Abstract
Background
The temporal and situational stability of personality has led generations of researchers to hypothesise that personality may have enduring effects on health, but the biological mechanisms of such relationships remain poorly understood. In the present study, we utilized a functional genomics approach to examine the relationship between the 5 major dimensions of personality and patterns of gene expression as predicted by ‘behavioural immune response’ theory. We specifically focussed on two sets of genes previously linked to stress, threat, and adverse socio-environmental conditions: pro-inflammatory genes and genes involved in Type I interferon and antibody responses.
Methods
An opportunity sample of 121 healthy individuals was recruited (86 females; mean age 24 years). Individuals completed a validated measure of personality; questions relating to current health behaviours; and provided a 5 ml sample of peripheral blood for gene expression analysis.
Results
Extraversion was associated with increased expression of pro-inflammatory genes and Conscientiousness was associated with reduced expression of pro-inflammatory genes. Both associations were independent of health behaviours, negative affect, and leukocyte subset distributions. Antiviral and antibody-related gene expression was not associated with any personality dimension.
Conclusions
The present data shed new light on the long-observed epidemiological associations between personality, physical health, and human longevity. Further research is required to elucidate the biological mechanisms underlying these associations.
Keywords: personality, gene expression, antiviral, antibody, immunity, pro-inflammatory
1 Introduction
Personality is commonly defined as a cluster of individual psychological attributes (e.g., thoughts, feelings and behaviours), which are typically stable across time and situations and give rise to enduring individual differences. Multivariate analyses have generally identified 5 major dimensions of human personality – Neuroticism, marked by a tendency toward negative affect; Extraversion characterized by high levels of sociability and appetitive motivation; Openness to Experience, which reflects general curiosity, creativity, and an orientation toward intellectual and aesthetic pursuits; Agreeableness, reflecting general likability and even temperament; and Conscientiousness, reflecting planfulness, caution, and harm avoidance (MCrae & Costa, 2004). The temporal and situational stability of personality has led generations of researchers to hypothesise that personality may have enduring effects on health (Eysenck, 1991; Friedman, 2008). Some large epidemiological studies have found associations between personality characteristics and measures of disease or longevity (Weiss & Costa, 2005; Nakaya et al., 2010; Chapman et al., 2011), but the biological mechanisms of such relationships remain poorly understood.
Several causal models have been invoked to explain associations between personality and health (Friedman, 2008), including common causation by underlying individual differences in genetics or early life experience (Cohen et al., 2003; MacMurray et al., 2013; Napolioni et al., 2014); effects of personality on health behaviours (e.g., smoking, alcohol consumption, physical activity: Turiano et al., 2012); differential proclivity to risky situations or environments (Cohen et al., 2003); effects of personality-related stress responses on biological function (Vedhara & Irwin, 2005; Miller et al., 2009a), and reverse causation of individual differences in behaviour by individual differences in health or inflammatory biology (Dantzer, et al., 2008; Eisenberger et al., 2010).
One body of theoretical analysis suggests that individual differences in the vigour of biological immune responses may come to be associated with individual differences in personality traits that serve as a sort of ‘behavioural immune response’ (Schaller & Murray, 2008; Thornhill et al., 2010; Schaller, 2011). According to this theoretical approach, individuals who have relatively weak biological immune responses are hypothesized to show stronger behavioural immune responses such as avoidance of strangers (i.e., Introversion), reduced exploratory behaviour (i.e., low Openness to experience), and greater harm-avoidant behaviour (i.e., Conscientiousness). Recent genetic association studies have supported this hypothesis in documenting increased levels of Introversion in people carrying immune response gene polymorphisms that confer increased vulnerability to infectious diseases (MacMurray et al., 2013; Napolioni et al., 2014). Conversely, allostatic physiology (Sterling, 2004) suggests that biological immune defences may be up-regulated in individuals who experience extended exposure to threat or stress and might thus experience an elevated risk of injury or infection, or in highly sociable individuals who face increased exposure to communicable diseases (Cole et al., 2011; Cole, 2013; Slavich & Cole, 2013).
Despite a wealth of theoretical explanation for links between personality and health, the biological mechanisms mediating those relationships remain poorly defined. Some studies have reported associations between measures of personality and specific endocrine and immune parameters such as catecholamine neurotransmitters from the sympathetic nervous system (SNS) and glucocorticoid hormones from the Hypothalamic-Pituitary-Adrenal (HPA)-axis (Miller et al., 1999; Rutledge, 2006; Molloy et al., 2008; Reinhard et al., 2012). Studies have also reported associations between some personality dimensions such as Extraversion and Conscientiousness and expression of pro-inflammatory mediators (e.g., cytokines) or biomarkers (e.g., C-reactive protein) that have been linked in particular to aging and longevity (Finch, 2007; Chapman et al., 2009; Sutin et al., 2010; Millar et al., 2013). However, little work has directly examined the leukocyte gene regulatory systems that govern immune cell function and thus regulate the inflammatory dynamics that contribute to disease (e.g., cardiovascular disease, cancer incidence and progression, neurodegenerative diseases) or the antibody- and antiviral gene expression programs that mediate host resistance to infection.
In the present study, we utilized a functional genomics approach to identify relationships between the 5 major dimensions of human individual differences in personality and broad patterns of gene expression in human leukocytes. Previous research has linked stress, threat, and adverse socio-environmental conditions to a conserved transcriptional response to adversity (CTRA) characterized by up-regulated transcription of pro-inflammatory genes and a complementary down-regulation of genes mediating the production of Type I interferon antiviral responses and IgG antibodies (Irwin & Cole, 2011; Antoni et al., 2012; Cole et al., 2012; Cole, 2013). This profile is hypothesized to represent an anticipatory immunological response to increased risk of injury during periods of experienced threat (Irwin & Cole, 2011; Antoni et al., 2012; Cole et al., 2012; Cole, 2013). Based on the ‘behavioural immune response’ theory (Schaller & Murray, 2008; Thornhill et al., 2010; Schaller, 2011), we hypothesized that pro-inflammatory gene expression would be up-regulated in extraverts and people with high levels of openness to experience (both of whom would be expected to experience elevated risk of injury/infection) and down-regulated in conscientious individuals with comparatively strong behavioural immune responses. Antiviral/antibody gene expression tends to be inversely associated with pro-inflammatory gene expression, so we hypothesised reduced expression of these genes in people with high levels of Extraversion and Openness, and elevated expression in Conscientious individuals. We tested these hypotheses in a healthy sample of young adults to limit possible reverse causation of social behaviour/personality by pre-existing illness (Eysenck, 1991) using the standard NEO Five Factor Inventory (NEO-FFI; McCrae & Costa, 2004) to assess the major dimensions of human personality.
2 Methods
2.1 Participants & Recruitment
An opportunity sample of 121 individuals was recruited from a UK higher education establishment. The cohort consisted of 86 females and 35 males with an average age of 24 years (range 18–59, standard deviation 7.7, 90% aged 18–33) and a mean body mass index of 23.3 (standard deviation 3.76). The cohort was ethnically diverse, including 49% (n = 61) who described their ethnic status as White, 39% (n = 47) who identified as Asian British, and other ethnic groups representing less than 5% of the sample. The vast majority of participants were students (n = 110), with the remainder describing themselves as ‘employed’. Individuals were recruited in response to posters, leaflets and emails promoting an opportunity to participate in a study looking at the relationship between personality and health. All interested participants received a participant information sheet and provided written informed consent prior to participation. Study participation criteria excluded individuals with concurrent acute or chronic illnesses and those who did not speak English. Illness was assessed by self-report on the day of study participation. All screened respondents met both inclusion criteria, and so no respondents were excluded. Participation was voluntary and no monetary compensation was provided. The study received ethical and regulatory approval from the Institute of Work, Health & Organisations ethics committee (University of Nottingham, UK) and the Department of Research and Innovation (Queen’s Medical Centre, Nottingham, UK). The authors assert that all procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
2.2 Procedure
All individuals who provided written informed consent were requested to complete a questionnaire booklet which contained the battery of instruments outlined below. This was followed by the collection of a blood sample by a trained phlebotomist. All blood samples were treated as described below and stored at −70°C until assayed in batch following the end of recruitment.
2.3 Materials
2.3.1 Personality
Personality was measured using the NEO-FFI (McCrae & Costa, 2004). This instrument is a 60 item measure of personality derived from the 240 item Revised NEO Personality Inventory (Costa & McCrae, 1992); and it measures the personality dimensions of Extraversion, Neuroticism, Openness, Agreeableness and Conscientiousness. The scale has been recommended for use in exploratory personality research, as in the present study (McCrae & Costa, 2004). Cronbach alphas were computed to examine the reliability of each subscale. The results revealed evidence of modest to good reliability for all dimensions (Openness: α = .63; Agreeableness: α = .69; Conscientiousness: α = .84; Neuroticism: α = .80; Extraversion: α = .78). Distributions were assessed for consistency with normality using quantile-quantile plots with normal scores correlation values > .97.
2.3.2 Health behaviours, medication use, and negative affect
Participants’ typical smoking, drinking and exercise behaviour was captured by items asking respondents to indicate their usual levels of these behaviours on a daily basis (e.g., On average, I smoke __ cigarettes per day / drink __ units per day / exercise __ hours per day). Current medication use was measured by free-response self-report of medication name and minor symptom indication (e.g., acetaminophen for headache, anti-histamines for hayfever, etc.). Negative affect was measured by the well-validated PANAS Scales (Watson, Clark & Tellegen, 1988).
2.3.3 Blood sampling and gene expression
The analysis of gene expression required the collection and preparation of a 5 ml sample of peripheral blood. All blood samples were diluted with Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma) and subject to Ficoll density gradient centrifugation to isolate peripheral blood mononuclear cells (PBMC). The resultant cell pellet was removed and suspended in 1.5 ml of Qiagen RNeasy Lysis Buffer (RLT) and stored at −70°C to ensure RNA stabilisation. Samples were then shipped in a batch on dry ice to the UCLA Social Genomics Core Laboratory for gene expression analysis. RNA was extracted (Qiagen QIAcube), tested for suitable mass (Nanodrop ND1000) and integrity (Agilent Bioanalyzer), converted to fluorescent cDNA (NuGEN PicoSL) and hybridized to Illumina Human HT-12 v4 BeadArrays following the manufacturer’s standard protocol in the UCLA Neuroscience Genomics Core Laboratory. Two samples yielded insufficient RNA for analysis. All remaining samples were assayed in a single batch and yielded valid results according to standard quality assurance methods (e.g., median probe fluorescence intensity > 100 units). The microarray-based transcriptome profiling approach used here does not require any normalization to a specific internal housekeeping control because the quantile-based data normalization employed at the outset of data analysis (see below) standardizes total assayed RNA levels across samples at the level of the whole transcriptome (Bolstad et al. 2003).
2.4 Statistical analysis
Gene expression values were quantile-normalized (Bolstad et al., 2003) and log2-transformed for primary general linear model analyses quantifying association of CTRA-related transcript expression with continuous (z-score) measures of each of the 5 NEO personality traits (treated as simultaneous / competing predictors, i.e., adjusted for one another) while controlling for age, sex, and race (white vs. non). Effect sizes for the resulting linear model coefficients (metric: difference in predicted gene expression per personality SD) were graphed as the range-spanning magnitude of difference in predicted gene expression values over the standardized range of observed continuous personality scores (i.e., comparing predicted gene expression at a value of +2 SD above mean personality score with that at a value of 2 SD below mean personality score, corresponding to predicted change in gene expression over the 4 SD range that captures 95% of observations in a normal distribution). Follow-up secondary analyses tested for contributions of health behaviours to personality-related differences in gene expression through additional control for body mass index (BMI), alcohol consumption (standard units per day), smoking (cigarettes per day), physical activity (hours per day), and minor symptoms (e.g., headache, hayfever). To determine whether personality effects might be mediated in part by differences in leukocyte subset composition of the PBMC pool (Cole, 2010), additional secondary analyses controlled for the prevalence of transcripts marking T lymphocytes subsets (CD3D, CD3E, CD4, CD8A), B lymphocytes (CD19), NK cells (CD16/FCGR3A, CD56/NCAM1), and monocytes (CD14). Gene expression data are publicly available as Gene Expression Omnibus series GSE60491.
Primary analyses focused on 2 a priori-defined sets of genes: 19 pro-inflammatory genes (IL1A, IL1B, IL6, IL8, TNF, PTGS1, PTGS2, FOS, FOSB, FOSL1, FOSL2, JUN, JUNB, JUND, NFKB1, NFKB2, REL, RELA, RELB), and 34 genes involved in Type I interferon responses (GBP1, IFI16, IFI27, IFI27L1-2, IFI30, IFI35, IFI44, IFI44L, IFI6, IFIH1, IFIT1-3, IFIT5, IFIT1L, IFITM1-3, IFITM4P, IFITM5, IFNB1, IRF2, IRF7-8, MX1-2, OAS1-3, OASL) and antibody synthesis (IGJ, IGLL1, IGLL3) (Fredrickson et al., 2013). For each gene set, we conducted a pooled average association analysis in which partial regression coefficients relating each transcript’s (log2) abundance to a given dimension of personality were averaged to produce a summary estimate of total CTRA gene set association (i.e., a simple sum of random variables in the form of regression coefficients (Ewens & Grant, 2005). That pooled association estimate was tested for statistically significant difference from 0 (null association) using a single-sample t-test with bootstrap standard errors (200 cycles of re-sampled residual vectors, which controls for any potential correlation among residuals across genes; Efron & Tibshirani, 1993).
Additional ancillary analyses sought to identify transcription control pathways that may mediate observed transcriptional differences associated with personality dimensions that reached statistical significance in primary analyses. Initial low level genome-wide analyses identified all transcripts showing a point estimate of ≥ 1.25-fold differential expression across the range −2 SD to +2 SD relative to mean personality z-score (each adjusted for the other major dimensions of personality and for the demographic, health behaviour, and minor symptom covariates listed above), and those putatively associated genes were subject to TELiS promoter-based bioinformatic analysis (Cole et al., 2005) to assess activity of NF- κB and Interferon Response Factor family transcription factors previously linked to CTRA transcriptional dynamics (TRANSFAC V$CREL_01, V$IRF1_01) and CREB and glucocorticoid receptor (GR) transcription factors (V$CREB_Q4, V$GR_Q6) mediating SNS and HPA axis signalling, respectively (Cole et al., 2005; Fredrickson et al., 2013) with results averaged over 9 parametric variations of MatInspector scan stringency and promoter length. Transcript Origin Analysis was also applied to the low-level association data to identify the specific PBMC subtypes mediating the observed differences in gene expression, as previously described (Cole et al., 2011). Transcript Origin Analysis seek to identify the specific leukocyte subsets (e.g., CD4+ or CD8+ T cells, B cells, NK cells, monocytes, plasmacytoid dendritic cells) that contribute to the observed transcriptome differences by quantifying the extent to which the genes that show empirical differential expression in the present study of a heterogeneous leukocyte pool are expressed predominately by a single type of cell in a previously conducted reference study of physically isolated cell types (Cole et al., 2011). Low-level transcript-phenotype associations (Supporting Dataset S1) were estimated solely to provide inputs into high-level TELiS and Transcript Origin Analysis gene set analyses and were not tested for statistical reliability at the level of individual genes. Ancillary analyses were conducted under a protected testing strategy in which specific personality dimensions were subject to additional TELiS and Transcript Origin analyses only if they reached statistical significance in one of the primary analyses focusing on the a priori proinflammatory and antiviral/antibody gene sets.
Missing gene expression data (n = 2) and ethnicity data (n = 2) reduced the primary analysed sample size to 117. Missing BMI data (n = 2) additionally reduced the sample size to 115 in analyses controlling for health behaviour. Throughout all analyses, the statistical significance level was set at p < .05, and analyses followed established statistical guidelines (Cao & Zhang, 2014) in controlling for multiple comparisons in exploratory analyses of related hypotheses (e.g., correlations among personality dimensions) but not in analyses of distinct substantive hypotheses (e.g., testing associations between specific personality dimensions and specific a priori predicted differences in gene expression).
3. Results
3.1 Participant characteristics
The analysed sample involved 121 currently healthy adults (no self-reported acute or chronic illness) who were predominately young (mean = 24.3 years; 90% between 18 and 33), female (73%), and ethnically diverse (51% non-white), with low levels of alcohol consumption and smoking and modest levels of exercise (approaching 1 hour per day). Table 1 shows the mean and standard deviation scores for the measures of personality and behaviour. Across all 5 personality dimensions, mean scores were just above the mid-point of the scales, with the lowest scores observed for Neuroticism and highest scores for Agreeableness and Conscientiousness. All 5 personality dimensions showed approximately normal distributions as determined by near-linearity of quantile-quantile plots and normal score correlations > .97.
Table 1.
Descriptive statistics for behaviour and the NEO FFI measures of personality
| Mean (Standard Deviation) | |
|---|---|
| Neuroticism (N) | 33.6 (7.9) |
| Extraversion (E) | 41.9 (6.8) |
| Openness (O) | 41.3 (6.4) |
| Agreeableness (A) | 45.1 (6.3) |
| Conscientiousness (C) | 44.9 (7.7) |
| Cigarettes per day | 0.4 (2.3) |
| Units of alcohol per day | 0.5 (0.9) |
| Hours of exercise per day | 0.8 (0.6) |
Table 2 reports correlations among the 5 major dimensions of personality. As expected, none of the dimensions was strongly correlated with any of the others, with the vast majority of correlations failing to exceed r = .30 (or a Bonferroni-corrected p < .05). However, Neuroticism showed a moderate inverse correlation with Extraversion (r = −.39, p < .0001) and with Conscientiousness (r = − .31, p = .001).
Table 2.
Pearson correlations among personality dimensions
| Extraversion | Openness | Agreeableness | Conscientiousness | |
|---|---|---|---|---|
| Neuroticism | −0.387 | 0.061 | −0.038 | −0.312 |
| p < .001 | p = .506 | p = .677 | p = .001 | |
| Extraversion | 0.220 | 0.267 | 0.191 | |
| p = .015 | p = .003 | p = .036 | ||
| Openness | 0.220 | 0.072 | −0.039 | |
| p = .015 | p = .434 | p = .674 | ||
| Agreeableness | 0.267 | 0.072 | 0.074 | |
| P = .003 | P = .434 | p = .421 | ||
| Conscientiousness | 0.191 | −0.039 | 0.074 | |
| p = .036 | p = .674 | p = .421 |
3.2 CTRA Gene Expression
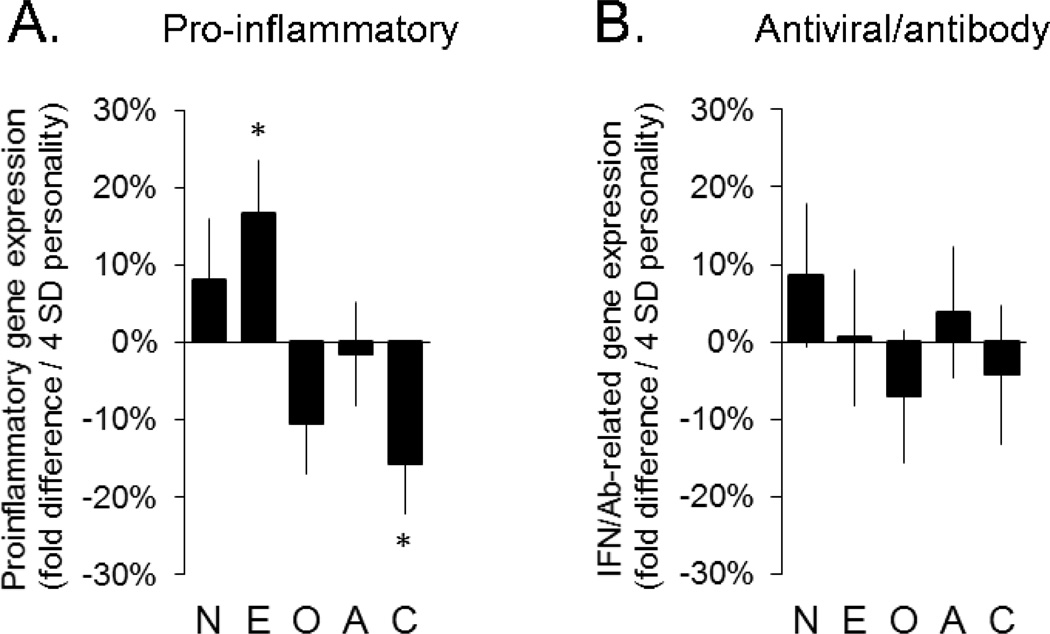
Primary analyses examined the relationships between continuously measured individual differences on the 5 major personality dimensions and the quantitative expression of 2 a priori-defined components of the CTRA gene expression profile (Fredrickson et al., 2013; Slavich & Cole, 2013): up-regulated expression of pro-inflammatory genes, and down-regulated expression of genes mediating Type I interferon antiviral responses and antibody synthesis. In analyses controlling for demographic characteristics (age, sex, race), Extraversion was associated with significantly greater expression of pro-inflammatory genes (p = .030; Figure 1). To provide an intuitive effect size for the magnitude of this continuous association, a hypothetical Extravert (2 SD above mean score) would be expected to show a 16.7% (± 7.9%) greater average expression of the 19 pro-inflammatory gene transcripts than a hypothetical Introvert (2 SD below mean score). Pro-inflammatory gene expression was also down-regulated in association with Conscientiousness (−15.7% ± 5.4% over the [−2SD, +2SD] range, p = .013). Openness to experience showed a non-significant trend toward decreased pro-inflammatory gene expression (−10.6% ± 5.7%, p = .087), and no significant pro-inflammatory gene expression association was observed for any of the other personality dimensions for which the behavioural immune system hypothesis makes no predictions (Neuroticism and Agreeableness, both p > .30; Figure 1). Expression of antiviral/antibody-related genes was not significantly associated with any personality dimension (Figure 1).
Figure 1.
Difference in predicted pro-inflammatory (1a) and antiviral/antibody-related (1b) gene expression at 2 SDs above vs. 2 SDs below the mean on each continuously measured dimension of personality (n = 121)
N = Neuroticism; E = Extraversion; O = Openness; A = Agreeableness; C=Conscientiousness
* p < .05
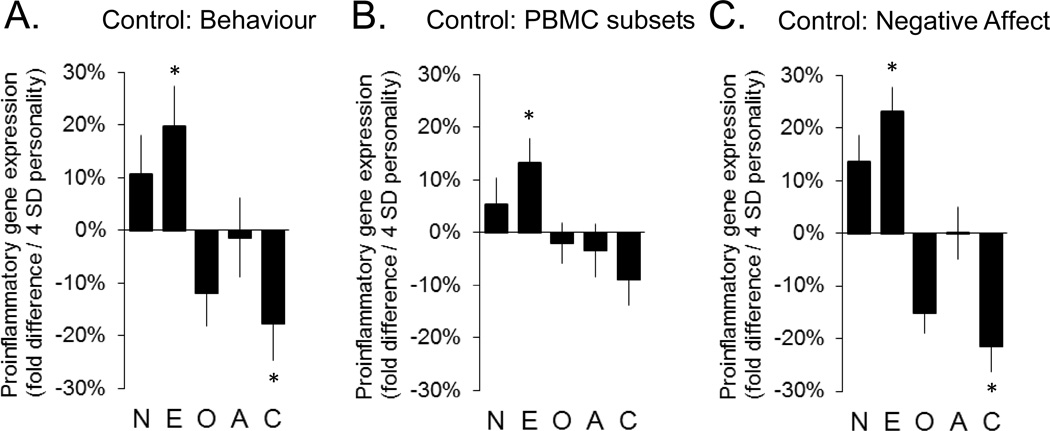
To determine whether individual differences in health behaviours might contribute to the observed associations between personality and pro-inflammatory gene expression, we conducted secondary analyses controlling for BMI, smoking, alcohol consumption, physical activity levels, and minor physical symptoms (e.g., headache, hayfever). Results (Figure 2a) continued to show a significant positive association with Extraversion (+19.9% ± 9.1%, p = .023) and a significant negative association with Conscientiousness (−17.6% ± 5.8%, p = .010). Openness also continued to trend toward reduced expression of pro-inflammatory genes (−11.9% ± 5.6%, p = .055), and Neuroticism and Agreeableness remained unassociated with gene expression (both p > .10).
Figure 2.
Association of pro-inflammatory gene expression with personality dimensions after additional control for health behaviours and minor physical symptoms (2a), leukocyte subsets (2b), and negative affect (2c)
N = Neuroticism; E = Extraversion; O = Openness; A = Agreeableness; C=Conscientiousness
* p < .05
To assess whether individual differences in leukocyte subset distributions might contribute to the observed differences in the aggregate PBMC transcriptome, an additional set of secondary analyses controlled for variations in the prevalence of mRNA markers for T lymphocytes (both total and CD4+ and CD8+ subsets; CD3D, CD3E, CD4, CD8A), B lymphocytes (CD19), NK cells (CD16/FCGR3A, CD56/NCAM1), and monocytes (CD14). Results (see Figure 2b) continued to link Extraversion to increased pro-inflammatory gene expression (+13.3% ± 5.2%, p = .013) and showed a marginally significant association of Conscientiousness with reduced pro-inflammatory gene expression (−9.0% ± 4.3%, p = .057). Neuroticism, Openness, and Agreeableness all remained unassociated in analyses controlling for leukocyte subsets (all p > .30).
As shown in Figure 2c, secondary analyses that additionally controlled for symptoms of stress, depression, anxiety, or other negative affective states (as measured by the PANAS Negative Affect scales) also continued to link Extraversion to increased pro-inflammatory gene expression (+17.4% ± 8.4%, p = .032) and Conscientiousness to reduced pro-inflammatory gene expression (−13.8% ± 5.9%, p = .049). Neuroticism, Openness, Agreeableness and negative affect were each unassociated in these analyses (all p > .10).
Similar results also emerged in secondary analyses that included 3 indicator covariates capturing the 2 most prevalent types of self-reported medication use (oral birth control pills: 12.7%, n = 17; antidepressants: 5.9%, n = 7) and a 3rd indicator representing miscellaneous other types of medications used (18.7%, n = 22; e.g. antihistamines for hayfever and oral or topical acne medications). Extraversion continued to associate with increased pro-inflammatory gene expression (+15.4% ± 8.1%, p = .047) and Conscientiousness to reduced pro-inflammatory gene expression (− 16.1% ± 5.6%, p = .014). Neuroticism, Openness, Agreeableness and all 3 medication indicators were unassociated with gene expression (all p > .10).
3.3 Cellular origins
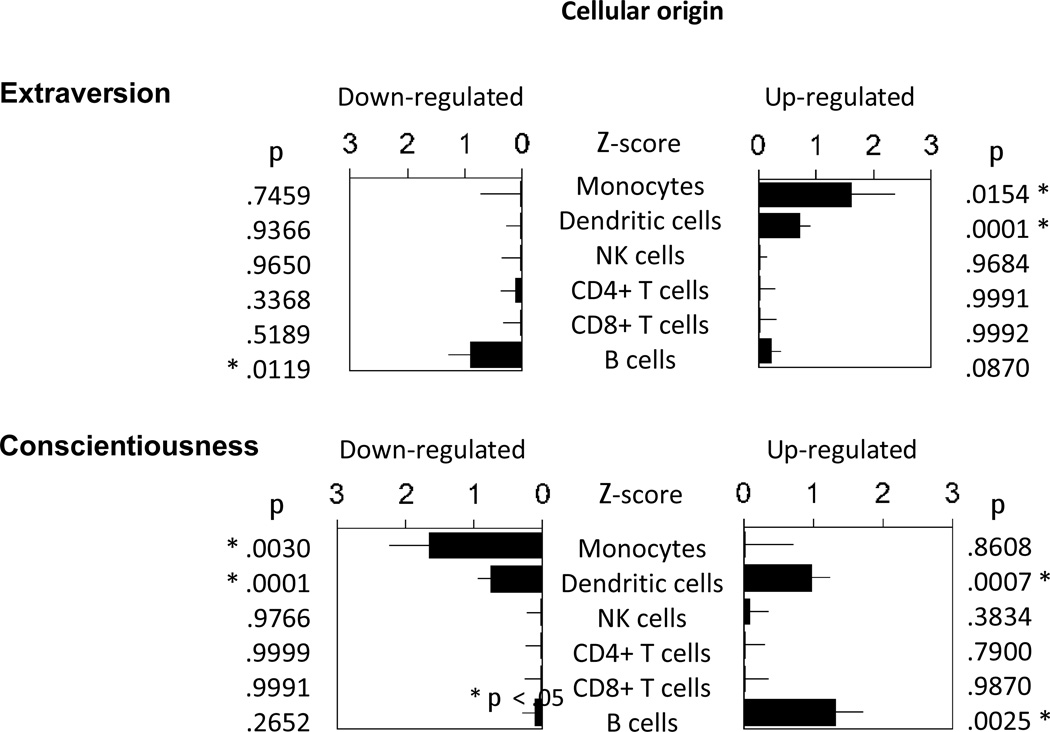
To determine whether personality-related transcriptome differences might occur within the same leukocyte subpopulations previously shown to mediate the CTRA transcriptional effects of adverse social environments (i.e., monocytes, dendritic cells, and to a lesser extent B lymphocytes: Miller et al., 2008; O’Donovan, 2011; Cole et al., 2011; Antoni et al., 2012; Cole et al., 2012), we conducted Transcript Origin Analysis (Cole et al., 2011) on all genes found to show associations that would imply ≥ 1.25-fold differential expression across the range −2 SD to +2 SD on Extraversion and Conscientiousness (Supporting Dataset S1). Results identified monocytes and plasmacytoid dendritic cells as primary carriers of genes up-regulated in association with Extraversion and of genes down- regulated in association with Conscientiousness (Figure 3). Genes down-regulated in association with Extraversion and up-regulated in association with Conscientiousness derived from B cells.
Figure 3.
Transcript Origin Analysis of genes showing ≥ 1.25-fold differential expression across the range −2 SD to +2 SD on Extraversion and Conscientiousness
3.4 Transcription control pathways
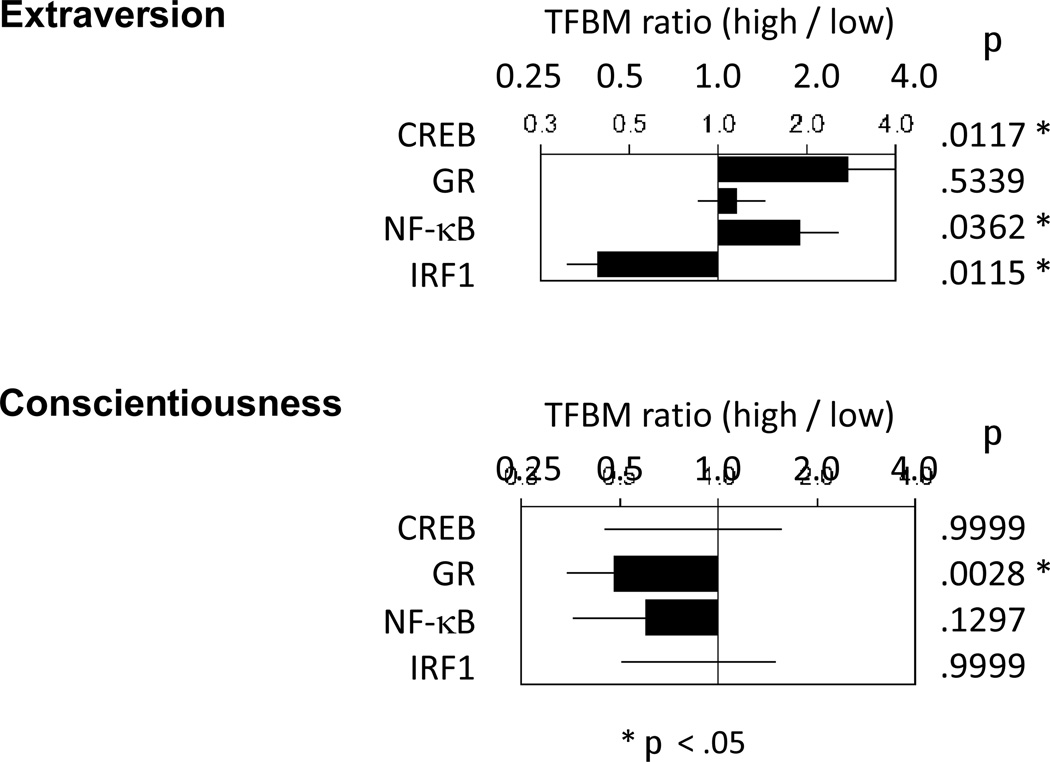
To assess the role of neuroendocrine-responsive and immunoregulatory transcription factors that have previously been linked to the CTRA gene expression profile (Bierhaus et al., 2003; Miller at al., 2008; Miller et al., 2009b; Antoni et al., 2012; Cole et al., 2012; Fredrickson et al., 2013), we conducted TELiS bioinformatics on genes differentially expressed in association with Extraversion and Conscientiousness (Figure 4). Consistent with a potential contribution from sympathetic nervous system signalling through beta-adrenergic receptors, results identified an increased prevalence of response elements for the CREB family of transcription factors in the promoters of genes up-regulated in association with Extraversion (p = .012). However, there was no significant association of Extraversion-related gene expression with promoter response elements for the glucocorticoid receptor (p = .5339). Consistent with the general association of Extraversion with a pro-inflammatory transcriptome shift, results also indicated elevated prevalence of promoter response elements for the key pro-inflammatory transcription factor NF-κB (p = .036) and reduced prevalence of promoter response elements for Interferon Response Factors (p = .012). Parallel analyses of Conscientiousness-related gene transcripts indicated no role for CREB but a significant down-regulation of glucocorticoid receptor activity (p = .003). Prevalence of promoter elements for the pro-inflammatory transcription factor NF- κB and the Interferon Response Factor family did not differ as a function of Conscientiousness (both p > .10).
Figure 4.
Results from TELiS bioinformatics on genes differentially expressed in association with Extraversion and Conscientiousness
4. Discussion
The present results identified systematic differences in leukocyte gene expression that correlate with individual differences on two major dimensions of human personality: Extraversion and Conscientiousness. Consistent with predictions from behavioural immune response theory (Schaller & Murray, 2008; Thornhill et al., 2010; Schaller, 2011), Extraversion was associated with up-regulated expression of pro-inflammatory genes, whereas Conscientiousness was associated with down-regulated expression of pro-inflammatory genes. These effects were independent of major health behavioural factors (BMI, smoking, alcohol consumption, physical activity); independent of variations in leukocyte subset prevalence; independent of negative affect; independent of minor physical symptoms and related medications; and independent of demographic characteristics as well as other major dimensions of human personality. In contrast, none of the major personality dimensions was significantly associated with differential expression of the other primary gene module involved in the CTRA profile – antiviral and antibody-related transcripts. In the context of previous data linking Extraversion and Conscientiousness to health and longevity (Chapman et al., 2011; Ferguson & Bibby, 2012), the present functional genomics findings may provide new insights into the molecular basis for such relationships.
The pattern of associations between leukocyte CTRA gene expression and specific dimensions of human personality are largely as would be predicted by the behavioural immune response hypothesis i.e., that stable individual differences in behaviour arise in response to stable individual differences in biological vulnerability to infection (Schaller & Murray, 2008; Thornhill et al., 2010; Schaller, 2011; MacMurray et al., 2013; Napolioni et al., 2014). Under this scenario, low levels of pro-inflammatory gene expression would be construed as a relatively weak biological immune defence that selects for relatively cautious behavioural tendencies including introverted behaviour (to reduce exposure to socially transmitted infections) and conscientious behaviour (to reduce exposure to risky situations or behaviours). However, the present pattern of results are also consistent with the converse neuro-immune allostasis theory which suggests that the immune system’s basal biological setpoint may adapt to individual differences in behaviour (rather than vice versa, as proposed by behavioural immune response theory: Cole et al., 2011; Irwin & Cole, 2011; Dhabar et al., 2012; Cole, 2013; Slavich & Cole, 2013 Powell et al., 2013). Under the neuro-immune allostasis theory, individuals chronically exposed to novel or risky situations (e.g., extraverts and impulsive/unconscientious people) activate nervous system-mediated signals that up-regulate the CTRA ‘defensive response’ in anticipation of injury: e.g., via SNS transcriptional induction of pro-inflammatory genes (Bierhaus et al., 2003; Cole et al., 2010; Irwin & Cole, 2011) or SNS-development and mobilization of specific leukocyte subsets into circulation and tissue (Dhabar et al., 2012; Powell et al. 2013). Given the key role of the SNS in mediating such immunologic anticipatory responses, it is notable that the SNS/beta-adrenergic-responsive CREB transcription factor family was implicated in structuring Extraversion-related gene expression differences. The indicated role of monocytes and dendritic cells in the present personality-related transcriptome differences is also consistent with previous data showing that the SNS can stimulate myelopoietic production of monocytes and dendritic cells (Powell et al., 2013). Such a dynamic would explain results from transcript origin analyses that implicate dendritic cells in both gene up-regulation (i.e., transcripts associated with developmental immaturity) and gene down-regulation (i.e., transcripts associated with developmental maturity and terminal differentiation). Ultimately, however, the cross-sectional design of this study cannot decisively discriminate among these two reciprocal hypotheses of behavioural immune response theory and allostatic immunology. Experimental manipulations of personality and/or pro-inflammatory gene expression would be required to definitively establish their relative causal roles.
The present data do not provide any results in support of a ‘disease-prone personality’ characterized by high levels of general negative affectivity (i.e., Neuroticism). Regardless of the nature of variables controlled for (demographic, behavioural, leukocyte subset individual differences, etc.), the results consistently failed to identify any significant associations between CTRA gene expression and Neuroticism. Moreover, a direct measure of negative affectivity in the PANAS negative affect scale also showed no significant association with gene expression. This lack of association does not rule out the possibility that Neuroticism or negative affect might associate with expression of other genes that lie outside the CTRA profile and remain to be identified in future analyses (as the present sample is not sufficiently powered for such genome-wide discovery analyses). However, the present data do suggest one potential reason why previous research may have found associations between Neuroticism and health (e.g., Hagger-Johnson, et al., 2012). Neuroticism shows a moderately strong inverse correlation with both of the personality dimensions that do show significant correlations with pro-inflammatory gene expression; Extraversion and Conscientiousness. To the extent that previous studies did not control for such associations, the biological and health correlates of Extraversion and Conscientiousness may have been misattributed to Neuroticism through confounding. These findings underscore the need to assess personality comprehensively (i.e., at the level of the ‘Big 5’ major personality dimensions) and assess its effects in multivariate analyses that control for potential confounding (Segerstrom, 2000). All of the present analyses control for confounding among major personality dimensions, and future research should do so as well because each major personality dimension showed at least one nominally statistically significant correlation with another major dimension of personality (Table 2). The absence of an association between pro-inflammatory gene expression and Neuroticism is also inconsistent with the hypothesis that individual differences in chronic inflammation play a major role in shaping human personality by regulating CNS function and promoting negative affective traits (Dantzer et al., 2008) and decreased sociability (Eisenberger et al., 2010). These data link elevated pro-inflammatory gene expression to comparatively low levels of Introversion (i.e., to Extraversion), rather than to the higher levels of Introversion and Neuroticism that would be predicted based on the known effects of pro-inflammatory cytokines on neural function and behaviour (Dantzer et al., 2008).
Beyond the cross-sectional nature of these results, the present findings are also limited in several other respects. First, these data come from a moderately sized convenience sample of UK university students, and the generality of these findings remains to be verified in other populations. Second, although data on health behaviours, medication use, and current acute or chronic illnesses were collected in this study, information on past health was not. Therefore, it remains unclear whether personality-related differences in minor illness risk might induce an association between gene expression and personality within this broadly healthy sample of young adults. Third, no direct measures of immune system functional activity were available in this sample (e.g., effector response to an immunologic challenge), and many post-transcriptional processes can modify the functional significance of differences in mRNA expression. As such, the functional immunologic significance and the human health significance of these gene expression dynamics remains to be determined in future research. However, the present results linking Introversion to altered leukocyte gene expression profiles are consistent with previous observations that Introverts show increased vulnerability to viral infections (Totman et al., 1980; Broadbent et al., 1984; Capitanio et al., 1999; Cohen et al., 2003); that Extraverts show comparatively high levels of inflammatory biomarkers (Millar et al., 2013; although other studies disagree, e.g., Chapman et al., 2009); and that Conscientious individuals show comparatively low levels of chronic inflammation (Sutin et al., 2010; Mottus et al., 2013; Turiano et al., 2013). Fourth, this study was not designed for de novo discovery of reliable associations between specific individual gene transcripts and personality dimensions. Thus, the low-level point estimates of association in Dataset S1 serve only as inputs into high-level bioinformatics analyses of CTRA-related cell types and transcription factors and should not be considered statistically reliable at the level of individual gene level. Although the high-level bioinformatic findings are consistent with previous direct analyses of target cell type and transcription factor activity (Irwin & Cole, 2011; Cole, 2012; Powell et al., 2013), these biological inferences require direct verification in future studies. The gene expression dynamics observed here can only be interpreted in the context of immune cells, and personality associations with transcriptomes in the central nervous system and other tissues remain an important topic for future research.
In summary, the present results support both behavioural immune response theory (Schaller, 2011) and neuro-immune allostatic theories (Cole, 2013) in identifying significant associations between leukocyte gene expression profiles and two major dimensions of human personality: Extraversion and Conscientiousness. These relationships were independent of major demographic dimensions (age, sex, race), independent of the effects of incident disease (which was controlled by sampling a young healthy university student population and additional statistical adjustments for any minor physical symptoms), independent of negative affective states, and independent of individual differences in the prevalence of leukocyte subsets within the PBMC pool. Although the biological mechanisms of these associations remain to be defined in future research, the present data may shed new light on the long-observed epidemiological associations between personality, physical health, and human longevity.
Supplementary Material
Highlights.
We examined the relationship between personality and patterns of gene expression
Extraversion was associated with increased expression of pro-inflammatory genes
Conscientiousness was associated with reduced expression of pro-inflammatory genes
The associations were independent of health behaviours and leukocyte subsets
Acknowledgments
We would like to thank Ms Nichola Farrar for technical assistance.
Financial support: This research was supported grants from the United States National Institutes of Health (P30 AG107265). The funders had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
Authors’ declarations
All the authors have materially participated in the research described in this manuscript and/or its preparation. The named authors have also reviewed and approved the final article. The following specific contributions have been made:
KV: the conception and design of the study, acquisition, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content and final approval of the submitted version.
SG: the design of the study, acquisition of data; drafting the article and final approval of the submitted version.
LE: the analysis and interpretation of data; revising the article critically for important intellectual content and; final approval of the submitted version.
BKC: KV: the design of the study, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content and final approval of the submitted version.
JMGA: the analysis and interpretation of data, (2) revising the article critically for important intellectual content and (3) final approval of the submitted version.
JM: the analysis and interpretation of data; revising the article critically for important intellectual content and final approval of the submitted version.
SWC: the conception and design of the study, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content and final approval of the submitted version.
References
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JM, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol. Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MH, Phillpotts RJ, Wallace J. Some further studies on the prediction of experimental colds in volunteers by psychological factors. J. Psychosom. Res. 1984;28:511–523. doi: 10.1016/0022-3999(84)90085-0. [DOI] [PubMed] [Google Scholar]
- Cao J, Zhang S. Multiple comparison procedures. J. Am. Med. Assoc. 2014;312:543–544. doi: 10.1001/jama.2014.9440. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Baroncelli S. The relationship of personality dimensions in adult male rhesus macaques to progression of simian immunodeficiency virus disease. Brain Behav. Immun. 1999;13:138–154. doi: 10.1006/brbi.1998.0540. [DOI] [PubMed] [Google Scholar]
- Chapman BP, Khan A, Harper M, Stockman D, Fiscella K, Walton J, Duberstein P, Talbot N, Lyness JM, Moynihan J. Gender, race/ethnicity, personality, and interleukin-6 in urban primary care patients. Brain Behav. Immun. 2009;23:636–642. doi: 10.1016/j.bbi.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Roberts B, Duberstein P. Personality and longevity: knowns, unknowns, and implications for public health and personalized medicine. J. Aging Res. 2011 doi: 10.4061/2011/759170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner R, Alper CM, Skoner DP. Sociability and susceptibility to the common cold. Psychol. Sci. 2003;14:389–395. doi: 10.1111/1467-9280.01452. [DOI] [PubMed] [Google Scholar]
- Cole SW. Elevating the perspective on human stress genomics. Psychoneuroendocrinology. 2010;35:955–962. doi: 10.1016/j.psyneuen.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. In: Social regulation of gene expression in the immune system. Segerstrom S, editor. The Oxford Handbook of Psychoneuroimmunology Oxford University Press; New York, USA: 2012. pp. 254–273. [Google Scholar]
- Cole SW. Social Regulation of Human Gene Expression: Mechanisms and Implications for Public Health. Am. J. Public Health. 2013:e1–e9. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene-social environment interaction at the human IL6 locus. Proc. Natl. Acad. Sci. USA. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. Proc. Natl. Acad. Sci. USA. 2012;109:20578–20583. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl. Acad. Sci. USA. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA. Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics. 2005;21:803–810. doi: 10.1093/bioinformatics/bti038. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO personality inventory (NEO-PU-R) and NEO five factor inventory (NEO-FFI) Odessa, FL, USA: Psychological assessment resources; 1992. Neo-PI-R professional manual. [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones. Psychoneuroendocrinology. 2012;37:1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. Chapman & Hall, New York, USA: 1993. [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav. Immun. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens WJ, Grant GR. Statistical methods in bioinformatics: An introduction. New York, USA: Springer; 2005. [Google Scholar]
- Eysenck HJ. Personality, stress and disease: an interactionist perspective. Psychol. Inq. 1991;2:221–232. [Google Scholar]
- Ferguson E, Bibby PA. Openness to experience and all-cause mortality: a meta-analysis and r-equivalent from risk ratios and odds ratios. Br. J. Health Psychol. 2012;17:85–102. doi: 10.1111/j.2044-8287.2011.02055.x. [DOI] [PubMed] [Google Scholar]
- Finch CE. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic Press; 2007. [Google Scholar]
- Fredrickson BL, Grewen KM, Coffey KA, Algoe SB, Firestine AM, Arevalo JM, Ma J, Cole SW. A functional genomic perspective on human well-being. Proc. Natl. Acad. Sci. USA. 2013;110:13684–13689. doi: 10.1073/pnas.1305419110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HS. The multiple linkages of personality and disease. Brain Behav. Immun. 2008;22:668–675. doi: 10.1016/j.bbi.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger-Johnson G, Roberets B, Boniface D, Sabia S, Batty D, Elbaz A, Singh-Manoux A, Deary IJ. Neuroticism and cardiovascular disease mortality: socioeconomic status modifies the risk in women (UK health and lifestyle survey) Psychosom. Med. 2012;74:596–603. doi: 10.1097/PSY.0b013e31825c85ca. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMurray J, Comings DE, Napolioni V. The gene-immune-behavioral pathway: Gamma-interferon (IFN-gamma) simultaneously coordinates susceptibility to infectious disease and harm avoidance behaviors. Brain Behav. Immun. 2013 doi: 10.1016/j.bbi.2013.09.012. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. A contemplated revision of the NEO five factor inventory. Pers. Individ. Dif. 2004;36:587–596. [Google Scholar]
- Millar K, Lloyd SM, McLean JS, Batty GD, Burns H, Cavanagh J, Deans KA, Ford I, McConnachie A, McGinty A, Mõttus R, Packard CJ, Sattar N, Shiels PG, Velupillai YN, Tannahill C. Personality, socio-economic status and inflammation: cross-sectional, population-based study. PLoS One. 2013;8:e58256. doi: 10.1371/journal.pone.0058256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Rabin BS, Skoner DP, Doyle WJ. Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain Behav. Immun. 1999;13:109–123. doi: 10.1006/brbi.1998.0545. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol. Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health Psychology: Developing biologically plausible models linking the social world and physical health. Annu. Rev. Psychol. 2009a;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signalling. Proc. Natl. Acad. Sci. USA. 2009b;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy GJ, Perkins-Porras L, Strike PC, Steptoe A. Type-D personality and cortisol in survivors of acute coronary syndrome. Psychosom. Med. 2008;70:863–868. doi: 10.1097/PSY.0b013e3181842e0c. [DOI] [PubMed] [Google Scholar]
- Mõttus R, Luciano M, Starr JM, Pollard MC, Deary IJ. Personality traits and inflammation in men and women in their early 70s: the Lothian Birth Cohort 1936 study of healthy aging. Psychosom. Med. 2013;75:11–19. doi: 10.1097/PSY.0b013e31827576cc. [DOI] [PubMed] [Google Scholar]
- Nakaya N, Bidstrup PE, Saito-Nakaya K, Frederiksen K, Koskenvuo M, Pukkala E, Kaprio J, Floderus B, Uchitomi Y, Johansen C. Personality traits and cancer risk and survival based on Finnish and Swedish registry data. Am. J. Epidemiol. 2010;172:377–385. doi: 10.1093/aje/kwq046. [DOI] [PubMed] [Google Scholar]
- Napolioni V, Murray DR, Comings DE, Peters WR, Gade-Andavolu R, MacMurray J. Interaction between infectious diseases and personality traits: ACP1*C as a potential mediator. Infect. Genet. Evol. 2014;26:267–273. doi: 10.1016/j.meegid.2014.06.002. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis. Markers. 2011;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA. 2013;110:16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard DA, Konrath SH, Lopez WD, Cameron HG. Expensive Egos: Narcissistic Males Have Higher Cortisol. PLoS ONE. 2012;7:e30858. doi: 10.1371/journal.pone.0030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge T. Defensive personality effects on cardiovascular health: A review of the evidence. In: Johns D, editor. Stress and its impact on society. Hauppauge, New York, USA: Nova Science Publishers; 2006. pp. 1–21. [Google Scholar]
- Schaller M. The behavioural immune system and the psychology of human sociality Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2011;366:3418–3426. doi: 10.1098/rstb.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, Murray DR. Pathogens, personality and culture: disease prevalence predicts worldwide variability in sociosexuality, extraversion, and openness to experience. J. Pers. Soc. Psychol. 2008;95:212–221. doi: 10.1037/0022-3514.95.1.212. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC. Personality and the immune system: models, methods and mechanisms. Ann. Behav. Med. 2000;22:180–190. doi: 10.1007/BF02895112. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Cole SW. The emerging field of human social genomics. Clinical Psychological Science. 2013 doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P. Principles of Allostasis: Optimal design, predictive regulation, pathophysiology, and rational therapeutics. In: Schulkin J, editor. Allostasis, homeostasis, and the costs of physiological adaptation. Cambridge, UK: Cambridge University Press; 2004. pp. Pp17–PP64. [Google Scholar]
- Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, Schlessinger D, Costa PT. High neuroticism and low conscientiousness are associated with interleukin-6. Psychol. Med. 2010;40:1485–1493. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill R, Fincher CL, Murray DR, Schaller M. Zoonotic and non-zoonotic diseases in relation to human personality and societal values: support for the parasite-stress model. Evol. Psychol. 2010;11:151–169. [PubMed] [Google Scholar]
- Totman R, Kiff J, Reed SE, Craig JW. Predicting experimental colds in volunteers from different measures of recent life stress. J. Psychosom. Res. 1980;24:155–163. doi: 10.1016/0022-3999(80)90037-9. [DOI] [PubMed] [Google Scholar]
- Turiano NA, Hill PL, Roberts BW, Spiro A, Mroczek DK. Smoking mediates the effect of conscientiousness on mortality: The veterans affairs normative aging study. J. Res. Pers. 2012;46:719–724. doi: 10.1016/j.jrp.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turiano NA, Mroczek DK, Moynihan J, Chapman BP. Big 5 personality traits and interleukin-6: evidence for “healthy Neuroticism” in a US population sample. Brain Behav. Immun. 2013;28:83–89. doi: 10.1016/j.bbi.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhara K, Irwin M. Human Psychoneuroimmunology. Oxford: Oxford University Press; 2005. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weiss A, Costa PT., Jr Domain and facet personality predictors of all-cause mortality among Medicare patients aged 65 to 100. Psychosom. Med. 2005;67:724–733. doi: 10.1097/01.psy.0000181272.58103.18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.