Abstract
Whether the number of chemotherapy cycles required to obtain a first morphological remission affects prognosis of patients with acute myeloid leukemia (AML) remains controversial. To clarify how achievement of early remission might influence outcome of allogeneic hematopoietic cell transplantation (HCT), we studied 220 consecutive adults with AML in first morphological remission who were transplanted following myeloablative or nonmyeloablative conditioning to investigate how the number of standard- or high-dose induction courses required to achieve remission influenced post-HCT outcome. Three-year estimates of overall survival were 65% (56-73%), 56% (43-67%), and 23% (6-46%) for patients requiring 1 course, 2 courses, or >2 courses of induction therapy; corresponding relapse estimates were 24% (17-31%), 43% (31-55), and 58% (30-78%), respectively. After covariate adjustment (MRD status, conditioning, age, cytogenetic disease risk, type of consolidation chemotherapy, pre-HCT karyotype, and pre-HCT peripheral blood count recovery), the hazard ratios for 2 or >2 induction courses vs. 1 induction were 1.16 (0.73-1.85, P=0.53) and 2.63 (1.24-5.57, P=0.011) for overall mortality, and 2.10 (1.27-3.48, P=0.004) and 3.32 (1.42-7.78, P=0.006), respectively, for relapse. These findings indicate that the number of induction courses required to achieve morphological remission in AML adds prognostic information for post-HCT outcome that is independent of other prognostic factors.
INTRODUCTION
For many patients with acute myeloid leukemia (AML) in first remission, allogeneic hematopoietic cell transplantation (HCT) is an effective consolidation therapy. Still, even in the absence of morphologically detectable disease at the time of transplantation, relapse remains a major cause of treatment failure,1,2 although it is widely appreciated that the risk of disease recurrence varies considerably among patients. Hence, there has been interest in understanding pre-transplant factors that could serve as predictors of adverse post-HCT outcome to inform patients accurately about likely treatment outcomes and to develop risk-stratified transplant regimens.
Recent attention has focused on the role of pre-transplant minimal residual disease (MRD) as indicator of increased risk of relapse following allogeneic HCT for patients with AML in morphological remission.3-6 However, other predictive factors have been recognized, including cytogenetic risk, white blood cell count at diagnosis, time of blast clearance, and the number of induction courses required to enter remission.7-13 The prognostic impact of early remission achievement (i.e. after the first cycle of chemotherapy), however, has not been fully clarified. Specifically, in a large study conducted by the United Kingdom Medical Research Council in the non-transplant setting, response after course 1 was strongly predictive of outcome.14 On the other hand, an analysis of 6 trials conducted by the Eastern Cooperative Oncology Group indicated that the outcome after induction therapy was not worse for patients if residual leukemia was present 10 to 14 days after the start of the first course of therapy if a second, similar cycle of treatment was given and patients subsequently achieved a remission.15 Nonetheless, achievement of an early first remission is recommended in a recent Working Party consensus statement of the European LeukemiaNet as one of the factors for AML risk assessment in the decision-making process regarding allogeneic HCT. With this, a better understanding on how early remission achievement might influence outcome of transplantation is imperative. To address this, we investigated to what degree, if any, the number of cycles of induction therapy required to achieve morphological remission was associated with post-transplant outcome after adjustment for other predictive factors including pre-HCT MRD in 220 consecutive patients who underwent allogeneic HCT for AML in first morphological remission at our institution.
PATIENTS AND METHODS
Study cohort
Adult AML patients ≥18 years of age were included in this retrospective study if they received induction therapy with “7+3” or high-dose cytarabine-based regimens, provided they met the criteria for morphological remission (i.e. <5% blasts by light microscopy without extramedullary disease). We included patients with or without complete peripheral blood count recovery and irrespective of the presence of flow cytometric or cytogenetic MRD at the time of HCT, underwent myeloablative or nonmyeloablative allogeneic HCT, and received peripheral blood or bone marrow as stem cell source. Patients were eligible for our analyses regardless of whether the treatment regimen was changed during re-induction therapy. We included all patients meeting these criteria between late April 2006 until April 2012. Analyses of the role of pre-HCT MRD on outcome have been published previously.16-18 We used the 2008 WHO criteria to define AML19 and the refined United Kingdom Medical Research Council (MRC) criteria to assign cytogenetic risk.20 Pretransplantation comorbidities were assessed retrospectively using the HCT-specific comorbidity index (HCT-CI).21,22 Treatment response criteria were used as proposed by the European LeukemiaNet.23 Information on typing at the HLA-A, B, C, DR, and DQ locus was collected. Criteria for diagnosis and grading of acute and chronic GVHD have been reported previously.24,25 Information on post-transplant outcomes was captured via the Long-Term Follow-Up Program through medical records from our outpatient clinic and local clinics that provided primary care for patients. All patients were treated on Institutional Review Board-approved protocols or standard treatment plans and gave consent to their data being used for research in accordance with the Declaration of Helsinki. Follow-up was current as of April 24, 2014.
Multiparameter flow cytometry (MFC) detection of MRD
Ten-color MFC was performed on bone marrow aspirates obtained as routine baseline assessment before HCT as described previously.16-18 The routine sensitivity of this assay was estimated at 0.1%, although a higher level of sensitivity was possible for a subset of leukemias featuring more frankly aberrant immunophenotypes. When identified, the abnormal population was quantified as a percentage of the total CD45+ white cell events. Any level of residual disease was considered MRDpos.16-18
Statistical analysis
Categorical patient characteristics were compared between patients requiring 1, 2, or >2 courses of induction therapy using Fisher's Exact tests, and continuous characteristics were compared with Kruskal Wallis tests. Unadjusted probabilities of overall survival (OS) and relapse-free survival (RFS) were estimated using the Kaplan-Meier method, and probabilities of NRM, relapse, and acute as well as chronic graft-versus-host disease (GVHD) were summarized using cumulative incidence estimates. NRM was defined as death without prior relapse and was considered a competing risk for relapse, while relapse was a competing risk for NRM; death was considered a competing risk for acute and chronic GVHD. All outcomes were treated as time-to-event endpoints. Outcomes between patients requiring 1, 2, or >2 courses of induction therapy were compared using Cox regression. Multivariate models included the following additional factors: presence of MRD by MFC (yes vs. no), type of conditioning regimen (nonmyeloablative vs. myeloablative), age at the time of HCT, HCT-CI, cytogenetic risk group at time of AML diagnosis (unfavorable vs. favorable/intermediate), type of AML at diagnosis (secondary vs. de novo), type of consolidation chemotherapy (none vs. high-dose cytarabine [HIDAC]-containing vs. non-HIDAC containing), karyotype at time of HCT (normalized vs. not normalized for patients presenting with abnormal karyotypes), and peripheral blood counts at the time of HCT (not recovered vs. recovered). Missing cytogenetic risk and karyotype were accounted for as separate categories. No adjustments were made for multiple comparisons, and all two-sided P-values from the regression models were derived from the Wald test. Statistical analyses were performed using STATA (StataCorp LP, College Station, TX).
RESULTS
Patient Characteristics
Our retrospective analyses included 220 patients undergoing first myeloablative (n=151) or nonmyeloablative (n=69) HCT from HLA-matched related or unrelated donors between April 2006 and April 2012 for AML in first morphological remission (i.e. <5% bone marrow blasts). Among these, 136 patients achieved a remission after 1 course of induction therapy, whereas 66 and 16 required 2 or >2 courses, respectively. In 41 of the 66 patients requiring 2 courses of induction chemotherapy, the therapeutic regimen was changed for re-induction, whereas the treatment regimen was changed at least once in all but one patient requiring >2 courses of therapy to achieve morphological remission. The characteristics of the study population, induction and consolidation chemotherapies, donors, and transplants stratified by number of induction courses are summarized in Table 1. While generally relatively well balanced across these patient strata, statistically significant differences were noted with regard to gender distribution (P=0.01), post-remission consolidative chemotherapy (P<0.001), remission duration before HCT (P=0.002), and proportion of patients with fully recovered peripheral blood counts at the time of HCT (P=0.009).
TABLE 1.
Pre-transplantation demographic and clinical characteristics of study cohort, stratified by number of induction courses before CR achievement
| All (n=220) | 1 Induction Course (n=138) | 2 Induction Courses (n=66) | >2 Induction Courses (n=16) | P-value | |
|---|---|---|---|---|---|
| Median Age at HCT (range), years | 51.9 (18.2-75.0) | 51.5 (18.2-75.0) | 51.9 (20.2-73.7) | 61.1 (18.2-67.3) | 0.22 |
| Male Gender | 57.3% | 50.0% | 66.7% | 81.3% | 0.01 |
| Median WBC at Diagnosis, ×103/μL | 5.0 (0.2-280) | 4.2 (0.3-238) | 5.7 (0.2-280) | 34.9 (0.6-145) | 0.31 |
| Cytogenetics | 0.40 | ||||
| Favorable | 4.6% | 6.5% | 1.5% | 0% | |
| Intermediate | 67.3% | 67.4% | 65.2% | 75.0% | |
| Adverse | 25.0% | 22.5% | 31.8% | 18.8% | |
| Missing | 3.2% | 3.6% | 1.5% | 6.3% | |
| Secondary AML | 37.7% | 42.0% | 30.3% | 31.3% | 0.25 |
| Consolidation Therapy | <0.001 | ||||
| No | 16.8% | 10.9% | 16.7% | 68.8% | |
| Yes (HiDAC-containing) | 70.9% | 74.6% | 75.8% | 18.8% | |
| Yes (not HiDAC-containing) | 12.3% | 14.5% | 7.6% | 12.5% | |
| Median CR Duration before HCT (range), days | 116 (16-788) | 121 (22-788) | 120 (26-465) | 59 (16-231) | 0.0014 |
| Recovered Peripheral Blood Counts before HCT* | 84.1% | 87.7% | 83.3% | 56.3% | 0.009 |
| Routine Cytogenetics before HCT | 0.23 | ||||
| Normalized karyotype | 48.2% | 50.7% | 45.5% | 37.5% | |
| Abnormal karyotype | 11.4% | 8.0% | 18.2% | 12.5% | |
| Missing/non-informative data | 40.5% | 41.3% | 36.4% | 50.0% | |
| HCT Comorbidity Index | 0.73 | ||||
| 0 | 15.0% | 13.0% | 19.7% | 12.5% | |
| 1-2 | 31.8% | 30.4% | 34.9% | 31.3% | |
| ≥3 | 52.7% | 55.8% | 45.5% | 56.3% | |
| Missing | 0.5% | 0.7% | 0% | 0% | |
| MRDpos at HCT by MFC | 19.6% | 16.7% | 22.7% | 31.3% | 0.24 |
| Myeloablative Conditioning | 68.6% | 67.4% | 75.8% | 50.0% | 0.12 |
| Unrelated Donor | 61.8% | 63.0% | 60.6% | 56.3% | 0.82 |
| Median Donor Age (range), years | 40.1 (18.1-76.6) | 40.1 (18.1-71.5) | 38.6 (18.6-76.6) | 43.7 (20.2-71.8) | 0.55 |
| HLA-Matching | 0.32 | ||||
| Matched/identical | 81.4% | 82.6% | 80.3% | 75.0% | |
| 1 locus mismatch | 16.8% | 16.7% | 15.2% | 25.0% | |
| ≥2 loci mismatch | 1.8% | 0.7% | 4.6% | -- | |
| Conditioning Regimen | |||||
| L-TBI ± Flu or Clo | 31.4% | 32.6% | 24.2% | 50.0% | |
| Bu/Cy ± L-TBI | 26.4% | 24.6% | 33.3% | 12.5% | |
| Bu/Flu | 14.1% | 15.9% | 12.1% | 6.3% | |
| H-TBI/Cy or H-TBI/Tepa/Flu | 7.3% | 3.6% | 10.6% | 25.0% | |
| Treo/Flu ± L-TBI | 18.6% | 21.7% | 15.2% | 6.3% | |
| Flu/Radiolabeled Ab/L-TBI ± Cy | 2.3% | 1.4% | 4.5% | 0% | |
| Source of Stem Cells | 0.07 | ||||
| PBSC | 81.4% | 84.1% | 72.7% | 93.8 | |
| BM | 18.6% | 15.9% | 27.3% | 6.3 | |
| GVHD Prophylaxis | |||||
| Calcineurin Inhibitor + Methotrexate | 55.5% | 54.3% | 59.1% | 50.0% | |
| Calcineurin Inhibitor + MMF | 32.3% | 32.6% | 27.3% | 50.0% | |
| Cy ± Calcineurin Inhibitor | 9.5% | 9.4% | 12.1% | 0% | |
| Other | 2.7% | 3.6% | 1.5% | 0% |
ANC ≥1,000/μL and platelets ≥100,000/μL Abbreviations: Ab, antibody; BM, bone marrow; Bu, busulfan; Clo, clofarabine; Cy, cyclophosphamide; Flu, fludarabine; H-TBI, high-dose total body irradiation; HCT, hematopoietic cell transplantation; HiDAC, high-dose cytarabine; L-TBI, low-dose total body irradiation; MFC, multiparameter flow cytometry; MMF, mycophenolate mofetil; PBSC, peripheral blood stem cells; Tepa; thiotepa; Treo, treosulfan
Acute and Chronic GVHD
The 120-day cumulative incidences of grade 3 or 4 acute GVHD differed slightly but not statistically significantly between patient strata, with estimates of 9% (5-15%), 17% (9-27%), and 13% (2-35%) for patients requiring 1 course, 2 courses, or >2 courses of induction therapy to achieve remission, respectively (P>0.13). Likewise, the 180-day cumulative incidences of chronic GVHD were not statistically significantly different between these patient cohorts: 49% (41-57%) for those who required 1 course of induction therapy, 53% (40-64%) for those requiring 2 courses, and 50% (25-71%) for those requiring >2 courses, respectively (P>0.07).
Association between Number of Induction Courses and Post-HCT Outcome
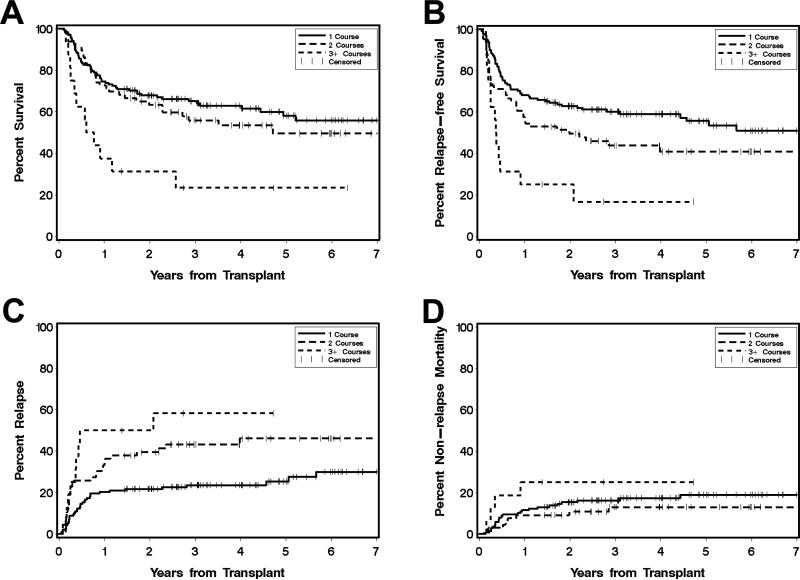
There were 95 deaths, 73 relapses, and 36 NRM events contributing to the probability estimates for OS, RFS, relapse, and NRM. The median follow-up after HCT among survivors was 4.0 [1.4-7.7] years, 4.0 [1.4-7.1] years, and 3.7 [1.4-6.3] years for patients requiring 1, 2, or >2 courses of induction therapy, respectively. The 3-year estimates of OS where 65% (56-73%), 56% (43-67%), and 23% (6-46%) for patients requiring 1 course, 2 courses, or >2 courses of induction therapy to achieve remission, respectively (Figure 1A). For RFS, the corresponding estimates were 60% (51-68%), 44% (31-56%), and 17% (3-39%) (Figure 1B). The 3-year estimate of relapse among patients who required 1 course of induction chemotherapy was 24% (17-31%), whereas for those requiring 2 and >2 courses of induction therapy, this risk was projected to be 43% (31-55) and 58% (30-78%), respectively (Figure 1C). Finally, the 3-year estimates of NRM where 16% (11-23%), 13% (6-23%), and 25% (8-47%) for patients requiring 1, 2, or >2 courses of induction therapy, respectively (Figure 1D).
Figure 1. Association between number of induction courses and post-HCT outcome for AML patients in CR1.
Estimates of overall survival (A), relapse-free survival (B), cumulative incidence of relapse (C), and cumulative incidence of non-relapse mortality (D) following myeloablative allogeneic HCT for AML in complete morphologic remission, shown individually for patients who required 1 course (n=138; black, solid line), 2 courses (n=66; grey, solid line), or >2 courses (n=16; grey, dashed line) of induction therapy to achieve CR1.
Relationship between Number of Induction Courses and Post-HCT Outcome
Univariate regression models for OS, RFS, relapse, and NRM were fit to assess the relevance of the number of induction courses as prognostic factor, and indicated an association between this pre-treatment covariate and post-HCT outcome. Specifically, as summarized in Supplemental Table 1, patients who required 2 induction courses had a shorter RFS (hazard ratio [HR] = 1.51 [95% confidence interval: 1.00-2.28], P=0.05) and increased risk of relapse (HR=1.97 [1.21-3.23], P=0.007) relative to those who required only 1 induction course, whereas OS and NRM were not statistically significantly different (OS: HR=1.20 [0.77-1.88], P=0.42; NRM: HR=0.82 [0.37-1.82], P=0.624). Moreover, requiring >2 induction courses to achieve CR1 was significantly associated with shorter OS (hazard ratio [HR] = 3.18 [95% confidence interval: 1.69-5.96], P<0.001) and RFS (HR=3.36 [1.83-6.17], P<0.001), an increased risk of relapse (HR=3.73 [1.78-7.83], P<0.001), and a trend toward increased NRM (HR=2.83 [0.97-8.25], P=0.06) relative to those requiring only 1 course of induction therapy.
Number of Induction Courses as Independent Prognostic Factor
Next, multivariate models were fitted for OS, RFS, relapse, and NRM to assess the potential role of the number of induction courses to achieve first remission (1 vs. 2 vs. >2) as an independent prognostic factor. We considered the following covariates: MRD status, HCT type, age at HCT, HCT-CI, cytogenetic disease risk at diagnosis, type of AML, type of consolidation chemotherapy before HCT, pre-HCT karyotype, and pre-HCT peripheral blood count recovery as covariates. Final models were built with inclusion of covariates that yielded P-values of <0.1 in univariate analyses (see Supplemental Table 1). After adjustment for these factors, the hazard ratios for 2 or >2 induction courses vs. 1 induction were 1.16 (0.73-1.85, P=0.53) and 2.63 (1.24-5.57, P=0.011) for overall mortality, 1.66 (1.09-2.54, P=0.018) and 2.84 (1.44-5.60, P=0.003) for failure for RFS, 2.10 (1.27-3.48, P=0.004) and 3.32 (1.42-7.78, P=0.006) for relapse, and 0.86 (0.38-1.99, P=0.73) and 1.79 (0.59-5.45, P=0.31) for NRM, respectively (Table 2).
TABLE 2.
Multivariate Cox Regression Models
| Overall Mortality | Failure for RFS | Relapse | NRM | |
|---|---|---|---|---|
| Induction Therapy | ||||
| 1 Induction course (n=138) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| 2 Induction courses (n=66) | 1.16 (0.73-1.85), P=0.532 | 1.66 (1.09-2.54), P=0.018 | 2.10 (1.27-3.48), P=0.004 | 0.86 (0.38-1.99), P=0.731 |
| >2 Induction courses (n=16) | 2.63 (1.24-5.57), P=0.011 | 2.84 (1.44-5.60), P=0.003 | 3.32 (1.42-7.78), P=0.006 | 1.79 (0.59-5.45), P=0.308 |
| MRD Status | ||||
| Negative (n=177) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Positive (n=43) | 2.97 (1.77-4.98), P<0.001 | 4.06 (2.43-6.77), P<0.001 | 4.86 (2.71-8.70), P<0.001 | 2.21 (0.88-5.58), P=0.092 |
| HCT Type | ||||
| Myeloablative (n=151) | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Nonmyeloablative (n=69) | 1.59 (0.97-2.61), P=0.068 | 1.68 (1.12-2.52), P=0.012 | 1.43 (0.87-2.35), P=0.159 | 1.89 (0.86-4.13), P=0.113 |
| Age (per 10 years) | 1.01 (0.82-1.23), P=0.960 | Not included | Not included | 1.26 (0.89-1.77), P=0.188 |
| Cytogenetic Risk Group | ||||
| Intermediate/favorable (n=158) | 1 (Reference) | 1 (Reference) | 1 (Reference) | Not included |
| Adverse (n=55) | 2.19 (1.23-3.91), P=0.008 | 2.06 (1.20-3.54), P=0.009 | 1.49 (0.87-2.56), P=0.150 | |
| Consolidation before HCT | ||||
| No (n=37) | 1 (Reference) | 1 (Reference) | 1 (Reference) | Not included |
| Yes, with HiDAC (n=156) | 1.22 (0.66-2.26), P=0.530 | 1.35 (0.75-2.43), P=0.313 | 1.26 (0.65-2.46), P=0.490 | |
| Yes, without HiDAC (n=27) | 1.06 (0.49-2.30), P=0.879 | 1.26 (0.61-2.59), P=0.526 | 0.94 (0.39-2.29), P=0.889 | |
| Pre-HCT Karyotype | ||||
| Normalized (n=106) | 1 (Reference) | 1 (Reference) | Not included | 1 (Reference) |
| Not normalized (n=25) | 1.35 (0.69-2.64), P=0.387 | 1.07 (0.57-2.02), P=0.830 | 2.01 (0.66-6.11), P=0.218 | |
| Pre-HCT Blood Counts* | ||||
| Recovered(n=185) | 1 (Reference) | 1 (Reference) | Not included | 1 (Reference) |
| Not recovered (n=35) | 1.32 (0.77-2.27), P=0.308 | 1.52 (0.91-2.53), P=0.111 | 1.63 (0.73-3.60), P=0.232 | |
Recovered: ANC ≥1,000/μL and platelets ≥100,000/μL; not recovered: ANC <1,000/μL and/or platelets <100,000/μL
Number of events: deaths=95; relapses=73; deaths without prior relapse=36
Included are covariates that yielded a P-value of <0.1 in univariate analyses.
DISCUSSION
It has become increasingly clear that post-treatment data bear important prognostic information that can significantly refine risk stratification in AML. For example, rapid clearance of peripheral blood blasts, determined either by manual differential blood counts or by flow cytometry, is predictive of remission achievement and survival.26-31 Moreover, the presence of submicroscopic amounts of residual AML, measured at various time points during and after therapy, identifies a subset of patients at particularly high risk of overt disease recurrence and poor outcome.3-6 This is also true for assessments before allogeneic HCT, a situation where MRD is now well recognized as a strong, independent predictor for adverse post-HCT outcome.3-6
Intrinsically, however, post-treatment information entails an assessment of the dynamic response of AML cells to anti-leukemia therapy, which may not be fully captured by single time-point analyses. This notion is illustrated by recent data from the Children's Oncology Group AAML03P1 trial, in which patients who cleared MRD early after initiation of chemotherapy and remained MRDneg at the end of therapy had significantly better outcomes than those who were similarly MRDneg at the end of therapy but had previously documented flow cytometric evidence of residual disease at some point during therapy.32 Thus, as a snap-shot assessment of the sensitivity of the patient's leukemia cells late in the course of treatment, it is plausible that the knowledge gained from this assessment could be refined by including additional data from earlier time points during the course of therapy to provide more dynamic response information.
The present findings support this assumption, demonstrating that the early response to induction therapy, as estimated by the number of induction courses required to achieve initial remission, is associated with post-transplant outcome independently of the pre-HCT MRD status. These results thus extend previous studies by Keating et al.9 showing that the time to remission maintains its prognostic relevance even after accounting for MRD, arguably one of the most important prognostic factors recognized to date for AML patients undergoing allogeneic HCT. Although the present findings may represent only a crude measure of chemosensitivity, data on the number of induction courses do provide additional prognostic information on expected outcomes beyond what can be gleaned from the MRD status, and, therefore, could be useful to educate patients. The data may also provide a more accurate and, perhaps, better guide to risk-stratified decision-making. Indeed, our study indicates that the constellation of early remission achievement and absence of MRD at the time of transplant can identify a subset of patients with excellent long-term outcome. For example, in our cohort, patients undergoing myeloablative HCT had an estimated 3-year OS of 80% (69-87%) and RFS of 79% (67-86%) if they required only one course of induction therapy to achieve remission and were MRDneg during the pre-HCT assessment.
As one potential limitation of our study, the majority of patients were referred to our institution for transplantation after having received induction and consolidation chemotherapy elsewhere. Thus, although patients generally received an anthracycline and cytarabine, many variations were used, and the decision and timing to initiate a second induction therapy cycle was not standardized. Because many patients were referred from elsewhere, information on molecular testing was not universally available and could thus not be included in our analyses; for example, data on NPM1 and FLT3-ITD status was only available for 51 and 55 patients, respectively. We also did not have MRD information available from time points other than the pre-transplant assessment, as systematic MRD measurements are not generally obtained outside of clinical trials, and such data are, thus, not typically available to the transplant physician. In the future, it is likely that sequential MRD assessments will become an integral part of the routine care of AML patients and may offer an optimized approach to the dynamic response monitoring. At least until then, information on the number of induction courses required to achieve initial remission may offer some value in the risk stratification of AML patients presenting for allogeneic transplantation while in remission.
Supplementary Material
HIGHLIGHTS.
We wondered how induction therapy influenced post-transplant outcome in adult AML
We studied 220 consecutive adults with AML in first remission
Need for ≥2 induction courses was associated with worse post-HCT outcome
Impact of induction therapy on post-HCT outcome is independent of other risk factors
Induction therapy data add prognostic information for AML patients undergoing HCT
Acknowledgments
Financial support: Research reported in this publication was supported by grants P01-CA078902, P01-CA018029, and R00-HL088021 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. R.B.W. is a Leukemia & Lymphoma Society Scholar in Clinical Research. C.D.G. and S.A.B. are recipients of a “Hematology Opportunity for the Next-Generation of Research Scientists” (HONORS) Award from the American Society of Hematology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: the authors declare no competing financial interests.
Authorship statement: R.B.W. and F.R.A. contributed to the conception and design of the study. B.M.S., J.M.P., M.L.S., H.J.D., R.S., and F.R.A. contributed to the provision of study material, patient recruitment, and acquisition of data. R.B.W., C.D.G., and S.A.B participated in the collection and assembly of data. R.B.W., B.E.S., and F.R.A. participated in the data analysis and interpretation. R.B.W. and F.R.A. participated in drafting of the manuscript. All authors revised the manuscript critically, and gave final approval to submit for publication.
Conflict of interest: the authors declare no competing financial interests.
REFERENCES
- 1.Hamilton BK, Copelan EA. Concise review: the role of hematopoietic stem cell transplantation in the treatment of acute myeloid leukemia. Stem Cells. 2012;30(8):1581–1586. doi: 10.1002/stem.1140. [DOI] [PubMed] [Google Scholar]
- 2.Cornelissen JJ, Gratwohl A, Schlenk RF, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012;9(10):579–590. doi: 10.1038/nrclinonc.2012.150. [DOI] [PubMed] [Google Scholar]
- 3.Dominietto A. Minimal residual disease markers before and after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Curr Opin Hematol. 2011;18(6):381–387. doi: 10.1097/MOH.0b013e32834bac7d. [DOI] [PubMed] [Google Scholar]
- 4.Buccisano F, Maurillo L, Del Principe MI, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012;119(2):332–341. doi: 10.1182/blood-2011-08-363291. [DOI] [PubMed] [Google Scholar]
- 5.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013;48(5):630–641. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 6.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10(8):460–471. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostrom B, Brunning RD, McGlave P, et al. Bone marrow transplantation for acute nonlymphocytic leukemia in first remission: analysis of prognostic factors. Blood. 1985;65(5):1191–1196. [PubMed] [Google Scholar]
- 8.McGlave PB, Haake RJ, Bostrom BC, et al. Allogeneic bone marrow transplantation for acute nonlymphocytic leukemia in first remission. Blood. 1988;72(5):1512–1517. [PubMed] [Google Scholar]
- 9.Keating S, Suciu S, de Witte T, et al. Prognostic factors of patients with acute myeloid leukemia (AML) allografted in first complete remission: an analysis of the EORTC-GIMEMA AML 8A trial. The European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell' Adulto (GIMEMA) Leukemia Cooperative Groups. Bone Marrow Transplant. 1996;17(6):993–1001. [PubMed] [Google Scholar]
- 10.Kern W, Haferlach T, Schoch C, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101(1):64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa H, Ikegame K, Kawakami M, et al. Impact of cytogenetics on outcome of stem cell transplantation for acute myeloid leukemia in first remission: a large-scale retrospective analysis of data from the Japan Society for Hematopoietic Cell Transplantation. Int J Hematol. 2004;79(5):495–500. doi: 10.1532/ijh97.03166. [DOI] [PubMed] [Google Scholar]
- 12.Kim DH, Sohn SK, Kim JG, et al. Parameters for predicting allogeneic PBSCT outcome of acute myeloid leukemia: cytogenetics at presentation versus disease status at transplantation. Ann Hematol. 2005;84(1):25–32. doi: 10.1007/s00277-004-0942-z. [DOI] [PubMed] [Google Scholar]
- 13.Walter RB, Pagel JM, Gooley TA, et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia. 2010;24(7):1276–1282. doi: 10.1038/leu.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107(1):69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 15.Rowe JM, Kim HT, Cassileth PA, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116(21):5012–5021. doi: 10.1002/cncr.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29(9):1190–1197. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122(10):1813–1821. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2014 doi: 10.1038/leu.2014.173. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 20.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 25.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Gianfaldoni G, Mannelli F, Baccini M, Antonioli E, Leoni F, Bosi A. Clearance of leukaemic blasts from peripheral blood during standard induction treatment predicts the bone marrow response in acute myeloid leukaemia: a pilot study. Br J Haematol. 2006;134(1):54–57. doi: 10.1111/j.1365-2141.2006.06100.x. [DOI] [PubMed] [Google Scholar]
- 27.Elliott MA, Litzow MR, Letendre LL, et al. Early peripheral blood blast clearance during induction chemotherapy for acute myeloid leukemia predicts superior relapse-free survival. Blood. 2007;110(13):4172–4174. doi: 10.1182/blood-2007-07-104091. [DOI] [PubMed] [Google Scholar]
- 28.Gianfaldoni G, Mannelli F, Bencini S, Leoni F, Baldini S, Bosi A. Peripheral blood blast clearance during induction therapy in acute myeloid leukemia. Blood. 2008;111(3):1746–1747. doi: 10.1182/blood-2007-10-121103. [DOI] [PubMed] [Google Scholar]
- 29.Lacombe F, Arnoulet C, Maynadie M, et al. Early clearance of peripheral blasts measured by flow cytometry during the first week of AML induction therapy as a new independent prognostic factor: a GOELAMS study. Leukemia. 2009;23(2):350–357. doi: 10.1038/leu.2008.296. [DOI] [PubMed] [Google Scholar]
- 30.Arellano M, Pakkala S, Langston A, et al. Early clearance of peripheral blood blasts predicts response to induction chemotherapy in acute myeloid leukemia. Cancer. 2012;118(21):5278–5282. doi: 10.1002/cncr.27494. [DOI] [PubMed] [Google Scholar]
- 31.Vainstein V, Buckley SA, Shukron O, et al. Rapid rate of peripheral blood blast clearance accurately predicts complete remission in acute myeloid leukemia. Leukemia. 2014;28(3):713–716. doi: 10.1038/leu.2013.341. [DOI] [PubMed] [Google Scholar]
- 32.Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood. 2012;120(8):1581–1588. doi: 10.1182/blood-2012-02-408336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.