 7KC (35 μM) for the indicated times in A or for 8 h in B in the absence or presence of the proteasome inhibitor MG-132 (10 μM). β-actin was used as a loading control. The bar graph represents densitometric analysis of the immunoblot data in B. C, Macrophages transfected with a plasmid encoding Bcl-2 was treated without (Con) or with IL-23 for 4 h in the presence of MG132. Bcl-2 was immunoprecipitated followed by immunoblotting for Bcl-2, phospho-serine (pSer), and ubiquitin (Ub). *, p<0.05 vs. control; #, p<0.05 vs. 7KC treatment; n.s., no significant difference. The data are representative of 3 independent experiments.
IL-23 down-regulates Bcl-2 and enhances apoptosis susceptibility by inducing MKP-1-mediated suppression of ERK
Phosphorylation of Bcl-2 is mediated by extracellular signal-related kinase (ERK)38, and so we tested whether the decrease in phospho-Bcl-2 by IL-23 is caused by a decrease in ERK activity. Consistent with this scenario, we observed that IL-23 treatment was associated with a decrease in the level of phospho-ERK (pERK), the active form of ERK (Figure 7A). Additionally, treatment of macrophages with an ERK inhibitor mimicked the effect of IL-23 on decreasing Bcl-2 protein (Online Figure XIXA). The decrease in pERK could be mediated by decreased phosphorylation by its upstream kinase MEK or by increased dephosphorylation by the phosphatases MKP-1 or MKP-3. Whereas the level of active phospho-MEK in IL-23 treated macrophages was similar to that in control cells (Online Figure XIXB), MKP-1 protein was increased in IL-23-treated macrophages (Figure 7B). MKP-3 levels were similar between the two groups of macrophages (data not shown).
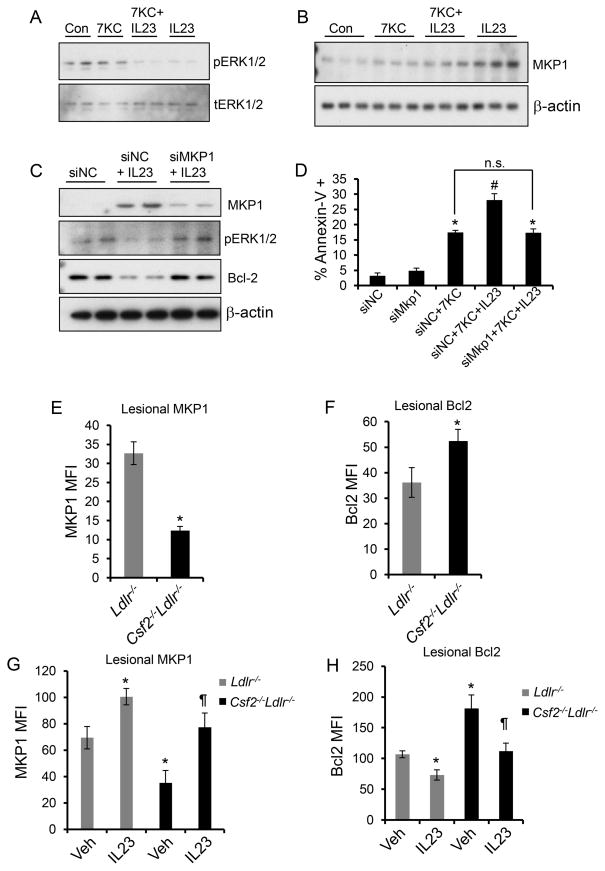
Figure 7. Evidence that an increase in MKP-1 links GM-CSF/IL-23 to decreased Bcl-2 and apoptosis in macrophages and in atherosclerotic lesions.
A, Macrophages treated with 7KC alone, IL-23 alone, or the combination of 7KC and IL-23 for 30 min were probed for phospho- or total ERK1/2 by immunoblotting. B, Immunoblot of MKP-1 in macrophages treated with 7KC alone, IL-23 alone, or the combination of 7KC and IL-23 for 4 h. C, Macrophages were transfected with negative control siRNA (siNC) or siRNA against MKP-1 (siMkp1) and then treated 48 h later without or with IL-23 for 8 h. Whole cell lysates were immunoblotted for MKP-1, pERK1/2, Bcl-2, and β-actin. D, Macrophages transfected with siNC or siMkp1 were treated with 7KC or a combination of 7KC and IL-23 for 18 h followed by quantification of apoptosis by microscopic analysis of annexin V-labeled macrophages. E-H, Immunofluorescence quantification (mean fluorescence intensity, MFI) of MKP-1 and Bcl-2 in F4/80+ macrophage-rich regions of aortic root atherosclerotic lesions. n=6 mice per group. In G and H, the indicated groups of mice were treated with saline (Veh) or rIL-23 (5 μg/kg). In D, the data are representative of 3 independent experiments. In E and F, n=6 mice per group, and in G and H, n=3 mice for the control group and n=6 mice for rIL-23-treated group; *, p<0.05 vs. control or Ldlr−/− mice; #, p<0.05 vs. 7KC treatment; ¶, p<0.05 vs. vehicle-treated Csf2−/−Ldlr−/− mice; n.s., no significant difference.
We next tested whether the increase in MKP-1 expression was causally related to ERK dephosphorylation, Bcl-2 degradation, and increased apoptosis susceptibility in IL-23-treated macrophages by using MKP-1 siRNA. As predicted by the hypothesis that MKP-1 is a key upstream mediator in the IL-23 pathway, silencing MKP-1 abrogated the decrease in pERK and Bcl-2 expression (Figure 7C). Most importantly, knockdown of MKP-1 protected macrophages from the increment in apoptosis observed in IL-23/7KC-treated macrophages compared with 7KC-treated macrophages (Figure 7D).
To test the relevance of the MKP-1 model to advanced atherosclerosis, we quantified lesional MKP-1 expression by immunohistochemistry. The data show a significantly lower level of MKP-1 in the lesions of GM-CSF-deficient Ldlr mice (Figure 7E and Online Figure XXA). As a control for the specificity of the antibody, we observed significantly lower expression of MKP-1 in macrophages transfected with siRNA against MKP-1 (Online Figure XXB). In addition, Western blotting for MKP-1 in extracts obtained from sections of aortic root demonstrated significantly lower expression of MKP-1 in the GM-CSF-deficient lesions (Online Figure XXC). Consistent with the decrease in MKP-1, the lesions of Csf2−/−Ldlr−/− mice demonstrated increased levels of Bcl-2 expression as measured by immunohistochemistry (Figure 7F and Online Figure XXI). Finally, both the decrease in lesional MKP-1 and the increase in lesional Bcl-2 in GM-CSF-deficient mice could be reversed by exogenous administration of rIL-23 (Figure 7G, 7F, and Online Figure XXII). In summary, IL-23 increases apoptosis susceptibility in 7KC-treated macrophages through up-regulation of MKP-1. MKP-1 decreases ERK-mediated phosphorylation of Bcl-2, leading to polyubiquitination and proteasomal degradation of Bcl-2 and a subsequent increase in apoptosis susceptibility.
The IL-23-MKP-1 pathway enhances ROS in 7KC-treated macrophages and in advanced atherosclerotic lesions
Oxidative stress and the generation of various reactive oxygen species (ROS) and ROS-modified proteins and lipids are key features of advanced plaque progression39, 40. In cultured primary macrophages exposed to athero-relevant factors, including 7KC, ROS mediated by NADPH oxidase promotes apoptosis29, 30. Interestingly, one of the mechanisms by which Bcl-2 can exert its anti-apoptotic activity is via its role as an anti-oxidant41, 42. In the context of these previous findings, we hypothesized that the IL-23-induced decrease in Bcl-2 might result in enhanced ROS generation, which in turn would further drive apoptosis susceptibility in macrophages exposed to athero-relevant pro-apoptotic factors. To address this hypothesis, we incubated macrophages with 7KC in the absence or presence of IL-23 and then probed the cells with CellROX Deep Red™, which fluoresces in the cytoplasm when exposed to ROS43. Similar to the apoptosis findings, IL-23 alone did not induce ROS in macrophages, but it enhanced ROS in the presence of 7KC (Figure 8A and Online Figure XXIIIA). In contrast, IL-23 did not affect 7KC-induced ROS in the mitochondria (data not shown), which was assayed using the mitochondrial ROS probe mitoSOX™40.
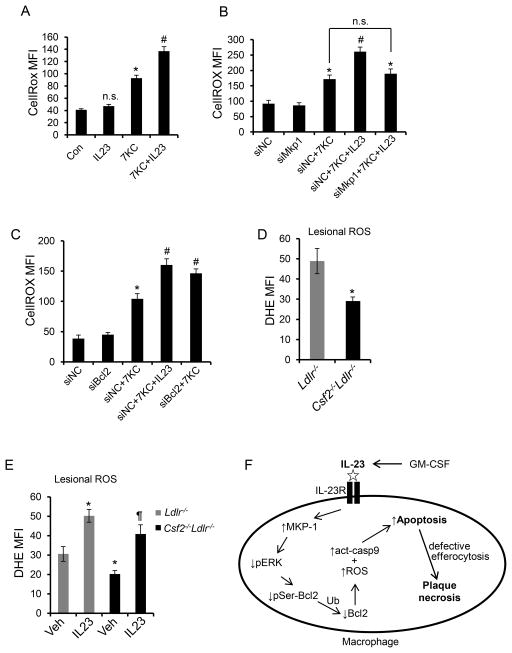
Figure 8. Lesional ROS is lower in GM-CSF-deficient Ldlr−/− mice and is reversed upon restoration of IL-23.
A–C, Flow-cytometric measurement of ROS using an ROS-sensitive probe, CellROX, in macrophages subjected to the indicated treatments for 6 h. D, Quantification of lesional ROS by microscopic analysis of DHE staining in 12-wk WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice and E, in Ldlr−/− and Csf2−/−Ldlr−/− mice treated with saline (Veh) or rIL-23 (5 μg/kg). n=3 mice for control group and n=6 mice for rIL-23 treated group; *, p<0.05 vs. control or Ldlr−/− mice; #, p<0.05 vs. the 7KC-treated group; ¶, p < 0.05 vs. Csf2−/−Ldlr−/− mice. MFI, mean fluorescence intensity. F, Schematic summary of the mechanism by which the GM-CSF-IL-23 pathway enhances apoptosis susceptibility in macrophages. Ub, ubiquitination. See text for details.
Next, to assess whether the increase in ROS upon IL-23 treatment was a consequence of the decrease in Bcl-241, 42, we blocked Bcl-2 degradation by using Mkp1 siRNA (above). We found that the increment in ROS that occurs when IL-23 is added to 7KC-treated macrophages was abrogated by silencing MKP-1 (Figure 8B and Online Figure XXIIIB). Conversely, silencing Bcl2 mimicked IL-23 in terms of its ability to increase the ROS response in 7KC-treated macrophages (Figure 8C and Online Figure XXIIIC and D). The question as to whether the IL-23-mediated increment in ROS is causally important in its ability to enhance apoptosis susceptibility in 7KC-treated macrophages is difficult to address, because blocking ROS in these cells, e.g., by using NOX2-deficient macrophages, blocks 7KC-induced apoptosis30, which by itself would negate the IL-23 effect. Nonetheless, we did find that IL-23 was unable to enhance apoptosis above the suppressed level seen in 7KC-treated NOX2-deficient macrophages (data not shown). Finally, to determine the relevance of these findings in atherosclerosis, we analyzed lesional ROS by staining aortic root sections obtained from WD-fed Ldlr−/− and Csf2−/−Ldlr−/− mice with the fluorescent superoxide sensor dihydroethidium (DHE). Consistent with the cultured macrophage data, we observed decreased DHE staining in the lesions of GM-CSF-deficient mice (Figure 8D and Online Figure XXIVA). Moreover, similar to the lesional apoptosis data in Figure 5D, this decrease in lesional ROS in the Csf2−/−Ldlr−/− mice was reversed by treating the mice with a restorative level of rIL-23 (Figure 8E and Online Figure XXIVB). These combined findings support a model in which the GM-CSF-IL-23-MKP-1 pathway promotes the degradation of Bcl-2, which increases apoptosis susceptibility by activating the mitochondrial-caspase 9 pathway of apoptosis as well as by enhancing ROS accumulation (Figure 8F).
DISCUSSION
Earlier studies examining the function of GM-CSF in atherosclerosis have focused on its roles in myeloid cell differentiation and proliferation. For example, GM-CSF was demonstrated to be essential for the proliferation of CD11chi cells in nascent atherosclerotic lesions44, which is consistent with the ability of GM-CSF to stimulate the differentiation of cultured DCs. However, a recent study demonstrated that GM-CSF is not necessary for differentiation of inflammatory DCs derived from monocytes45. Thus, it is possible that GM-CSF affects a specific subset of resident conventional DCs in the subendothelial space of healthy arteries or the intima of very early atherosclerotic lesions. Consistent with this idea, we observed only a modest decrease in CD11chiMHC-IIhi DCs in established atherosclerotic lesions of GM-CSF deficient mice.
In terms of atherosclerosis per se, the role of GM-CSF appears to be influenced by the model used and the focus of the study. In particular, studies using mice that completely lack apolipoprotein E (apoE) in all cells or in bone marrow-derived cells, which is known to affect immune cell function46, 47 and hematopoietic stem cell proliferation48 have shown complex effects that may be specific to models lacking apoE. As an example of the complexity, exogenous administration of GM-CSF to Apoe−/− mice was reported to increase atherosclerotic lesion size14, whereas deficiency of GM-CSF in an Apoe−/− background was also associated with larger lesion size and increased macrophage content, which was attributed to a decrease in macrophage PPAR-γ and ABCA17. In contrast, in WD-fed Ldlr−/− model used here, which has lipoprotein profiles similar to dyslipidemic humans and do not have adverse immune effects, GM-CSF deficiency did not affect macrophage Pparg, Abca1, or Abcg1 expression in lesional macrophages (unpublished data). Moreover, in WD-fed Ldlr−/− mice, we found that GM-CSF deficiency had no significant effect on aortic root lesion size per se, which agrees in principle with another group showing only a modest effect in females but not males13. Rather, as demonstrated here, the dominant effect of GM-CSF in Ldlr−/− mice is enhancement of macrophage apoptosis in advanced atherosclerosis by a specific mechanism related to its ability to induce IL-23 production.
The results of the current study underscore the importance of the cytokine-inducing role of GM-CSF in atherosclerosis, which in this case involves a particular cytokine, IL-23, that promotes macrophage apoptosis. Under physiologic conditions, GM-CSF-induced production of IL-23 and subsequent macrophage apoptosis may act as a feedback mechanism to control immune cell populations or to prevent excessive inflammation. In that setting, the apoptotic macrophages would be rapidly cleared by neighboring phagocytes (efferocytosis), which prevents both secondary necrosis and generation of pro-inflammatory damage-associated molecular patterns (DAMPS) and also activates anti-inflammatory signaling pathways in the efferocytes themselves49. However, in advanced atherosclerotic lesions, efferocytosis is defective50, and so processes that increase apoptosis promote necrosis and inflammation, which, as demonstrated here, is the case with GM-CSF-induced IL-23.
The link between GM-CSF and IL-23 has been explored most extensively in the setting of autoimmune disorders, where a GM-CSF/IL-23/Th17 axis has been demonstrated to play a major role in disease exacerbation3, 24. Accordingly, anti-GM-CSF, anti-IL-23, and anti-IL-17 therapies are currently under investigation for treatment of these diseases12, 51. In these disorders, mechanistic studies have focused on the role of IL-23 in promoting Th17 cell survival and Th17-mediated IL-17 production. In advanced atherosclerosis, however, the pathogenic effect of IL-23 appears to be largely independent of IL-17 generation, as neutralization of IL-17 activity did not block IL-23-induced macrophage apoptosis or plaque necrosis. Moreover, IL-23, but not IL-17, increased apoptosis in 7KC-treated macrophages. IL-23 has been shown previously to induce apoptosis in self-reactive thymocytes27, and, at high concentration, in B-acute lymphoblastic leukemia cells (B-ALL)28. In B-ALL cells, like macrophages, the pro-apoptotic mechanism of IL-23 involves down-regulation of Bcl-2. In B-ALL cells, however, Bcl-2 down-regulation is mediated by a microRNA, miR15a28, while in macrophages, Bcl-2 down-regulation is mediated by the proteasome following MKP-1-mediated Bcl-2 dephosphorylation.
Our lab has previously shown that atherosclerosis-prone mice lacking macrophage-Bcl-2 have increased lesional macrophage apoptosis and increased necrotic area52, which demonstrates that Bcl-2 is critical for macrophage survival in advanced atherosclerosis. The current study provides a pathophysiolgically relevant context for this effect, namely, GM-CSF/IL-23-mediated down-regulation of macrophage Bcl-2. The classic role of Bcl-2 is suppression of the mitochondrial-caspase-9 pathway of apoptosis37, but our data as well as previous studies41, 42 suggest that Bcl-2 can also suppress intracellular oxidant stress. Given the role of ROS in macrophage apoptosis18, we propose the GM-CSF/IL-23 pathway, through destabilizing Bcl-2, promotes apoptosis susceptibility in macrophages by increasing both caspase-9 activity and intracellular ROS. The precise mechanism through which Bcl-2 regulates intracellular ROS in other models is not well understood, but there is evidence that it may involve up-regulation of superoxide dismutase and catalase42 as well as direct binding to the anti-oxidant GSH53. How Bcl-2 regulates cytosolic ROS in macrophages in the setting of advanced atherosclerosis will require further study, but we speculate it may involve suppression of NADPH oxidase30, 54.
Stimuli other than GM-CSF might also increase the generation of IL-23 by macrophages and DCs in atherosclerotic lesions. For example, IL-23 production by DCs is induced by the endoplasmic reticulum (ER) stress effector CHOP, which binds to a specific promoter region on IL-23 gene55. We have previously demonstrated that ER stress-induced CHOP plays an important role in macrophage apoptosis and plaque necrosis in advanced atherosclerotic lesions56. Whether, the pro-apoptotic effects of ER stress on lesional macrophages is mediated, in part, via induction of IL-23 remains to be explored. Additionally, Ataxia telangiectasia mutated (ATM) kinase represses production of IL-23 by DCs57, and atherosclerosis is exacerbated in mice lacking ATM58 and in humans with single nucleotide polymorphisms (SNPs)59 or mutations in ATM60. The potential link between these observations and excessive IL-23 production thus represents another area of future investigation based on the new findings and pathway described herein.
The results of several pre-clinical studies and a small human study raise the possibility that exogenously administered rGM-CSF might be pro-atherogenic and promote CAD9, 10, 14. The current data raise the possibility that GM-CSF-induced IL-23 could be involved in these findings. Indeed, a small human study comparing serum IL-23 and IL-17 levels in patients with CAD vs. healthy subjects demonstrated that serum IL-23 levels were associated strongly with CAD61. This association requires replication and could simply reflect a non-causative, marker-related phenomenon, but the data herein raise the possibility that IL-23 itself could be causative. With these studies in mind, and given the mechanistic insight of the current study, it would be interesting to determine whether patients receiving anti-IL-23 therapy for the treatment of rheumatoid arthritis accrue additional cardiovascular benefits due to decreased plaque necrosis. If so, one could imagine a future therapeutic strategy in which IL-23 or its downstream pro-apoptotic mediators are neutralized in high-risk patients with the goal of preventing plaque necrosis and subsequent acute cardiovascular clinical events.
Supplementary Material
Novelty and Significance.
What Is Known?
Granulocyte macrophage colony stimulating factor (GM-CSF) is a growth-factor for myeloid cells and can also induce myeloid cells to produce the cytokine IL-23.
In the setting of early atherosclerosis in mice, GM-CSF deficiency surprisingly has only a modest effect on total lesional cellularity and lesion area.
What New Information Does This Article Contribute?
Deficiency of GM-CSF in the setting of advanced atherosclerosis in mice decreases the expression of IL-23 by lesional myeloid cells and suppresses both advanced lesional myeloid-cell apoptosis and plaque necrosis, indicating associations among GM-CSF, IL-23, and apoptosis in atherosclerosis.
The protective effect of GM-CSF deficiency in advanced plaque progression is abrogated by administration of IL-23, suggesting that IL-23 links GM-CSF to lesional myeloid-cell apoptosis.
IL-23 enhances the sensitivity of cultured macrophages and dendritic cells to apoptosis by down-regulating the anti-apoptotic protein Bcl-2 and by increasing oxidative stress, and advanced lesions of GM-CSF-deficient atherosclerosis-prone mice have lower levels of Bcl2 and increased oxidative stress.
The types of atherosclerotic plaques that cause acute cardiovascular events in humans are characterized by prominent areas of lesional cell apoptosis, but the mechanisms remain to be fully explored. Our current study reveals a new apoptosis pathway in which an endogenous molecule in lesions, GM-CSF, induces IL-23 in lesional myeloid cells, which then enhances the sensitivity of these cells to apoptosis. IL-23 enhances apoptosis by down-regulating the anti-apoptotic protein Bcl-2 and by increasing oxidative stress. In view of the implementation of anti-IL-23 therapy for other disorders, its focused use in high-cardiovascular risk subjects may provide a novel means to delay the formation of vulnerable plaques.
Acknowledgments
We thank Dr. Bruce Trapnell (University of Cincinnati College of Medicine) for providing Csf2−/− mice and Dr. Aldons (Jake) Lusis (UCLA) for helpful discussion and for providing lesion sections from Ldlr−/− and Csf2−/−Ldlr−/− mice for a pilot study. We also thank George Kuriakose for providing excellent technical support in the execution of this project.
SOURCES OF FUNDING
This study was supported by National Institutes of Health grants to E. Thorp (R01HL122309), I. Tabas (R01HL075662, R01HL106019) and Diabetes and Endocrinology Research Core (5P30DK063608).
Nonstandard Abbreviations and Acronyms
- GM-CSF
granulocyte-macrophage colony stimulating factor
- DC
dendritic cell
- WD
Western-type diet
- 7KC
7-ketocholesterol
- ROS
reactive oxygen species
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- MFI
mean fluorescence intensity
- NOX
NADPH oxidase
Footnotes
In September, 2014, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.29 days
DISCLOSURES
None.
References
- 1.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brissette WH, Baker DA, Stam EJ, Umland JP, Griffiths RJ. Gm-csf rapidly primes mice for enhanced cytokine production in response to lps and tnf. Cytokine. 1995;7:291–295. doi: 10.1006/cyto.1995.0035. [DOI] [PubMed] [Google Scholar]
- 3.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, Kopf M. Gm-csf mediates autoimmunity by enhancing il-6-dependent th17 cell development and survival. J Exp Med. 2008;205:2281–2294. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD. Granulocyte-macrophage colony-stimulating factor (csf) and macrophage csf-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for csf blockade in inflammation. J Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- 5.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biwa T, Hakamata H, Sakai M, Miyazaki A, Suzuki H, Kodama T, Shichiri M, Horiuchi S. Induction of murine macrophage growth by oxidized low density lipoprotein is mediated by granulocyte macrophage colony-stimulating factor. J Biol Chem. 1998;273:28305–28313. doi: 10.1074/jbc.273.43.28305. [DOI] [PubMed] [Google Scholar]
- 7.Ditiatkovski M, Toh BH, Bobik A. Gm-csf deficiency reduces macrophage ppar-gamma expression and aggravates atherosclerosis in apoe-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2337–2344. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- 8.Plenz G, Koenig C, Severs NJ, Robenek H. Smooth muscle cells express granulocyte-macrophage colony-stimulating factor in the undiseased and atherosclerotic human coronary artery. Arterioscler Thromb Vasc Biol. 1997;17:2489–2499. doi: 10.1161/01.atv.17.11.2489. [DOI] [PubMed] [Google Scholar]
- 9.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–1642. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Shindo J, Ishibashi T, Yokoyama K, Nakazato K, Ohwada T, Shiomi M, Maruyama Y. Granulocyte-macrophage colony-stimulating factor prevents the progression of atherosclerosis via changes in the cellular and extracellular composition of atherosclerotic lesions in watanabe heritable hyperlipidemic rabbits. Circulation. 1999;99:2150–2156. doi: 10.1161/01.cir.99.16.2150. [DOI] [PubMed] [Google Scholar]
- 11.Metcalf D. The colony-stimulating factors and cancer. Nat Rev Cancer. 2010;10:425–434. doi: 10.1038/nrc2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-csf and gm-csf as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:554–559. doi: 10.1038/nrrheum.2009.178. [DOI] [PubMed] [Google Scholar]
- 13.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- 15.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 16.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, Runge MS. P47phox is required for atherosclerotic lesion progression in apoe(−/−) mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett MR. Reactive oxygen species and death: Oxidative DNA damage in atherosclerosis. Circ Res. 2001;88:648–650. doi: 10.1161/hh0701.089955. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 22.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subramanian M, Tabas I. Dendritic cells in atherosclerosis. Semin Immunopathol. 2014;36:93–102. doi: 10.1007/s00281-013-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeachy MJ. Gm-csf: The secret weapon in the t(h)17 arsenal. Nat Immunol. 2011;12:521–522. doi: 10.1038/ni.2044. [DOI] [PubMed] [Google Scholar]
- 25.Zhu F, Wang Q, Guo C, Wang X, Cao X, Shi Y, Gao F, Ma C, Zhang L. Il-17 induces apoptosis of vascular endothelial cells: A potential mechanism for human acute coronary syndrome. Clin Immunol. 2011;141:152–160. doi: 10.1016/j.clim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Liao YH, Xia N, Zhou SF, Tang TT, Yan XX, Lv BJ, Nie SF, Wang J, Iwakura Y, Xiao H, Yuan J, Jevallee H, Wei F, Shi GP, Cheng X. Interleukin-17a contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. 2012;59:420–429. doi: 10.1016/j.jacc.2011.10.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Wu Q, Yang P, Cua D, Hsu H-C, Mountz J. Il-23 induces apoptosis of self-reactive thymocytes in thymic negative selection by an rorc dependent mechanism. J Immunol. 2011:186. [Google Scholar]
- 28.Cocco C, Canale S, Frasson C, Di Carlo E, Ognio E, Ribatti D, Prigione I, Basso G, Airoldi I. Interleukin-23 acts as antitumor agent on childhood b-acute lymphoblastic leukemia cells. Blood. 2010;116:3887–3898. doi: 10.1182/blood-2009-10-248245. [DOI] [PubMed] [Google Scholar]
- 29.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Scull C, Ozcan L, Tabas I. Nadph oxidase links endoplasmic reticulum stress, oxidative stress, and pkr activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belladonna ML, Renauld JC, Bianchi R, Vacca C, Fallarino F, Orabona C, Fioretti MC, Grohmann U, Puccetti P. Il-23 and il-12 have overlapping, but distinct, effects on murine dendritic cells. J Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 32.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via abcg1. Proc Natl Acad Sci U S A. 2007;104:15093–15098. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger cd36-tlr2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng X, Taleb S, Wang J, Tang TT, Chen J, Gao XL, Yao R, Xie JJ, Yu X, Xia N, Yan XX, Nie SF, Liao MY, Cheng Y, Mallat Z, Liao YH. Inhibition of il-17a in atherosclerosis. Atherosclerosis. 2011;215:471–474. doi: 10.1016/j.atherosclerosis.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Biasi F, Leonarduzzi G, Vizio B, Zanetti D, Sevanian A, Sottero B, Verde V, Zingaro B, Chiarpotto E, Poli G. Oxysterol mixtures prevent proapoptotic effects of 7-ketocholesterol in macrophages: Implications for proatherogenic gene modulation. FASEB J. 2004;18:693–695. doi: 10.1096/fj.03-0401fje. [DOI] [PubMed] [Google Scholar]
- 36.Pop C, Timmer J, Sperandio S, Salvesen GS. The apoptosome activates caspase-9 by dimerization. Mol Cell. 2006;22:269–275. doi: 10.1016/j.molcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 38.Breitschopf K, Haendeler J, Malchow P, Zeiher AM, Dimmeler S. Posttranslational modification of bcl-2 facilitates its proteasome-dependent degradation: Molecular characterization of the involved signaling pathway. Mol Cell Biol. 2000;20:1886–1896. doi: 10.1128/mcb.20.5.1886-1896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ Res. 2001;88:733–739. doi: 10.1161/hh0701.088684. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappab-mediated inflammation in macrophages. Circ Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 42.Deng X, Gao F, May WS., Jr Bcl2 retards g1/s cell cycle transition by regulating intracellular ros. Blood. 2003;102:3179–3185. doi: 10.1182/blood-2003-04-1027. [DOI] [PubMed] [Google Scholar]
- 43.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Critical role for the nlrp3 inflammasome during acute lung injury. J Immunol. 2014;192:5974–5983. doi: 10.4049/jimmunol.1400368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. Gm-csf regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, Bogunovic M, Gautier EL, Miller J, Leboeuf M, Lu G, Aloman C, Brown BD, Pollard JW, Xiong H, Randolph GJ, Chipuk JE, Frenette PS, Merad M. Gm-csf controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curtiss LK, Boisvert WA. Apolipoprotein e and atherosclerosis. Curr Opin Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Wang M, Subramanian M, Abramowicz S, Murphy AJ, Gonen A, Witztum J, Welch C, Tabas I, Westerterp M, Tall AR. Interleukin-3/granulocyte macrophage colony-stimulating factor receptor promotes stem cell expansion, monocytosis, and atheroma macrophage burden in mice with hematopoietic apoe deficiency. Arterioscler Thromb Vasc Biol. 2014;34:976–984. doi: 10.1161/ATVBAHA.113.303097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy AJ, Akhtari M, Tolani S, Pagler T, Bijl N, Kuo CL, Wang M, Sanson M, Abramowicz S, Welch C, Bochem AE, Kuivenhoven JA, Yvan-Charvet L, Tall AR. Apoe regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121:4138–4149. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving tgf-beta, pge2, and paf. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: The importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 51.Tausend W, Downing C, Tyring S. Systematic review of interleukin-12, interleukin-17, and interleukin-23 pathway inhibitors for the treatment of moderate-to-severe chronic plaque psoriasis: Ustekinumab, briakinumab, tildrakizumab, guselkumab, secukinumab, ixekizumab, and brodalumab. J Cutan Med Surg. 2014;18:1–14. doi: 10.2310/7750.2013.13125. [DOI] [PubMed] [Google Scholar]
- 52.Thorp E, Li Y, Bao L, Yao PM, Kuriakose G, Rong J, Fisher EA, Tabas I. Brief report: Increased apoptosis in advanced atherosclerotic lesions of apoe−/− mice lacking macrophage bcl-2. Arterioscler Thromb Vasc Biol. 2009;29:169–172. doi: 10.1161/ATVBAHA.108.176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA. Glutathione binding to the bcl-2 homology-3 domain groove: A molecular basis for bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in apoe−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 55.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS. Endoplasmic reticulum stress-induced transcription factor, chop, is crucial for dendritic cell il-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of apoe−/− and ldlr−/− mice lacking chop. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q, Franks HA, Lax SJ, El Refaee M, Malecka A, Shah S, Spendlove I, Gough MJ, Seedhouse C, Madhusudan S, Patel PM, Jackson AM. The ataxia telangiectasia mutated kinase pathway regulates il-23 expression by human dendritic cells. J Immunol. 2013;190:3246–3255. doi: 10.4049/jimmunol.1201484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. Atm-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Zhang L, Chen T, Tian B, Deng X, Zhao Z, Yuan P, Dong B, Zhang Y, Mo X. Functional polymorphism rs189037 in the promoter region of atm gene is associated with angiographically characterized coronary stenosis. Atherosclerosis. 2011;219:694–697. doi: 10.1016/j.atherosclerosis.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 60.Su Y, Swift M. Mortality rates among carriers of ataxia-telangiectasia mutant alleles. Ann Intern Med. 2000;133:770–778. doi: 10.7326/0003-4819-133-10-200011210-00009. [DOI] [PubMed] [Google Scholar]
- 61.Qi Y, Feng W, Song A, Song H, Yan S, Sun Q, Yang P. Role of serum il-23/il-17 axis in the relationship between periodontitis and coronary heart disease. Int J Periodontics Restorative Dent. 2013;33:185–191. doi: 10.11607/prd.1327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.