Summary
Dietary restriction (DR) without malnutrition encompasses numerous regimens with overlapping benefits including longevity and stress resistance, but unifying nutritional and molecular mechanisms remain elusive. In a mouse model of DR-mediated stress resistance, we found that sulfur amino acid (SAA) restriction increased expression of the transsulfuration pathway (TSP) enzyme cystathionine γ-lyase (CGL), resulting in increased hydrogen sulfide (H2S) production and protection from hepatic ischemia reperfusion injury. SAA supplementation, mTORC1 activation, or chemical/genetic CGL inhibition reduced H2S production and blocked DR-mediated stress resistance. In vitro, the mitochondrial protein SQR was required for H2S-mediated protection during nutrient/oxygen deprivation. Finally, TSP-dependent H2S production was observed in yeast, worm, fruit fly and rodent models of DR-mediated longevity. Together, these data are consistent with evolutionary conservation of TSP-mediated H2S as a novel mediator of DR benefits with broad implications for clinical translation.
Introduction
Dietary restriction (DR) encompasses a variety of nutritional interventions with overlapping functional benefits including increased stress resistance and extended longevity in a number of organisms across evolutionary boundaries (Fontana et al., 2010). In mammals, dietary regimens associated with these benefits are diverse, including reduced daily food intake, intermittent fasting and reduced protein or essential amino acid intake. While common features exist amongst DR regimens, including reduced adiposity and improved sensitivity to growth factors, differences exist as well. Thus, whether overlapping functional benefits of DR regimens share a common underlying nutritional and/or molecular basis remains unclear. Despite strong evidence of DR benefits in humans (Levine et al., 2014), difficulties with compliance prevent widespread clinical applications. Uncovering common mechanisms is thus of great significance for targeted dietary and/or pharmacological interventions.
Mitohormesis represents a potential unifying molecular hypothesis of DR action (Sinclair, 2005). Based on the concept of hormesis (Calabrese and Mattson, 2011), in which low level stressors promote adaptive changes resulting in stress resistance, the mitohormesis hypothesis of DR posits that increased reactive oxygen and nitrogen species (RONS) derived from increased mitochondrial fatty acid oxidation induce mild oxidative stress, thus driving adaptive mechanisms of antioxidant protection (Tapia, 2006). SKN1/NRF2 has emerged as a candidate mediator of adaptive protection based on its activation by RONS and function as a key transcription factor in the cytoprotective Phase II antioxidant and detoxification response (Hine and Mitchell, 2012). Gene targets of NRF2 include heme oxygenase-1 (HO-1), NAD(P)H dehydrogenase quinone 1 (NQO-1), glutathione transferases (GSTs) and additional genes that utilize glutathione (GSH) for resolving oxidative stress. SKN1/NRF2 in worms is required for stress resistance and longevity benefits of DR (Bishop and Guarente, 2007). In mammals, NRF2 is required for DR-mediated protection from chemically induced carcinogenesis but not for DR-mediated longevity (Pearson et al., 2008). Functionally, mitohormesis can be blocked by Vitamin C, Vitamin E, and/or N-acetyl cysteine (NAC), presumably due to their antioxidant capacity (Ristow and Schmeisser, 2011).
Restriction of sulfur amino acids (SAA) methionine (Met) and cysteine (Cys) is common to numerous DR regimens across evolutionary boundaries and is thus a potential shared nutritional trigger of DR benefits. In yeast, Met restriction extends longevity (Ruckenstuhl et al., 2014). In flies, restriction of essential amino acids (EAA), and in particular Met, controls DR longevity benefits (Grandison et al., 2009). Dietary Met further interacts with overall protein levels in longevity control (Lee et al., 2014). In rodents, diets lacking the non-essential amino acid (NEAA) Cys and restricted for Met (Met restriction, or MetR) extend longevity and increase hepatic stress resistance (Miller et al., 2005; Orentreich et al., 1993).
The TSP controls the conversion of Met into Cys and is required for DR-mediated lifespan extension in flies (Kabil et al., 2011a). Two key enzymes of this evolutionarily conserved pathway are cystathionine β-synthase (CBS) and cystathionine γ-lyase (CGL). Under normal physiological conditions, CBS converts serine and homocysteine, a product of Met methyl transfer, into cystathionine. Cystathionine is then converted into α-ketobutyrate and Cys by CGL (Kabil et al., 2011b). Although Cys is required for de novo synthesis of GSH, thus potentially linking TSP to NRF2-mediated antioxidant responses, the molecular mechanism underlying the genetic requirement for a functional TSP in DR-mediated benefits is unknown.
A product of the TSP with potential to mediate physiological benefits including stress resistance and extended longevity is the water and fat-soluble gas H2S (Cuevasanta et al., 2012; Zhang et al., 2013). While toxic at high levels, H2S produced at low concentrations by degradation of Cys or homocysteine by CGL or CBS acts on the vasculature and the brain as a signaling molecule to reduce blood pressure (Yang et al., 2008) and prevent neurodegeneration (Paul and Snyder, 2012). Exogenous H2S can also extend lifespan of worms (Miller and Roth, 2007) and induce suspended animation in mammals (Blackstone et al., 2005). Although diet can impact H2S production (Predmore et al., 2010), neither the dietary requirements for increased endogenous H2S production, nor the potential role of H2S in the benefits of DR, are currently known.
Ischemia reperfusion injury (IRI) is initiated by lack of nutrients and oxygen due to occlusion of blood flow (ischemia) followed by activation of pro-oxidation pathways and inflammatory mediators in damaged tissues upon return of blood flow (reperfusion). IRI represents a major clinical concern in controlled (tissue resection, organ transplantation) and uncontrolled settings (stroke, heart attack). Various short-term (3–14 days) DR regimens improve outcome in models of kidney, liver and brain IRI (Harputlugil et al., 2014; Mitchell et al., 2010; Peng et al., 2012; Varendi et al., 2014). Here we used dietary preconditioning against hepatic IRI as a model system to probe dietary and molecular mechanisms underlying protection.
Results
NAC, but not NRF2 deficiency, abrogates benefits of DR against IRI
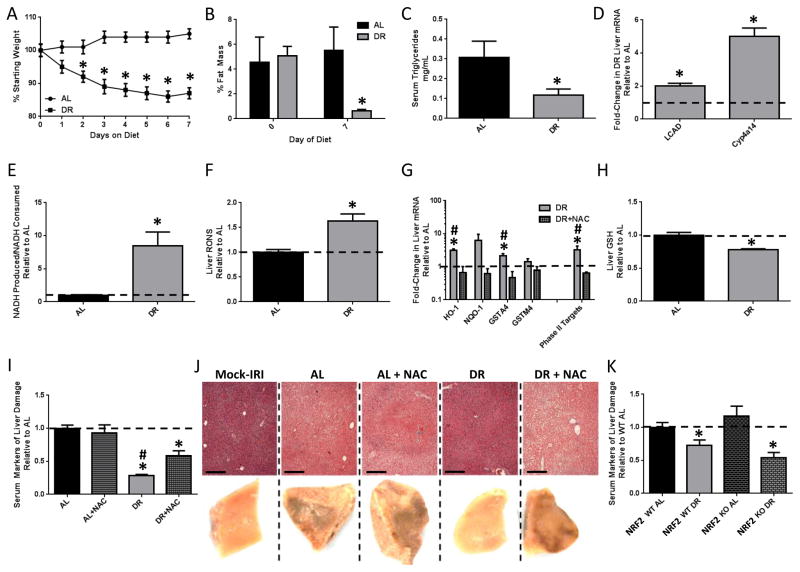
50% DR for 7 days significantly decreased body weight, % fat mass, serum triglycerides (TG) (Figure 1A–C) and blood glucose (BG) (Supplemental Figure 1A) while increasing hepatic expression of FAO-related genes (Figure 1D) and the rate of peroxisomal FAO (Figure 1E) relative to the ad libitum (AL) fed group. Consistent with the mitohormesis hypothesis, hepatic RONS and NRF2 target gene expression were increased (Figure 1F,G), and the latter blocked by NAC administration during the DR period. Total GSH was also decreased upon DR (Figure 1H).
Figure 1. NAC abrogates benefits of DR against acute stress independent of NRF2.
(A–H) Effects of 1wk of 50% DR vs. AL feeding +/− NAC as indicated on body weight (n=15–17/group, A); %fat mass (n=5/group, B); serum triglycerides (n=4–7/group, C); hepatic FAO-associated gene expression (n=3/group, D); peroxisomal FAO capacity in liver extracts (n=3/group, E); hepatic RONS (n=6–8/group, F); hepatic NRF2 target gene expression (n=4/group, G); and hepatic GSH levels (n=7/group, H). (I, J) Combined serum markers of liver damage normalized to the average of the AL control group (I) and liver pathology from injured left liver lobes on the microscopic level stained with hematoxylin and eosin (250μm scale bar, above) and the macroscopic level (1x magnification, below) showing fixed tissue 24hrs after reperfusion (J) in mice (n=5–6/group) preconditioned as indicated before hepatic IRI or mock injury. Asterisk indicates the significance of the difference between AL and DR, and pound sign between DR and DR+NAC; */#p<0.05. (K) Serum markers of liver damage in WT (n=5–6/group) or NRF2KO (n=7–12/group) preconditioned as indicated. Asterisk indicates the significance of the difference between AL and DR within genotype; *p<0.05. See also Suppl. Fig. 1.
The functional relevance of increased RONS and NRF2 activation in DR-mediated stress resistance was tested in a model of hepatic IRI. Wildtype (WT) mice were preconditioned on AL or DR regimens +/− NAC for 1wk prior to IRI. NAC treatment was halted 24hrs prior to IRI to avoid any direct antioxidant effects of this short-lived compound on outcome. Neither DR nor NAC had any significant effect on liver damage markers in serum prior to IRI (data not shown). After reperfusion, liver damage markers remained significantly lower in DR serum indicative of protection from injury (Figure 1I). NAC had no effect on outcome in the AL group, but significantly reduced protection in the DR group. Macroscopic and histological analysis of hemorrhagic necrosis in livers excised 24hrs after reperfusion (Figure 1J, Supplemental Figure 1B) were consistent with serum damage markers (Figure 1I).
To test the requirement for the NRF2-dependent Phase II antioxidant response, we compared NRF2 knockout (KO) mice to wildtype (WT) littermate controls. NRF2KO mice subject to DR had decreased hepatic GSH similar to WT (Supplemental Figure 1C), but failed to upregulate Phase II antioxidant response genes as expected (Supplemental Figure 1D). Surprisingly, benefits of DR against hepatic IRI did not require NRF2, with similar reductions in liver damage markers in serum (Figure 1K) and macroscopic evidence of hemorrhage 24hrs post reperfusion upon DR in both WT and NRF2KO mice (Supplemental Figure 1E). To confirm and extend this result, we tested the requirement for NRF2 in DR-mediated protection from renal IRI. While AL-fed NRF2KOs had slightly elevated damage and decreased renal function upon IRI relative to WT mice as reported previously (Liu et al., 2009), both gained similar benefits upon DR (Supplemental Figure 1F).
Sulfur amino acids control the benefits of DR and PR
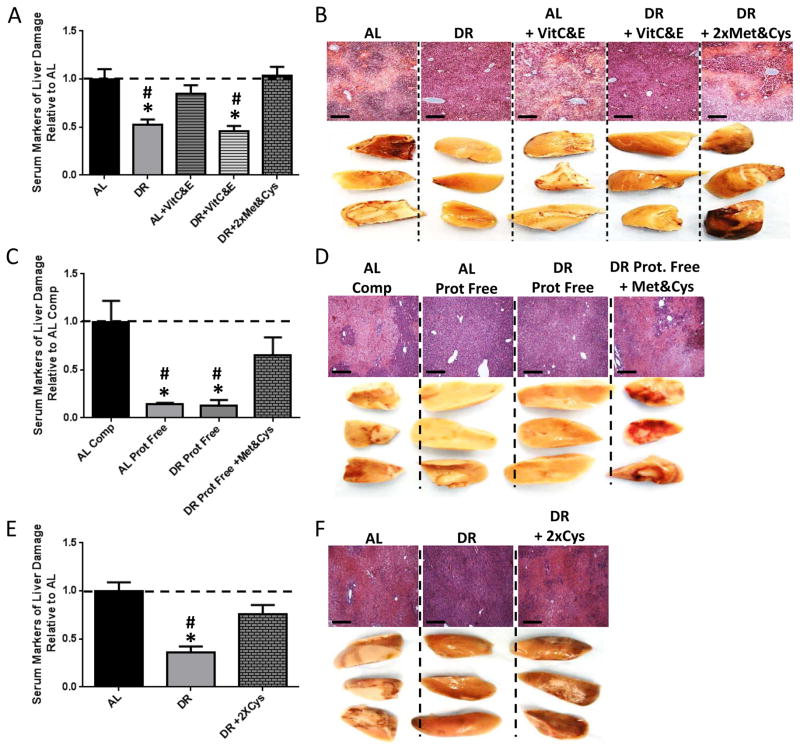
NRF2 independence of DR benefits despite abrogation by NAC is consistent either with an alternate RONS-dependent mechanism of DR-mediated protection, or an antioxidant-independent activity of NAC. NAC is also a source of Cys, and NAC supplementation to the DR group above serendipitously returned dietary Cys content close to AL levels. To separate the functional consequences of antioxidant capacity from SAA content, we supplemented restricted diets separately with either antioxidants (Vitamin C and E, VitC&E) or a 2x concentration of Met and Cys (2xMet&Cys) for 1wk prior to hepatic IRI. Following reperfusion, protection afforded by DR was maintained despite VitC&E antioxidant treatment, but abrogated by 2xMet&Cys (Figure 2A, B) without affecting food intake, weight loss or expression of FOXO target genes (Supplemental Figure 2A, B, C), consistent with the relative importance of the SAA aspect of NAC in abrogation of DR benefits.
Figure 2. Sulfur amino acids control the benefits of DR and PR.
(A, B) Serum markers of liver damage (A) and liver pathology after reperfusion (B) in mice (n=5/group) preconditioned on complete diets fed AL or 50% DR +/− supplementation with vitamins C&E or Met&Cys as indicated. Asterisk indicates the significance of the difference vs. AL and pound sign vs. DR+2xMet&Cys; */#p<0.05. (C, D) Serum markers of liver damage (C) and liver pathology after reperfusion (D) in mice (n=5/group) preconditioned on complete (Comp) or protein free (Prot. Free) diets fed AL or 35% DR with Met&Cys addition as indicated prior to hepatic IRI. Asterisk indicates the significance of the difference vs. AL complete and pound sign vs. DR Prot. Free+Met&Cys; */# p<0.05. (E, F) Serum markers of liver damage (E) and liver pathology after reperfusion (F) in mice (n=5/group) preconditioned on complete diets fed AL or 50% DR with 2xCys added as indicated. Asterisk indicates the significance of the difference vs. AL and pound sign vs. DR+2xCys; */#p<0.05. See also Suppl. Fig. 2.
Protein and carbohydrates are isocaloric and partially interconvertible as dietary energy sources, rendering distinctions between calorie restriction and protein/amino acid restriction upon DR challenging. To test the relative contribution of energy restriction, protein restriction (PR), and specifically SAA restriction to DR-mediated protection against hepatic IRI, we preconditioned mice on a complete or isocaloric protein-free diet fed either AL or 35% restricted +/− 2xMet&Cys. Following hepatic IRI, PR mice displayed significant protection from liver damage (Figure 2C, D) independent of total calorie intake or weight loss (Supplemental Figure 2D, E). Importantly, 2xMet&Cys abrogated significant benefits of combined protein/energy restriction (Figure 2C, D) without affecting weight loss (Supplemental Figure 2E). Because MetR regimens offering overlapping functional benefits with DR are also restricted or deficient in Cys, we also tested Cys reconstitution alone. Following reperfusion, protection observed in the DR group was abrogated upon 2xCys supplementation (Figure 2E, F), consistent with PR and Cys restriction as critical aspects of DR-mediated resistance to hepatic IRI.
DR stimulates endogenous H2S production via repression of mTORC1 and activation of the TSP
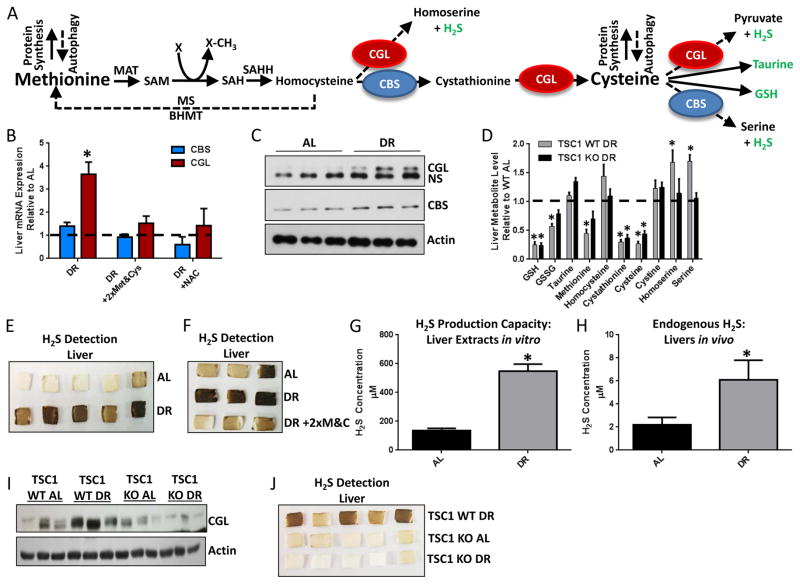
Expression and activity of TSP genes CBS and CGL increase when Cys is low (Stipanuk and Ueki, 2011) to allow de novo synthesis from Met (Figure 3A). Hepatic CGL and CBS to a lesser degree were increased on the mRNA and protein levels upon 1wk DR (Figure 3B, C), and this was abrogated by 2xMet&Cys or NAC supplementation (Figure 3B).
Figure 3. DR stimulates endogenous H2S production via the TSP.
(A) Model of the transmethylation and TSP. Arrows trace sulfur from Met to Cys and downstream cellular processes via Cystathionine Beta-Synthase (CBS) and Cystathionine Gamma-Lyase (CGL). Metabolites in green (taurine, GSH and H2S) have demonstrated potential to protect against IRI. MAT: methionine adenosyl transferase, SAM: S-Adenosylmethionine, SAH: S-Adenosylhomocysteine, SAHH: S-adenosylhomocysteine hydrolase, MS: Methionine synthase, BHMT: Betaine homocysteine methyltransferase. (B) Hepatic CBS and CGL gene expression upon DR +/− 2xMet&Cys or NAC as indicated; n=3/group. (C) Immunoblots of CGL, CBS and actin in liver extracts from AL or DR mice as indicated; NS, non-specific protein band. (D) Liver metabolites in WT or LTsc1KO mice fed 35% DR on a protein free diet relative to the AL fed complete diet group; n=5/group. (E, F) H2S production capacity from liver extracts of AL or DR mice (E) +/− 2xMet&Cys (F) as detected by the black precipitate, lead sulfide. (G) H2S production capacity as in (E), but using a micro-sulfide probe to detect H2S. (H) Endogenous hepatic H2S in mice on the indicated diet using a micro-sulfide probe inserted into liver lobes; n=3 mice/group, 2 lobes/mouse. Asterisk indicates the significance of the difference vs. AL; *p<0.05. (I, J) Immunoblots of liver CGL (I) and H2S production (J) in WT and LTsc1KO mice fed AL or 35% DR on a protein free diet. See also Suppl. Fig. 3.
A metabolomics approach was used to identify candidate TSP metabolites involved in DR-mediated protection. To facilitate identification of potentially causative vs. correlative changes, we included a genetic model that fails to gain the benefits of DR against hepatic IRI due to liver-specific genetic ablation of the mTORC1 repressor, tuberous sclerosis complex 1 (TSC1) (Harputlugil et al., 2014) (Figure 3D, Supplemental Figure 3A). GSH and taurine are two downstream metabolites with potential to protect against IRI, however neither was increased by DR specifically in WT mice (Figure 3D). A third protective TSP metabolite is hydrogen sulfide (H2S) (Kang et al., 2009). Although not detected in our metabolomic screen, associated by-products homoserine and serine (Kabil et al., 2011b) were significantly increased upon DR in WT but not TSC1KO livers (Figure 3D).
For direct measurement of H2S, we leveraged the specific reaction between H2S and lead acetate to form a black precipitate (lead sulfide) that can be trapped and visualized on filter paper containing lead acetate (Supplemental Figure 3B). In the presence of excess CGL/CBS substrate Cys and co-factor pyridoxal-5′-phosphate (PLP), H2S gas produced by liver extracts was greater from DR than AL fed mice (Figure 3E). This phenomenon was independent of NRF2 status (Supplemental Figure 3C). Met&Cys add-back during DR blocked the DR-mediated increase in H2S production capacity (Figure 3F), correlating with a loss of protection against hepatic IRI (Figure 2A) and normalization of hepatic GSH levels (Supplemental Figure 3D).
Using a less specific but more easily quantifiable sulfide probe-based method calibrated against known H2S concentrations (Supplemental Figure 3E, F), liver extracts of DR mice displayed a 4-fold increase in H2S production capacity compared to AL mice (Figure 3G). Endogenous hepatic H2S levels measured by insertion of the sulfide probe into the right and median lobes of anesthetized mice were in the low micromolar range relative to the appropriate standard curve (Supplemental Figure 3G, H), and nearly 3-fold higher in the livers of DR relative to AL fed mice (Figure 3H).
Consistent with a link between DR, TSP, H2S and protection from IRI, DR failed to increase hepatic CGL protein and H2S production capacity in livers of TSC1KO mice (Figure 3I, J), suggesting that constitutive mTORC1 activation blocks DR-mediated TSP upregulation.
H2S is necessary and sufficient to confer DR-mediated stress resistance in vivo
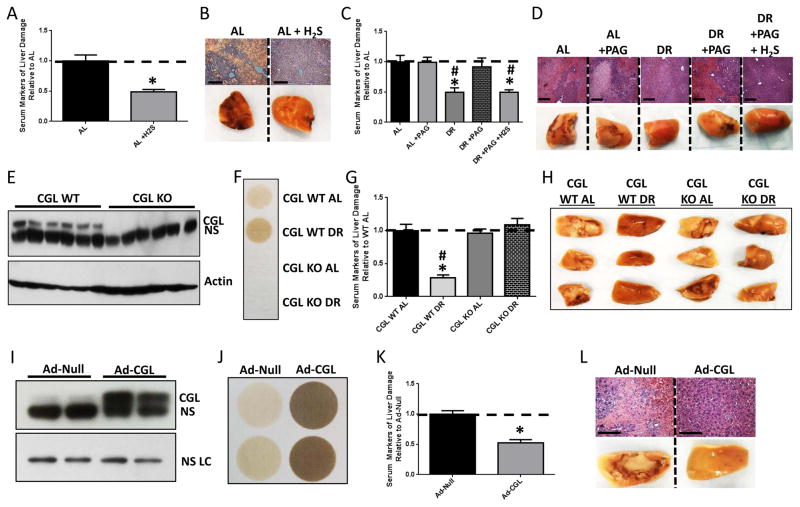
We next determined if exogenous H2S is sufficient to confer protection in our model of hepatic IRI. AL-fed mice injected with the H2S precursor NaHS or vehicle 30min prior to ischemia had decreased liver damage relative to vehicle-treated controls (Figure 4A, B). Similarly, non-invasive H2S delivery in the drinking water for 1wk prior to hepatic IRI using the slow releasing/long-lived donor GYY4137 (Lee et al., 2011) (but not the fast releasing/short-lived donor NaHS) decreased liver damage without affecting animal weight, food intake or water consumption (Supplemental Figure 4A, B, C, D). Thus, exogenous H2S is sufficient to protect against hepatic IRI.
Figure 4. H2S is necessary and sufficient to confer DR benefits against hepatic IRI.
(A, B) Serum markers of liver damage (A) and liver pathology after reperfusion (B) in mice (n=4–5/group) treated with vehicle or H2S 30 min prior to surgery. Asterisk indicates the significance of the difference vs. AL; *p<0.05. (C, D) Serum markers of liver damage (C) and liver pathology after reperfusion (D) in mice (n=3–4/group) treated with PAG during AL or DR preconditioning with a single H2S injection prior to surgery as indicated. Asterisk indicates the significance of the difference vs. AL and pound sign vs. DR+PAG; */#p<0.05. (E) Immunoblot of CGL in liver extracts of WT or CGLKO mice as indicated. NS, non-specific protein. (F) H2S production capacity of liver extracts from WT or CGLKO mice preconditioned as indicated. (G, H) Serum markers of liver injury (G) and liver pathology after reperfusion (H) in WT or CGLKO mice (n=5–8/group) preconditioned as indicated. Asterisk indicates the significance of the difference vs. WT AL and pound sign vs. CGLKO DR; */#p<0.05. (I) Immunoblot of CGL in liver extracts from WT mice infected with control (Ad-Null) or CGL-overexpressing (Ad-CGL) adenovirus as indicated; NS LC, non-specific loading control protein. (J) H2S production capacity of liver extracts of Ad-Null or Ad-CGL infected mice. (K, L) Serum markers of liver damage (K) and liver pathology after reperfusion (L) in Ad-Null or Ad-CGL infected mice (n=6/group). Asterisk indicates the significance of the difference vs. Ad-Null; *p<0.05. See also Suppl. Fig. 4.
A specific inhibitor of CGL, DL-propargylglycine (PAG), was employed to determine if endogenous H2S is necessary for DR-mediated stress resistance. PAG treatment blocked hepatic H2S production capacity (Supplemental Figure 4E) and decreased endogenous hepatic H2S levels upon DR (Supplemental Figure 4F), consistent with the dominant role of CGL in hepatic H2S production. PAG treatment had no effect on IRI outcome in AL mice, but abrogated benefits of DR (Figure 4C, D). Re-addition of H2S via IP injection of 30min prior to induction of ischemia in DR+PAG-treated mice restored protection (Figure 4C, D).
Two genetic models were used to test the specificity of the TSP and H2S in protection against hepatic IRI (Figure 4E–L). The necessity of CGL in DR-mediated benefits was tested with whole-body CGLKO mice (Yang et al., 2008). CGLKO mice responded to DR like WT mice with respect to changes in body weight, food intake, body composition and blood glucose (Supplemental Figure 4G–J) despite the lack of CGL protein in liver or capacity of liver extracts to produce H2S (Figure 4E, F). However, while the absence of CGL had no apparent impact on the outcome of hepatic IRI in AL fed mice, CGLKO mice failed to gain protection upon DR (Figure 4G, H). To test the sufficiency of increased TSP activity and endogenous H2S production, CGL was overexpressed via adenoviral-mediated gene delivery (Ad-CGL). Hepatic CGL protein levels and H2S production capacity were both significantly increased (Figure 4I, J) independent of body mass or food intake relative to control adenovirus (Ad-Null) infected mice (Supplemental Figure 4K, L). Ad-CGL infection resulted in improved outcome after hepatic IRI relative to Ad-Null infected mice (Figure 4K, L). Together, these results suggest that increased TSP activity and endogenous H2S production via CGL are sufficient, and in the case of DR, necessary, for protection against the acute surgical stress of hepatic IRI.
H2S is necessary and sufficient to confer DR-mediated stress resistance in vitro via sulfhydration and antioxidant properties
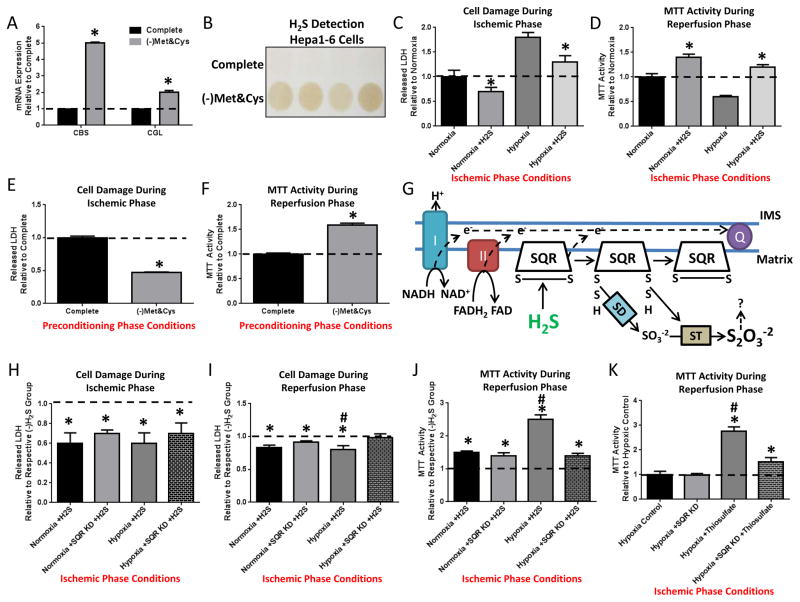
To determine if TSP gene expression and H2S production can be induced in a cell autonomous manner by SAA restriction, we cultured mouse hepatocyte-derived Hepa1-6 cells overnight in complete media or media lacking SAA (–Met&Cys). Similar to DR in vivo, SAA deprivation increased CBS and CGL expression and H2S production capacity in vitro (Figure 5A, B).
Figure 5. Mitochondrial SQR is required for cytoprotective effects of H2S during ischemia.
(A, B) Cell autonomous increase in TSP enzymes and H2S production. (A) CBS and CGL gene expression in Hepa1-6 cells cultured overnight in complete or -Met&Cys media. Asterisk indicates the significance of the difference vs. complete; *p< 0.05. (B) H2S production in live Hepa1-6 cells preconditioned in complete or -Met&Cys media in quadruplicate for 16–24hrs as indicated before readdition of Cys and PLP for H2S detection. (C, D) Cell autonomous effects of exogenous H2S on simulated IRI in Hepa1-6 cells in vitro; H2S was added during the ischemic phase and removed upon simulated reperfusion. (C) LDH release during the ischemic phase consisting of 3hr incubation in saline (simulated nutrient/energy deprivation) under normoxic or hypoxic (simulated ischemia) conditions +/− exogenous H2S as indicated. (D) MTT activity during the reperfusion phase consisting of readdition of complete media under normoxic conditions (simulated reperfusion). (E, F) Cell autonomous effects of overnight Met&Cys withdrawal (preconditioning phase conditions) on simulated IRI in primary hepatocyes in vitro. LDH release (E) during simulated ischemia and MTT activity (F) upon simulated reperfusion. (G) Schematic of H2S oxidation to thiosulfate by SQR in mitochondria. Q, Coenzyme Q; IMS, inner membrane space; I and II, Complex I and II of the mitochondrial ETC; SD, Sulfur Dioxygenase; ST, Sulfur Transferase; S2O3−2, thiosulfate. (H–J) Effects of exogenous H2S on simulated IRI upon SQR KD in Hepa1-6 cells. LDH release during the ischemic phase (H) in saline under the indicated normoxic or hypoxic conditions +/− SQR KD, and during the reperfusion phase (I) in complete media expressed relative to respective group not receiving H2S during the ischemic phase; and MTT activity (J) during the reperfusion phase. Asterisk indicates the significance of the difference in the indicated group +/− H2S treatment; hash mark indicates the significance of the difference between control and SQR KD within a given treatment group; */#p<0.05. (K) Cell autonomous effects of exogenous thiosulfate on MTT activity during the reperfusion phase following simulated IRI in Hepa1-6 cells +/− SQR KD. Asterisk indicates the significance of the difference in the indicated group +/− thiosulfate treatment; hash mark indicates the significance of the difference between control and SQR KD within a given treatment group; */#p<0.05. See also Suppl. Fig. 5.
To determine if exogenously added H2S can protect cultured Hepa1-6 cells against simulated IRI, warm ischemia was simulated by replacing growth media with saline and incubating in a 37°C hypoxic chamber for up to 4hrs, and reperfusion by replacing the growth media under normoxic conditions. Addition of H2S decreased Hepa1-6 cell damage under both normoxic and hypoxic conditions during the ischemic phase (Figure 5C) and restored MTT activity during the reperfusion phase (Figure 5D), indicating that exogenous H2S is sufficient to induce significant protection against both nutrient/energy deprivation and hypoxia in vitro.
To assess if endogenous H2S induced by SAA restriction afforded similar protection, primary hepatocytes were cultured +/− Met&Cys overnight prior to simulated IRI. LDH release during ischemia was decreased (Figure 5E) and MTT activity during reperfusion increased in hepatocytes preconditioned in Met&Cys deficient media (Figure 5F). Similar protection afforded by overnight preconditioning of Hepa1-6 cells in -Met&Cys media was blocked by addition of PAG during the preconditioning period and was partially rescued by exogenous addition of H2S during ischemia (Supplemental Figure 5A).
H2S has been proposed to prevent cell damage/death under stress in part by sulfhydration of target proteins (Paul and Snyder, 2012) including the mitochondrial inner membrane component Sulfide Quinone Oxidoreductase (SQR), which allows transfer of electrons from H2S to Coenzyme Q and the electron transport chain (ETC) (Módis et al., 2013; Olson et al., 2013) (Figure 5G). To test the potential role of the SQR in H2S-mediated protection, we partially knocked down the SQR in Hepa1-6 cells (Supplementary Figure 5B) prior to simulated IRI +/− exogenous H2S. H2S addition reduced LDH release during the ischemic phase and upon reperfusion (Figure 5H, I); the latter was significantly altered upon SQR KD (Figure 5I). Additionally, the increase in MTT activity during the reperfusion phase in the presence of H2S was significantly reduced by SQR knockdown (KD) (Figure 5J). SQR KD did not significantly alter the effects of H2S addition on nutrient/growth factor withdrawal in the absence of hypoxia (Figure 5H–J).
Because oxidation of H2S by SQR results in production of thiosulfate (S2O3−2) (Hildebrandt and Grieshaber, 2008), which itself can be utilized in mammalian tissues under hypoxic conditions for further H2S production (Olson et al., 2013), we next tested if this downstream metabolite could afford similar protection against simulated IRI in Hepa1-6 cells. While thiosulfate had a negative impact on LDH release upon nutrient/growth factor withdrawal under normoxia, increasing thiosulfate concentrations decreased cell damage during the ischemic phase under hypoxia (Supplemental Figure 5C) and improved MTT activity during the reperfusion phase (Supplemental Figure 5D). SQR KD partially blocked thiosulfate-mediated protection against simulated IRI (Figure 5K and Supplemental Figure 5E), suggesting that the protective effect of thiosulfate during hypoxia can be partially attributed to its conversion to H2S.
H2S also has antioxidant properties (Calvert et al., 2010) that could contribute to cytoprotection independent of target protein sulfhydration. At 100μM but not 10μM final concentration, H2S protected Hepa1-6 cells against acute oxidative stress delivered by H2O2 exposure (Supplemental Figure 5F, G). Similar protection was found with 100μM H2S against H2O2 exposure in primary smooth muscle cells (Supplemental Figure 5H, I). Thus, H2S protects from acute ischemic insult and oxidative stress via multiple mechanisms.
Increased endogenous H2S production in diet-induced longevity extension models across evolutionary boundaries
While exogenous H2S can extend longevity in worms, the potential role of endogenous H2S in DR-mediated lifespan extension has not been reported. To test the hypothesis that increased endogenous H2S is a common feature of DR regimens that lead to extended longevity across evolutionary boundaries, we measured H2S production capacity in four different organisms subject to species-appropriate DR longevity regimens.
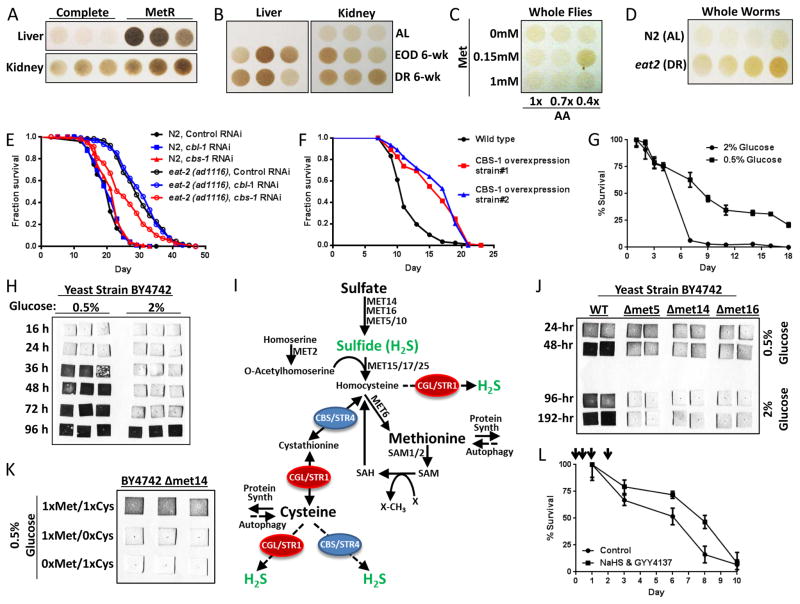
Adult mice subject to long-term MetR (Figure 6A), EOD fasting or 20–30% DR (Figure 6B) had increased H2S production capacity in liver and kidney extracts compared to AL-fed complete diet controls. In mice subject to 1wk 50% DR, kidney, spleen and carotid artery additionally demonstrated increased H2S production capacity (Supplemental Figure 6A), while brain, skeletal muscle, heart and aorta tested under the same conditions did not (Supplemental Figure 6B). Nonetheless, primary smooth muscle cells prepared from aorta displayed increased H2S production capacity upon Met&Cys depletion (Supplemental Figure 6C), suggesting tissue/cell type specificity in the regulation of the TSP in response to diet. Although we failed to observe an increase in H2S production capacity in mouse brain upon 1wk 50% DR, 3 days of water-only fasting increased H2S production capacity (Supplemental Figure 6D).
Figure 6. Increased endogenous H2S production in diet-induced longevity extension models crosses evolutionary boundaries.
(A–B) H2S production capacity of liver and kidney extracts from mice with AL access to complete or MetR diets as indicated for 4mos (A) and in mice with AL, every-other-day (EOD) fasting or 20–30% restricted diets for 6wks (B). Each circle represents H2S production from an individual animal, normalized for extract protein content. (C) H2S production capacity of whole fly lysates normalized for protein content from populations subject to the indicated dietary amino acid (AA) and Met concentrations. Maximal H2S production capacity correlated with the optimal diet for longevity extension (0.4x AA with 0.15mM Met) (Lee et al., 2014). (D) H2S production capacity of N2 and eat-2 whole worm lysates in quadruplicate. (E) Lifespan of N2 and eat-2 worms subject to RNAi-mediated KD of the TSP genes cbs-1 or cbl-1 as indicated. (F) Lifespan analysis of WT or transgenic worms overexpressing CBS-1. (G) Chronological lifespan of budding yeast grown in 2% or 0.5% glucose as indicated. (H) H2S production in yeast cultures grown in 2% or 0.5% glucose as detected by black lead sulfide accumulation on lead acetate papers inserted into the caps of the growth flasks and removed at the indicated time point. (I) Schematic of transmethylation, TSP and H2S production in budding yeast. (J) H2S production in WT and sulfur assimilatory pathway mutant strains grown in 2% or 0.5% glucose for the indicated time. (K) H2S production in the met14 sulfur assimilatory mutant grown in 0.5% glucose and the indicated relative amount of Met or Cys. (L) Chronological lifespan in 2% glucose +/− H2S donors NaHS and GYY4137 added during early culture growth at indicated time points (arrows). See also Suppl. Fig. 6.
In the fruit fly D. melanogaster, maximal H2S production capacity of whole-body extracts of flies subject to varying levels of dietary protein and Met restriction (Figure 6C) correlated with maximal lifespan extension (Lee et al., 2014).
In the nematode worm C. elegans, the eat-2 mutant serves as a genetic model of lifespan extension by DR due to defects in pharyngeal function resulting in decreased food intake (Lakowski and Hekimi, 1998). Extracts of eat-2 mutants produced more H2S than extracts of age-matched WT N2 worms (Figure 6D). To assess the genetic requirements for TSP factors in longevity extension, we performed individual RNAi-mediated KD of the two worm CGL homologs CTH-1 and CTH-2, the CBS homolog CBS-1, and a fourth worm TSP gene, CBL-1. Individual RNAi KD of CBL-1 and CBS-1 had no effect on longevity (Supplemental Figure 6E), but RNAi KD specifically of CBS-1 significantly decreased eat-2 mediated lifespan extension, while overexpression of CBS-1 significantly extended median lifespan of WT N2 controls (Figure 6F).
Finally, DR-mediated extension of chronological lifespan in the yeast S. cerevisiae can be accomplished by reducing glucose in the growth media from 2% to 0.5% (Figure 6G) (Wu et al., 2013). Under these conditions, H2S production from the glucose-restricted cultures was detected within 24–48hrs and continued to increase for up to 96hrs in three different yeast strains (Figure 6H and Supplemental Figure 6F, G). As the majority of H2S production in yeast occurs via an assimilatory pathway not present in metazoans in which extracellular sulfate is converted to sulfide by the combined enzymatic activities of MET14, MET16, and MET5/10 (Figure 6I), we next asked if increased H2S upon DR occurred through this assimilatory pathway or the conserved TSP pathway in yeast. Deletion of MET5, 14, or 16 resulted in inhibition of H2S production under 2.0% glucose, but did not block H2S induction (Figure 6J) or chronological longevity extension (Supplemental Figure 6H) upon glucose restriction. To further confirm Met and/or Cys, rather than inorganic sulfur, as the primary source of increased H2S under conditions upon glucose restriction, we examined H2S production in a Δmet14 line lacking a functional assimilatory pathway, with differing concentrations of Met and Cys in the media. Complete deprivation of either Met or Cys resulted in reduced H2S production (Figure 6K). Thus, under conditions of glucose restriction, H2S is produced from SAAs via the TSP. Because TSP-deficient CGL/STR1 and CBS/STR4 deletion strains grow abnormally in culture, it was not possible to test their requirement for increased H2S production or extended chronological longevity under low glucose conditions. However, exogenous H2S donors NaHS and GYY4137 added early after inoculation were sufficient to significantly increase chronological lifespan (Figure 6L).
Discussion
H2S as unifying mechanism of DR regimens and benefits
As presented in the model in Figure 7, multiple DR regimens, including total food restriction, PR and SAA restriction/MetR, confer overlapping functional benefits in a number of diverse organisms from yeast (Johnson and Johnson, 2014) to worms (Cabreiro et al., 2013) to flies (Grandison et al., 2009; Troen et al., 2007) to rodents (Miller et al., 2005; Orentreich et al., 1993). In each of these experimental organisms, we found an increase in TSP-mediated H2S production upon application of species-appropriate longevity regimens, ranging from EOD and MetR in mice to glucose restriction in yeast. Furthermore, in rodents and worms we demonstrated that genetic repression of TSP components blocked DR benefits, while overexpression could mimic benefits in the absence of any dietary intervention. In yeast we found the TSP, rather than the assimilatory pathway, to be a major source of H2S production upon glucose restriction. Although future studies are required to directly demonstrate the necessity of H2S production for various DR benefits, our data demonstrate increased H2S production via the TSP is an evolutionarily conserved response to DR from yeast to mammals with the potential to mediate multiple DR benefits including stress resistance and longevity. We note that the ability of SAA restriction to confer benefits in no way rules out other dietary triggers or downstream mechanisms of DR benefits, and that future studies will be required to determine if benefits attributed to different DR regimens, including Trp restriction, impact the TSP or H2S production.
Figure 7. Model of DR-mediated benefits through increased H2S production.
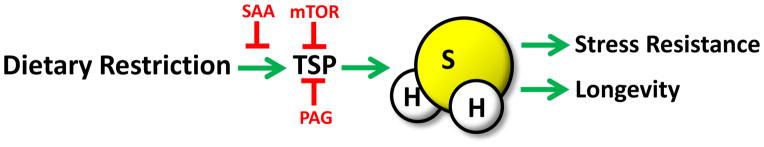
DR regimens leading to extended longevity across evolutionary boundaries include PR, SAA restriction and Cys restriction, leading to increased TSP activity via CGL and/or CBS family members, and increased endogenous H2S production. Specific SAA addition, increased activity of the nutrient/energy sensing kinase, mechanistic target of rapamycin (mTOR), or the pharmacological agent, PAG, can block TSP upregulation and H2S production. H2S can readily diffuse through tissues and has pleiotropic, overlapping beneficial effects on the cellular, tissue and organismal levels with the potential to contribute to stress resistance and longevity benefits of DR.
Regulation of TSP gene expression and H2S production
Cys deprivation triggers increased TSP gene expression possibly via the integrated stress response through GCN2 activation, eIF2α phosphorylation and ATF4 stabilization (Lee et al., 2008). We confirmed the ability of DR to increase hepatic CGL expression in vivo, and Cys or NAC addback to block this induction. Whether or not GCN2 is required for this remains to be determined; however, our data suggest a novel role for mTORC1 in CGL regulation, as constitutive mTORC1 expression in the TSC1KO livers blocked diet-induced CGL expression.
Because the logic underlying TSP activation upon Cys deprivation is presumably to increase Cys biosynthesis, the notion that Cys degradation into H2S increases when Cys concentrations are limiting is potentially counterintuitive. However, the in vivo source and identity of substrates for CGL or CBS-derived H2S upon DR are not known. Free Cys released upon autophagy, rather than that produced de novo by TSP, could be a major fuel source for H2S production. Interestingly, the benefits of MetR on longevity in yeast require autophagy (Ruckenstuhl et al., 2014), but whether autophagy plays a role in H2S production remains to be determined.
Potential mechanisms of H2S action in DR
By what mechanism(s) could endogenous H2S give rise to pleiotropic DR benefits? We found that resistance to simulated IRI in vitro required the mitochondrial ETC component SQR. The SQR is thought to be of eubacterial origin and conserved from the early evolution of eukaryotes in an anoxic and sulfidic world (Theissen et al., 2003). H2S could interact with SQR to lend protection against ischemia by donating electrons to the ETC via coenzyme Q, thus potentially serving as a source of electrons during ischemia. Interestingly, thiosulfate generated as a product of H2S oxidation via SQR can undergo further chemical modification in a thioredoxin reductase dependent reaction using glutathione to produce sulfite and/or sulfate (Olson et al., 2013), which are used in some unicellular organisms as terminal electron acceptors for ATP production instead of oxygen, resulting in H2S generation (Muyzer and Stams, 2008). Whether this can occur in mammalian cells remains to be explored; however, the addition of thiosulfate to hypoxic mammalian tissues results in the production of H2S by unknown mechanisms (Olson et al., 2013).
Vasodilatory effects of H2S could contribute to healthspan and lifespan extension in mammals by counteracting atherosclerosis, hypertension, and trauma. Exogenous H2S protects the vasculature from oxidative damage (Yan et al., 2006), while a lack of CGL and/or endogenous H2S production leads to hypertension (Yang et al., 2008). Fasting and PR protect the vasculature against intimal hyperplasia as a result of carotid focal stenosis (Mauro et al., 2014), correlating with increased H2S production capacity upon DR in the carotid artery in vivo and vascular smooth muscle cells in vitro. Fasting protects the brain against IRI in rat models of transient middle cerebral artery occlusion (Varendi et al., 2014) and increased H2S production capacity in mouse brain (Supplemental Figure 6D). Future studies are required to address the specific role of increased TSP activity and H2S production in DR-mediated effects on the vasculature and other organ systems.
Clinical Significance
The use of exogenous H2S as a DR mimetic is appealing for applications in which patient compliance with restricted food intake is an issue, but nonetheless remains challenging due to the short half-life of the gas in vivo and risks of toxicity at high levels. Modulation of endogenous H2S production by diet offers advantages in terms of safety and continuous delivery in planned applications with brief duration, for example in the context of preconditioning prior to elective surgery. In this context, H2S may have pleiotropic advantages on surgical outcome, including vasodilatory and anti-inflammatory effects on the systemic level, as well as benefits against ischemic injury that may arise as part of the procedure itself or an unintended complication including heart attack or stroke.
Conclusion
H2S was in vogue for centuries past as a cure-all before being viewed as a poisonous toxin with little or no acceptable level of exposure. Recently, H2S has re-emerged as a potential therapeutic agent addressing numerous health issues (Zhang et al., 2013). Here, we identified DR as a trigger of increased endogenous H2S production, and H2S as a molecular mediator of pleiotropic DR benefits including longevity and stress resistance. These findings have broad implications for our basic understanding of DR and its potential clinical applications.
Material and Methods
Mice
All experiments were performed with IACUC approval. 8–10 week old male B6D2F1 hybrids (Jackson Laboratories) were used for all experiments unless otherwise indicated. Male and female NRF2 KO and littermate controls on a mixed 129/C57BL/6 background (Martin et al., 1998), LTsc1KO and littermate controls on a C57BL/6 background (Harputlugil et al., 2014) and CGLKO and control mice on a mixed 129/C57BL/6 background (Yang et al., 2008) were described previously.
Preconditioning Regimens
Dietary
Mice were given AL access to food and water unless otherwise indicated. Experimental diets were based on Research Diets D12450B with approximately 18% of calories from protein (hydrolyzed casein or individual crystalline amino acids (Ajinomoto) in the proportions present in casein), 10% from fat and 72% from carbohydrate. AL food intake was monitored for several days and used to calculate 50% DR. Mice were fed daily with fresh food between 6 and 7pm.
Pharmacological
NAC (600mg/kg/day total in food and drinking water) and VitC&E (1000 and 250mg/kg/day, respectively, in food and drinking water) supplementation was halted 16–24hrs prior to organ harvest and/or induction of IRI. NaHS (1mM) or GYY4137 (260μM final) were delivered in the drinking water; PAG (10mg/kg) and NaHS (3–5mg/kg) were administered by IP injection.
Adenoviral-mediated gene delivery
CGL was overexpressed by IV injection of 1010 PFUs of Ad-CMV-CGL (ADV-256305) or control Ad-CMV-Null virus (Vector Biolabs) 7 days prior to hepatic IRI.
Cells
In vitro DR was performed in mouse Hepa1-6 cells, primary hepatocytes or primary aortic smooth muscle cells by incubating in DMEM lacking Met and Cys (Invitrogen) with 10% dialyzed FBS for up to 20hrs.
Ischemia reperfusion models
In vivo
Warm hepatic or renal IRI was induced and damage assessed as previously described (Peng et al., 2012). ALT, AST, and/or LDH values were normalized to the experimental control, usually the WT group fed a complete diet AL, for each time point (3, 24hrs post reperfusion) and combined into a single value per animal.
In vitro
For simulated ischemia, growth or preconditioning media was replaced with saline to mimic nutrient/energy deprivation and placed in an airtight chamber flushed with nitrogen gas at 37°C for 4hrs. Saline supernatant was removed after simulated ischemia for LDH measurement. Reperfusion was simulated by adding back fresh complete DMEM-12320 (Invitrogen) containing 0.5 mg/mL MTT under normoxia at 37°C for an additional 3–4hrs.
Metabolic Parameters
Body composition was determined with an Echo MRI. Blood glucose was measured with an Easy Step Glucometer (Home Aide Diagnostics), serum triglycerides with a Serum Triglyceride Determination Kit (Sigma), liver RONS and GSH from left hepatic lobes normalized by wet weight using OxiSelect In Vitro Green Fluorescence ROS/RNS Assay Kit (Cell Biolabs) and the fluorimetric Glutathione Assay Kit (Sigma), and liver metabolites via mass spectrometry and normalized to total protein content. Peroxisome β-oxidation of lipids was performed on freshly harvested liver (Hu et al., 2005; Lazarow, 1981).
mRNA and Protein Analysis
Total RNA was isolated from tissues and cells using miRNeasy Mini Kit (Qiagen) and cDNA synthesized by random hexamer priming with the Verso cDNA kit (Thermo). qRT-PCR was performed with SYBR green dye (Lonza) and TaqPro DNA polymerase (Denville). Fold changes were calculated by the ΔΔCt method using Hprt and/or Rpl13 as standards and normalized to the experimental WT AL control. Protein expression was analyzed in tissues homogenized in passive lysis buffer (Promega), separated by SDS-PAGE, transferred to PVDF membrane (Whatman) and blotted for CGL (ab151769 Abcam), CBS (ab135626 Abcam) or Actin (13E5 Cell Signaling) followed by HPRT conjugated secondary anti-rabbit antibody (Dako).
H2S measurements
Lead sulfide method
H2S production capacity was measured in 100mg fresh tissue, or 100–300μg freeze-thaw homogenate in passive lysis buffer (Promega) of tissues, flies or worms, in PBS supplemented with 10mM Cys and 10μM-6mM PLP. A lead acetate H2S detection paper (Sigma; or made by soaking blotting paper (VWR) in 20mM lead acetate (Sigma) and then vacuum drying) was placed above the liquid phase in a closed container (mirocentrifuge tube or covered 96 well plate) and incubated 2–5hrs at 37°C until lead sulfide darkening of the paper occurred. In live Hepa1-6 cells in 96-well plates, growth media was supplemented with 10mM Cys and 10μM PLP, and a lead acetate paper placed over the plate for 2–24hrs of further incubation in a CO2 incubator at 37°C. In live yeast culture, lead acetate papers were placed above liquid cultures at the time of inoculation.
Sulfide probe method
A micro-sulfide ion electrode probe (Lazar Research Laboratories) and volt meter (Jenco) were used to measure H2S production capacity in extracts in vitro and endogenous H2S concentrations in liver in vivo upon direct insertion into median and left lobes of anesthetized mice prior to sacrifice.
Fly Conditions
D. melanogaster were maintained as described previously (Lee et al., 2014). Flies used in the H2S production capacity assay were held on the designated diet and transferred to fresh vials without anesthesia every 3 days until 18 days of age.
Worm Conditions
N2 and DA1116 (eat-2(ad1116) II.) strains were grown following standard procedures, and all lifespan experiments carried out as previously described (Mair et al., 2011). RNAi clones were from the Ahringer RNAi library. CBS transgenic strains overexpressing the cbs-1 isoform a (ZC373.1a) from the ubiquitous sur-5 promoter and unc-54 3′UTR were generated by microinjection into gonads of young adult hermaphrodites.
Yeast Conditions
Experiments were carried out in WT strains BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0), W303 (MATa leu2-3,112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15), and DBY746 (MATα leu2-3, 112 his3Δ1 trp1-289 ura 3-52 GAL+). Met deletion strains in BY4742 (met5, met14 and met16) were purchased from EUROSCARF. For chronological aging experiments, cells were inoculated to an OD600 of 0.05, and grown at 28°C in SCD media and cell survival determined by clonogenicity at the given time points in at least 3 independent cultures and values normalized to survival on day one. Longevity effects of exogenous H2S were tested by adding 100μM GYY4137 (in DMSO) to BY4742 cultures at the time of inoculation and 5μM NaHS at 6, 24 and 48hrs after inoculation, with DMSO-treated BY4742 as a control.
Statistical Analyses
Data are displayed as means +/− SEM and statistical significance assessed in GraphPad Prism using Student’s t tests to compare values and one-sample t test to compare means to a hypothetical value of 1 or 100 when data was normalized to the average value of the control group. A P-value of 0.05 or less was deemed statistically significant.
A more detailed Materials and Methods section is included in Supplemental Materials.
Supplementary Material
(A) Blood glucose following 1wk of preconditioning on the indicated diet; n=5/group. (B) Photographs of liver lobes (1x magnification) fixed 24hrs after reperfusion from mice fed ad libitum (AL) or 50% restricted (DR) with or without NAC supplementation as indicated. (C) GSH levels in livers of NRF2 WT and KO mice on AL or DR regimens as indicated; n=3–4/group. (D) Hepatic expression of NRF2 gene targets after 1wk of DR in WT or NRF2 KO animals as indicated and expressed relative to the same genotype fed AL; n=4/group. (E) Photographs of liver lobes (1x magnification) fixed 24hrs after reperfusion from WT or NRF2KO mice preconditioned on AL or DR regimens as indicated; n=7–12/group (F) Serum urea after renal IRI in WT or NRF2KO mice preconditioned on AL or DR regimens as indicated; n=3–4/group. Asterisk indicates the significance of the difference compared to WT AL, and pound sign compared to NRF2KO AL; */#p<0.05.
(A) Food intake during the 7d preconditioning period on the indicated complete diet fed ad libitum (AL) or 50% restricted (DR) with or without supplementation of 2xMet&Cys as indicated and expressed as grams food eaten per gram of mouse body weight; n=5/group. Asterisk indicates the significance of the difference vs. the AL group; *p<0.05. (B) Average daily weight of mice on the indicated diet during the 7d preconditioning period; n=5/group. (C) Changes in FOXO target gene expression in DR and DR +2xMet&Cys diet groups expressed relative to the AL group; n=3–4/group. (D) Food intake during the 7d preconditioning period on the indicated complete or protein free diets fed AL or restricted 35% (DR) with or without supplementation of Met&Cys as indicated and expressed as grams eaten per gram of mouse; n=5/group. Asterisk indicates the significance of the difference vs. the AL group on a complete diet; *p<0.05. (E) Average daily weight of mice on the indicated diet during the 7d preconditioning period; n=5/group.
(A) Liver metabolite levels in LTsc1KO mice on AL complete diet expressed relative to the average value of that metabolite in the WT AL group; n=5/group. (B) Lead sulfide standard curve generated with NaHS dissolved in water at the indicated concentration for detection of H2S using lead acetate paper. (C) H2S production from NRF2KO mice fed AL or restricted 50% (DR) for 1wk as indicated using the lead sulfide method. Each sample represents an individual mouse; n=4/group. (D) Hepatic GSH levels of mice on the indicated diet for 1wk relative to the AL group; n=3–5/group. Asterisk indicates the significance of the difference relative to the AL group, and the pound sign relative to DR +2xMet&Cys; */#p<0.05. (E, F) Microsulfide probe-based mV reading of H2S production from extracts of livers from mice on the indicated diet for 1wk (E) and the corresponding standard curve using NaHS dissolved in water at the indicated concentration (F). (G, H) Microsulfide probe-based mV reading of endogenous H2S present in liver lobes of mice on the indicated diet (n=3 mice/group and 2 lobes/animal, G) and the corresponding standard curve using NaHS dissolved in water at the indicated concentration (H). Asterisk indicates the significance of the difference relative to the AL group; *p<0.05.
(A–D) Combined serum markers of liver damage 3 and 24hrs after reperfusion (A), animal weight (B), food intake (C) and water intake (D) of mice (n=4/group) treated with extended slow-release H2S donor GYY4137 or short-lived NaHS in the drinking water as indicated for 1wk prior to hepatic IRI. Asterisk indicates the significance of the difference vs. the Control water group; *p<0.05. (E) H2S production using the lead sulfide method in extracts of livers prepared 24hrs after hepatic IRI from animals preconditioned on AL or DR regimens with or without PAG as indicated for 1wk. Each circle represents H2S production from an individual animal. (F) Microsulfide probe-based mV readings of endogenous H2S in livers of animals preconditioned on AL or DR regimens with PAG as indicated for 1wk; n=4/group. Asterisk indicates the significance of the difference vs. the AL group, and pound sign relative to DR +PAG; */#p<0.05. (G–J) Body mass (G), food intake (H), changes in body composition (I) and blood glucose (J) in in WT or CGLKO mice preconditioned on the indicated diet for 1wk; n=5–8/group. (K, L) Body mass (K) and food intake (L) of AL fed animals infected with the indicted virus prior to and up to 7d post infection; n=6/group.
(A) LDH release during the ischemic phase in Hepa1-6 cells preconditioned overnight in complete media or media lacking Met, Cys and serum (-Met&Cys) with or without PAG as indicated; H2S was added during the ischemic phase where indicated. Asterisk indicates the significance of the difference relative to the Complete group, and the pound symbol indicates the significance of the difference relative to the –Met&Cys +PAG group; */# p<0.05. (B) Immunoblot of SQR in Hepa1-6 cells 48hr after siRNA targeting mouse SQR or scrambled control. (C, D) Cell autonomous effects of exogenous thiosulfate (S2O3−2) on simulated IRI in Hepa1-6 cells. Thiosulfate was added during the ischemic phase and removed during the reperfusion phase. LDH release (C) during 3hr incubation in saline (simulated nutrient/energy deprivation) under normoxic or hypoxic (simulated ischemia) conditions in the presence of the indicated concentration of thiosulfate; MTT activity (D) upon readdition of complete media without thiosulfate under normoxic conditions (simulated reperfusion). Asterisk indicates the significance of the difference between the indicated thiosulfate dose and no thiosulfate addition within normoxic or hypoxic treatment groups; *p<0.05. (E) LDH release from Hepa1-6 cells with or without SQR KD during simulated ischemia +/− exogenous thiosulfate (S2O3−2). Pound sign indicates the significance of the difference between control and SQR KD within a given treatment group; #p<0.05. (F–G) Effects of the indicated H2S concentration on LDH release (F) and MTT activity (G) following 5mM H2O2 treatment of Hepa1-6 cells. (H–I) Effects of 100uM H2S addition on LDH release (H) and MTT activity (I) following 1mM H2O2 treatment of primary mouse vascular smooth muscle cells. Pound sign indicates the significance of the difference between H2O2 alone vs. H2O2 plus H2S; #p<0.05.
(A–B) H2S production capacity in extracts of kidney, spleen, carotid artery, brain, skeletal muscle, heart, and aorta from mice with AL or restricted access (DR) to complete or protein free (PF) diets as indicated. Extracts were normalized for protein content or organ weight; each circle represents H2S production from an individual animal as measured by the lead sulfide method. (C) H2S production from primary aorta smooth muscle cells after overnight preconditioning in complete or media lacking Met&Cys. (D) H2S production from brains after AL feeding or 3d water-only fasting. (E) Effects of RNAi-mediated knockdown of individual TSP components on longevity of wildtype N2 worms compared to empty vector (EV). (F–G) H2S production from three different wildtype yeast strains as a function of glucose concentration and time in culture as detected by black lead sulfide accumulation on lead acetate strips placed in the growth flasks (F) or square lead acetate papers inserted into the caps of the growth flasks for the indicated time (G). (H) Chronological lifespans of the indicated WT or sulfur assimilatory pathway mutants grown in 2.0% (left) or 0.5% (right) glucose.
Highlights.
Sulfur amino acid restriction is a key nutritional determinant of dietary restriction
Cysteine restriction enhances endogenous CGL activity and H2S production
Increased H2S is necessary and sufficient for DR-mediated stress resistance
Increased H2S is shared by DR regimens in yeast, worms, flies and rodents
Acknowledgments
We thank Bruce Kristal and Jaan-Olle Andressoo for critical reading of the manuscript; Silvia Dichtinger, Manmeet Gujral and Jason Li for technical assistance; and Paul Ney for sharing the NRF2KO mice. This work was supported by grants from NIH (DK090629, AG036712) and the Glenn Foundation to JRM; CH was supported by T32CA0093823; FM was supported by the Austrian Science Fund FWF (LIPOTOX, I1000, P23490-B12, and P24381-B20); WBM was supported by 1R01AG044346; VNG was supported by R01AG021518, CKO was supported by American Heart Association 12GRNT9510001 and 12GRNT1207025.
Footnotes
The authors claim no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science. 2005;308:518. doi: 10.1126/science.1108581. [DOI] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cochemé HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP. Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Coetzee WA, Lefer DJ. Novel insights into hydrogen sulfide--mediated cytoprotection. Antioxid Redox Signal. 2010;12:1203–1217. doi: 10.1089/ars.2009.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevasanta E, Denicola A, Alvarez B, Möller MN. Solubility and permeation of hydrogen sulfide in lipid membranes. PLoS One. 2012;7:e34562. doi: 10.1371/journal.pone.0034562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harputlugil E, Hine C, Vargas D, Robertson L, Manning BD, Mitchell JR. The TSC Complex Is Required for the Benefits of Dietary Protein Restriction on Stress Resistance In Vivo. Cell Rep. 2014;8:1160–1170. doi: 10.1016/j.celrep.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- Hine CM, Mitchell JR. NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction. J Clin Exp Pathol. 2012:S4. doi: 10.4172/2161-0681.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Foxworthy P, Siesky A, Ficorilli JV, Gao H, Li S, Christe M, Ryan T, Cao G, Eacho P, et al. Hepatic peroxisomal fatty acid beta-oxidation is regulated by liver X receptor alpha. Endocrinology. 2005;146:5380–5387. doi: 10.1210/en.2005-0591. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Johnson FB. Methionine restriction activates the retrograde response and confers both stress tolerance and lifespan extension to yeast, mouse and human cells. PLoS One. 2014;9:e97729. doi: 10.1371/journal.pone.0097729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil H, Kabil O, Banerjee R, Harshman LG, Pletcher SD. Increased transsulfuration mediates longevity and dietary restriction in Drosophila. Proc Natl Acad Sci U S A. 2011a;108:16831–16836. doi: 10.1073/pnas.1102008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011b;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Zhao M, Jiang H, Tan G, Pan S, Sun X. Role of hydrogen sulfide in hepatic ischemia-reperfusion-induced injury in rats. Liver Transpl. 2009;15:1306–1314. doi: 10.1002/lt.21810. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow PB. Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol. 1981;72:315–319. doi: 10.1016/s0076-6879(81)72021-4. [DOI] [PubMed] [Google Scholar]
- Lee BC, Kaya A, Ma S, Kim G, Gerashchenko MV, Yim SH, Hu Z, Harshman LG, Gladyshev VN. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nature communications. 2014;5:3592. doi: 10.1038/ncomms4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Dominy JE, Sikalidis AK, Hirschberger LL, Wang W, Stipanuk MH. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol Genomics. 2008;33:218–229. doi: 10.1152/physiolgenomics.00263.2007. [DOI] [PubMed] [Google Scholar]
- Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- Martin F, van Deursen JM, Shivdasani RA, Jackson CW, Troutman AG, Ney PA. Erythroid maturation and globin gene expression in mice with combined deficiency of NF-E2 and nrf-2. Blood. 1998;91:3459–3466. [PubMed] [Google Scholar]
- Mauro CR, Tao M, Yu P, Trevino-Villerreal JH, Longchamp A, Kristal BS, Ozaki CK, Mitchell JR. Preoperative dietary restriction reduces intimal hyperplasia and protects from ischemia-reperfusion injury. Journal of vascular surgery. 2014 doi: 10.1016/j.jvs.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, Chu T, Forrer F, Müller C, de Jong M, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010;9:40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, Stams AJ. The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol. 2008;6:441–454. doi: 10.1038/nrmicro1892. [DOI] [PubMed] [Google Scholar]
- Módis K, Coletta C, Erdélyi K, Papapetropoulos A, Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- Olson KR, Deleon ER, Gao Y, Hurley K, Sadauskas V, Batz C, Stoy GF. Thiosulfate: a readily accessible source of hydrogen sulfide in oxygen sensing. Am J Physiol Regul Integr Comp Physiol. 2013;305:R592–603. doi: 10.1152/ajpregu.00421.2012. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Paul BD, Snyder SH. H S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, Charlip A, Chu T, Mitchell JR. Surgical stress resistance induced by single amino Acid deprivation requires gcn2 in mice. Sci Transl Med. 2012;4:118ra111. doi: 10.1126/scitranslmed.3002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predmore BL, Alendy MJ, Ahmed KI, Leeuwenburgh C, Julian D. The hydrogen sulfide signaling system: changes during aging and the benefits of caloric restriction. Age (Dordr) 2010;32:467–481. doi: 10.1007/s11357-010-9150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Ruckenstuhl C, Netzberger C, Entfellner I, Carmona-Gutierrez D, Kickenweiz T, Stekovic S, Gleixner C, Schmid C, Klug L, Sorgo AG, et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. 2014;10:e1004347. doi: 10.1371/journal.pgen.1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med Hypotheses. 2006;66:832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Theissen U, Hoffmeister M, Grieshaber M, Martin W. Single eubacterial origin of eukaryotic sulfide:quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times. Mol Biol Evol. 2003;20:1564–1574. doi: 10.1093/molbev/msg174. [DOI] [PubMed] [Google Scholar]
- Troen AM, French EE, Roberts JF, Selhub J, Ordovas JM, Parnell LD, Lai CQ. Lifespan modification by glucose and methionine in Drosophila melanogaster fed a chemically defined diet. Age (Dordr) 2007;29:29–39. doi: 10.1007/s11357-006-9018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varendi K, Airavaara M, Anttila J, Vose S, Planken A, Saarma M, Mitchell JR, Andressoo JO. Short-term preoperative dietary restriction is neuroprotective in a rat focal stroke model. PLoS One. 2014;9:e93911. doi: 10.1371/journal.pone.0093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liu SQ, Huang D. Dietary restriction depends on nutrient composition to extend chronological lifespan in budding yeast Saccharomyces cerevisiae. PLoS One. 2013;8:e64448. doi: 10.1371/journal.pone.0064448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;351:485–491. doi: 10.1016/j.bbrc.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol. 2013;33:1104–1113. doi: 10.1128/MCB.01215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Blood glucose following 1wk of preconditioning on the indicated diet; n=5/group. (B) Photographs of liver lobes (1x magnification) fixed 24hrs after reperfusion from mice fed ad libitum (AL) or 50% restricted (DR) with or without NAC supplementation as indicated. (C) GSH levels in livers of NRF2 WT and KO mice on AL or DR regimens as indicated; n=3–4/group. (D) Hepatic expression of NRF2 gene targets after 1wk of DR in WT or NRF2 KO animals as indicated and expressed relative to the same genotype fed AL; n=4/group. (E) Photographs of liver lobes (1x magnification) fixed 24hrs after reperfusion from WT or NRF2KO mice preconditioned on AL or DR regimens as indicated; n=7–12/group (F) Serum urea after renal IRI in WT or NRF2KO mice preconditioned on AL or DR regimens as indicated; n=3–4/group. Asterisk indicates the significance of the difference compared to WT AL, and pound sign compared to NRF2KO AL; */#p<0.05.
(A) Food intake during the 7d preconditioning period on the indicated complete diet fed ad libitum (AL) or 50% restricted (DR) with or without supplementation of 2xMet&Cys as indicated and expressed as grams food eaten per gram of mouse body weight; n=5/group. Asterisk indicates the significance of the difference vs. the AL group; *p<0.05. (B) Average daily weight of mice on the indicated diet during the 7d preconditioning period; n=5/group. (C) Changes in FOXO target gene expression in DR and DR +2xMet&Cys diet groups expressed relative to the AL group; n=3–4/group. (D) Food intake during the 7d preconditioning period on the indicated complete or protein free diets fed AL or restricted 35% (DR) with or without supplementation of Met&Cys as indicated and expressed as grams eaten per gram of mouse; n=5/group. Asterisk indicates the significance of the difference vs. the AL group on a complete diet; *p<0.05. (E) Average daily weight of mice on the indicated diet during the 7d preconditioning period; n=5/group.
(A) Liver metabolite levels in LTsc1KO mice on AL complete diet expressed relative to the average value of that metabolite in the WT AL group; n=5/group. (B) Lead sulfide standard curve generated with NaHS dissolved in water at the indicated concentration for detection of H2S using lead acetate paper. (C) H2S production from NRF2KO mice fed AL or restricted 50% (DR) for 1wk as indicated using the lead sulfide method. Each sample represents an individual mouse; n=4/group. (D) Hepatic GSH levels of mice on the indicated diet for 1wk relative to the AL group; n=3–5/group. Asterisk indicates the significance of the difference relative to the AL group, and the pound sign relative to DR +2xMet&Cys; */#p<0.05. (E, F) Microsulfide probe-based mV reading of H2S production from extracts of livers from mice on the indicated diet for 1wk (E) and the corresponding standard curve using NaHS dissolved in water at the indicated concentration (F). (G, H) Microsulfide probe-based mV reading of endogenous H2S present in liver lobes of mice on the indicated diet (n=3 mice/group and 2 lobes/animal, G) and the corresponding standard curve using NaHS dissolved in water at the indicated concentration (H). Asterisk indicates the significance of the difference relative to the AL group; *p<0.05.
(A–D) Combined serum markers of liver damage 3 and 24hrs after reperfusion (A), animal weight (B), food intake (C) and water intake (D) of mice (n=4/group) treated with extended slow-release H2S donor GYY4137 or short-lived NaHS in the drinking water as indicated for 1wk prior to hepatic IRI. Asterisk indicates the significance of the difference vs. the Control water group; *p<0.05. (E) H2S production using the lead sulfide method in extracts of livers prepared 24hrs after hepatic IRI from animals preconditioned on AL or DR regimens with or without PAG as indicated for 1wk. Each circle represents H2S production from an individual animal. (F) Microsulfide probe-based mV readings of endogenous H2S in livers of animals preconditioned on AL or DR regimens with PAG as indicated for 1wk; n=4/group. Asterisk indicates the significance of the difference vs. the AL group, and pound sign relative to DR +PAG; */#p<0.05. (G–J) Body mass (G), food intake (H), changes in body composition (I) and blood glucose (J) in in WT or CGLKO mice preconditioned on the indicated diet for 1wk; n=5–8/group. (K, L) Body mass (K) and food intake (L) of AL fed animals infected with the indicted virus prior to and up to 7d post infection; n=6/group.
(A) LDH release during the ischemic phase in Hepa1-6 cells preconditioned overnight in complete media or media lacking Met, Cys and serum (-Met&Cys) with or without PAG as indicated; H2S was added during the ischemic phase where indicated. Asterisk indicates the significance of the difference relative to the Complete group, and the pound symbol indicates the significance of the difference relative to the –Met&Cys +PAG group; */# p<0.05. (B) Immunoblot of SQR in Hepa1-6 cells 48hr after siRNA targeting mouse SQR or scrambled control. (C, D) Cell autonomous effects of exogenous thiosulfate (S2O3−2) on simulated IRI in Hepa1-6 cells. Thiosulfate was added during the ischemic phase and removed during the reperfusion phase. LDH release (C) during 3hr incubation in saline (simulated nutrient/energy deprivation) under normoxic or hypoxic (simulated ischemia) conditions in the presence of the indicated concentration of thiosulfate; MTT activity (D) upon readdition of complete media without thiosulfate under normoxic conditions (simulated reperfusion). Asterisk indicates the significance of the difference between the indicated thiosulfate dose and no thiosulfate addition within normoxic or hypoxic treatment groups; *p<0.05. (E) LDH release from Hepa1-6 cells with or without SQR KD during simulated ischemia +/− exogenous thiosulfate (S2O3−2). Pound sign indicates the significance of the difference between control and SQR KD within a given treatment group; #p<0.05. (F–G) Effects of the indicated H2S concentration on LDH release (F) and MTT activity (G) following 5mM H2O2 treatment of Hepa1-6 cells. (H–I) Effects of 100uM H2S addition on LDH release (H) and MTT activity (I) following 1mM H2O2 treatment of primary mouse vascular smooth muscle cells. Pound sign indicates the significance of the difference between H2O2 alone vs. H2O2 plus H2S; #p<0.05.
(A–B) H2S production capacity in extracts of kidney, spleen, carotid artery, brain, skeletal muscle, heart, and aorta from mice with AL or restricted access (DR) to complete or protein free (PF) diets as indicated. Extracts were normalized for protein content or organ weight; each circle represents H2S production from an individual animal as measured by the lead sulfide method. (C) H2S production from primary aorta smooth muscle cells after overnight preconditioning in complete or media lacking Met&Cys. (D) H2S production from brains after AL feeding or 3d water-only fasting. (E) Effects of RNAi-mediated knockdown of individual TSP components on longevity of wildtype N2 worms compared to empty vector (EV). (F–G) H2S production from three different wildtype yeast strains as a function of glucose concentration and time in culture as detected by black lead sulfide accumulation on lead acetate strips placed in the growth flasks (F) or square lead acetate papers inserted into the caps of the growth flasks for the indicated time (G). (H) Chronological lifespans of the indicated WT or sulfur assimilatory pathway mutants grown in 2.0% (left) or 0.5% (right) glucose.