Abstract
The human oral microbiome is known to play a significant role in human health and disease. While less well studied, the feline oral microbiome is thought to play a similarly important role. To determine roles oral bacteria play in health and disease, one first has to be able to accurately identify bacterial species present. 16S rRNA gene sequence information is widely used for molecular identification of bacteria and is also useful for establishing the taxonomy of novel species.
The objective of this research was to obtain full 16S rRNA gene references sequences for feline oral bacteria, place the sequences in species-level phylotypes, and create a curated 16S RNA based taxonomy for common feline oral bacteria.
Clone libraries were produced using “universal” and phylum-selective PCR primers and DNA from pooled subgingival plaque from healthy and periodontally diseased cats. Bacteria in subgingival samples were also cultivated to obtain isolates. Full-length 16S rDNA sequences were determined for clones and isolates that represent 171 feline oral taxa. A provisional curated taxonomy was developed based on the position of each taxon in 16S rRNA phylogenetic trees.
The feline oral microbiome curated taxonomy and 16S rRNA gene reference set will allow investigators to refer to precisely defined bacterial taxa. A provisional name such as “Propionibacterium sp. feline oral taxon FOT-327” is an anchor to which clone, strain or GenBank names or accession numbers can point. Future next-generation-sequencing studies of feline oral bacteria will be able to map reads to taxonomically curated full-length 16S rRNA gene sequences.
Keywords: feline, oral, bacteria, microbiome, 16S rRNA, taxonomy, phylogeny
1. Introduction
Most of what we currently know about the oral bacteria of mammals, including humans, cats and dogs, is based on 16S rRNA gene sequences from isolates and clone libraries. Unfortunately, 16S rRNA gene sequences in GenBank rarely have full or accurate taxonomic attribution. Thus, the individual bacterial species in the microbiomes of mammals, for which many bacteria have not yet been formally named, are nearly impossible to reference specifically in scientific communications. The Bacterial Code requires full phenotypic and genetic characterization of multiple isolates in axenic culture to formally name a bacterial species (Lapage et al., 1992). Uncultivated bacteria can be given formal Candidatus status if extensively characterized, but this requires substantially more than simple 16S rRNA sequence determination. Thus, microbiologists have the difficult task of communicating the identity of bacteria known only as 16S rRNA gene sequence-based phylotypes. Carefully curated provisional taxonomic schemes have been established for human and canine oral microbiomes (Dewhirst et al., 2010; Dewhirst et al., 2012). The goal of this research is to establish a carefully curated 16S rRNA gene based provisional taxonomy for bacteria present in the oral cavities of cats. This taxonomic scheme will enable investigators to reference feline oral bacterial species as clearly defined phylotypes with Feline Oral Taxon numbers and species level taxonomic attribution linked to full 16S rRNA gene reference sequences. This study was not designed to examine the differences between the microbiomes of cats with and without periodontal disease, but simply to identify bacteria from cats in each group for future next generation sequencing studies.
Some phyla described in this research that may be unfamiliar to some readers. The phyla Chlorobi, Chloroflexi, SR1, Synergistetes, and TM7 have been discussed extensively in a recent publication on host-associated lesser known phyla and candidate divisions (Camanocha and Dewhirst, 2014).
Understanding the species comprising the oral microbiome of the cat is important for at least two reasons. Firstly, it is likely that as in humans, the feline oral microbiome has a profound influence on the oral and systemic health of cats. Secondly, associating oral bacterial species with specific feline health or disease conditions requires knowledge of the bacterial species present and having the means to precisely and rapidly identify them. This is easily accomplished when a 16S rRNA gene based taxonomy and reference sequence set have been established.
2. Materials and Methods
2.1 Subject recruitment and collection of dental plaque
Twenty cats domestic short hair cats between the ages of 1 and 10 years were recruited to the study from a UK population of client owned cats that presented at a veterinary dental referral clinic for periodontal disease or other dental problems. Ten of the cats were periodontally healthy and 10 had periodontitis. This ensured representation of bacterial species from a broad spectrum of disease states. The healthy group cats had clinically healthy gingiva with no more than low levels of localized (gingivitis 1) (Wiggs and Lobprise, 1997). The periodontal disease group cats each had a minimum of 4 sites displaying periodontitis of at least stage 3 (>25% attachment loss), (Wiggs and Lobprise, 1997). Exclusion criteria were: less than one year old; being a pure bred animal; and having received antibiotics in the previous 3 months. For isolating feline oral bacterial species, samples were taken from 5 clinically healthy domestic short hair cats (aged between 1 and 7 years old) from the WALTHAM Centre for Pet Nutrition undergoing routine dental treatment. These studies were approved by the WALTHAM Centre for Pet Nutrition ethical review committee.
Animals were sampled under anesthesia during their normal dental treatment. Each cat was given a premedication of 0.02 mg/kg acepromazine (ACP 2 mg/ml) and 0.02 mg/kg buprenorphine (Vetergesic 0.3 mg/ml) given intramuscularly. Anaesthesia was induced with 4 mg/kg propofol (Rapinovet 10 mg/ml) given intravenously, and then maintained on inhalational isoflurane and oxygen. Initially supragingival and gingival margin plaque and calculus were removed using a Gracey curette to prevent contamination of the subgingival sample. A sterile periodontal probe was then used to obtain a subgingival plaque sample from each sampling site (4–10 per cat). In the cats with periodontitis, samples were taken from the sites with periodontal pockets, typically the maxilla 3rd premolars, the mandibular 1st molars and the canines. Samples were taken from the same tooth types in the healthy cats. All the samples from each cat were combined and suspended in 350 µL of 50 mM Tris (pH 7.6), 1 mM EDTA (pH 8.0), 0.5% Tween 20 and immediately stored at −20°C prior to DNA extraction.
2.2 DNA extraction and 16S rDNA amplification
DNA extraction was performed using a DNeasy Tissue Kit (Qiagen) following the manufacturer’s instructions for the isolation of genomic DNA from Gram-positive bacteria. Two DNA pools, one from 10 periodontally healthy and one from 10 periodontally diseased cats, were each amplified to make clone libraries using “universal” primers F24 5’-GAGTTTGATYMTGGCTCAG-3’ (9–27 forward) and Y36 5’-GAAGGAGGTGWTCCADCC-3’(1525–1541 reverse) as previously described (Dewhirst et al., 2012). The pooled DNA from the diseased cats was also amplified with Spirochaetes-Synergistetes selective primer pair F24 / M98 3’-GTTACGACTTCACCCYCCT-3’ (1483–1501 reverse) and the Bacteroidetes selective primer pair F24 / F01 5’-CCTTGTTACGACTTAGCCC-3’ (1487–1505 reverse) for making phylum selective libraries. The size and amount of the amplicons were examined by electrophoresis on a 1% agarose gel. DNA was stained with SYBER Safe DNA gel stain (Invitrogen, Carlsbad, CA) and visualized under UV light. A preparative gel was run after determining that a strong amplicon of the correct size had been produced. The appropriate amplicon band was cut out and purified using a Qiagen Gel Extraction kit (Qiagen, Valencia, CA).
2.3 Cloning procedures and library screening
Purified DNA was cloned using a TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Transformation was done using competent E. coli TOP10 cells provided by the manufacturer. The transformed cells were plated onto Luria-Bertani agar plates supplemented with kanamycin (50 µg/ml) and incubated overnight at 37°C. Each white colony was placed into a tube containing 40 µL of 10 mM Tris-HCl pH 8.0. One µL of the cell suspension was used directly as template for PCR with an M13 (−21) forward primer and an M13 reverse primer (Invitrogen). The amplicon was checked for correct size by electrophoresis on a 1% agarose gel. DNA from clones was purified to remove primer and dNTPs by treatment with exonuclease and shrimp alkaline phosphatase (Amersham, Pittsburg, PA).
Clones were screened by sequencing with primer Y31, 5’-TKACCGCGGCTGCTG-3’ (519–533 reverse). If the clone sequence differed by more than 7 bases from previously identified feline oral reference sequences, the remaining amplicon DNA was purified using QIAquick PCR purification kits (Qiagen, Valencia, CA) and fully sequenced with additional primers (Dewhirst et al., 2012).
2.4 Strain isolation
Subgingival plaque samples were placed in 300 µL of maximum recovery diluent (Sigma) and dilutions of plaque (neat to 10−5) were spread onto agar plates. Strains were routinely grown for 2 days for aerobes and up to 14 days for anaerobes, at 38°C on Columbia Blood Agar (CBA) plates (OXOID). For isolating anaerobic strains, CBA was supplemented after autoclaving with Hemin (5 µg/ml) and Menadione (1 µg/mL) (Sigma). For liquid culture, Brain Heart Infusion (BHI) broth (OXOID) supplemented with yeast extract (5 g/L, Sigma) and cysteine hydrochloride (0.75 g/L, Sigma) and adjusted to a pH of 8.5. Hemin and Menadione were added to cooled broth after autoclaving, as above. Anaerobic strains were incubated in a MACS1000 anaerobic workstation (Don Whitley, UK) with an atmosphere of 85% N2, 10% CO2, 5% H2. After observation for growth, single colonies were sub-cultured onto fresh CBA plates (Hemin and Menadione for anaerobes as above). Following further incubation at 38°C as described above, colonies on agar plates were examined for purity. Cultures were then harvested for 16S rDNA sequencing to confirm their identity and the isolates stored at −80°C in BHI with 15% glycerol.
2.5 16S rRNA gene sequencing
Purified 16S rDNA was fully sequenced on both strands using at least 6 primers as previously described (Dewhirst et al., 2012). Full 16S rDNA sequences were assembled from the ABI electropherogram files using Sequencher (Gene Codes Corporation, Ann Arbor, MI).
Sequences were checked for the possibility of being chimeric by examining mismatches with the aligned members of closely related taxa and by comparing trees generated using the first 600 bases against those generated with the last 900 bases. Taxa that changed position significantly were considered suspect and further examined using Mallard (Ashelford et al., 2006). Chimeric sequences were discarded.
2.6 Phylogenetic analyses and provisional taxonomy
Sequences were aligned in a custom 16S rRNA gene database created by F.E. Dewhirst (Dewhirst et al., 2010). Sequences were provisionally identified and taxonomically placed by BLASTN analysis against several 16S rRNA gene databases: the Canine Oral Microbiome reference set (Dewhirst et al., 2012), the Human Oral Microbiome Database (Dewhirst et al., 2010), National Center for Biotechnology Information (Altschul et al., 1990), Ribosomal Database Project (Cole et al., 2014) and Greengenes (DeSantis et al., 2006). Neighbor Joining trees were generated for all feline clone and strain sequences by phyla, including sequences for relevant reference species. Sequences were assigned to genera or higher taxonomic level based on analysis of tree branching rather than simple BLAST similarity. A cutoff of approximately 95% sequence similarity was generally used for genus assignment. However, some genera such as Treponema are substantially broader. In all cases, a sequence was placed in a genus only if it had higher similarity to that genus than the similarity between members of neighboring genera. Sequences that could not be placed in a genus were assigned to the most specific higher taxonomic level.
Sequences were clustered into phylotypes with a definition of 98.5% similarity for full 16S rRNA gene sequences. Each phylotype, whether corresponding to a named taxon or not, was given a Feline Oral Taxon (FOT) number in the range of 001 to 370. The numbering is discontinuous as some FOT numbers were removed when taxa with greater than 98.5% similarity were merged or chimeras identified.
Trees for use in this publication were created by export of aligned sequences from our RNA sequence database into MEGA version 6 (Tamura et al., 2013). The evolutionary history was inferred using the neighbor-joining method (Saitou and Nei, 1987). Bootstrap test (500 replicates) were performed using the method of Felsenstein (Felsenstein, 1985). The evolutionary distances were computed using the Jukes-Cantor method (Jukes and Cantor, 1969). Missing data (aligned sequence columns) were excluded on a pairwise basis. Trees were exported from MEGA into Microsoft Word in vector format and text edited to create the final figures.
2.7 Feline oral 16S rRNA reference set
The full 16S rRNA gene sequences of 207 clones and 41 isolates representing 171 feline oral taxa were deposited in GenBank under BioProjectID PRJNA260360 and individual accession numbers KM461942-KM462187. Accession numbers are also included for each clone or isolate in the phylogenetic trees in Figs. 1–4.
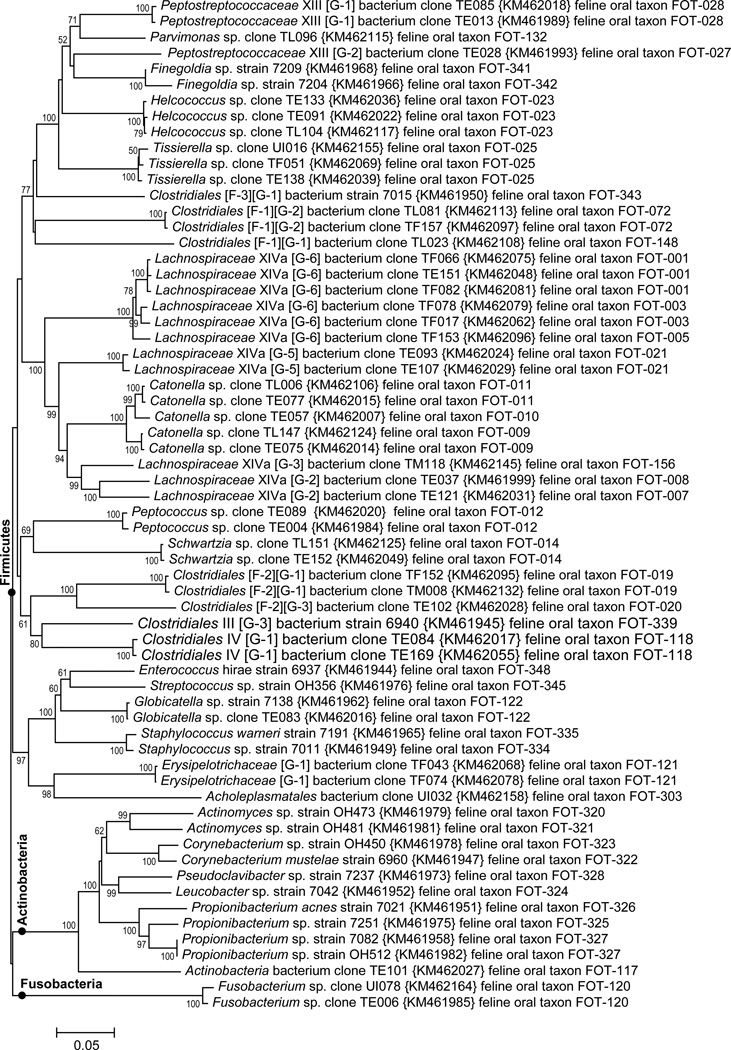
Fig. 1. Neighbor-joining tree for phyla Firmicutes, Actinobacteria and Fusobacteria.
The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei, 1987). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches (Felsenstein, 1985). The evolutionary distances were computed using the Jukes-Cantor method (Jukes and Cantor, 1969). The scale equals 0.05 substitutions per site. Evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013). The branch position of clades representing the three phyla are marked with ‘●’.
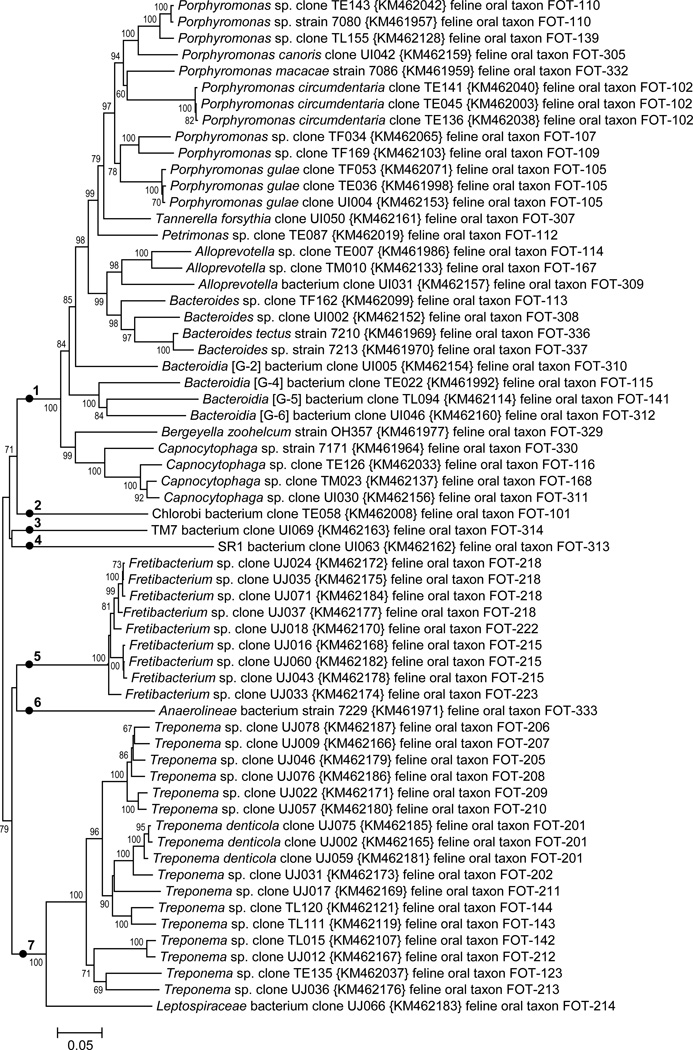
Fig. 4. Neighbor-joining tree for Bacteroidetes, Spirochaetes, Synergistetes and other phyla.
The tree was constructed as described in Fig 1. The phyla are indicated by numbers as follows: Bacteroidetes, 1; Chlorobi, 2; TM7, 3; SR1, 4; Synergistetes, 5; Chloroflexi, 6; and Spirochaetes, 7.
3. Results
3.1 Clone library and isolate analyses
Four 16S rDNA libraries were created from amplicons using “universal” primers and one library each was created from the Spirochaetes-Synergistetes and the Bacteroidetes selective primers. Approximately 160 colonies were picked from each “universal” library and 75 from each phylum selective library for a total of 777 clones analyzed by initially sequencing approximately 500 bases at the 5’end of the 16S rDNA. Twenty-two clones were eliminated from further analysis for being chimeric or having mixed or poor reads, yielding 755 clone sequences. Full 16S rDNA sequences were obtained for 207 potentially novel taxa. These sequences were collapsed into 133 16S rRNA gene phylotypes based on a 98.5% sequence similarity definition as has been done for the human and canine oral microbiomes (Dewhirst et al., 2010; Dewhirst et al., 2012). These full length 16S rDNA sequences formed the initial core of our feline oral microbiome taxonomic database. Feline oral isolates were obtained from WALTHAM cats and were identified by 16S rDNA sequencing and BLASTN analysis using this feline oral microbiome reference sequence set. Sequences from 41 out of 236 isolates did not match this reference set and represented 38 additional feline oral taxa for the current total of 171 16S rRNA gene based feline oral species and phylotypes.
3.2 Taxonomic analysis
The taxonomic position of each 16S rDNA sequence was determined by BLASTN analysis of multiple 16S rRNA gene databases and by constructing phylogenetic trees. Figures 1–4 show neighbor-joining trees for 248 feline reference sequences representing 171 feline oral taxa Where sequences for a phylotype were >98.5% similar to the sequence of the type strain of a named organism, the phylotype was designated as that species. Phylotypes that fell into named genera were designated “Genus sp.”. Those which could be identified only at a higher taxonomic level were designated “Higher-taxon bacterium”. Each phylotype, whether formally named or not, was given a Feline Oral Taxon (FOT) number. A full provisional six taxonomic level scheme (phylum, class, order, family, genus, and species) for feline oral bacteria is presented in Supporting Table S1.
Feline oral taxa in eleven phyla were identified in this study: Firmicutes, 72; Proteobacteria, 38; Bacteroidetes, 26; Spirochaetes, 16; Actinobacteria, 10; Synergistetes, 4; Chlorobi, 1; Chloroflexi, 1; Fusobacteria, 1; SR1, 1; and TM7, 1.
3.3 Firmicutes, Actinobacteria and Fusobacteria
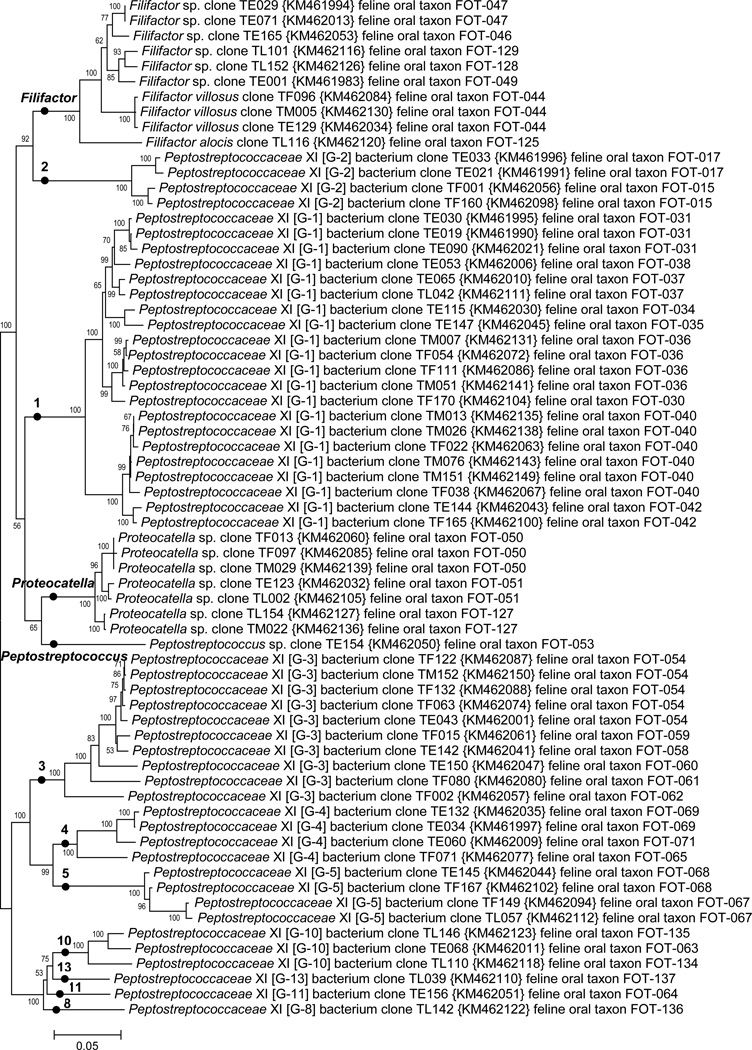
Feline oral bacterial taxa in phyla Firmicutes, Actinobacteria and Fusobacteria are shown in Figs. 1 & 2. The unnamed genera in each family are given designations such as “[G-1]” to indicate it is an unnamed genus in that family. Roman numerals after a family name refer to the Collins nomenclature for Clostridial families (Collins et al., 1994). Thus, “Peptostreptococcaceae XIII [G-1] bacterium” refers to a taxon in an unnamed genus ‘G-1’ in Collins Clostridial family XIII (see top Fig. 2). Within the Firmicutes there are 15 named genera: Catonella, Clostridium, Enterococcus Filifactor, Finegoldia, Globicatella, Helcococcus, Parvimonas, Peptococcus, Peptostreptococcus, Proteocatella, Schwartzia, Staphylococcus, Streptococcus, and Tissierella, and 26 genus level clades which are currently unnamed. Within the Actinobacteria named genera include: Actinomyces, Corynebacterium, Propionibacterium, Leucobacter and Pseudoclavibacter. All but one of the 10 taxa identified came from sequencing isolates rather than from clone libraries. The Fusobacteria phyla had only one genus detected, Fusobacterium. The class Peptostreptococcaceae XI is shown in Fig. 2. The three named and nine unnamed genera level clades are designated with filled circles. The unnamed genera are marked using genus numbers which exactly corresponds to the genus numbering scheme used for canine oral taxa (Dewhirst et al., 2012).
Fig. 2. Neighbor-joining tree for class Peptostreptococcaceae XI of phylum Firmicutes.
The tree was constructed as described in Fig. 1. The 12 genus level clades are labeled by genus name or unnamed genus designation as discussed in the text.
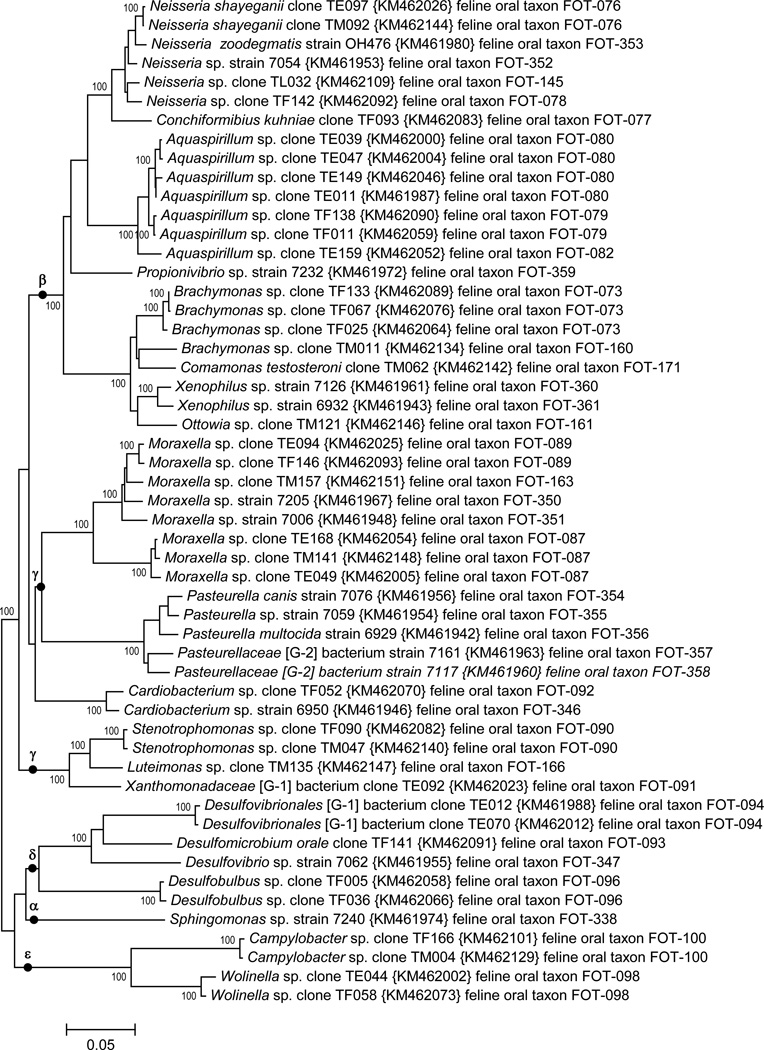
3.4 Proteobacteria
A phylogenetic tree for feline oral bacteria in the phylum Proteobacteria is shown in Fig. 3. The five major classes of Proteobacteria are marked with Greek letters. There are taxa in eight named genera of Betaproteobacteria: Aquaspirillum, Brachymonas, Comamonas, Conchiformibius, Neisseria, Ottowia, Proprionivibrio, and Xenophilus. There are taxa in five named genera of Gammaproteobacteria: Cardiobacterium, Luteimonas, Moraxella, Pasteurella, and Stenotrophomonas, as well as two unnamed genera. Sphingomonas sanguinis is the sole representative of the Alphaproteobacteria. The Deltaproteobacteria contains members in the named genera Desulfobublus, Desulfomicrobium, and Desulfovibrio, as well as in one unnamed genus in the family Desulfovibrionales. The Epsilonproteobacteria includes two members of the genera Campylobacter and Wolinella.
Fig. 3. Neighbor-joining tree for phylum Proteobacteria.
The tree was constructed as described in Fig 1. The five Proteobacteria classes are marked with Greek letters.
3.5 Bacteroidetes, Chlorobi, TM7, and SR1
A phylogenetic tree for the Bacteroidetes and six other phyla is shown in Fig. 4. The phylum level clades are marked with filled circles and numbered ‘1’ through ‘7’. Named genera in the phylum Bacteroidetes, ‘1’, include Alloprevotella, Bacteroides, Bergeyella, Capnocytophaga, Petrimonas, Porphyromonas, and Tannerella. Taxa in five unnamed Bacteroidetes genera were also identified. Seven of 26 taxa were identified through use of the Bacteroidetes-selective primers. Single taxa were identified representing phyla Chlorobi, ‘2’, TM7, ‘3’, and SR1, ‘4’.
3.6 Synergistetes, Chloroflexi, and Spirochaetes
The Synergistetes phylum is, ‘5’, contains four taxa which are all members of the genus Fretibacterium. The human oral bacterium Fretibacterium fastidiosum is the type species of the genus (Vartoukian et al., 2013). The Chloroflexi phylum, ‘6’, is represented by a single taxon in the class Anaerolineae (Yamada et al., 2006) The phylum Spirochaetes contains fifteen taxa which are members of the genus Treponema, and one taxon which is a deeply branching member of an unnamed genus in the family Leptospiraceae. Thirteen of 16 taxa identified in the Spirochaetes came from analysis of the library produced using Spirochaetes-Synergistetes selective PCR primers.
4. Discussion
A provisional naming scheme for feline oral taxa has been proposed based on analysis of full length 16S rRNA gene based phylotypes. Few bacterial species isolated from cats have been formally named, but we currently have molecular tools that will eventually allow investigators to identify several hundred feline oral taxa. It may take many years to formally name the most prevalent 200 feline oral bacteria, but in the meantime, investigators need a means of precisely communicating the identity of bacteria identified in feline molecular microbiological studies. A provisional name such as “Propionibacterium sp. feline oral taxon FOT-327” is an anchor to which clone, strain or GenBank names or accession numbers can point. The Human Oral Microbiome Database (www.homd.org) provides a provisional taxonomic scheme that has been widely by investigators working on the human oral microbiome, and serves as the model for investigators doing feline and canine microbiology.
Many current molecular studies of bacteria in host-associated environments involve next generation sequencing on 454 Pyrosequencing or Illumina platforms. Depending on the length and region of the 16S rRNA gene sequenced, varying levels of taxonomic resolution can be achieved. Reads of 200–400 bases are often classified only to genus or higher taxonomic level using RDP classifier or operational taxonomic units (OTU) based methods. Studies of human and animal diseases have identified particular bacterial species as having causative roles and thus being able to identify clones or strains to a species level taxon is critically important for many types of investigations. Species level identification and classification is significantly facilitated by having a taxonomically curated reference sequence set. The feline oral microbiome 16S rRNA gene reference set for the 171 phylotypes described here has been deposited in GenBank. A file of aligned reference sequences in phylogenetic order (the same order as in Figs. 1–4) is available from F.E Dewhirst in FASTA format. Files for classification using MOTHUR (Schloss et al., 2009) are also available from F.E. Dewhirst by request.
Molecular studies which create 16S rDNA clone libraries most often use “universal” primers. All primers are now recognized as having biases such that libraries produced do not include clones representing input phyla evenly. For many diseases, such as periodontitis, phyla such as the Spirochaetes and Bacteroidetes have been documented to contain key pathogens, but members of these phyla are not always well represented libraries made using “universal” primers. The use of phyla-specific or phyla-selective primers can facilitate identifying novel taxa, as was demonstrated in the current study. Twelve of 16 Spirochaetes (mostly of the genus Treponema) and all Synergistetes (genus Fretibacterium), and several members of the Bacteroidetes were identified from single libraries where only approximately 70 clones were examined. Culture methods are also an important complement to molecular methods for identifying novel taxa. In this report, all but one of the Actinobacteria taxa were identified from isolates. Since Actinobacteria species are known to be poorly represented in standard “universal” primer generated libraries (de Lillo et al., 2006), cultivation or Actinobacteria-selective PCR primers should be used in future studies to identify novel taxa in host-associated microbiomes. Use of primers developed for making phylum-selective clone libraries of lesser-known phyla (Camanocha and Dewhirst, 2014) could be useful for seeking taxa in phyla found in dogs and humans that have not been found in cats, as well as increasing the number of taxa in several phyla with few representatives.
The feline oral microbiome 16S rRNA gene reference set presented here is an important step in describing feline oral bacteria. Author F.E. Dewhirst intends to expand the feline oral microbiome database in the future with taxa identified in ongoing studies. Investigators with full 16S rRNA gene sequences representing novel feline oral bacterial taxa can have these taxa and their reference sequence added to this dataset by contacting the corresponding author. A future step for feline oral microbiome studies will be to make BLAST analysis and other tools available to the research community through the web as has been done for the Human Oral Microbiome Database (www.homd.org).
Supplementary Material
Highlights.
The feline oral microbiome has been examined using molecular and cultivation methods
A curated provisional taxonomic scheme is presented for unnamed feline oral bacteria
246 full length 16S rRNA reference sequence are presented for 171 feline oral taxa
Future next generation sequencing studies can anchor reads to a curated taxonomy
Acknowledgements
This work was supported by the WALTHAM Centre for Pet Nutrition. Research reported in this publication was supported in part by The National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R37DE016937 (F.E. Dewhirst). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. We thank Sara Barbuto for her assistance in DNA sequencing and Lisa Milella for plaque sample collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have the following interests. SJH, JMC and ZVM-J are employees of the WALTHAM Centre for Pet Nutrition. M-LB was an employee of the WALTHAM Centre for Pet Nutrition during the course of this study and is currently at Mars Pet Care Europe. FED, EAK were employees of the Forsyth Institute during the course of this study. FED has been a consultant for WALTHAM. There are no other patents, products in development or other marketed products to declare.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl Environ Microbiol. 2006;72:5734–5741. doi: 10.1128/AEM.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camanocha A, Dewhirst FE. Host-associated bacterial taxa from Chlorobi, Chloroflexi, GN02, Synergistetes, SR1, TM7, and WPS-2 Phyla/candidate divisions. J Oral Microbiol. 2014;6 doi: 10.3402/jom.v6.25468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic acids Res. 2014;42:D633–D642. doi: 10.1093/nar/gkt1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow JA. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- de Lillo A, Ashley FP, Palmer RM, Munson MA, Kyriacou L, Weightman AJ, Wade WG. Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Micro Immun. 2006;21:61–68. doi: 10.1111/j.1399-302X.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Klein EA, Thompson EC, Blanton JM, Chen T, Milella L, Buckley CM, Davis IJ, Bennett ML, Marshall-Jones ZV. The canine oral microbiome. PLoS One. 2012;7:e36067. doi: 10.1371/journal.pone.0036067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. Evolution of protein molecules. New York: Academic Press; 1969. [Google Scholar]
- Lapage SP, Sneath PHA, Lessel EF, Skerman VBD, Seeliger HPR, Clark WA. Chapter 3, Rules of Nomenclature with Rocommendations. Washington (DC): ASM Press; 1992. International Code of Nomenclature of Bacteria. Bacteriological Code 1990 Revision. [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform373 independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartoukian SR, Downes J, Palmer RM, Wade WG. Fretibacterium fastidiosum gen. nov., sp. nov., isolated from the human oral cavity. Int J Syst Evol Microbiol. 2013;63:458–463. doi: 10.1099/ijs.0.041038-0. [DOI] [PubMed] [Google Scholar]
- Wiggs RB, Lobprise HB. Periodontology. In: Niemiec BA, editor. Veterinary Dentistry, Principles and Practice. Philadelphia, USA: Lippincotte Raven; 1997. pp. 186–231. [Google Scholar]
- Yamada T, Sekiguchi Y, Hanada S, Imachi H, Ohashi A, Harada H, Kamagata Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int J Syst Evol Microbiol. 2006;56:1331–1340. doi: 10.1099/ijs.0.64169-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.