Abstract
The diagnosis of patients with pulmonary infiltrates and human immunodeficiency virus (HIV) infection remains a challenge. In current clinical practice the gold standard for Pneumocystis jirovecii pneumonia (PCP) diagnosis remains the identification of the organism in broncoalveolar lavage (BAL) using microscopy (e.g., silver stain). (1->3)-β -D-glucan (BG) is a polysaccharide that is present within the cell wall of Pneumocystis and other fungi. We analyzed serum and BAL lavage fluid from a cohort of 119 patients that did have HIV, a diagnosis of pneumonia and underwent bronchoscopy (FOB) for diagnosis of PCP. The discriminative power of serum BG for the diagnosis of PCP in this group of patients was very high. Using a cutoff of 300 pg/mL, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 91%, 92%, 89% and 93% respectively. A model for ROC with just serum BG (N = 108) had an AUC of 0.95. Serum procalcitonin (PCT) and BAL BG were not as accurate for the diagnosis of PCP. For BAL BG using a cutoff of 783 pg/mL, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 72%, 79%, 72% and 79% respectively. The differences between the medians for serum PCT between the group with a without PCP did not reach statistical significance (p=0.6137). The measurement of serum BG should be incorporated in the diagnostic work up of HIV positive patients with dyspnea and infiltrates on chest X ray. Our study confirms the diagnostic value of serum BG previously reported by others but we add a cutoff value that we believe is more accurate for patients with AIDS and suspicion of PCP.
INTRODUCTION
The diagnosis of patients with pulmonary infiltrates and human immunodeficiency virus (HIV) infection remains a frequent challenge.1 Very often the only way to distinguish between Pneumocystis jirovecii pneumonia (PCP), community-acquired pneumonia (CAP) and other entities is by fiberoptic bronchoscopy (FOB), an invasive procedure used to collect microbiologic samples. Many times patients with PCP lack a productive cough and so sputum cannot be obtained.2 Sputum samples have a much lower sensitivity for detection of PCP than bronchoalveolar lavage (BAL) samples even with adequate induction. A meta-analysis3 found an overall sensitivity of 55.5% and specificity of 98.6% for sputum induction; there was a difference in sensitivity when immunofluorescence was used compared to cytochemical stains (67.1 versus 43.1%). In current clinical practice the gold standard for PCP diagnosis remains the identification of the organism in BAL using microscopy (e.g., silver stain).4,5 More recently, polymerase chain reaction (PCR) has been used for diagnosis and quantification of fungal load but may be limited by the detection of colonization.1
In recent years a promising serologic test for the detection of invasive fungal infections has been established. Serum measurement of (1->3)-β -D-glucan (BG) is based on the level of this polysaccharide that is present within the cell wall of Pneumocystis and other fungi.6
The first description of elevated BG, both in serum and BAL, of HIV positive patients with PCP was in 1996.7 Since that time several studies have been reported. Using plasma samples from a previous study, Sax et al.8 found in 252 patients with HIV, that BG had a sensitivity of 92% and a specificity of 65% for the diagnosis of PCP, using a cutoff value of 80 pg/mL. In another study comparing 28 serum samples of patients with HIV or hematological malignancy vs. 28 control patients, the sensitivity and specificity of BG for PCP were 100% and 96.4%, respectively.9 A prospective study from Japan in immunocompromised patients,10 using 40 BAL and 107 sputum samples of patients with clinical suspicion of PCP, found a sensitivity and specificity of 100% and 80% using a cutoff of 15 pg/mL of BG for the diagnosis of definitive PCP (positive silver staining). The values in probable PCP (negative silver staining but clinical presentation compatible) were 76.2% and 73.3% at a cutoff value of 6 pg/mL. In the Japanese study the kit used for BG quantification was different from the kit currently employed in the US. They did use the BG test WACO that has a recommended cutoff value of 11 pg/mL and uses different reagents for quantification, we did use for our study the Fungitell® assay, from Associates of Cape Cod, Inc. that has a recommended cutoff value of 80 pg/mL. A small prospective study from the Netherlands in HIV-negative patients11 also showed excellent diagnostic accuracy of serum BG levels for the diagnosis of PCP. Using the same samples of Sax et al.8, but restricting the study to patients with respiratory symptoms, Wood et al.12 were able to achieve a positive predictive value of 96.3% using a cutoff of 80 pg/mL for the diagnosis of PCP. A recent study from Portugal by Esteves et al.13 employed serum BG and serum lactate dehydrogenase for the diagnosis of PCP in 100 HIV-positive patients and 50 healthy blood donors. They propose a cutoff of 400 pg/mL for serum BG as optimal.
Several meta-analysis for the use of serum BG for the diagnosis of invasive fungal infections have been published. A recent one from He14 included 4214 patients but did focus mainly on invasive infections by Candida and Aspergillus. More recently Karageorgopoulos et al.15 published an excellent meta-analysis about the accuracy of BG for the diagnosis of PCP. In their analysis they did include 14 studies, with 357 patients that had PCP and 1723 controls. Their definition of PCP did include the detection of the organism in BAL but also in sputum or by PCR. Most of those patients were HIV negative and did have another cause for immunosupresion. Their overall sensitivity and specificity was 94.7% and 86.3%. They suggest that a negative serum BG could reasonably exclude PCP in patients with low or moderate pretest probability for the disease.
BG was measured in BAL and serum in 109 immunocompromised patients with possible fungal infections in a recent study from the Mayo Clinic.16 Of 8 patients that were diagnosed with PCP, 7 had positive serum and BAL levels for BG. In those patients with PCP the median values for BG in serum and BAL were 406 pg/mL and 500 pg/mL, respectively. When BG has been measured in BAL17,18 the data appear to indicate that it does not add to the diagnostic accuracy of serum levels alone. Of note, both studies involved ventilator-associated pneumonia patients, not HIV patients with acute pulmonary infiltrates. A more recent paper19 studying the utility of BAL BG testing in invasive fungal infections did conclude that this measurement has poor specificity and reproducibility. Of note the measurement of BG in BAL is not validated for the Fungitell® assay. The lack of standardization in the BAL technique could be in part a reason for this poor reproducibility.
Another serologic marker, procalcitonin (PCT), has been shown to be quite valuable and more specific than previous markers for bacterial infections.20 It is the peptide precursor of the hormone calcitonin and is ubiquitously produced in response to endotoxin and bacterial mediators. PCT levels strongly correlate with the extent and severity of illness in several types of bacterial infections.21 More specifically, the use of procalcitonin in bacterial pneumonia has proven to help with antibiotic guidance and improve outcomes in several randomized controlled trials.22,23
PCT levels were assessed in a 2006 study from South Africa24 involving 266 patients admitted with a clinical diagnosis of CAP. In 169 of those a microbiological diagnosis was made; 44 patients were found to have tuberculosis, 31 PCP, and 35 bacterial pneumonia. The mean PCT levels for tuberculosis, PCP and bacterial pneumonia were 4.164 ng/mL (95% CI 1.749–6.579), 1.138 ng/mL (95% CI 0.543–1.734) and 19.479 ng/mL (95% CI 8.021–30.938), respectively. Moreover, a well-designed prospective study from 200725 included 107 consecutive patients with various reasons for their immune-compromise state. In that study, serum PCT had an area-under-the-curve of 0.746 (95% CI 0.602 to 0.889) for the prediction of bacterial infection, defined as positive bacteriologic results in the BAL. In this later study only 8 patients had HIV infection and bacterial infection was established in 25% of the HIV group.
The definition of Pneumocystis colonization has been debated; it has been proposed based on the presence of Pneumocystis microorganism or its DNA in the BAL of asymptomatic individuals26. It is unclear if there is a threshold of fungal load to cause disease. Recently, Costa et al.27, using stored serum and BAL samples from 63 patients, found a clear association between BAL fungal load (measured by quantitative RT-PCR) and BG levels. They also were able to demonstrate excellent sensitivity and specificity of BG for the diagnosis of PCP in their study population.
The purpose of this study was to find the diagnostic accuracy of the levels of serum BG, BAL BG and serum PCT in a cohort of patients with HIV and respiratory symptoms who were hospitalized and underwent FOB. The hypothesis was that symptomatic HIV patients with BAL- proven PCP infection would have elevations in the level of BG, both in sera and BAL, and possibly lower levels of PCT when compared to the PCP-negative patients.
MATERIALS AND METHODS
Serum and bronchoalveolar fluid (BAL) samples from the Louisiana State University (LSU) HIV Translational Research Biorepository were utilized for this study. Those samples were collected between 2007 and 2010 and frozen for preservation; we did analyze them in 2013. The Biorepository banks excess material from clinically indicated and acquired samples, along with a peripheral blood collection, linked to a database of clinical variables. The inclusion criteria were consecutive adult patients admitted to the hospital with HIV and a diagnosis of pneumonia by a physician who underwent FOB as part of their care. Those patients who refused to give informed consent were excluded. A clinical diagnosis of PCP required microbiologic confirmation with methenamine silver or direct fluorescent antibody staining. The LSU HIV Translational Research Biorepository protocols were approved by the LSU Health Sciences Center Institutional Review Board and all subjects provided informed consent. The use of those samples for the current study was approved by the Tulane University Biomedical IRB in New Orleans, LA reference number 13-420174E.
Commercially available assays for the measurement of BG (Fungitell® test, Associates of Cape Cod, Inc.) and PCT (Vidas B.R.A.H.M.S test, bioMerieux, Inc.) were performed on banked samples per the manufacturers’ standard protocols. The manufacturers’ thresholds for positive results are 80 pg/mL and 0.5 ng/mL, respectively. The laboratory technician performing the assay was unaware of the clinical diagnoses of the patients.
The distributions of serum and BAL measures (serum BG, serum PCT, and BAL BG) were positively skewed and therefore not amenable to normalizing transformation, so these data were summarized using medians and inter-quartile ranges. Differences in concentrations were assessed using the Wilcoxon Rank Sum test. Spearman correlation coefficients (rho) were used to assess associations between the three measures. Receiver operating characteristic (ROC) curves were computed using PCP+ or PCP- as the clinical diagnosis and BG (serum and BAL) and PCT as the test variables. All analyses were conducted in SAS version 9.3.
RESULTS
Study population
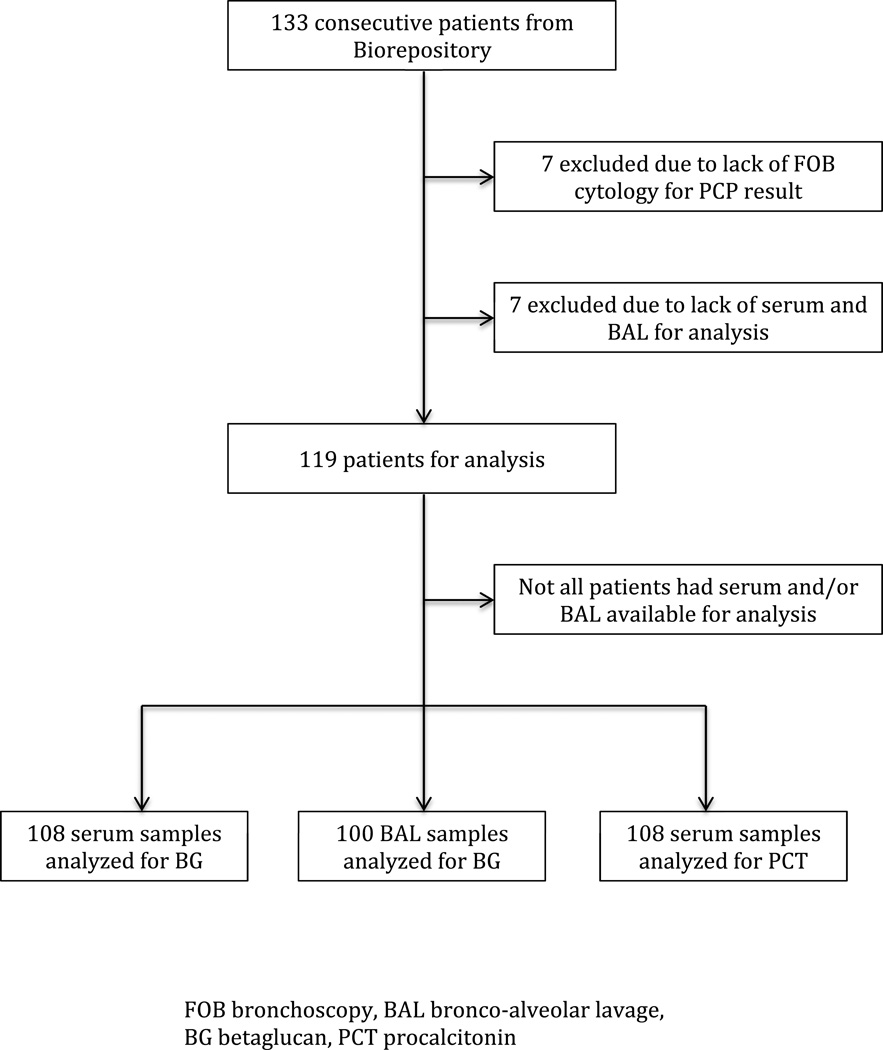
The LSU HIV Translational Research Biorepository had 133 samples from consecutive patients, of those 119 had FOB cytology results confirming PCP status and serum/BAL for analysis. Among the 119 samples we were able to analyze serum BG level in 108, BAL BG level in 100 and serum PCT in 108. Figure 1 illustrates this. Baseline characteristics from the cohort are presented in Table 1. We analyzed 49 PCP+ pantients, the prevalence of PCP in this group was 41%. This cohort had profound HIV-related immunosuppression, was predominantly male and most of the patients denied a prior history of PCP. Interestingly the description of oral candidiasis was quite similar between the PCP- and PCP+ group. Comparing our PCP+ patients with the PCP- the first group had significantly lower CD4 counts and was less likely to be using antiretroviral treatment or taking prophylaxis for PCP.
Figure 1.
Patient-samples flow in the study
FOB bronchoscopy, BAL bronco-alveolar lavage, BG betaglucan, PCT procalcitonin 7 excluded due to lack of FOB cytology for PCP result
Table 1.
Baseline Characteristics of Study Participants
| PCP+ (n = 49) |
PCP− (n = 70) |
P | |
|---|---|---|---|
| Age (mean + SD) | 42.9 + 7.6 (n = 49) |
45.0 + 8.8 (n = 65) |
0.1665 |
| CD4 (mean + SD) (median, (IQR)) |
39.7 + 60.9 16 (39) (n = 23) |
131.3 + 139.9 90 (153) (n = 44) |
0.0048 |
| Male % (n) | 69.4 (n = 49) | 68.6 (n = 70) | 0.9246 |
| Using ART % (n) | 8.2 (n = 49) | 40.0 (n = 70) | <0.0001 |
| Prior PCP % (n) | 18.6 (n = 43) | 23.8 (n = 63) | 0.5232 |
| Taking proph % (n) | 11.1 (n = 45) | 38.2 (n = 68) | 0.0016 |
| Oral candida % (n) | 50.0 (n = 48) | 54.4 (n = 68) | 0.6393 |
In this table n represents in each cell the number of patients with data available for analysis.
Chi-square test used to assess differences in frequencies.
t-test used to assess differences in age and log(CD4). Natural log of CD4 was used for analysis because of skew in data, but results were also verified with the Wilcoxon Rank Sum Test on untransformed values.
Analysis of biomarkers
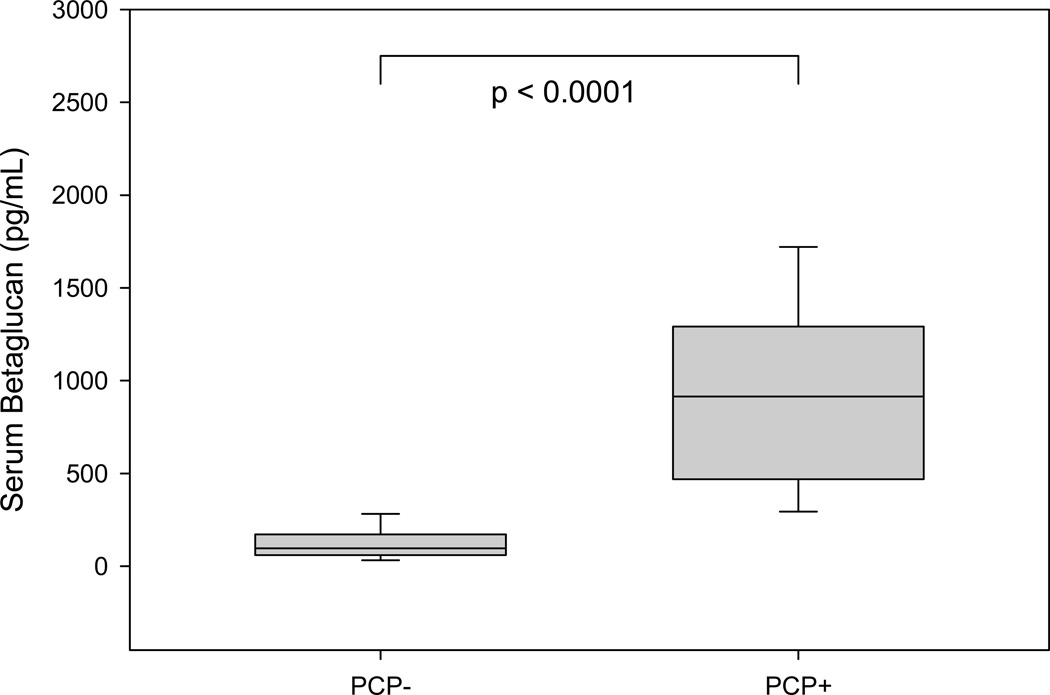
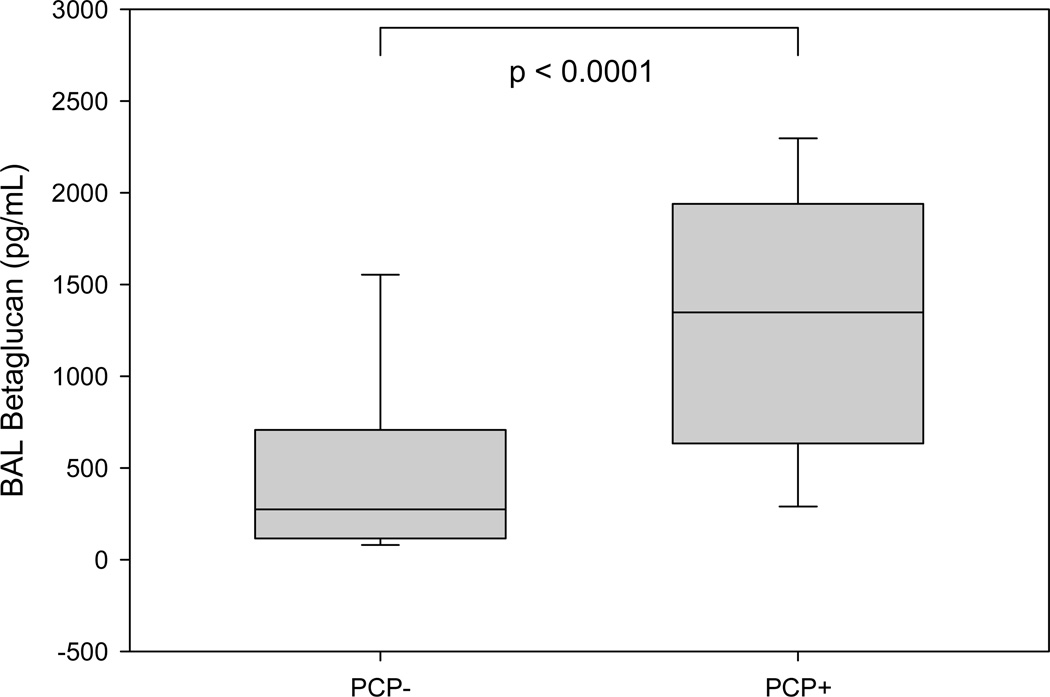
The discriminative power of serum BG for the diagnosis of PCP in this group of patients was very high. Using a cutoff of 300 pg/mL, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were 91%, 92%, 89% and 93% respectively. Table 2 illustrates how serum BG and BAL BG performed at different cutoff levels. When the PCP+ group was compared to the PCP- group, the differences in the values of serum and BAL BG in both cases were statistically significant (p < 0.0001), as seen in Figures 2 and 3.
Table 2.
Test performance for serum and BAL BG at different cutoff levels
| Variables and cutoffs |
Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Serum BG, pg/mL | ||||
| 80 | .98 (.94, 1.0) | .39 (.27, .51) | .54 (.44, .65) | .96 (.88, 1.0) |
| 300 | .91 (.83, .99) | .92 (.85, .99) | .89 (.81, .98) | .93 (.87, 1.0) |
| 490 | .74 (.61, .87) | .95 (.90, 1.0) | .92 (.83, 1.0) | .83 (.74, .92) |
| BAL BG, pg/mL | ||||
| 241 | .95 (.89, 1.0) | .39 (.26, .51) | .54 (.43, .65) | .92 (.81, 1.0) |
| 783 | .72 (.59, .86) | .79 (.68, .90) | .72 (.59, .86) | .79 (.68, .90) |
| 2241 | .12 (.02, .21) | .95 (.89, 1.0) | .63 (.29, .96) | .59 (.49, .69) |
BG = Betaglucan; PPV = positive predictive value; NPV = negative predictive value
Figure 2.
Whisker and Box plot comparing serum BG between PCP− and PCP+
Figure 3.
Whisker and Box plot comparing BAL BG between PCP− and PCP+
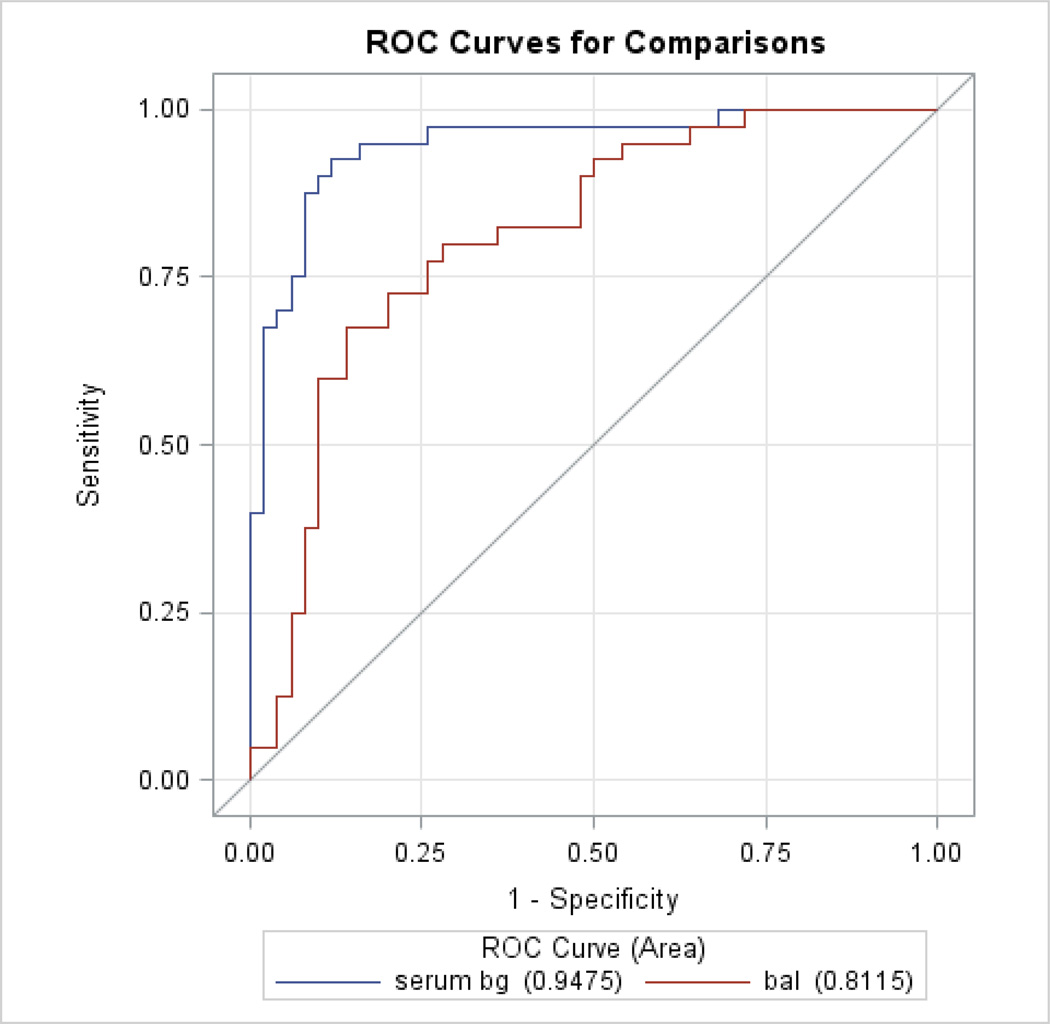
A model for ROC curves using both serum and BAL BG was created, with N = 90 for serum BG, the area under the curve (AUC) was 0.95, for BAL BG it was 0.81. In this case serum BG was significantly better than BAL BG (p < 0.01). A model with just serum BG (N = 108) had an AUC of 0.95. On the other hand, serum PCT was not a significant predictor of PCP (p=0.53), the AUC for serum PCT was 0.53. Figure 4 demonstrate the ROC curves for serum and BAL BG levels.
Figure 4.
Receiver Operating Characteristic (ROC) curves for Serum and BAL BG
Table 3 summarizes the results for our three biomarkers when only patients with FOB cytology results were included. A statistical difference (p<0.0001) was achieved when PCP+ was compared to PCP- for serum and BAL BG but not for serum PCT. The following results were obtained when correlation between the biomarkers was assessed: serum BG and BAL BG rho = 0.49 p < 0.0001; serum BG and serum PCT rho = −0.09 p = 0.3463.
Table 3.
Median values for BAL BG, serum BG and serum PCT in patients PCP+ and PCP−
| PCP+ (n = 46) |
PCP− (n = 62) |
p | |
|---|---|---|---|
| BAL BG* | 1348 (634, 1939) | 274 (118, 1939) | <0.0001 |
| Serum BG | 915 (479, 1267) | 97 (61, 169) | <0.0001 |
| Serum PCT | 0.16 (0.06, 0.44) | 0.13 (0.05, 0.52) | 0.6137 |
Data are median (25th percentile, 75th percentile)
PCP+ n = 43 and PCP− n = 57 differences assessed using the Wilcoxon Test
Other fungal organisms
It is well know that BG is present in the cell wall of many fungal organisms, so when BG is used for the diagnosis of a specific fungal disease (PCP in our case) other fungal elements might cause false positive results. In the 119 patients that we analyzed 33 had fungal smear of culture positive for Candida or yeast. Among those, using a cutoff for serum BG of 300 pg/mL we just found one false positive case. In the group with fungal smear of culture positive for Candida or yeast the false positive rate was 3% (1/33), whereas in the remaining patients the false positive rate was 5% (4/86). Based on this it seems like in our study the presence of Candida or yeast in the BAL did not cause a significant problem with false positives for serum BG in the detection of PCP. Clinically, as seen in table 1, oral candidiasis was reported in around half of all our patients, with no significant differences between the PCP+ and PCP- groups. It is important to notice that in general bronchoscopes are introduced nasally, so if candida is really confined to the mouth it might not contaminate the instrument.
DISCUSSION
Our data support the hypothesis that serum BG has excellent discriminatory power for the diagnosis of PCP in patients with AIDS. Serum BG has the advantage of avoiding invasive FOB, and based on our results was a better predictor of PCP than BAL BG. The measurement of serum BG should be incorporated in the diagnostic work up of HIV positive patients with dyspnea and infiltrates on chest X ray. It is unlikely for patients in this setting and BG values below 300 pg/mL to have PCP. The clinician should use all elements available (CD4 count, CXR findings, signs and symptoms, use of ART, use of PCP prophylaxis) to make a rational decision about the likelihood of infection in each particular case. The recommended threshold value for the Fungitell® assay is 80 pg/mL. Based on the results from this study, we propose that a threshold of 300 pg/mL for the diagnosis of PCP in patients with AIDS may be more appropriate.
On the contrary, serum PCT did not correlate with PCP status in our study. Our initial hypothesis was that PCT, employed as a marker of bacterial infection, might be low in patients with an active fungal infection such as PCP. The study from Nyamande et al.24 showed significantly lower PCT levels in patients with PCP compared to those with bacterial pneumonia. A recent study in HIV-negative patients28 also showed the value of a low serum PCT in differentiating pulmonary tuberculosis from bacterial pneumonia, but did not include patients with PCP. A small study of patients after lung transplantation29 found a relation between bronchial tree colonization by PCP and increased serum PCT. In our study the serum PCT level did not add information for the diagnosis of PCP. It is possible that bacterial organisms in the alveolar space that were not measured in our study affected our serum PCT results.
The diagnosis of patients with AIDS and pulmonary infiltrates on chest X ray remains a clinical challenge. Recent guidelines for diagnostic FOB from the British Thoracic Society30 still define BAL as the gold standard for the diagnosis of PCP. Its sensitivity before antibiotic administration ranges between 90–98%,31,32 but after antibiotics is reduced to around 64%.33 Nevertheless, FOB is not completely devoid of complications and adds cost and duration to the patient’s hospital stay.34
There are just 3 previous publications that did study BG as a diagnostic tool for PCP exclusively in HIV patients. Watanabe35 did evaluate 111 patients, Sax8 did evaluate 252 and Esteves13 did analyze 100 HIV positives and 50 controls. None of them did study like we did BAL BG or serum procalcitonin. Just Esteves did propose a new cut off value for the test in this specific population, like we do. Is also different the group from where the samples came in those previous studies; in Sax it was patients with HIV and acute opportunistic infections of all types, in Watanabe all patients did have PCP and in Esteves there were healthy controls and patients with HIV and respiratory symptoms. Our study did include inpatients with HIV and diagnosis of pneumonia, a group that more closely resembles the population where a clinician can use serum BG to help with the diagnosis of PCP.
Another important aspect of this research is the fact that patients with suspicion of pulmonary infections are usually started on empiric antimicrobials well before bronchoscopies are performed. Several studies have shown that this practice impairs the diagnostic yield of BAL in HIV+ patients with PCP, bacterial pneumonias and tuberculosis.33,36,37 If appropriate serum biomarkers are included in the early evaluation of HIV-associated pneumonia patients, the impact of this limitation could be minimized.
One limitation to the application of BG testing in the care of HIV-associated pneumonia is the “pan-fungal” character of the BG test.9 Positive results could be related to other fungal infections (candida, aspergillus, histoplasma, etc.). While this is certainly relevant in a broader population of immunosuppressed patients, within the clinical context of HIV-related immunosuppression this is likely less important. Other false-positive BG results can occur in patients on hemodialysis if cellulose membranes are used, Gram negative bacteremia, severe mucositits, exposure of samples to gauze, use of intravenous immunoglobulins as well perhaps the use of some antimicrobials.12,38 Some hospitals in the US that process BG in their laboratories can give results in one day; therefore that could impact clinical decision-making. Research to develop a rapid in-house BG test is in progress. An interesting paper from Austria39 recently shown a validation for the detection of candidemia using a rapid single sample in house BG test that provides results in less than one hour.
Another issue is the role of quantitative PCR (qPCR) for the diagnosis of PCP.40 There is still a debate about what levels define colonization versus infection.41 It is possible that in certain settings qPCR for PCP might become the gold standard for diagnosis, and then the correlation between that marker and serum levels of BG will need further study. A recent retrospective single-center study from France27 found a positive serum BG level, using a 80 pg/mL threshold, in 25 of 26 samples that were BAL microscopy-negative but qPCR positive for PCP. Another recent study42 in 46 patients concluded that a combination of qPCR and serum BG (using a cutoff of 100 pg/mL) could differentiate between PCP infection and colonization.
A final factor that could have influenced our results is the timing of administration of antimicrobials for PCP. Likely the majority of our patients were empirically treated for PCP at the time of the FOB. Very few papers have investigated the kinetics of serum BG after the initiation of antifungal treatment.43,44 However, it appears that the decline of serum BG after treatment is slow and the value should remain above the cut off for positivity for many days. A prospective study with clearly defined timing of FOB and initiation of treatment could overcome this limitation.
Another element that could have had some impact on our results is the interaction during colonization of bacterial and fungal organisms in the alveolar space. In a study by Friaza et al.45 the authors were able to show an antagonistic relationship between PCP and bacteria in a group of patients with interstitial lung disease. The patients with PCP colonization did have less bacterial microbiota diversity. The authors hypothesized that the fungal elements create an inflammatory status that can interfere with bacterial colonization or that increases its alveolar clearance. This type of research has not yet been conducted in patients with AIDS. Also it is important to notice that our study was performed in an area of low tuberculosis prevalence, we do not know what impact will have in the biomarkers that we measured using a group of patients from an area with endemic tuberculosis.
One advantage of our study is the fact that our BG levels were quantified even above 500 pg/mL. Some previous studies have censored those values. Another important aspect of our research is how well defined our population was. Some previous reports have tested serum BG in a broader mix of immunocompromised patients (HIV, hematological malignancies, transplant recipients) and looked for any type of invasive fungal infection.9,16,46,47 Thus our study design could translate into better application of our results into clinical practice.
Our study is not suggesting dichotomizing the care of patient with AIDS and suspicion of PCP based on results of serum BG. The clinician should use all elements available (CD4 count, CXR findings, signs and symptoms, use of ART, use of PCP prophylaxis) to make a rational decision about the likelihood of infection in each particular case. In the future, using a larger prospective study it might be feasible to create a clinical prediction rule for PCP in AIDS that could incorporate serum BG and all the other elements from laboratory, image and clinical data. It is also important to mention that patients with HIV and PCP have higher burden of pathogens than those with other types of immunosuppression and PCP,15 and this might reflect in the BG levels. So is quite possible that BG will be a better diagnostic tool in the first group.
CONCLUSIONS
The measurement of serum BG should be incorporated into the diagnostic work up of HIV-positive patients with dyspnea and infiltrates on chest X-ray. Using a cutoff of 300 pg/mL, the sensitivity and specificity are above 90% and the NPV is 93% for the diagnosis of PCP. It is unlikely for patients in this setting and BG values below our proposed cutoff have PCP. The clinician should use all elements available (CD4 count, CXR findings, signs and symptoms, use of ART, use of PCP prophylaxis) to make a rational decision about the likelihood of infection in each particular case. Our study confirms the diagnostic value of serum BG previously reported by others but we add a cutoff value that we believe is more accurate for patients with AIDS and suspicion of PCP.
The gold standard for PCP diagnosis remains the identification of the organism in BAL.
BG is a polysaccharide that is present within the cell wall of Pneumocystis and other fungi.
The discriminative power of serum BG for the diagnosis of PCP in our study was very high.
Serum BG should be incorporated in the diagnostic work up of patients with HIV and pneumonia.
Our study adds a new cutoff value more accurate for patients with HIV and pneumonia.
ACKNOWLEDGEMENTS
Role of sponsors:
Supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health which funds the Louisiana Clinical and Translational Science Center. Supported in part by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers P60 AA009803 and R24 AA019661 and the National Heart, Lung, and Blood Institute / NIH P01 HL076100.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other Contributions:
We are grateful to Malcolm Finkelman, PhD from Associates of Cape Cod, Inc. and Vince Tuminello from bioMerieux, Inc. for the donation of kits for the measurement of BG and PCT. We would also like to thank Joseph Lasky, MD for the critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Calderon EJ, Gutierrez-Rivero S, Durand-Joly I, et al. Pneumocystis infection in humans: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:683–701. doi: 10.1586/eri.10.42. [DOI] [PubMed] [Google Scholar]
- 2.Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-beta-Dglucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2012;50:7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruciani M, Marcati P, Malena M, et al. Meta-analysis of diagnostic procedures for Pneumocystis carinii pneumonia in HIV-1-infected patients. Eur Respir J. 2002;20:982–989. doi: 10.1183/09031936.02.01372002. [DOI] [PubMed] [Google Scholar]
- 4.Taylor IK, Coker RJ, Clarke J, et al. Pulmonary complications of HIV disease: 10 year retrospective evaluation of yields from bronchoalveolar lavage, 1983–93. Thorax. 1995;50:1240–1245. doi: 10.1136/thx.50.12.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Cattamanchi A, Davis JL, et al. HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc. 2011;8:294–300. doi: 10.1513/pats.201009-062WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris AM, Masur H. A serologic test to diagnose pneumocystis pneumonia: are we there yet? Clin Infect Dis. 2011;53:203–204. doi: 10.1093/cid/cir348. [DOI] [PubMed] [Google Scholar]
- 7.Yasuoka A, Tachikawa N, Shimada K, et al. (1-->3) beta-D-glucan as a quantitative serological marker for Pneumocystis carinii pneumonia. Clin Diagn Lab Immunol. 1996;3:197–199. doi: 10.1128/cdli.3.2.197-199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sax PE, Komarow L, Finkelman MA, et al. Blood (1->3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis. 2011;53:197–202. doi: 10.1093/cid/cir335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desmet S, Van Wijngaerden E, Maertens J, et al. Serum (1–3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol. 2009;47:3871–3874. doi: 10.1128/JCM.01756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura Y, Ito Y, Iinuma Y, et al. Quantitative real-time PCR and the (1-->3)-beta-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect. 2012;18:591–597. doi: 10.1111/j.1469-0691.2011.03605.x. [DOI] [PubMed] [Google Scholar]
- 11.de Boer MG, Gelinck LB, van Zelst BD, et al. beta-D-glucan and Sadenosylmethionine serum levels for the diagnosis of Pneumocystis pneumonia in HIV-negative patients: a prospective study. J Infect. 2011;62:93–100. doi: 10.1016/j.jinf.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Wood BR, Komarow L, Zolopa AR, et al. Test performance of blood beta-glucan for Pneumocystis jirovecii pneumonia in patients with AIDS and respiratory symptoms. AIDS. 2013;27:967–972. doi: 10.1097/QAD.0b013e32835cb646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteves F, Lee CH, de Sousa B, et al. (1–3)-Beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2014 doi: 10.1007/s10096-014-2054-6. [DOI] [PubMed] [Google Scholar]
- 14.He S, Hang JP, Zhang L, et al. A systematic review and meta-analysis of diagnostic accuracy of serum 1,3-beta-d-glucan for invasive fungal infection: Focus on cutoff levels. J Microbiol Immunol Infect. 2014 doi: 10.1016/j.jmii.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Karageorgopoulos DE, Qu JM, Korbila IP, et al. Accuracy of beta-D-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect. 2013;19:39–49. doi: 10.1111/j.1469-0691.2011.03760.x. [DOI] [PubMed] [Google Scholar]
- 16.Theel ES, Jespersen DJ, Iqbal S, et al. Detection of (1, 3)-beta-D-glucan in bronchoalveolar lavage and serum samples collected from immunocompromised hosts. Mycopathologia. 2013;175:33–41. doi: 10.1007/s11046-012-9579-y. [DOI] [PubMed] [Google Scholar]
- 17.Duflo F, Debon R, Monneret G, et al. Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology. 2002;96:74–79. doi: 10.1097/00000542-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Linssen CF, Bekers O, Drent M, et al. C-reactive protein and procalcitonin concentrations in bronchoalveolar lavage fluid as a predictor of ventilator-associated pneumonia. Ann Clin Biochem. 2008;45:293–298. doi: 10.1258/acb.2007.007133. [DOI] [PubMed] [Google Scholar]
- 19.Rose SR, Vallabhajosyula S, Velez MG, et al. The utility of bronchoalveolar lavage beta-D-glucan testing for the diagnosis of invasive fungal infections. J Infect. 2014;69:278–283. doi: 10.1016/j.jinf.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9:107. doi: 10.1186/1741-7015-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gogos CA, Drosou E, Bassaris HP, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 22.Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 23.Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 24.Nyamande K, Lalloo UG. Serum procalcitonin distinguishes CAP due to bacteria, Mycobacterium tuberculosis and PJP. Int J Tuberc Lung Dis. 2006;10:510–515. [PubMed] [Google Scholar]
- 25.Stolz D, Stulz A, Muller B, et al. BAL neutrophils, serum procalcitonin, and Creactive protein to predict bacterial infection in the immunocompromised host. Chest. 2007;132:504–514. doi: 10.1378/chest.07-0175. [DOI] [PubMed] [Google Scholar]
- 26.Calderon EJ. Pneumocystis infection: seeing beyond the tip of the iceberg. Clin Infect Dis. 2010;50:354–356. doi: 10.1086/649870. [DOI] [PubMed] [Google Scholar]
- 27.Costa JM, Botterel F, Cabaret O, et al. Association between circulating DNA, serum (1->3)-beta-D-glucan, and pulmonary fungal burden in Pneumocystis pneumonia. Clin Infect Dis. 2012;55:e5–e8. doi: 10.1093/cid/cis412. [DOI] [PubMed] [Google Scholar]
- 28.Ugajin M, Miwa S, Shirai M, et al. Usefulness of serum procalcitonin levels in pulmonary tuberculosis. Eur Respir J. 2011;37:371–375. doi: 10.1183/09031936.00011910. [DOI] [PubMed] [Google Scholar]
- 29.Zeglen S, Wojarski J, Wozniak-Grygiel E, et al. Procalcitonin serum concentration during Pneumocystis jiroveci colonization or Pseudomonas aeruginosa infection/colonization in lung transplant recipients. Transplant Proc. 2009;41:3225–3227. doi: 10.1016/j.transproceed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68(Suppl 1):i1–i44. doi: 10.1136/thoraxjnl-2013-203618. [DOI] [PubMed] [Google Scholar]
- 31.Orenstein M, Webber CA, Cash M, et al. Value of bronchoalveolar lavage in the diagnosis of pulmonary infection in acquired immune deficiency syndrome. Thorax. 1986;41:345–349. doi: 10.1136/thx.41.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golden JA, Hollander H, Stulbarg MS, et al. Bronchoalveolar lavage as the exclusive diagnostic modality for Pneumocystis carinii pneumonia. A prospective study among patients with acquired immunodeficiency syndrome. Chest. 1986;90:18–22. doi: 10.1378/chest.90.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Gracia JD, Miravitlles M, Mayordomo C, et al. Empiric treatments impair the diagnostic yield of BAL in HIV-positive patients. Chest. 1997;111:1180–1186. doi: 10.1378/chest.111.5.1180. [DOI] [PubMed] [Google Scholar]
- 34.Jin F, Mu D, Chu D, et al. Severe complications of bronchoscopy. Respiration. 2008;76:429–433. doi: 10.1159/000151656. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe T, Yasuoka A, Tanuma J, et al. Serum (1-->3) beta-D-glucan as a noninvasive adjunct marker for the diagnosis of Pneumocystis pneumonia in patients with AIDS. Clin Infect Dis. 2009;49:1128–1131. doi: 10.1086/605579. [DOI] [PubMed] [Google Scholar]
- 36.Dotson RG, Pingleton SK. The effect of antibiotic therapy on recovery of intracellular bacteria from bronchoalveolar lavage in suspected ventilatorassociated nosocomial pneumonia. Chest. 1993;103:541–546. doi: 10.1378/chest.103.2.541. [DOI] [PubMed] [Google Scholar]
- 37.Souweine B, Veber B, Bedos JP, et al. Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments. Crit Care Med. 1998;26:236–244. doi: 10.1097/00003246-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Marty FM, Lowry CM, Lempitski SJ, et al. Reactivity of (1-->3)-beta-d-glucan assay with commonly used intravenous antimicrobials. Antimicrob Agents Chemother. 2006;50:3450–3453. doi: 10.1128/AAC.00658-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pruller F, Wagner J, Raggam RB, et al. Automation of serum (1-->3)-beta-D-glucan testing allows reliable and rapid discrimination of patients with and without candidemia. Med Mycol. 2014;52:455–461. doi: 10.1093/mmy/myu023. [DOI] [PubMed] [Google Scholar]
- 40.Finkelman MA. Pneumocystis jirovecii infection: Cell wall (1-->3)-beta-D-glucan biology and diagnostic utility. Crit Rev Microbiol. 2010;36:271–281. doi: 10.3109/1040841X.2010.484001. [DOI] [PubMed] [Google Scholar]
- 41.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25:297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damiani C, Le Gal S, Da Costa C, et al. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-beta-D-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol. 2013;51:3380–3388. doi: 10.1128/JCM.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Held J, Wagner D. beta-d-Glucan kinetics for the assessment of treatment response in Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2011;17:1118–1122. doi: 10.1111/j.1469-0691.2010.03452.x. [DOI] [PubMed] [Google Scholar]
- 44.Koo S, Baden LR, Marty FM. Post-diagnostic kinetics of the (1 -->3)-beta-Dglucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin Microbiol Infect. 2012;18:E122–E127. doi: 10.1111/j.1469-0691.2012.03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friaza V, la Horra C, Rodriguez-Dominguez MJ, et al. Metagenomic analysis of bronchoalveolar lavage samples from patients with idiopathic interstitial pneumonia and its antagonic relation with Pneumocystis jirovecii colonization. J Microbiol Methods. 2010;82:98–101. doi: 10.1016/j.mimet.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 46.Issa NC, Koo S, Lynch RC, et al. Serum galactomannan and (1->3)-beta-D-glucan assays for patients with multiple myeloma and Waldenstrom's macroglobulinemia. J Clin Microbiol. 2012;50:1054–1056. doi: 10.1128/JCM.06295-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onishi A, Sugiyama D, Kogata Y, et al. Diagnostic accuracy of serum 1,3-beta-Dglucan for pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol. 2011;50:7–15. doi: 10.1128/JCM.05267-11. [DOI] [PMC free article] [PubMed] [Google Scholar]