Abstract
Background
Acetylation of Hsp90 regulates downstream hormone signaling via the glucocorticoid receptor (GR), but the role of this molecular mechanism in stress homeostasis remains poorly understood. We tested whether acetylation of Hsp90 in the brain predicts and modulates the behavioral sequelae of a mouse model of social stress.
Methods
Mice subjected to chronic social defeat stress (CSDS) were stratified into resilient and vulnerable subpopulations. HPA axis function was probed using a DEX/CRF test. Hsp90 acetylation, Hsp90-GR interactions and GR translocation were measured in the dorsal raphe nucleus (DRN). To manipulate Hsp90 acetylation, we pharmacologically inhibited Hdac6, a known deacetylase of Hsp90 or overexpressed a point-mutant that mimics the hyperacetylated state of Hsp90 at lysine K294
Results
Lower acetylated Hsp90, higher GR-Hsp90 association and enhanced GR translocation were observed in DRN of vulnerable mice after CSDS. Administration of ACY-738, an Hdac6-selective inhibitor, led to Hsp90 hyperacetylation in brain and in neuronal culture. In cell-based assays, ACY-738 increased the relative association of Hsp90 with FKBP51 versus FKBP52 and inhibited hormone-induced GR translocation. This effect was replicated by overexpressing the acetylation-mimic point-mutant of Hsp90. In vivo, ACY-738 promoted resilience to CSDS and serotonin-selective viral overexpression of the acetylation-mimic mutant of Hsp90 in raphe neurons reproduced the behaviroral effect of ACY-738.
Conclusions
Hyperacetylation of Hsp90 is a predictor and causal molecular determinant of stress resilience in mice. Brain-penetrant Hdac6 inhibitors increase Hsp90 acetylation and modulate GR chaperone dynamics offering a promising strategy to curtail deleterious socioaffective effects of stress and glucocorticoids.
Keywords: Stress, Resilience, Acetylation, Hsp90, HDAC6, ACY-738, Raphe, Serotonin, Social Defeat
Introduction
Stressful life events play a significant role in the precipitation of affective disorders, but only a subset of individuals develop lasting psychological sequelae after severe stress (1–4). The glucocorticoid receptor (GR) and several components of its multiprotein chaperone complex have been implicated as a point of convergence for genetic, epigenetic and environmental factors shaping stress vulnerability (5–9). Converging data from psychiatric genetics and animal studies indicate that mechanisms affecting the stoichiometry and interactions of proteins within the GR chaperone heterocomplex profoundly influence the physiology of the hypothalamic-pituitary-adrenal (HPA) axis (10–12) as well as stress coping (13–18). However, how molecular regulation of GR chaperone dynamics translates into emergent neurobehavioral traits defining vulnerability or resilience is not well understood.
Heat shock protein 90 (Hsp90) is a core component of the GR chaperone complex. Through direct interactions with the GR and co-chaperones (including immunophilins FKBP51 and FKBP52), Hsp90 affects multiple aspects of GR’s response, including hormone binding (19), nuclear mobility (20), DNA binding (21) and clearance (22).
Post-translational modifications of Hsp90 are a potentially key mechanism allowing flexible and circuit-specific downstream regulation of steroid signaling (23; 24). Lysine N-acetylation has emerged in recent years as key biochemical switch regulating protein-protein interactions of Hsp90 with several nuclear receptors (25–28) and cochaperones (29; 30). The lysine deacetylase Hdac6 is the best-characterized regulator of Hsp90 acetylation. We and others have previously shown that genetic or pharmacological inhibition of Hdac6 leading to Hsp90 hyperacetylation reduces the neurobehavioral impact of emotional stressors and exogenous glucocorticoids (31–34). Although Hdac6 is found in tissue homogenates from virtually all GR-expressing brain regions, our previous results indicate that the enzyme shows a striking enrichment in serotonin (5-HT) neurons of the dorsal raphe nucleus (DRN), where we identified post-stress down regulation of Hdac6 mRNA as an adaptive molecular signature correlating with resilience and antidepressant response (31).
This study was designed to extend our analysis of Hdac6-dependent regulation of GR chaperone dynamics in stress homeostasis and resilience. We asked whether Hsp90 acetylation is regulated by chronic social defeat stress (CSDS), a murine model of traumatic stress, and tested whether this molecular mark predicts the behavioral and neuroendocrine impact of CSDS in individual mice. We next used novel pharmacological and viral-mediated approaches to test the causal influence of Hsp90 acetylation in resilience. Finally, we used tissue culture to examine how Hsp90 acetylation influences GR chaperone dynamics and hormone-induced GR nuclear trafficking in serotonin neurons.
Data in this report lend support to the hypothesis that Hdac6-dependent regulation of GR chaperone dynamics is a critical mechanism mediating emotional adaptations during psychosocial stress and is a viable target for pro-resilient pharmacological interventions.
Materials and Methods
A more detailed description of all methods used is provided in supplemental material.
Animals
All mice were 8–12 weeks of age, housed on a 12 hour light cycle with food and water available ad libitum. All studies were conducted according to protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee in accordance with institutional guidelines.
Repeated social defeat and social interaction testing
The chronic social defeat stress (CSDS), social interaction test, and stratification into vulnerable and resilient were completed as previously described (31; 36; 37), using TopScan video-tracking software (CleverSys, Reston, VA).
Corticosterone measurements
Corticosterone (CORT) response was measured using a commercial ELISA kit (AssayPro LLC, St. Charles, MO). Detailed timelines are presented in figure 1A.
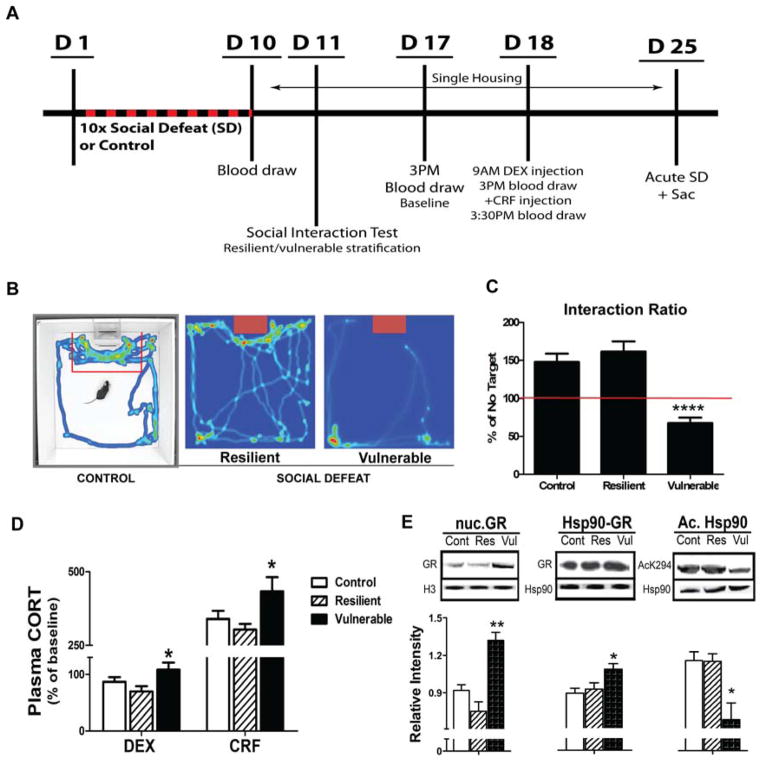
Figure 1. Vulnerability to social defeat (CSDS) is associated with corticosterone nonsupression in the combined DEX/CRF test and altered GR response in the DRN.
(A) Experimental timeline. Mice were subjected to CSDS for 10 days. Control mice were exposed to empty novel cages. On D11, 24 h after last CSDS, mice were tested for social interaction and stratified into resilient and vulnerable subgroups. DEX/CRF test was conducted 7–8 days after last CSDS. On day 25, 14 days after last CSDS, mice were subjected to an additional 10 min physical defeat, followed by 30 min of sensory contact with the aggressor, until sacrifice and dissection. (B) Videotracking heat-maps depicting variations of approach/avoidance behaviors in CSDS-exposed mice. Red denotes areas where mice spent the most time. Note a preference for corners opposite to the social target in vulnerable mice. (C) Average interaction ratios (IR) after stratification of resilient and vulnerable mice using an IR cutoff of 100%. Vulnerable mice displayed significantly lower average IR than controls, while resilient animals did not (**** p< 0.0001, n=19/group). (D) Percent changes in plasma corticosterone (CORT) during a combined DEX/CRF test conducted a week after last CSDS exposure. In contrast to control and resilient mice, which showed relative decreases in plasma CORT (−13% and −34% respectively) 6 hours following administration of a low dose of dexamethasone (DEX; 0.05 mg/kg), vulnerable mice were characterized by nonsupression (* p<0.05 vs. control and resilient groups). Group differences were further amplified after corticotropin-releasing factor (CRF) challenge (* p<0.05, n=14/group). (E) GR protein in nuclear fraction (left), GR-Hsp90 interactions measured by Hsp90 co-IP (center) and Hsp90 acetylation (right) were differentially regulated in the DRN of vulnerable mice compared to resilient and control. (*p< 0.05, **p< 0.01, n=5/group).
Measurements of nuclear GR, Hsp90-GR association and Hsp90 acetylation
Two weeks after behavioral testing for social avoidance, mice were subjected to an additional 10 minutes CSDS exposure. Brains were dissected 30 minutes after the end of the physical interaction, from animals kept in sensory contact with aggressor. Punches were taken of the DRN using a brain matrix. Nuclear fractions were prepared using a commercially available kit (BioVision, Milpitas, CA). Standard Western blotting procedures were used and blots were analyzed on Li-COR Odyssey system and quantified using Image J software. The Hsp90-GR complex was immunoprecipitated from DRN punch homogenates using mouse monoclonal HSP 90α/β F-8 Antibody (sc-13119, Santa Cruz Biotechnology).
In vivo drug treatments
The Hdac6 inhibitor ACY-738 (provided by Acetylon Pharmaceuticals, Boston, MA) was administered at a dose of 5 mg/kg by i.p. injection 10 days prior to the beginning of the CSDS procedure and continued throughout the stress period for a total of 20 days (Fig 2B). Data from our laboratory indicate that the IC50 of ACY-738 for Hdac6 is 1.7nM, and selectivity over Class 1 HDACs is between 60 and 120 fold, depending on the isoform. At the dose used in present study, ACY-738 does not elevate histone acetylation in brain (34).
Figure 2. The brain-penetrant selective Hdac6 inhibitor, ACY-738, increases Hsp90 acetylation and promotes the CSDS resilient behavioral phenotype.
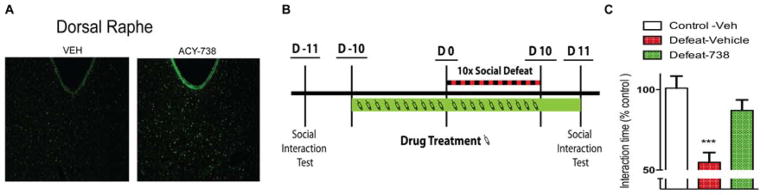
(A) Fluorescent immunohistochemical staining for acetylated Hsp90 at lysine 294 was increased in the DRN of mice treated chronically with ACY-738 (5 mg/kg, IP). (B) Timeline of experiment. Mice received daily IP injections of ACY-738 (5 mg/kg) starting 10 days prior to CSDS exposure and continuing throughout the stress procedure. (C) Mice receiving vehicle had reduced average social interaction after CSDS and ACY-738 treatment prevented the development of social avoidance (***p<0.001, n=11–20/group).
Expression vectors
Human Hsp90 WT and K294A point mutant were obtained from Len Neckers (National Cancer Institute, Rockville MD). GR-GFP plasmid was obtained from Edwin Sanchez (University of Toledo College of Medicine, Toledo, OH). Flag-tagged human FKBP51 and FKBP52 expression vectors were obtained from Theo Rein (Max Planck Institute for Psychiatry, Munich, Germany).
Virus construction and stereotaxic surgery
Flag-tagged Hsp90 construct containing a lysine to alanine mutation at position 294 (K294A) was cloned into a Cre-dependent AAV2 FLEX backbone (38) under control of the human synapsin promoter (AAV.hSynap.Flex.SV40) and packaged into AAV 2.9 viral particles and injected into the DRN of mice expressing Cre-recombinase in a serotonin selective manner driven by the Pet1 promoter. A timeline of these experiments is presented in figure 3A.
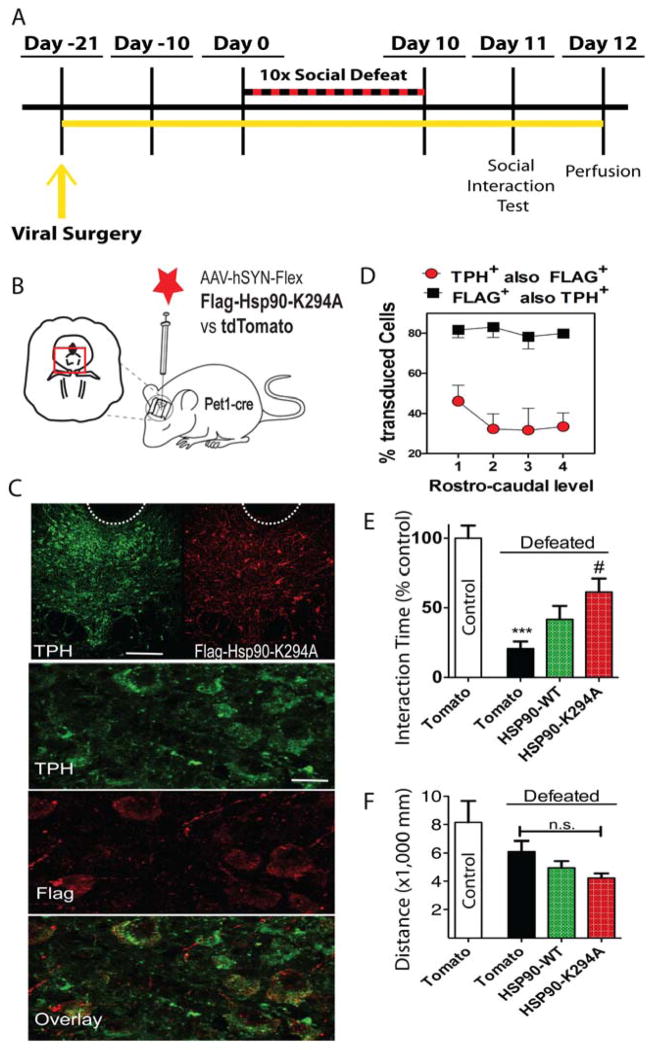
Figure 3. Viral-mediated overexpression of Hsp90 K294A in DRN serotonin neurons induces resilience.
(A) Timeline of experiment. Three weeks prior to the beginning of the CSDS procedure mice were stereotaxically injected into the DRN with AAV vectors expressing Flag-Hsp90 WT or Flag-Hsp90 K294A or tdTomato. (B) Strategy for cell-type specific viral-mediated overexpression of Hsp90 WT, K294A point mutant or tdTomato in serotonin neurons of the DRN. Mice expressing Cre-recombinase under control of the Pet1 promoter were injected stereotaxically in the DRN with an AAV 2.9 FLEX vector expressing Flag-tagged Hsp90 WT, K294A or tdTomato control protein. (C) Verification of transduction efficiency and selectivity by IHC for the serotonergic marker tryptophan hydroxylase (TPH, green) and Flag-tag (red). On the top row, the dotted line on the coronal view of the ventral brainstem depicts the localization of the cerebral aqueduct. Scale bar 100 μm. Lower rows show high magnification views of DRN. Note high degrees of colocalization between TPH and Flag expressing cells. Scale bar 25 μm. (D) Double-labeled cells were counted across 4 coronal levels spanning the entire rostro-caudal extent of the DRN. In targeted animals, approximately 40% of TPH+ cells expressed the Flag-tag and over 80% of Flag+ cells were labeled for TPH, n= 6. (E) Mice expressing tdTomato protein in DRN displayed typical social avoidance behavior after defeat (***p<0.001). Serotonergic overexpression of the acetyl mimic Hsp90 K294A mutant enhanced social approach behavior to a level significantly higher than tdTomato (#p<0.01). Mice overexpressing WT Hsp90 display an intermediate avoidance behavior following social defeat. (F) Total distance traveled during the no target trial was not affected by virus treatment (n=5–13/group).
Immunohistochemistry
Mice were perfused with 4% PFA, and brains were processed using standard single or dual immunolabeling methods as previously reported (31).
Cell-based assays
RN46A-B14 cells, an immortalized rat raphe cell line, were used for all tissue culture assays. For GR translocation cells treated with either vehicle (0.75% DMSO), 2.5μM tubastatin A (39),or 2.5μM ACY-738 (34) for 1 hour, followed by 1μM dexamethsone (Sigma-Aldrich) or vehicle (2% EtOH) for 30 minutes. Cells were fixed in 4% paraformaldehyde (PFA) and immunostained for GFP (Aves, Tigard, OR) then imaged and ratios of GR-GFP signal inside and outside the nucleus were obtained in a high-throughput manner using the ImageXpress Micro system (Molecular Devices, Sunnyvale, CA).
Data analysis
All variables were distributed normally and were analyzed using parametric statistics with t-test or one-way ANOVA followed by Fisher’s PLSD or Tukey post hoc tests where appropriate. Correlations between pairs of variables were examined using linear regressions and proportions were compared using the Fisher exact test. Statistical significance was defined as a P value < 0.05, and all data is presented as the mean ± SEM.
Results
Chronic social defeat stress results in resilient and vulnerable populations
Mice exposed to the CSDS paradigm displayed a significantly lower interaction ratio compared to undefeated controls (t(54)=2.787, p=0.0073) and were divided into two distinct subpopulations using an interaction ratio (IR) cutoff of 100% (Fig. 1B and C). As a group, resilient mice showed average interaction ratios similar to control mice while vulnerable mice showed significantly lower interaction ratios (main effect of group F2,53 = 22.3, p< 0.00001; post hoc vulnerable different from both control and resilient p< 0.00001).
Differential effects of CSDS on HPA regulation in resilient and vulnerable mice
All mice exposed to CSDS had an increase in plasma CORT, with hormone levels returning to baseline at 120 min post CSDS (main effect of time F2,42 = 3.88, p= 0.03, Fig. S1A). On day 10, 90-minutes after social defeat challenge, CORT levels were increased in defeated mice compared to undefeated control (73 ± 21ng/ml) mice, with no difference between resilient (280 ± 36ng/ml) and vulnerable (312 ± 48ng/ml) (F2,24 = 6.225, p= 0.0066). At baseline, 8 days after the last defeat exposure, no significant differences in plasma CORT after CSDS were observed between control mice (75 ± 11ng/ml) and mice stratified as resilient (113 ± 19ng/ml) or vulnerable (91 ± 16ng/ml; F2,35 = 1.561, p= 0.2242). However, differences were observed in response to pharmacological DEX/CRF test (Fig. 1D). Vulnerable mice displayed an attenuated response to DEX compared to resilient animals (main effect of group F2,34 = 4.29, p= 0.02; post hoc p= 0.01). They also exhibited a greater elevation relative to baseline following CRF treatment (main effect of group F2,34 = 3.94, p= 0.03; post hoc p= 0.02).
Differential Hsp90 acetylation and GR chaperone dynamics in DRN of resilient and vulnerable mice
In a preliminary experiment using a small cohort of mice exposed to a single social defeat (SD), we examined the temporal dynamics of GR translocation, Hsp90 association, and Hsp90 acetylation in parallel with plasma CORT. We focused on the dorsal raphe nucleus (DRN) because of previous evidence that this brain region has the highest level of expression of Hdac6 in the mouse brain (31) and also expresses high density of GR (40; 41). Results indicated that GR translocation peaks in the DRN approximately 30 min post CSDS, corresponding with the peak in plasma CORT (Fig. S1B). To evaluate changes in Hsp90 acetylation and Hsp90-GR association we immunoprecipitated Hsp90 and blotted with antibodies against acetylated Hsp90 or GR. We observed a peak of acetylation that was delayed compared to that of plasma CORT, occurring at 2 hours post CSDS (Fig. S1C). There was an overall negative correlation between Hsp90 acetylation and GR-Hsp90 association (R2 = 0.32, p= 0.007). Having determined the temporal dynamics of GR response, we evaluated the same variables in the DRN of control, resilient and vulnerable mice sacrificed 30 min post CSDS (Fig. 1E). Total levels of GR and Hsp90 were unchanged (data not shown) but GR binding to Hsp90 was significantly elevated in vulnerable mice (main group effect, F2,14 = 4.27, p= 0.04; post hoc vul vs. res p<0.05). This coincided with reduced Hsp90 acetylation in vulnerable mice (main group effect F2,10 = 7.51, p= 0.01 post hoc p<0.05 vs res and cont). Further, we observed a significant increase in nuclear GR in vulnerable mice (F2,10 = 8.78, p= 0.01; post hoc p<0.01 vul vs res).
Selective inhibition of Hdac6 increases Hsp90 acetylation in the brain and prevents social avoidance
We have shown previously that chronic administration of the brain-penetrant selective Hdac6 inhibitor ACY-738 promotes a resilient phenotype in mice subjected to CSDS (34). In this study we asked whether this effect is associated with increased acetylation of Hsp90 in the DRN, as detected in spontaneously resilient mice (Fig. 1E). We evaluated acetylation of Hsp90 (acHsp90) in DRN by IHC using a previously validated site-specific antibody against acetylated lysine 294 (K294). We focused on this conserved residue because its acetylation levels are observed to be higher in resilient versus vulnerable mice following social defeat stress as well as in Hdac6 KO mice (31). Furthermore, acetylation at K294 is known to reduce the interactions of Hsp90 with the immunophilin FKBP52 (27), a cochaperone that promotes GR signaling. Mice were administered selective Hdac6 inhibitor ACY-738 (21 days) and perfused 30 min after the last drug injection. 24 hours prior to perfusion, we confirmed by testing the mice behaviorally that the treatment promoted resilience. As expected we observed a significant decrease in social interaction after CSDS in mice treated with vehicle (Fig. 2C) (main effect of treatment, F2,36 = 6.14, p=0.005; post hoc control/veh vs. defeat/veh, p<0.05), while ACY-738 treated mice maintained interaction ratios comparable to undefeated controls (defeat/veh vs. defeat/ACY-738, p<0.01). Increased acHsp90 signal was observed widely across the brain but was most striking in the DRN (Fig. 2A) and ventromedial prefrontal cortex (not shown). We also found that chronic ACY-738 treatment did not alter CORT response following DEX/CRF challenge (not shown).
Viral overexpression of acetyl-mimic Hsp90 mutant promotes resilient phenotype
We have shown previously that serotonin-specific KO of Hdac6 (flHdac6Pet1Cre) increases acetylation of Hsp90 at K294 and enhances the proportion of resilient mice after CSDS (31) similar to ACY-738 in present study. These results suggest acetylation of Hsp90 may be key in mediating the antidepressant/pro-resilient activity of Hdac6 KO and inhibition. Since Hdac6 has multiple known substrates that could be necessary to produce this pro-resilient effect, we asked whether Hsp90 hyperacetylation at K294 is sufficient to recapitulate the resilient phenotype. We tested this using a genetic strategy commonly used to study acetylation, whereby lysine residues are mutated to alanine (K to A) to mimic an acetylated state. We hypothesized that expression of this lysine to alanine mutant (K294A) would act similarly to ACY-738 or Hdac6 KO and decrease stress vulnerability through a reduction of GR signaling. We expressed Flag-tagged Hsp90 K294A mutant in brain in vivo using viral-mediated gene transfer. We used a FLEX AAV (38) to conditionally express Hsp90 WT or K294A in a Cre-recombinase-dependent manner. A vector conditionally expressing the fluorescent tdTomato protein was used as a control. Viral vectors were injected into the DRN of mice that express Cre-recombinase under the serotonin-specific Pet1 promoter (Fig. 3B). Double label IHC confirmed serotonin-specific targeting of the transgene. On average, 40% of serotonin DRN cells were positive for Flag-Hsp90 indicating acceptable transduction efficiency and up to 90% of neurons expressing the transgene were positive for TPH2, indicating high expression selectivity (Fig. 3C & D). Cell counting at different rostro-caudal levels revealed a slight targeting bias towards the rostral portion of the DRN (Fig. 3D).
After 3-4 weeks of recovery, injected mice were exposed to 10 days of CSDS, followed by a social interaction test (Fig. 3E). There was a main effect of CSDS and an interaction with Hsp90 virus (F3,36 = 9.942, p<0.0001). Defeated mice receiving the tdTomato vector developed the social avoidance typically observed after CSDS, as indicated by lower time interacting with the unfamiliar social target compared to undefeated control mice (post hoc p<0.001). In contrast, mice receiving the acetyl-mimic mutant of Hsp90 showed no significant difference from undefeated control mice and significantly higher social interaction than mice receiving the control virus (post hoc Hsp90-K294A vs tdTomato p<0.01), indicating that overexpression of Hsp90-K294A prevents the effect of CSDS. Importantly, in contrast to Hsp90-K294A, mice overexpressing WT Hsp90 displayed a significant decrease in interaction time after CSDS (post hoc p<0.01) indicating that acetylation status of Hsp90 is a determining factor. However, mice injected with WT Hsp90 interacted significantly more than those expressing tdTomato (post hoc p<0.01), so overexpression of Hsp90 per se is not devoid of effect. Total distance traveled was not affected by viral treatment (Fig. 3F).
Effects of Hdac6 inhibition and Hsp90-K294 point-mutation on GR nuclear transport and co-chaperone interactions
To determine the functional consequence of Hsp90 K294 acetylation in serotonin neurons, we tested the effects of Hsp90 mutation and Hdac6 inhibition on hormone-induced GR translocation in a serotonin cell line (42) (Fig. 4A&B). The two selective Hdac6 inhibitors, Tubastatin A and ACY-738, both significantly reduced GR translocation induced by the GR agonist dexamethasone (DEX), a result in line with our previous observations with other Hdac6 inhibitors (31) (main effect of treatment F3,62 = 5.2, p= 0.003; post hoc p<0.05 vs. vehicle, Fig 4C). Next, to establish that chaperone-dependent regulation of GR translocation in our system was in line with mechanisms previously reported in the literature, we evaluated the effects on two well-characterized co-chaperones, FKBP51 and FKBP52, on GR translocation. In agreement with previous studies (13), we found that overexpression of FKBP51 inhibited, while overexpressed FKBP52 potentiated, GR translocation in response to DEX (FKBP52 vs empty vector p<0.05, FKBP51 vs empty vector p<0.01) (Fig. 4D).
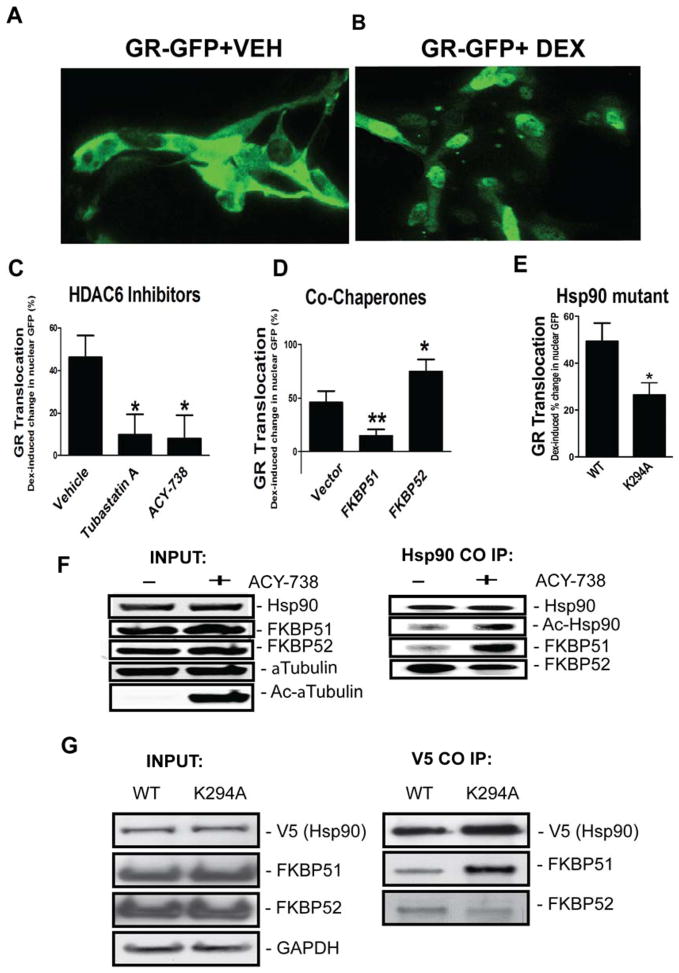
Figure 4. Hsp90 acetylation inhibits GR translocation and increases the relative association of Hsp90 with cochaperone FKBP51 versus FKBP52.
Cells from the RN46A neuronal cell line were transfected transiently with a GR-GFP construct and stimulated with DEX for 30 min. Example depiction of the distribution of GR-GFP signal 30 min after treatment with vehicle (A) (VEH) or (B) dexamethasone (DEX). Note the shift from primarily cytoplasmic to primarily nuclear GFP distribution after DEX. GR translocation was measured using high-content imaging of relative nuclear/cytoplamic fluorescence. Effect of Hdac6 inhibitors, overexpressed FKBP51/52 co-chaperones, and Hsp90 lysine to alanine point mutation at residue 294 were all tested. (C) Pretreatment with Hdac6 inhibitors, Tubastatin A (2.5 uM) or ACY-738 (2.5 uM), inhibited DEX-induced GR translocation (* p<0.05 compared to vehicle). (D) Overexpression of FKBP51 antagonized, while overexpression of FKBP52 potentiated GR translocation in response to DEX (* p<0.05, ** p<0.01 vs empty vector). (E) The acetyl-mimic (K294A) point mutation of Hsp90 reduced GR translocation in response to DEX (* p=0.09, **p<0.01 compared to WT Hsp90 vector), n = 10–23 images/condition. (F) Co-immunoprecipitation experiments testing the effect of ACY-738 on Hsp90 acetylation and effect on interactions of Hsp90 with FKBP51/52 co-chaperones. Compared with vehicle, ACY-738 (2.5 uM) increased the acetylation of α-tubulin and Hsp90. These effects coincided with an increased relative association of Hsp90 with FKBP51 and reduced interaction of Hsp90 with FKBP52. (G) Co-immunoprecipitation experiments testing the effect of acetyl-mimic point mutation of Hsp90 on interactions of V5-tagged Hsp90 with FKBP51/52 co-chaperones. Compared with WT, K294A point mutation of Hsp90 increased the relative association of Hsp90 with FKBP51 and reduced interaction with FKBP52, recapitulating the effect of ACY-738.
We next examined the effect of the acetyl-mimic (K294A) point-mutant of Hsp90. In accordance with earlier studies evaluating the effects of Hsp90 acetylation mutants on signaling by nuclear receptors (26–28; 30; 43), we found that the K294A mutation inhibits GR translocation relative to WT Hsp90 (t(68)=2.362, p=0.0211) (Fig. 4E).
Hsp90 acetylation may regulate GR translocation and stress reactivity by influencing the relative incorporation of the antagonistic immunophilins FKBP51 and FKBP52 in the chaperone complex, which respectively inhibit and facilitate translocation (13; 44; 45). To test this hypothesis we evaluated how pharmacological inhibition of Hdac6 or point mutations of Hsp90 influence interactions of Hsp90 with FKBP51/52 co-chaperones. We expressed Flag-tagged forms of FKBP51/52 proteins in the RN46 serotonin cell line and carried out co-immunoprecipitation experiments from cells treated with ACY-738 or vehicle (Fig. 4F), or co-transfected with V5-tagged forms of WT or mutant Hsp90 (Fig. 4G). As expected, treatment with ACY-738 led to an increase in the acetylation of α-tubulin detected in input samples using anti-acetylated α-tubulin antibody and also led to an increased acetylation of Hsp90 at lysine 294 (Fig. 4F). These effects coincided with an increased association of Hsp90 with FKBP51 and reduced interaction with FKBP52. A similar effect was observed after overexpressing the acetyl-mimic mutant of Hsp90 (Fig. 4G). After pull-down of V5 and blotting for Flag-FKBPs, we observed an increased association of Hsp90 with FKBP51 and reduced interaction with FKBP52 in cells expressing acetyl-mimic K294A, compared to WT Hsp90.
Discussion
Our data support the hypothesis that Hdac6-dependent regulation of GR chaperone dynamics in the DRN is a critical mechanism underlying resilience to psychosocial stress. We show that vulnerable mice have higher nuclear GR after exposure to CSDS coinciding with a reduced acetylation of Hsp90. Furthermore, we demonstrate that both genetic and pharmacological enhancement of Hsp90 acetylation, which reduce GR response in serotonin neurons, protect against the development of social avoidance in the CSDS paradigm.
Role of Hsp90 acetylation in Hdac6 mediated resilience
We found that Hsp90 acetylation is regulated dynamically and region-specifically during psychosocial stress. In the DRN, this molecular mark correlated negatively with post-stress levels of nuclear GR and positively with resilience. In accordance with this observation, viral overexpression of a mutant protein mimicking the hyperacetylated state of Hsp90, seen in Hdac6 KO, was sufficient to replicate the resilience phenotype of Hdac6 KO mice. Interestingly, the overexpression of WT Hsp90 also partially prevented CSDS effect on social behavior, although more subtly that that of acetyl-mimic mutant. This observation might be explained by previous evidence that WT Hsp90 becomes hyperacetylated at K294 when overexpressed in vivo and thereby enhances the overall levels of acetylated Hsp90 (27; 23). Although our experiments have not explored the identity of acetyltransferases acting on Hsp90 in brain, p300 (46) or pCAF (47) are two potential candidates known to promote Hsp90 acetylation in other tissues. Manipulation of these proteins could be a potential avenue for further exploration of the involvement of Hsp90 acetylation in stress resilience.
Our results showing that ACY-738 stimulates the association of Hsp90 with FKBP51 are intriguing given its status as a validated risk gene for PTSD and other stress-related disorders (7; 48). Considering previous data implicating Hdac6 (49; 50) and FKBP51/52 (13) in the regulation of microtubule dynamics and dynein-mediated retrograde transport, it will be important to determine how these mechanisms intersect in the context of neuroendocrine regulation and stress resilience.
A question that remains open for future studies is whether Hsp90 hyperacetylation, in addition to preventing the behavioral sequalae of CSDS, also mediates the ability of HDAC6 blockade to prevent CSDS-induced changes in neuronal morphology (31). This possibility is supported by previous evidence that HDAC6-mediated deacetylation of Hsp90 modulates growth factor-induced actin-remodeling (51). Furthermore, Hsp90 has been implicated in neural differentiation and axonal elongation (52) as well as in the control of neurotransmitter release and glutamatergic receptors cycling at synapses (53). Altogether these results point to a number of potential mechanisms whereby Hsp90 could affect neuroplastic adaptations.
Although our results implicate residue K294 as a key site in Hsp90, over a dozen additional acetylated lysines residues have been identified after treatment with HDAC inhibitors (46; 54). It is thus possible that at least some of the other acetylated residues in Hsp90 might interact to affect the GR-mediated plasticity and stress resilience. Of interest are sites on Hsp90 that regulate its extracellular localization (46) and could be involved in modulating synaptic and neuroplastic effects of glucocorticoids through non-canonical GR signaling at the membrane.
DRN specificity of Hdac6-mediated regulation of GR signaling
The glucocorticoid receptor hypothesis of depression postulates that a key pathophysiological mechanism in major depression is an impaired negative feedback control of the HPA axis, resulting in progressively unrestrained cortisol release and cellular endangerment, reflected in morphologic alterations in a number of brain regions including the hippocampus, frontal cortex and amygdala. Glucocorticoid-mediated toxicity is also thought to contribute to impaired cognitive and affective function commonly reported in stress-related conditions (55). The functional and behavioral significance of GR signaling in serotonin neurons in the context of this hypothesis remains an open question. Glucocorticoid fluctuations have been shown to regulate TPH2 expression in the raphe (56) and dexamethasone, despite reportedly limited central activity, has been shown to reduce TPH2 expression in the DRN, as well as serotonin synthesis (48; 49; 54), and to increase the expression of the serotonin transporter (50). Conversely, the antidepressant fluoxetine reduced GR expression and increased TPH2 in the dorsal raphe (55), and combined treatment with a GR antagonist has been shown to potentiate fluoxetine’s effects (56). The high levels of GR transcript in serotonin neurons, as well as the high density of other steroid receptors, such as estrogen and progesterone receptors, that share common intracellular signaling pathways, suggest that regulation of Hsp90 chaperone complexes in this area is likely to play a major behavioral role, as supported by our present data.
The fact that Hdac6 is particularly enriched in serotonin neurons as well as in a few other regions including the lateral septum, but does not appear to be expressed at particularly high levels in classical HPA regulating regions (31; 32), suggests that the use of Hdac6 inhibitors to modulate GR signaling may offer a way to pharmacologically dissociate components of GR signaling relevant to socioaffective regulation (such as CRF expression in brainstem and septum) from other physiological roles of GR mediated by different circuits, such as the regulation of peripheral glucocorticoids, metabolism, and feeding.
Translational relevance of HPA dysregulation in CSDS-susceptible mice
In the present study we describe a novel, translationally-relevant physiological correlate of social avoidance in our CSDS paradigm (36; 57). Indeed we find that stratification for social avoidance predicts higher plasma CORT levels in a combined DEX/CRF pharmacological challenge. Interestingly, changes described in CSDS vulnerable mice but absent in resilient mice resemble non-suppression consistently reported in depression in a similar DEX/CRH test (58; 59). Previous studies have shown that responses to this test are affected by psychosocial stress in rodents and reflect molecular mechanisms affecting GR chaperoning (60).
The combined DEX/CRF test has been developed to probe the dynamic status of the HPA-axis and overcome the relative lack of specificity of the simple dexamethasone suppression test. In this test, CRH is administered to stimulate ACTH release after DEX pre-treatment and an 80–90% sensitivity for detecting depression has been reported (59; 61). Interestingly, although differences were observed between vulnerable and resilient mice in response to DEX/CRF, our results indicate that there were no differences across these subpopulations regarding plasma corticosterone rise and decay following a social defeat challenge. This indicates that, although DEX/CRF detects stable differences in HPA dynamics and GR signaling that are predictive of the behavioral impact of a stressor, the test is not necessarily predictive of peripheral hormone regulation in response to psychogenic stimuli. Although dexamethasone is thought to suppress plasma cortisol primarily via GR located in the pituitary (62), questions remain regarding the exact functional significance DEX/CRF test, and the possible contribution of brainstem circuits (63). Our observation that the HDAC6 inhibitor ACY-738 restores social approach behaviors without changing peripheral hormone responses in the DEX/CRF test suggests that circuits mediating ACY-738 GR-dependent action are dissociable from those involved in GR-mediated regulation of peripheral glucocorticoids. This view is in line with recent observations indicating that ablation of GR in dopaminergic and serotonergic systems enhances socioaffective responses without major effects on regulation of stress-induced hormone responses (64; 65).
In summary, this report provides further support to the hypothesis that Hdac6-dependent regulation of GR chaperone dynamics in the DRN is a critical mechanism mediating emotional adaptations during psychosocial stress. We confirm Hdac6 as a viable therapeutic target and identify Hsp90 acetylation as a molecular mechanism for the pro-resilience phenotype observed with Hdac6 loss of function. More specifically our results implicate modulation of interactions between Hsp90 and the GR co-chaperone FKBP51 as a mechanism underlying the activity of HDAC6 inhibitors.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health Grants MH087581 from the U.S. National Institute of Mental Health, NIH, and by an award from the International Mental Health Research Organization (IMHRO). The authors thank M. Jarpe and Acetylon Pharmaceuticals Inc. for providing ACY-738 and A. Kozikowski for providing Tubastatin A. Thanks to I. Lucki for his comments during preparation of the manuscript.
Footnotes
Conflict of Interest Disclosure
O. Berton is inventor on pending patent application # 61713014 “Pyrimidine hydroxyamide compounds as protein deacetylase inhibitors and methods of use thereof. ” All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 2.Grinage BD. Diagnosis and management of post-traumatic stress disorder. Am Fam Physician. 2003;68:2401–2408. [PubMed] [Google Scholar]
- 3.Duclot F, Kabbaj M. Individual differences in novelty seeking predict subsequent vulnerability to social defeat through a differential epigenetic regulation of brain-derived neurotrophic factor expression. Journal of Neuroscience. 2013;33:11048–11060. doi: 10.1523/JNEUROSCI.0199-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almli LM, Fani N, Smith AK, Ressler KJ. Genetic approaches to understanding post-traumatic stress disorder. Int J Neuropsychopharm. 2014;17:355–370. doi: 10.1017/S1461145713001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rossum EFC, Binder EB, Majer M, Koper JW, Ising M, Modell S, et al. Polymorphisms of the glucocorticoid receptor gene and major depression. BPS. 2006;59:681–688. doi: 10.1016/j.biopsych.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann P, Brückl T, Nocon A, Pfister H, Binder EB, Uhr M, et al. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zuiden M, Geuze E, Willemen HLDM, Vermetten E, Maas M, Amarouchi K, et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2012;71:309–316. doi: 10.1016/j.biopsych.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Murani E, Reyer H, Ponsuksili S, Fritschka S, Wimmers K. A substitution in the ligand binding domain of the porcine glucocorticoid receptor affects activity of the adrenal gland. PLoS ONE. 2012;7:e45518. doi: 10.1371/journal.pone.0045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Isoda F, Yen K, Kleopoulos SP, Janssen W, Fan X, et al. Hypothalamic Fkbp51 is induced by fasting, and elevated hypothalamic expression promotes obese phenotypes. Am J Physiol Endocrinol Metab. 2012;302:E987–91. doi: 10.1152/ajpendo.00474.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab. 2011;22:481–490. doi: 10.1016/j.tem.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeng S, Hunsberger JG, Pearson B, Yuan P, Wang Y, Wei Y, et al. BAG1 plays a critical role in regulating recovery from both manic-like and depression-like behavioral impairments. Proc Natl Acad Sci USA. 2008;105:8766–8771. doi: 10.1073/pnas.0803736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology. 2013;38:616–627. doi: 10.1038/npp.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djordjevic A, Adzic M, Djordjevic J, Radojcic MB. Stress type dependence of expression and cytoplasmic-nuclear partitioning of glucocorticoid receptor, hsp90 and hsp70 in Wistar rat brain. Neuropsychobiology. 2009;59:213–221. doi: 10.1159/000223733. [DOI] [PubMed] [Google Scholar]
- 17.Shen H-Y, Zhao Y, Chen X-Y, Xiong R-P, Lu J-L, Chen J-F, et al. Differential alteration of heat shock protein 90 in mice modifies glucocorticoid receptor function and susceptibility to trauma. J Neurotrauma. 2010;27:373–381. doi: 10.1089/neu.2009.0926. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson D, Lin S, Sapolsky R. Viral vector-mediated blockade of the endocrine stress-response modulates non-spatial memory. Neurosci Lett. 2008;437:1–4. doi: 10.1016/j.neulet.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratt WB, Morishima Y, Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem. 2008;283:22885–22889. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci USA. 2004;101:2876–2881. doi: 10.1073/pnas.0400116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway-Campbell BL, George CL, Pooley JR, Knight DM, Norman MR, Hager GL, Lightman SL. The HSP90 Molecular Chaperone Cycle Regulates Cyclical Transcriptional Dynamics of the Glucocorticoid Receptor and Its Coregulatory Molecules CBP/p300 During Ultradian Ligand Treatment. Molecular Endocrinology. 2011;25:944–954. doi: 10.1210/me.2010-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echeverría PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien-Pilipuk G, Galigniana MD. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Molecular and Cellular Biology. 2009;29:4788–4797. doi: 10.1128/MCB.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim Biophys Acta. 2012;1823:648–655. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furay AR, Murphy EK, Mattson MP, Guo Z, Herman JP. Region-specific regulation of glucocorticoid receptor/HSP90 expression and interaction in brain. Journal of Neurochemistry. 2006;98:1176–1184. doi: 10.1111/j.1471-4159.2006.03953.x. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs JJ, Murphy PJM, Gaillard S, Zhao X, Wu J-T, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Molecular Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 26.Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson JB, Wang Z. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Molecular Endocrinology. 2009;23:1963–1972. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoyagi S, Archer TK. Modulating molecular chaperone Hsp90 functions through reversible acetylation. Trends Cell Biol. 2005;15:565–567. doi: 10.1016/j.tcb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Kekatpure VD, Dannenberg AJ, Subbaramaiah K. HDAC6 modulates Hsp90 chaperone activity and regulates activation of aryl hydrocarbon receptor signaling. J Biol Chem. 2009;284:7436–7445. doi: 10.1074/jbc.M808999200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Murphy PJM, Morishima Y, Kovacs JJ, Yao T-P, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- 30.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Molecular Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espallergues J, Teegarden SL, Veerakumar A, Boulden J, Challis C, Jochems J, et al. HDAC6 regulates glucocorticoid receptor signaling in serotonin pathways with critical impact on stress resilience. Journal of Neuroscience. 2012;32:4400–4416. doi: 10.1523/JNEUROSCI.5634-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukada M, Hanai A, Nakayama A, Suzuki T, Miyata N, Rodriguiz RM, et al. Loss of Deacetylation Activity of Hdac6 Affects Emotional Behavior in Mice. PLoS ONE. 2012;7:e30924. doi: 10.1371/journal.pone.0030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JB, Wei J, Liu W, Cheng J, Feng J, Yan Z. Histone deacetylase 6 gates the synaptic action of acute stress in prefrontal cortex. The Journal of Physiology. 2012;590:1535–1546. doi: 10.1113/jphysiol.2011.224907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jochems J, Boulden J, Lee BG, Blendy JA, Jarpe M, Mazitschek R, et al. Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability. Neuropsychopharmacology. 2014;39:389–400. doi: 10.1038/npp.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golden SA, Covington HE, Berton O, Russo SJ. Nature Protocols. Vol. 6. Nature Publishing Group; 2011. A standardized protocol for repeated social defeat stress in mice; pp. 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. Journal of Neuroscience. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler KV, Kalin J, Brochier C, Vistoli G, Langley B, Kozikowski AP. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J Am Chem Soc. 2010;132:10842–10846. doi: 10.1021/ja102758v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaouloff F. Serotonin, stress and corticoids. J Psychopharmacol (Oxford) 2000;14:139–151. doi: 10.1177/026988110001400203. [DOI] [PubMed] [Google Scholar]
- 41.Wylie CJ, Hendricks TJ, Zhang B, Wang L, Lu P, Leahy P, et al. Distinct transcriptomes define rostral and caudal serotonin neurons. Journal of Neuroscience. 2010;30:670–684. doi: 10.1523/JNEUROSCI.4656-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White LA, Eaton MJ, Castro MC, Klose KJ, Globus MY, Shaw G, Whittemore SR. Distinct regulatory pathways control neurofilament expression and neurotransmitter synthesis in immortalized serotonergic neurons. J Neurosci. 1994;14:6744–6753. doi: 10.1523/JNEUROSCI.14-11-06744.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, et al. Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res. 2007;13:4882–4890. doi: 10.1158/1078-0432.CCR-06-3093. [DOI] [PubMed] [Google Scholar]
- 44.Davies TH, Ning Y-M, Sánchez ER. A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- 45.Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, et al. Role of Acetylation and Extracellular Location of Heat Shock Protein 90 in Tumor Cell Invasion. Cancer Research. 2008;68:4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SM, Bae JH, Kim MJ, Lee HS, Lee MK, Chung BS, et al. Bcr-Abl-Independent Imatinib-Resistant K562 Cells Show Aberrant Protein Acetylation and Increased Sensitivity to Histone Deacetylase Inhibitors. Journal of Pharmacology and Experimental Therapeutics. 2007;322:1084–1092. doi: 10.1124/jpet.107.124461. [DOI] [PubMed] [Google Scholar]
- 48.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–95. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 51.Gao Y-S, Hubbert CC, Lu J, Lee Y-S, Lee J-Y, Yao T-P. Histone Deacetylase 6 Regulates Growth Factor-Induced Actin Remodeling and Endocytosis. Molecular and Cellular Biology. 2007;27:8637–8647. doi: 10.1128/MCB.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quintá HR, Maschi D, Gomez-Sanchez C, Piwien-Pilipuk G, Galigniana MD. Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth. Journal of Neurochemistry. 2010;115:716–734. doi: 10.1111/j.1471-4159.2010.06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerges NZ. Independent Functions of hsp90 in Neurotransmitter Release and in the Continuous Synaptic Cycling of AMPA Receptors. Journal of Neuroscience. 2004;24:4758–4766. doi: 10.1523/JNEUROSCI.0594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 55.Davidson RJ, Mcewen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malek ZS, Sage D, Pévet P, Raison S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology. 2007;148:5165–5172. doi: 10.1210/en.2007-0526. [DOI] [PubMed] [Google Scholar]
- 57.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 58.Wahlberg K, Ghatan PH, Modell S, Nygren A, Ingvar M, Asberg M, Heilig M. Suppressed neuroendocrine stress response in depressed women on job-stress-related long-term sick leave: a stable marker potentially suggestive of preexisting vulnerability. Biol Psychiatry. 2009;65:742–747. doi: 10.1016/j.biopsych.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heuser I, Yassouridis A, Holsboer F. The combined dexamethasone/CRH test: a refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 60.Touma C, Gassen NC, Herrmann L, Cheung-Flynn J, Büll DR, Ionescu IA, et al. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70:928–936. doi: 10.1016/j.biopsych.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, et al. Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? BPS. 2007;62:47–54. doi: 10.1016/j.biopsych.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 62.Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–167. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 63.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
- 65.Vincent MY, Jacobson L. Glucocorticoid receptor deletion from the dorsal raphé nucleus of mice reduces dysphoria-like behavior and impairs hypothalamic-pituitary-adrenocortical axis feedback inhibition. Eur J Neurosci. 2014;39:1671–1681. doi: 10.1111/ejn.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.