Abstract
Purpose
Cancer cells are highly dependent on folate metabolism, making them susceptible to drugs that inhibit folate receptor activities. Targeting overexpressed folate receptor alpha (FRα) in cancer cells offers a therapeutic opportunity. We investigated the functional mechanisms of MORAB-003 (farletuzumab), a humanized monoclonal antibody against FRα, in ovarian cancer models.
Experimental Design
We first examined FRα expression in an array of human ovarian cancer cell lines and then assessed the in vivo effect of MORAB-003 on tumor growth and progression in several orthotopic mouse models of ovarian cancer derived from these cell lines. Molecular mechanisms of tumor cell death induced by MORAB-003 were investigated by cDNA and protein expression profiling analysis. Mechanistic studies were performed to determine the role of autophagy in MORAB-003–induced cell death.
Results
MORAB-003 significantly decreased tumor growth in the high-FRα IGROV1 and SKOV3ip1 models but not in the low-FRα A2780 model. MORAB-003 reduced proliferation but had no significant effect on apoptosis. Protein expression and cDNA microarray analyses showed that MORAB-003 regulated an array of autophagy-related genes. It also significantly increased expression of LC3 isoform II and enriched autophagic vacuolization. Blocking autophagy with hydroxychloroquine or bafilomycin A1 reversed the growth inhibition induced by MORAB-003. In add, alteration of FOLR1 gene copy number significantly correlated with shorter disease-free survival in patients with ovarian serous cystadenocarcinoma.
Conclusions
MORAB-003 displays prominent antitumor activity in ovarian cancer models expressing FRα at high levels. Blockade of folate receptor by MORAB-003 induced sustained autophagy and suppressed cell proliferation.
Keywords: Folate receptor α, MORAB-003, ovarian cancer, autophagy, cell death
Introduction
The folate receptor 1 gene (FOLR1) encodes a 38-kDa GPI-anchored protein (folate receptor alpha, or FRα) that binds folic acid with high affinity and transports folate by receptor-mediated endocytosis (1). FRα is selectively overexpressed in approximately 90% of non-mucinous ovarian carcinomas but is largely absent from normal tissues (2, 3). Its expression is maintained on metastatic foci and recurrent tumors (4). Studies that have examined the functional significance of FRα in ovarian cancer cells have shown that higher receptor expression is associated with greater biological aggressiveness (5).
Previous studies showed that anti-FRα antibodies such as LK26, Mov18, and Mov19 have reached development stages and been tested for clinical relevance (6, 7). MORAB-003 (farletuzumab), which is a humanized anti-FRα monoclonal antibody derived from optimization of the LK26 molecule; it maintains an affinity similar to the original murine LK26 antibody (approximately 2 nM) and a tissue binding profile consistent with FRα distribution (5, 8). It has been shown to inhibit cell growth and activate antibody-dependent, cell-mediated cytotoxicity (5, 8, 9). Although MORAB-003 has been shown to act through antibody-dependent cellular cytotoxicity in vitro (10), additional mechanisms underlying its function are likely. Herein, we reported that sustained treatment with MORAB-003 antibody in xenograft models of human ovarian cancers or in 3-D cultured ovarian cancer models expressing high levels of FRα induced enrichment of late-stage autophagic events, eventually leading to cell death and tumor growth inhibition.
Methods
Cell lines and cultures
Human ovarian cancer cells (A2780, HeyA8, SKOV3ip1, and IGROV1) were cultured in RPMI 1640 cell culture medium supplemented with 15% FBS and 0.5% gentamicin (11, 12). All cell lines were purchased from ATCC and authenticated by the Cell Line Core Facility at The University of Texas MD Anderson Cancer Center and routinely tested to confirm the absence of Mycoplasma.
For the 3-D models, cells were grown on non-adhesive optical plates (MatTek Corporation, Ashland, MA) and then brought to the glass surface, which was precoated with 200 µL of growth factoRreduced Matrigel (BD Biosciences, San Diego, CA). Approximately 18,000~20,000 spheroid-like cells were plated in each well of a 24-well plate and incubated at 37°C for 1 hour to allow cells to seed gradually. Spheroids were cultured with full medium containing 2% Matrigel (13). The culture conditions and growth of 3-D spheroids were monitored daily in preparation for drug treatment.
Reagents and transfection
The pEGFP-RFP-LC3 plasmid in lentiviral vector was derived from Addgene plasmid 21074 (Addgene, Cambridge, MA)(14). The PEA-15 siRNA (ggaagacauccccagcgaatt) was described previously (15). The EGFP-LC3 plasmid was derived from Addgene 11546(16). The siRNAs were applied to cells at a final concentration of 100 nmol/L for 48 hours. The shRNAs against BECN-1,29-mer mix (human) were purchased from Origene, Inc. (Rockville, MD). Transfections were performed according to the manufacturer’s instructions.
Analysis of FRα expression
Cellular expression of FRα was determined by FACS analysis of cells stained with fluorescein isothiocyanate–labeled MORAB-003. The mean fluorescence value was 1 in cells that do not express FRα, such as CHO-parental cells. The expression of FRα was validated with FRα Antibody PA5-27465 (Thermo Pierce, Rockford, IL).
Animal
The female athymic nude mice used for this experiment were cared for according to guidelines set forth by the American Association for Accreditation of Laboratory Animal Care and the U.S. Public Health Service policy on Humane Care and Use of Laboratory Animals. All mouse studies were approved and supervised by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee. Development and characterization of the orthotopic mouse models of advanced ovarian cancer has been described (11, 17).
In vivo MORAB-003 therapeutic dose-finding
To determine the optimal dose and schedule of MORAB-003 therapy, 5 mice were injected intraperitoneally with SKOV3ip1 cells to induce tumors. One week later, treatment was initiated with either control IgG or MORAB-003 (5, 25, or 50 mg/kg) twice or thrice weekly for 28 days. Mean tumor weights were recorded after 4 weeks of therapy for each treatment group.
In vivo murine ovarian cancer models
Mice were injected with tumor cells (1×106 cells/mL (SKOV3ip1 and A2780) or 2×106 cells/mL (IGROV1) in Hank balanced salt solution) to induce tumors; one week later, tumor-bearing mice were randomized into 4 groups (n=10 mice/group for SKOV3ip1 or IGROV1 models, and n=5 for A2780 model or control) and treated with intraperitoneal injections of the following agents: 1) control IgG (5.0 mg/kg twice weekly), 2) MORAB-003 (5.0 mg/kg twice weekly), 3) control IgG (5.0 mg/kg twice weekly) plus docetaxel (35 µg/mouse weekly), or 4) MORAB-003 (5.0 mg/kg twice weekly) plus docetaxel (35 µg/mouse weekly). Mice were monitored daily for adverse effects and killed if they became moribund. After 4 to 5 weeks of therapy, all of the mice were killed and necropsies were performed. For each mouse, body and tumor weight, tumor distribution, number of tumor nodules, and amount of ascites were recorded at necropsy. Tissue specimens were fixed in formalin for paraffin embedding or snap frozen in optimal cutting medium (Miles, Inc., Elkhart, IN) for frozen slide preparation.
Tandem mRFP/mGFP-LC3 fluorescence microscopy
pGFP-RFP-LC3 (Addgene) plasmids (14) or EGFP-LC3 plasmids (16) were stably transfected into epithelial ovarian cancer cells (HeyA8, SKOV3). After 3-D culture on glass-bottom optical 35-mm2 dishes (MatTek Corporation), expression of EGFP and RFP was visualized with a laser scanning multiphoton confocal microscope (TCS SP5 MP; Leica, Buffalo Grove, IL).
Apoptosis assay
Apoptosis was measured by using the AnnexinV-7AAD apoptosis dectection kit I (BD Biosciences, San Jose, CA). Twenty-four hours and 7 days following treatment with MORAB-003, SKOV3ip1 cells were washed twice with cold PBS and then resuspended in 1× Binding Buffer at a concentration of 1×106 cells/mL. One hundred microliters of the solution (1×105 cells) was then transferred to a 5-mL culture tube. AnnexinV and 7-AAD (5 µL each) were added to the solution. Cells were subjected to gentle vortexing and then incubated for 15 minutes at room temperature (25°C) in the dark. 1× binding buffer (400 µL) was added to each tube. Samples were analyzed by flow cytometry within 1 hour.
Immunohistochemistry for PCNA and CD31 and TUNEL assay
Formalin-fxed, paraffin-embedded tumor tissues collected from the mice after treatment were sectioned (5 µm) for detection of PCNA and for TUNEL assay according to previous protocol(18). For quantification of PCNA, microvessel density or TUNEL expression, the numbers of PCNA-or TUNEL-positive tumor cells or CD31-positive endothelial cells were counted in 10 random fields at ×200 magnification.
Immunoblotting
Immunoblotting analyses were performed as previously described (11).
Cell viability and proliferation
To determine the in vitro effects of MORAB-003 on ovarian cancer cell viability, SKOV3ip1 cells were cultured in folate-free medium supplemented with 10% dialyzed FBS and 0.1% gentamicin sulfate for 5 days. Cells were then plated in medium supplemented with 5-MTF (i.e., physiologic folate conditions), based on previous protocol (19). Experimental conditions were set in triplicate.
To assess cell viability, 50 µL per well of 0.15% MTT (Sigma) was added a 96-well plate, and the plate was incubated for 2 hours at 37°C. The medium/MTT preparation was then removed from each well, and 100 µL of dimethyl sulfoxide (Sigma) was added. After incubation for 20 minutes, absorbance at 570 nm was read (Ceres UV 900C; Bio-Tek Instrument, Inc., Winooski, VT) within 30 minutes. Each treatment was repeated in 8 individual replicas.
To assess in vitro cell proliferation, the percentages of proliferative cells were measured by using Click-iT EdU Flow Cytometry Assay Kits (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The 2-D cultured cells were incubated with 10 mM EdU for 2 hours and the trypsinized cells fixed with 4% paraformaldehyde before staining and flow cytometry. For 3-D cultured spheroids, cells were extracted with Accutase solution (Sigma) to dissociate the cells from Matrigel and 0.05% trypsin-EDTA (Lonza), and the assays were performed with the same fixation and staining as for FACS analysis. Each treatment was repeated in 6 individual replicates.
Acridine orange staining and flow cytometry
The formation of AVOs was visualized by AO (20). In AO-stained cells, the cytoplasm and nucleolus fluorescence bright green and dim red, whereas acidic compartments fluoresce bright red. The volume of the cellular acidic compartment can be quantified, therefore, by the intensity of the red fluorescence. Cells were stained with AO as described previously (20). Green (510–530 nm) and red (>650 nm) fluorescence emissions from 1×104 cells illuminated with blue (488 nm) excitation light were measured with a FACSCalibur from Becton Dickinson (Franklin Lakes, NJ) and analyzed by CellQuest software (BD Biosciences).
Transmission electronic microscopy
3-D cultured SKOV3ip1 spheroids were fixed and processed as previously described (21).
Reverse-phase protein arrays
Proteins lysates were extracted and submitted to the RPPA Process Core Facility at MD Anderson Cancer Center (http://bioinformatics.mdanderson.org/OOMPA)(22). Slides were probed with 263 validated antibodies, then were scanned and analyzed to quantitatively measure spot density to generate a fitted curve for each condition. We included only the data for the 172 individual antibodies with QC Scores higher than 0.80 in the heat maps for the SKOV3ip1 array and the 214 antibodies higher than 0.80 for the IGROV1 array. The fitted curve was plotted with the log2-concentration of proteins versus spot density. All data presented reflect fold-change compared to the baseline (i.e., to control-treated cells). Positive fold-change was calculated by dividing each linear value >1.0 by the average control linear value for each antibody tested, while negative fold-change (for linear values <1.0) was also calculated (by using the following formula: [−1/linear fold-change]) and plotted in a heat map as a log 2.0 value. Original data for RPPA was provided in Supplemental Table 1.
Microarray analysis
SKOV3ip1 3-D spheroids with and without MORAB-003 treatment were subjected to microarray analysis under the following conditions as previously reported (19): 1) cells plated in folate-free medium (7 days), 2) cells plated in medium with physiologic levels of folic acid (2–10 nm) (7 days), 3) cells plated with physiologic levels of folic acid (7 days) and MORAB-003 (24 hours), and 4) cells plated with physiologic levels of folic acid (7 days) and control antibody (24 hours). For each condition, a single cell growth was carried out and an RNA sample was extracted. Three aliquots from each RNA sample were then hybridized to Affymetrix U133 2 arrays, for a total of 15 arrays. Integrated pathway analysis networks were generated by using Core analysis with indicated data cutoffs for up/downregulated genes and using direct interactions, with the cutoff for network size at 70. The highest scoring 5 networks were merged and exported as IPEG files. All network plottings were carried out according to the method described previously (23). The original microarray data was uploaded to GEO with accession number GSE61513 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE61513).
The Cancer Genome Altas database analysis
Data from the TCGA were evaluated for expression of the FOLR1 gene in samples from patients with ovarian serous cystadenocarcinoma and expression of cluster immunoreactive signatures (n=110) (TCGA) in relation to the patients’ overall survival and disease-free survival. We also queried data from patients with uterine corpus endometrial carcinoma (n=82) www.cbioportal.org) (24, 25
Statistical analysis
All results are expressed as mean ± SD unless indicated otherwise. Continuous variables were compared by using the Student t-test (between two groups) or ANOVA (for all groups). For non-normally distributed data sets (as determined by the Kolmogrov–Smirnov test), the Mann-Whitney rank sum test was used. A P <0.05 was considered statistically significant. At least 3 independent replicas were included for each test.
Results
In vivo antitumor effects of MORAB-003 in human ovarian cancer
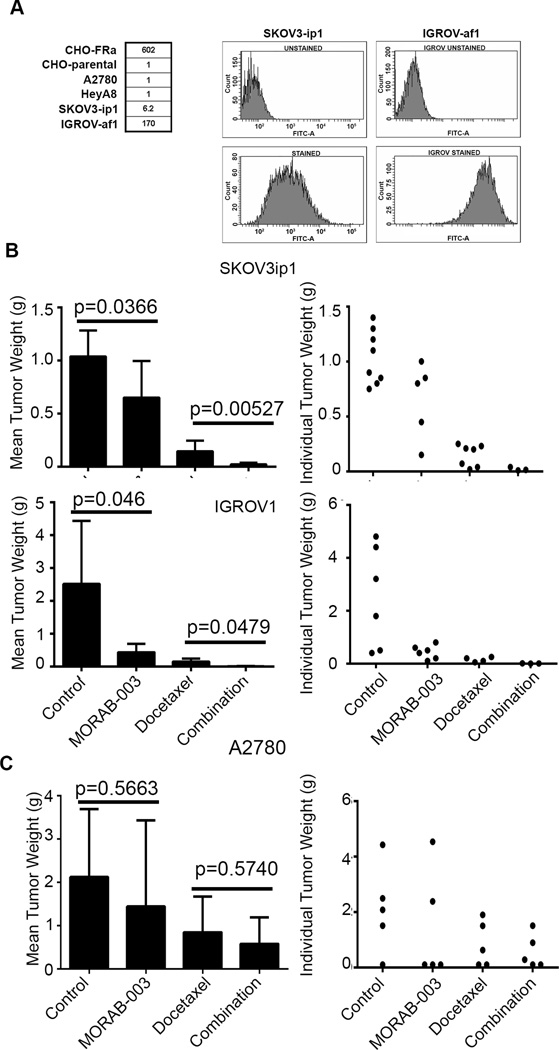
We first characterized FRα expression of human ovarian cancer cell lines by using fluorescence-activated cell sorting (FACS) analysis. Compared to a mean fluorescence value of 1 in cells that do not express FRα (CHO parental cells, A2780 or HeyA8 cells), FRα expression was 602-fold higher in the transfected cell line CHO-FRα, 170-fold higher in IGROV1 cells, and 6.2-fold higher in SKOV3ip1 cells (Fig. 1A). We confirmed the expression of FRα in an array of ovarian cancer cell lines by Western blots with FRα antibody (Supplemental Fig. 1A). We also determined the basal levels of autophagic flux as measured by isoform II of the microtubule-associated protein 1 light chain (LC3II), but did not observe significant differences across the cell lines regardless of levels of FRα.
Figure 1. In vivo therapeutic effects of MORAB-003 on growth of human ovarian cancer.
(A) Left panel: FACS analysis revealed the absence of FRα expression in CHO parental cells, while high expression was confirmed in the transfected cell line CHO-FRα. Numbers represent relative fluorescence. Right panel: FACS plot showed that both SKOV3ip1 and IGROV1 cell lines expressed high levels of FRα.
(B) Effects of MORAB-003 and docetaxel on tumor growth in SKOV3ip1 and IGROV1 orthotopic mouse models. Left panel: SKOV3ip1: P <0.05 (control vs. MORAB-003); P <0.001(control vs. MORAB-003+docetaxel [Combination]). IGROV1: P <0.05 (control vs. MORAB-003); P <0.001 (control vs. MORAB-003+docetaxel). Right panel: Weights of individual tumors from each mouse are shown.
(C) Effects of MORAB-003 and docetaxel on tumor growth in the low-FRα A2780 orthotopic mouse model. Left panel: P >0.05 (control vs. MORAB-003). Right panel: Weights of the tumors from each mouse are shown. Error bars represent SEM.
We first performed a dose-finding experiment using the SKOV3ip1 orthotopic mouse model to determine the optimal dosage. A dose of 5 mg/kg twice weekly was chosen (Supplemental Fig. 1B) since no additional benefit was noted at higher doses; this dose is within clinically-relevant doses (26). We then tested the therapeutic effect of MORAB-003 in orthotopic mouse models of IGROV1, SKOV3ip1, and A2780 tumors. Since taxanes are frequently used for treatment of newly diagnosed or relapsed ovarian cancer, we also tested combinations of MORAB-003 with docetaxel. Compared to the control, MORAB-003 monotherapy reduced tumor weight by 44% in the SKOV3ip1 model (P <0.05) and 84% in the IGROV1 model (P <0.05; Fig. 1B). Furthermore, the combination of MORAB-003 and docetaxel significantly reduced tumor weight in both SKOV3ip1 and IGROV1 tumor-bearing mice compared to docetaxel monotherapy. MORAB-003 monotherapy also reduced the numbers of tumor nodules in the SKOV3ip1 and IGROV1 models in comparison with the control group (P<0.05, Supplemental Figs. 1C & 1D). However, neither tumor weight (Fig. 1C) nor number of tumor nodules was reduced by MORAB-003 treatment in the A2780 model (Supplemental Fig. 1E). In all of these experiments, there was no significant difference in feeding behavior or average mouse weight among the various treatment groups, suggesting that MORAB-003 has no systemic toxicity (Supplemental Fig. 1F, 1G &1H).
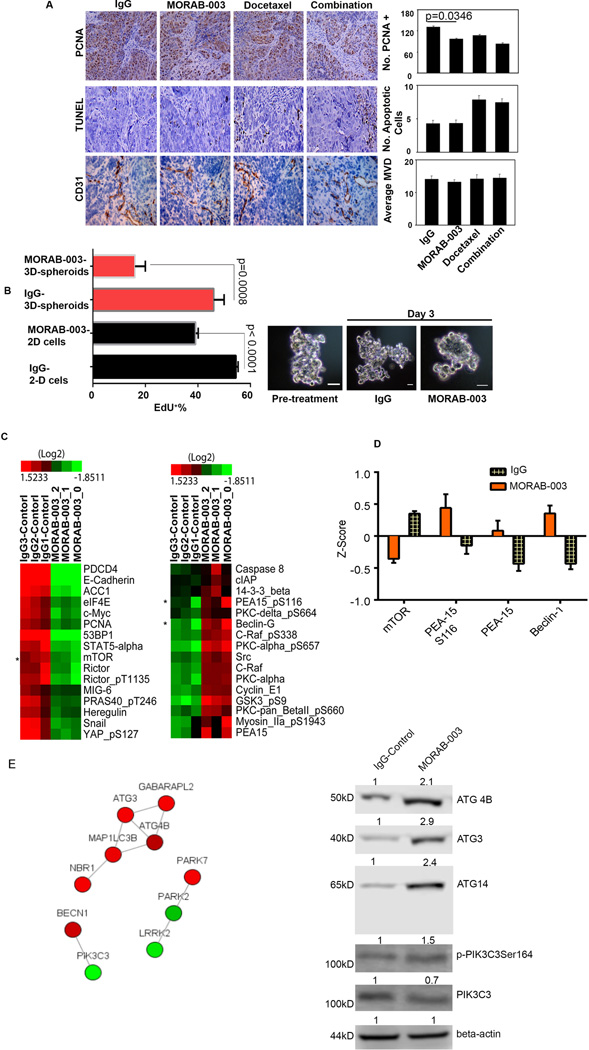
We then examined tumor sections from different treatment groups for markers of proliferation (proliferating cell nuclear antigen [PCNA]), apoptosis (terminal deoxyribonucleotide transferase-mediated nick-end labeling [TUNEL]), and angiogenesis (CD31). Treatment with MORAB-003 reduced tumor cell proliferation by 20–27% in comparison with untreated controls (P <0.05), but had no significant effects on angiogenesis or tumor cell apoptosis (Fig. 2A).
Figure 2. Effects of MORAB-003 in in vivo or in 3-D ovarian cancer spheroids and 2-D monolayer cultured ovarian cancer cells.
(A) In the in vivo IGROV1 tumor model, treatment with MORAB-003 resulted in a 27% decrease in tumor cell proliferation by PCNA staining; P <0.001 (control vs. MORAB-003). However, MORAB-003 treatment did not significantly affect angiogenesis (i.e., microvessel density [MVD]), as detected by CD31 staining, or tumor cell apoptosis, as detected by TUNEL assay.
(B) Left panel: Treatment with MORAB-003 decreased cell proliferation (as measured by EdU incorporation) by 20% in 2-D cultured SKOV3ip1 cells and by 80% in 3-D SKOV3ip1 spheroids P <0.001 (control vs. MORAB-003). Right panel: Representative images show 3-D SKOV3ip1 spheroids prior to treatment or after treatment with control IgG or MORAB-003 antibody for 72 hours.
(C) Left panel: Heat map clustered from RPPA analysis in tumor samples of SKOV3ip1 model revealed that an array of autophagic factors, including mTOR (marked by asterisk) and RICTOR, were downregulated by MORAB-003. Right panel: Other autophagic factors, including S116-PEA-15 and beclin-1 (marked by asterisks), were upregulated by MORAB-003 treatment.
(D) Normalized fold-changes in Z-score indicate that mTOR, S116-PEA-15, and beclin-1 were significantly regulated by MORAB-003 treatment.
(E) Left panel: Network Pathway analysis in SKOV3ip1 3-D spheroids treated with control antibody or MORAB-003. Different colors represent different interactions: red, gene regulation; green, reactome interaction. Right panel: Western blots show that treatment of 3-D SKOV3ip1 spheroids with MORAB-003 led to elevated levels of ATG4B, ATG3, an ATG14-containing PIK3C3 complex with phosphorylated PIK3C3(S164), and ATG14. Level of unphosphorylated PIK3C3 was decreased upon MORAB-003 treatment. Fold-density is shown at the top of each panel, and control IgG was normalized as 1.
Blocking FRα with MORAB-003 induces sustained autophagy
To recapitulate the in vivo inhibitory effects of MORAB-003, we performed in vitro analyses in both 2-dimensional (2–D) cultured monolayer SKOV3ip1 cells and 3-dimensional (3–D) cultured SKOV3ip1 spheroid-like models (27). A 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay showed that MORAB-003 (5 µg/mL) treatment decreased proliferation of 3-D cultured SKOV3ip1 spheroids by ~40% in comparison with untreated controls (P <0.001; Fig. 2B), while it decreased proliferation of 2-D cultured SKOV3ip1 cells by only ~18% (Fig. 2B, P <0.001). Given the substantial difference between the effects of MORAB-003 in 2-D and 3-D conditions, we reasoned that the in vivo inhibitory mechanism of MORAB-003 may be recapitulated by 3-D cultured cancer spheroids (28), as shown by representative images for spheroids treated with IgG or MORAB-003 (Fig. 2B).
To determine if the inhibitory mechanism of MORAB-003 is related to apoptosis, we first performed FACS analysis with Annexin-V and propidium iodide (PI) staining in 3-D SKOV3ip1 spheroids. We observed no significant increase in cell apoptosis in treated cells, particularly not late-stage apoptosis (Annexin-V+PI+). The percentage of Annexin-V+PI− cells in the cells treated with control IgG (9.28%±0.98) was not significantly different than that in cells treated with MORAB-003 (12.0%±0.25) (Supplementary Figs. 2A & 2B). This indicates that the inhibitory effect of MORAB-003 on cell proliferation of 3-D SKOV3ip1 spheroids is likely not mediated through an apoptotic mechanism. We then tested various doses of MORAB-003 on the viability of cells in 3-D SKOV3ip1 spheroids and found that 5 µg/mL of MORAB-003 significantly reduced viability compared to controls (P <0.001, Supplementary Fig. 2C).
Next, reverse-phase protein array (RPPA) analysis was performed to determine the protein expression profile of SKOV3ip1 tumors. A number of autophagy factors were regulated by treatment with 5 µg/mL of MORAB-003, including mTOR and rapamycin-insensitive companion of mTOR (RICTOR), were downregulated (Fig. 2C), and S116-phosphorylated phosphoprotein enriched in astrocytes (S116-PEA-15; a factor involved in activation of autophagic cell death in cancer cells (29)), and beclin-1 (central mediator for formation of autophagosomes; Fig. 2C), were upregulated. RICTOR and mTOR are known to activate autophagy in response to environmental stress (30, 31). Levels of beclin-1 (32) and S116-PEA-15 were significantly increased (by >2-fold) by MORAB-003 treatment (Fig. 2D). The same screening by RPPA was performed in IGROV1 tumors, and the results also indicated that S116-PEA-15 and beclin-1 were upregulated by MORAB-003 treatment (Supplemental Fig. 3A).
We then analyzed the transcriptional profiles of SKOV3ip1 tumor cells using a cDNA microarray. Using a data-biased network analysis (23), we found that treatment with MORAB-003 significantly upregulated a number of genes involved in autophagy initiation and progress, including LC3II (MAP1LC3B), ATG3, ATG4B, and BECN1 (Fig. 2E, left panel), supporting the findings of the RPPA. To further confirm the regulation of autophagy signaling by MORAB-003, we determined the levels of ATG4B, ATG3, S164-phosphorylated PIK3C3, and its partner ATG14 (33), in 3-D cultured SKOV3ip1 spheroids. We found that these signaling cascades were upregulated by MORAB-003 treatment in comparison with controls (Fig. 2E, right panel). We also found that MORAB-003 treatment slightly decreased levels of the non-autophagic PIK3C3 complex.
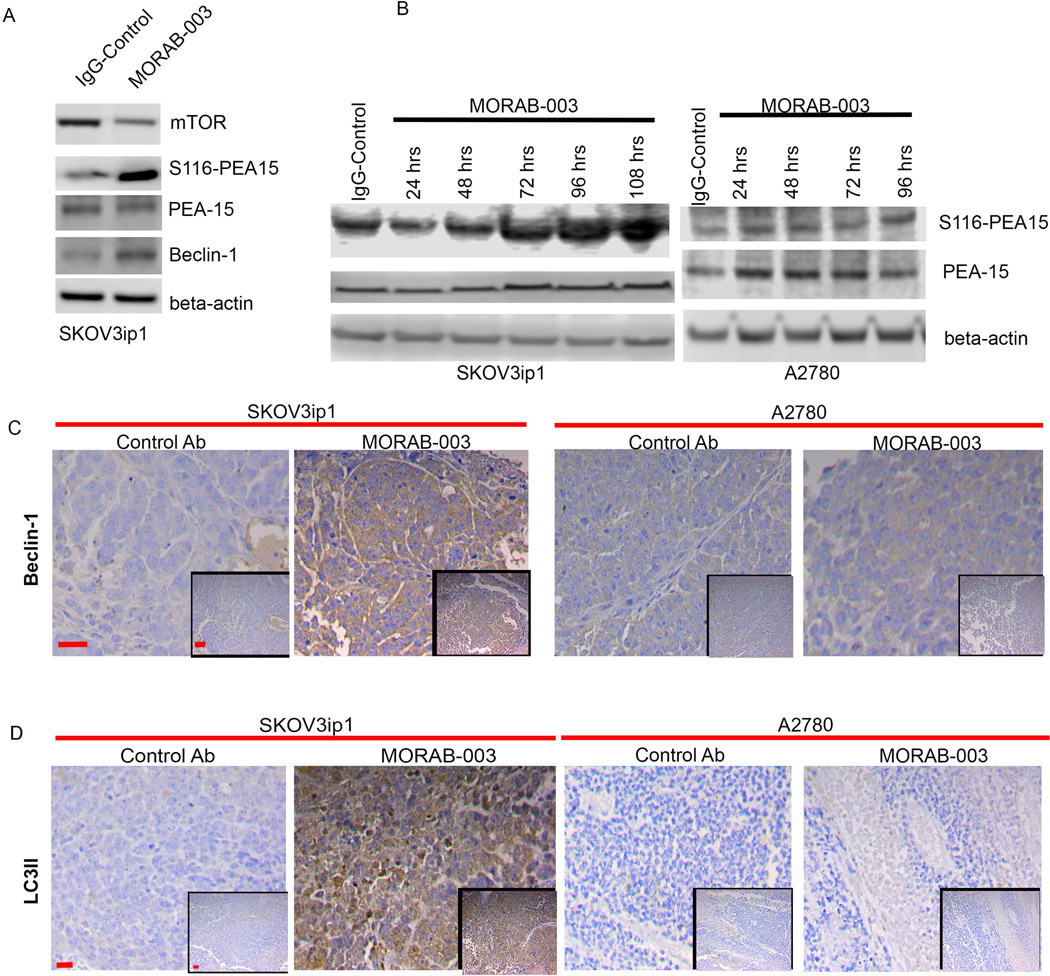
So far, little is known about the phosphorylation status of PEA-15 at Ser116 in tumors or the impact of this phosphorylation on the function of PEA-15 during tumor progression. Our studies discovered that S116-PEA-15 may play a tumor-suppressive role during MORAB-003–induced autophagy. This finding was validated by western blot analysis of lysates of SKOV3ip1 tumors, showing that the tumors of mice treated with MORAB-003 had significantly greater levels of S116-PEA-15 and beclin-1 and lower levels of mTOR than tumors from mice treated with IgG (Fig. 3A). Next, we examined the dynamic regulation of S116-PEA-15 during MORAB-003–induced autophagy. Accumulation of S116-PEA-15, rather than total PEA-15, reached its highest levels following treatment with MORAB-003 in 3-D SKOV3ip1 spheroids but not in A2780 spheroids. This finding indicated a role for activated S116-PEA-15 during MORAB-003–induced autophagy (Fig. 3B). We validated this finding in the IGROV1 3-D spheroids and found that knockdown of PEA-15 with siRNA blocked the autophagic flux, as measured by conversion of LC3I to LC3II and elevation of cleaved caspase-3 level (Supplemental Fig. 3B.) This indicated that MORAB-003–induced autophagy contributes to caspase-3–activated cell death.
Figure 3. Autophagic factors induced by MORAB-003 in 3-D ovarian cancer spheroids and in vivo tumor models.
(A) Expression of mTOR, S116-PEA-15, PEA-15, and beclin-1 was determined by immunoblotting lysates of 3-D SKOV3ip1 spheroids treated with IgG or MORAB-003. Beta-actin was applied as loading control.
(B) Time dynamics of S116-PEA-15 expression in 3-D SKOV3ip1 and A2780 spheroids. Beta-actin was applied as loading control.
(C) Representative tissue images show different expression patterns of beclin-1 in the SKOV3ip1 and A2780 tumor models. Treatment with MORAB-003 substantially increased beclin-1 expression in the SKOV3ip1 tumor model but not in the A2780 model.
(D) Representative tissue images show different expression patterns of LC3B in the SKOV3ip1 and A2780 tumor models. Treatment with MORAB-003 substantially increased the levels of LC3B in the SKOV3ip1 tumor model but not in the A2780 model.
Next, we determined the expression of selected autophagic markers, including beclin-1 and LC3II, in sections of SKOV3ip1 and A2780 tumors from the mouse models. Treatment with MORAB-003 raised the levels of beclin-1 substantially in the SKOV3ip1 tumors (Fig. 3C). Levels of LC3II, a marker for autophagic processing (16), were also elevated in SKOV3ip1 tumors treated with MORAB-003 (Fig. 3D). However, no increases of beclin-1 or LC3II levels were observed in the low-FRα A2780 tumors (Figs. 3C & 3D). We also examined the role of beclin-1 in MORAB-003–induced autophagy in vitro in the 3-D cultured IGROV1 cells. Knockdown of beclin-1 in these cells by a short-hairpin (shRNA) complex against BECN1 (Supplemental Fig. 3C) blocked the accumulation of LC3II and abolished the MORAB-003–induced autophagic flux. Knockdown of beclin-1 also reduced the MORAB-003–induced elevations in cleaved caspase-3 levels (Supplemental Fig. 3D). This result indicated that beclin-1 mediates MORAB-003–induced autophagy and plays a role in crosstalk for this autophagy with caspase-3–activated cell death.
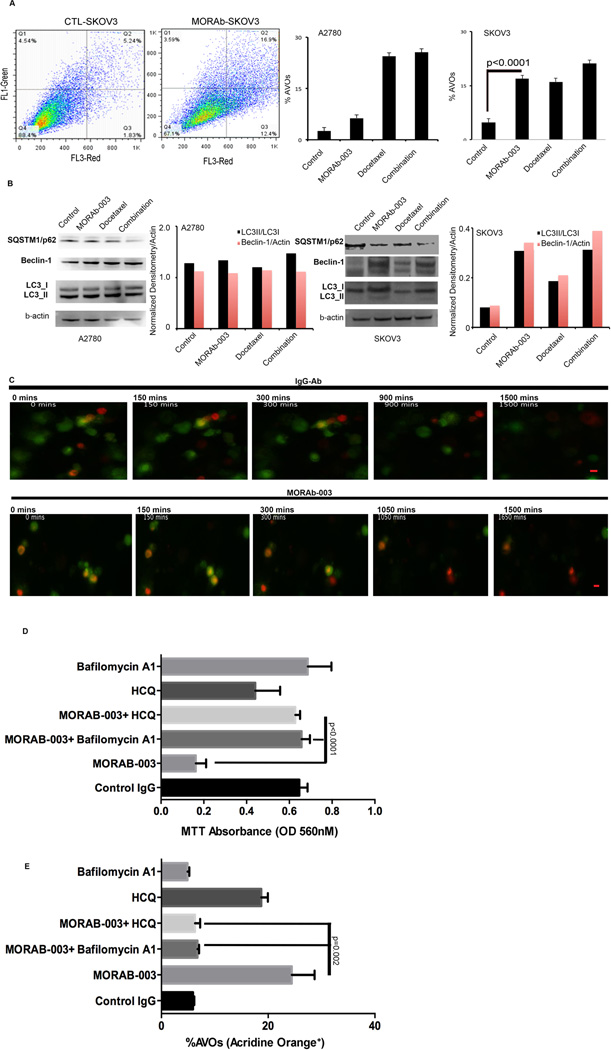
Thirdly, we quantitatively measured the accumulation of acidic vesicular organelles (AVOs) in cytoplasm using FACS with acridine orange (AO) staining (34). Treatment with MORAB-003, alone or in combination with docetaxel, significantly raised the percentages of AVOs (i.e., the AO+ population) in 3-D SKOV3ip1 spheroids (P <0.001, MORAB-003 vs. controls; P <0.001, MORAB-003+doxcetaxel vs. controls; Fig. 4A). However, MORAB-003 did not significantly increase the percentage of AVOs in 3-D A2780 spheroids (P >0.5, MORAB-003 vs. controls; Fig. 4A). We then determined the levels of autophagic markers, including SQSTM1/p62 (35), beclin-1, and conversion of LC3I to LC3II, in A2780 and SKOV3ip1 3-D spheroids by western blot. MORAB-003 treatment increased levels of beclin-1 and LC3II (converted from LC3I) in SKOV3ip1 cells but not in A2780 cells (Fig. 4B). The densitometry of beclin-1 and LC3II (converted from LC3I) in these blots was analyzed by Image J software (NIH). MORAB-003 treatment decreased the level of SQSTM1/p62, a specific autophagy substrate during autophagy maturation (36, 37), in the SKOV3ip1 model but not in A2780 model. This indicates a possibility that ubiquitin-protein degradation processes linked with lysosome fusion were initiated by MORAB-003.
Figure 4. Induction of sustained autophagy by MORAB-003.
(A) Upper panel: Representative FACS plot shows the percentage of AVOs, i.e., late-stage autophagosomes, as detected by AO staining, in SKOV3ip1 cells treated with control (CTL), MORAB-003 alone, docetaxel, or a combination of MORAB-003 with docetaxel. Lower panel: Percentage of AVOs in A2780 and SKOV3ip1 cells treated with MORAB-003 alone or in combination with docetaxel. Only in SKOV3ip1 cells, which express high levels of FRα, did MORAB-003 induce prominent increases in the AO+ population, which as late-stage autophagosomes lead to programmed cell death P <0.001 (control vs. MORAB-003).
(B) Expression of factors for autophagic processing, including SQSTM1/p62, conversion of LC3I to LC3II, and beclin-1, was measured by immunoblotting lysates from 3-D cultured A2780 and SKOV3ip1 cells. The upregulation of autophagy was validated by elevated levels of beclin-1 and LC3II in SKOV3ip1 cells but not in A2780 cells. Beta-actin was applied as loading control. The densitometry of beclin-1 (normalized to beta-actin) and LC3II (converted from LC3I) in each condition was determined by Image J software.
(C) Autophagic flux in 3-D SKOV3ip1 spheroids was observed by real-time videomicroscopy. 3-D SKOV3ip1 spheroids expressing pGFP-RFP-LC3 were treated with control IgG or MORAB-003 and the accumulation of late-stage autolysosomes was monitored by quantifying RFP+LC3 vesicles. Representative photomicrographs indicate the effect of IgG (upper panel) or MORAB-003 (lower panel) at approximate elapsed time of 0 minutes (min), 150 mins, 300 mins, 900 mins, and 1500 mins, showing the conversion of GFP+andRFP+LC3 to RFP+onlyLC3 in MORAB-003–treated spheroids and the destabilized GFP+LC3 in IgG-treated spheroids. Scale bar, 10 µm for all images. Larger magnified fields and high-resolution images are provided in Supplemental Figure 9A.
(D) The cytotoxicity of MORAB-003 was assessed by MTT assay in SKOV3ip1 cells cultured under 3-D conditions. After cells were exposed to physiologic levels of folic acid (7 days), they were treated with control IgG (72 hours), MORAB-003 (5 µg/mL, 72 hours), bafilomycin A1 (0.16 nM, 24 hours) plus MORAB-003 (72 hours), HCQ (1 nM, 24 hours) plus MORAB-003 (72 hours), HCQ alone (1 nM, 24 hours), or bafilomycin A1 alone (0.16 nM, 24 hours). Differences between groups were compared by unpaired t-test. Values are mean + SEM (n=6).
(E) The percentage of AVOs (measured by FACS with AO staining) in each group.
We then performed real-time videomicroscopy of 3-D SKOV3ip1 spheroids expressing a monomeric pGFP-RFP-LC3 plasmid (14, 38) to dynamically visualize LC3-labeled cytoplasmic vacuolation. The pGFP-RFP–tagged LC3 plasmid was stably transfected into 3-D SKOV3ip1 cells prior to treatment with MORAB-003 or IgG control (14). The GFP protein is acid sensitive and is degraded following fusion of autophagosomes with lysosomes, while the mRFP is relatively stable through this fusion. Since GFP-LC3 is degraded in a step-wise fashion in the autolysosome and RFP-LC3 is relatively stable in the acidic vacuoles enriched in autolysosomes, we used this method to monitor autophagic flux. SKOV3ip1 3-D spheroids treated with IgG antibody maintained stable expression of GFP-LC3 throughout the filming (Fig. 4C, upper panel). However, SKOV3ip1 3-D spheroids treated with MORAB-003 had detectable autophagic LC3 puncta with GFP/RFP-merged signals in the cytoplasm, indicating the formation of autophagosomes (RFP+andGFP+LC3, Fig. 4C, lower panel). More importantly, as a result of quenching of the acid-sensitive GFP signal by the low pH in the lysosomes, red puncta containing RFP+onlyLC3 (RFP+GFP−) became strongly visible in cells treated with MORAB-003 but not in cells treated with IgG control (Fig. 4C, Supplemental movies 1&2). Larger magnified images are presented in Supplemental Figure 4A.
We then performed confocal microscopy to locate the cytoplasmic LC3 puncta in 3-D SKOV3ip1 spheroids. We found distinct patterns of RFP-LC3 accumulation in the cytoplasm in MORAB-003–treated SKOV3ip1 cells compared with the IgG control (Supplemental Fig. 4B). These results indicate that in SKOV3ip1 3-D spheroids, MORAB-003 caused enrichment of late-stage autolysosomes containing RFP−LC3 only. To confirm the specificity of GFP fluorescence signaling, we also used a pGFP-LC3–only plasmid stably expressed in SKOV3ip1 cells to monitor the accumulation of GFP-LC3 in cytoplasm at the initiation stage of autophagy; it diminished at the elongation stage (Supplemental movie 3).
MORAB-003 induced autophagy associated with cell death
To determine if MORAB-003–induced autophagy links with cell death, we employed inhibitors of late-stage autophagy, including bafilomycin A1 (39) or hydroxychloroquine (HCQ) (40), to block the autophagic vacuolization and fusion. We found that both bafilomycin A1 and HCQ reversed the MORAB-003–induced reduction of cell viability in the SKOV3ip1 3-D spheroids (Fig. 4D). Addition of bafilomycin A1 to MORAB-003 or a single treatment with bafilomycin A1 decreased the percentages of AVOs in SKOV3ip1 3-D spheroids, indicating the blockade of MORAB-003–induced autophagosome formation (Fig. 4E). Addition of HCQ to MORAB-003 also decreased the percentages of AVOs in comparison with HCQ or MORAB-003 alone, possibly because accumulation of late-stage autolysosomes induced by MORAB-003 was blocked by HCQ through inhibition of lysosome acidification (Fig. 4E). Together, these results in 3-D cancer spheroids suggest that MORAB-003 treatment has potential for inhibiting tumor cell growth by sustaining late-stage autophagy.
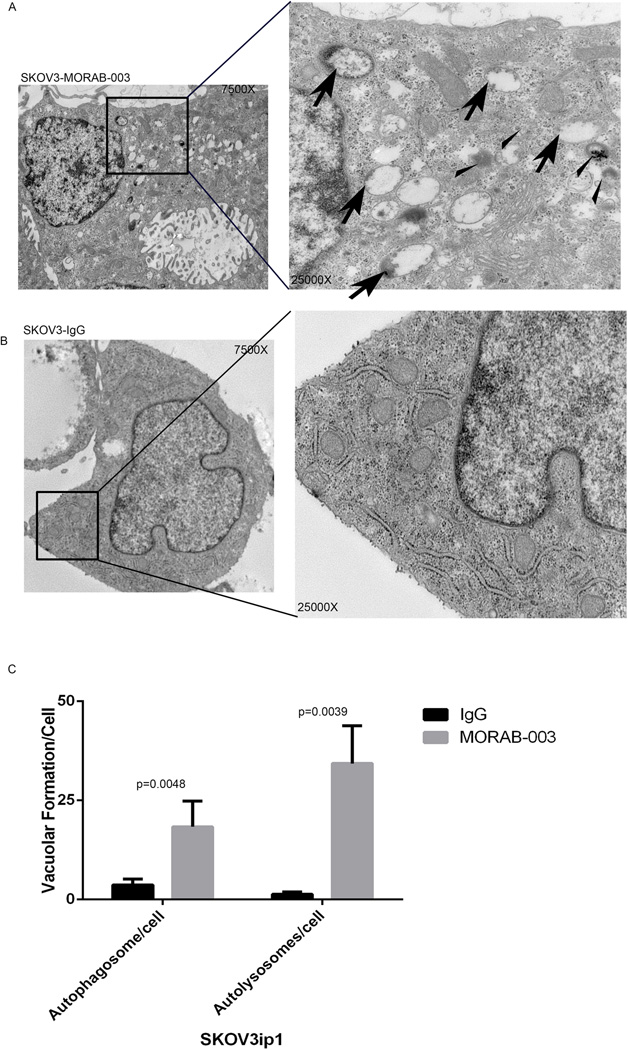
We then employed transmission election microscopy to analyze vacuole localization in SKOV3ip1 3-D spheroids. MORAB-003 induced large amounts of autophagosomes (also called early autophagic compartments), containing morphologically intact cytosol or organelles (shown by red arrows, Fig. 5A), and late-stage autophagy vacuoles, or autolysosomes, containing partially degraded cytoplasmic as well as organelle material (shown by yellow arrows, Fig. 5A). Furthermore, the MORAB-003–treated SKOV3ip1 spheroids had intact nuclear membranes and no apoptosomes (Fig. 5A). We did not observe the same amounts of autophagosomes or autolysosomes in spheroids treated with IgG (Fig. 5B). Vacuolar localization in SKVO3ip1 spheroids was quantified in 15 individual cells in 3 different sections, and the numbers of autophagosomes and autolysosomes in MORAB-003–treated cells were significantly higher than in controls (P < 0.05, Fig. 5C).
Figure 5. Vacuolar localization and autophagosome formation induced by MORAB-003 treatment.
Representative images from transmission electron microscopy show autophagic vesicles formed in SKOV3ip1 3-D spheroids treated with MORAB-003 (A) or IgG control (B). Substantial amounts of autophagosomes (Big arrows) and autolysosomes (Small arrows) were identified in the cytoplasm of the MORAB-003–treated spheroids, while no autophagosomes were observed in IgG-treated spheroids. Substructure was observed under 7500× and 25,000× magnification. Ultra-thin sections in representative areas from 10 fields are shown.
(C) Vacuolar formation, including autophagosomes and autolyosomes, was evaluated in 15 cells from 3 independent sections.
Association of FOLR1 with clinical outcome in human ovarian cancer
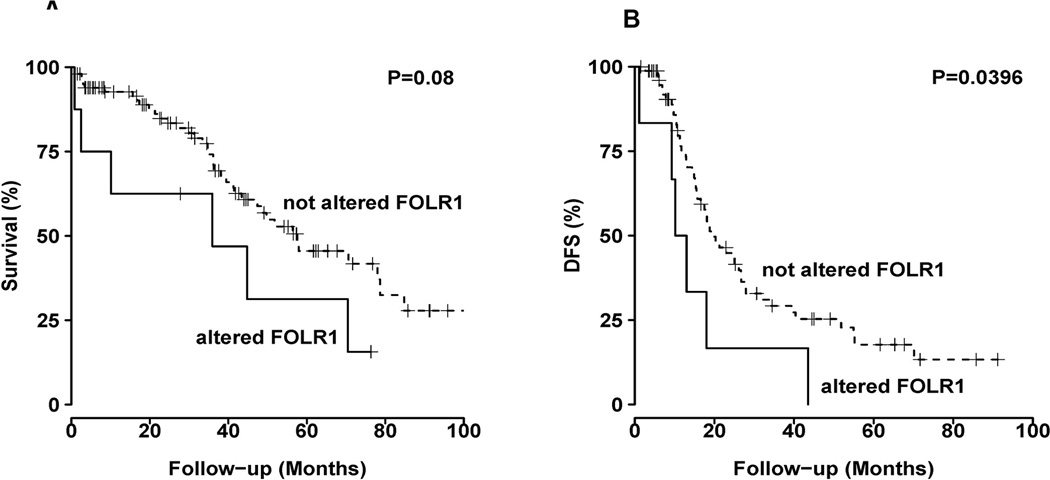
Given the paucity of data regarding the association between clinical outcome and FRα expression, we analyzed data from the Cancer Genome Atlas (TCGA) (24) to determine the correlation between copy numbers of FOLR1 gene and survival in patients with serous ovarian cystoadenocarcinoma. For patients with this immunoreactive tumor type, the Kaplan-Meier estimate for disease-free survival was significantly shorter in those whose tumor had an altered copy-number of the FOLR1 gene (n=6) than in those whose tumor expressed the unaltered FOLR1 gene (Fig. 6A & 6B, n=83, P=0.039, www.cbioportal.org) (TCGA, (24). We also examined FOLR1 gene expression in data from the Broad Institute’s CCLE cell lines. The epithelial cancer cells with the highest FOLR1 mRNA expression are endometrial and ovarian (Supplemental Fig. 4C). We then queried for alterations of FOLR1 gene copy-number in a set of patients with uterine corpus endometrial carcinoma (www.cbioportal.org) (TCGA,(25)). As in the ovarian cancer analysis, patients whose tumor had an altered FOLR1 gene copy-number had a significantly shorter life span than those whose tumor expressed unaltered FOLR1 (P=0.001855, Supplemental Fig. 4D). These data point to the potential clinical importance of alternation of FOLR1 gene copy number during development of serous ovarian cancer.
Figure 6. Clinical significance of FRα in patients with high-grade serous ovarian cancer.
(A) Overall survival in serous ovarian cancer patients whose tumors were of the immunoreactive subtype and expressed altered FOLR1 (n=8) or unaltered FOLR1 (n=102).
(B) Disease-free survival (DFS) in serous ovarian cancer patients whose tumors were of the immunoreactive subtype and expressed altered FOLR1 (n=6) or unaltered FOLR1 (n=83).
Discussion
In this study, we discovered that MORAB-003, a humanized monoclonal antibody against FRα, significantly reduced growth of human ovarian cancers in xenograft mouse models. Specifically, MORAB-003 significantly decreased tumor growth in the high-FRα IGROV1 and SKOV3ip1 models but not in the low-FRα A2780 model, and these effects were not related to apoptosis. MORAB-003 regulated an array of autophagy-related genes and significantly increased expression of LC3 isoform II and enriched autophagic vacuolization. Blocking autophagy with an inhibitor (HCQ or bafilomycin A1) reversed the growth inhibition induced by MORAB-003. These findings constitute in vivo and in vitro evidence that MORAB-003 induced sustained autophagy in SKOV3ip1 tumors, leading to growth inhibition.
Miotti and colleagues demonstrated that FRα modulated activities of signaling molecules including lyn kinase and G protein subunits (3). They showed that the active (phosphorylated) form of lyn kinase associated with FRα was translocated out of lipid rafts when FRα was inhibited by LK26 (precursor of MORAB-003) or the rate of cell division was reduced by over-confluence (3). These results suggest a role for FRα in intracellular signaling associated with tumor cell proliferation. Our study provides a new understanding of the in vivo effects of FRα inhibition (i.e., by MORAB-003) on ovarian cancer. The level of FRα expression was correlated with the extent of MORAB-003 therapeutic effect. We also discovered a correlation between level of FRα and the inhibitory effect of MORAB-003. These findings are consistent with previous studies showing that FRα confers a growth advantage to cells (19, 41, 42).
Our results further point to a previously unrecognized mechanism whereby blockade of FRα by MORAB-003 inhibits tumor growth via induction of cell death associated with sustained autophagy in FRα-positive cells. Typically induced by prolonged stress, sustained autophagy eventually leads to cell death when protein and organelle turnover overwhelm the capacity of the cell (21). It interrupts the metabolic system in proliferative tumor cells, serving as an alternative or complementary mechanism to tumor suppression through induction of type II programmed cell death (43) (44). This cell growth inhibition via sustained autophagy mediated by FRα inhibition is a novel mechanism for cell death (21) and an important addition to the previous reports on cell cycle– and apoptosis-regulatory mechanisms, including inhibition of phosphatidylinositol-3 kinase and Akt during antibody-mediated tumor inhibition by transtuzumab (Herceptin) (45) and the immune effects of rituximab (46). Our studies also showed that MORAB-003 significantly augmented the antitumor effect of docetaxel through an autophagy-associated mechanism. Given that lysosomotropic agents such as HCQ and chloroquine have been reported widely to inhibit autophagy in cancer treatment (47), this study provides new insight into the use of combinations of taxane-based regimens with MORAB-003 in improving therapeutic efficacy for ovarian cancer through induction of late-stage autophagy. The finding of association between autophagy induced by MORAB-003 and tumor growth inhibition is consistent with previous reports where such autophagic events eventually lead to type II programmed cell death and growth inhibition in tumor cells (48–50). In addition, PEA-15 is an acidic, serine-phosphorylated phosphoprotein and an endogenous substrate for calcium-calmodulin-dependent protein kinase II at ser116 position (51). However, the role of phosphorylation on PEA-15 that switches it from tumor suppressor to tumor promoter remains controversial. Our studies indicates that S116-PEA-15 plays a critical role during MORAB-003 induced autophagy that leads to cell death. Further studies are needed to characterize the roles of S116-PEA-15, S164-PIK3C3 and ATG14 complex in ovarian cancer during MORAB-003 mediated induced autophagy.
In summary, our findings indicate that the in vivo antitumor effects of MORAB-003 were mediated by late-stage autophagy in cancer cells expressing FRα and that targeting FRα with MORAB-003 is highly effective in reducing tumor growth in orthotopic ovarian cancer models. We conclude that the mechanism of FRα blockade–induced inhibition of tumor growth is autophagy-related cell death. Moreover, these data identify potential biomarkers related to autophagy that could be useful during clinical development of MORAB-003.
Supplementary Material
Translational Relevance.
In this study, we discovered that targeting folate receptor alpha (FRa) in ovarian cancer cells with specific humanized antibody MORAB-003 (farletuzumab) resulted in prominent antitumor activity. Our studies revealed that MORAB-003 inhibited tumor growth via induction of autophagy-associated cell death. This inhibitory effect of MORAB-003 can be recapitulated in three-dimensional in vitro models. Blocking autophagy reversed the growth inhibition induced by MORAB-003. Moreover, data from the Cancer Genome Atlas revealed that copy-number gains of the FOLR1 gene significantly correlated with shorter disease-free survival in patients with the immunoreactive subtype of ovarian serous carcinoma. Taken together, our results revealed a novel functional mechanism of MORAB-003 in inhibiting ovarian cancer growth.
Acknowledgements
We thank Mr. Kenneth Dunner, Jr., and Dr. Robert Langley for support in electronic microscopy and immunohistochemistry. We thank Ms. Kathryn L. Hale for editorial review.
Financial support:
This work was supported in part by grants from the US National Institutes of Health (P50CA083639, P50CA098258, P50 CA083639, CA109298, U54 CA151668, CA140933, CA177909, UH2TR000943, T32CA101642, and CA16672) and the Department of Defense (OC120547 and OC093416), by a Program Project Development Grant from the Ovarian Cancer Research Fund (CPRIT RP110595), and by the Bettyann Asche Murray Distinguished Professorship, the Chapman Foundation, the Meyer and Ida Gordon Foundation, the Gilder Foundation, and the RGK Foundation. This work was also supported by an award from the Blanton-Davis Ovarian Cancer Research Program to Y.W and A.K.S. Y.W. is supported in part by the NIH 5 P50 SPORE CDP Award CA116199, the Marsha Rivkin Center, and the Foundation for Women’s Cancer. W.M.M., Y.G.L., A.M.N., and W.A.S. were supported by a National Cancer Institute T32 Training Grant (T32 CA101642).
Footnotes
Statement of potential conflicts of interest: We report no conflicts of interest.
References
- 1.Rijnboutt S, Jansen G, Posthuma G, Hynes JB, Schornagel JH, Strous GJ. Endocytosis of GPI-linked membrane folate receptor-alpha. The Journal of cell biology. 1996;132:35–47. doi: 10.1083/jcb.132.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagnoli M, Canevari S, Figini M, Mezzanzanica D, Raspagliesi F, Tomassetti A, et al. A step further in understanding the biology of the folate receptor in ovarian carcinoma. Gynecol Oncol. 2003;88:S140–S144. doi: 10.1006/gyno.2002.6705. [DOI] [PubMed] [Google Scholar]
- 3.Miotti S, Bagnoli M, Ottone F, Tomassetti A, Colnaghi MI, Canevari S. Simultaneous activity of two different mechanisms of folate transport in ovarian carcinoma cell lines. J Cell Biochem. 1997;65:479–491. [PubMed] [Google Scholar]
- 4.Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecologic oncology. 2008;108:619–626. doi: 10.1016/j.ygyno.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. International journal of cancer. 1997;74:193–198. doi: 10.1002/(sici)1097-0215(19970422)74:2<193::aid-ijc10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani LT, Miotti S, Menard S, Canevari S, Raspagliesi F, Bottini C, et al. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19. European journal of cancer. 1994;30A:363–369. doi: 10.1016/0959-8049(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 7.Leamon ALJCP. Targeted drug strategies for cancer and inflammation. New York: Springer; 2011. p. 162. BOOK. [Google Scholar]
- 8.Garin-Chesa P, Campbell I, Saigo PE, Lewis JL, Jr, Old LJ, Rettig WJ. Trophoblast and ovarian cancer antigen LK26. Sensitivity and specificity in immunopathology and molecular identification as a folate-binding protein. Am J Pathol. 1993;142:557–567. [PMC free article] [PubMed] [Google Scholar]
- 9.Kalli KR. MORAb-003, a fully humanized monoclonal antibody against the folate receptor alpha, for the potential treatment of epithelial ovarian cancer. Current opinion in investigational drugs. 2007;8:1067–1073. [PubMed] [Google Scholar]
- 10.Lin J, Spidel JL, Maddage CJ, Rybinski KA, Kennedy RP, Krauthauser CL, et al. The antitumor activity of the human FOLR1-specific monoclonal antibody, farletuzumab, in an ovarian cancer mouse model is mediated by antibody-dependent cellular cytotoxicity. Cancer biology & therapy. 2013;14:1032–1038. doi: 10.4161/cbt.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halder J, Kamat AA, Landen CN, Jr, Han LY, Lutgendorf SK, Lin YG, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–4924. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Lu Y. Optimizing a 3D Culture System to Study the Interaction between Epithelial Breast Cancer and Its Surrounding Fibroblasts. Journal of Cancer. 2011;2:458–466. doi: 10.7150/jca.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 15.Bartholomeusz C, Gonzalez-Angulo AM, Kazansky A, Krishnamurthy S, Liu P, Yuan LX, et al. PEA-15 inhibits tumorigenesis in an MDA-MB-468 triple-negative breast cancer xenograft model through increased cytoplasmic localization of activated extracellular signal-regulated kinase. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1802–1811. doi: 10.1158/1078-0432.CCR-09-1456. [DOI] [PubMed] [Google Scholar]
- 16.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. The EMBO journal. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 18.Landen CN, Jr, Lu C, Han LY, Coffman KT, Bruckheimer E, Halder J, et al. Efficacy and antivascular effects of EphA2 reduction with an agonistic antibody in ovarian cancer. J Natl Cancer Inst. 2006;98:1558–1570. doi: 10.1093/jnci/djj414. [DOI] [PubMed] [Google Scholar]
- 19.Ebel W, Routhier EL, Foley B, Jacob S, McDonough JM, Patel RK, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer immunity. 2007;7:6. [PMC free article] [PubMed] [Google Scholar]
- 20.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, et al. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 21.Wen Y, Zand B, Ozpolat B, Szczepanski MJ, Lu C, Yuca E, et al. Antagonism of tumoral prolactin receptor promotes autophagy-related cell death. Cell reports. 2014;7:488–500. doi: 10.1016/j.celrep.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Molecular cancer therapeutics. 2006;5:2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 23.Komurov K, White MA, Ram PT. Use of data-biased random walks on graphs for the retrieval of context-specific networks from genomic data. PLoS computational biology. 2010;6 doi: 10.1371/journal.pcbi.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancer Genome Atlas Research N, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:5288–5295. doi: 10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 27.Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecologic oncology. 2009;113:143–148. doi: 10.1016/j.ygyno.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 28.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nature reviews Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 29.Steffensen KD, Alvero AB, Yang Y, Waldstrom M, Hui P, Holmberg JC, et al. Prevalence of epithelial ovarian cancer stem cells correlates with recurrence in early-stage ovarian cancer. Journal of oncology. 2011;2011:620523. doi: 10.1155/2011/620523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welberg L. RICTOR in acti(o)n. Nature reviews Neuroscience. 2013;14:225. doi: 10.1038/nrn3478. [DOI] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Current biology : CB. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell death and differentiation. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan HX, Russell RC, Guan KL. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy. 2013;9:1983–1995. doi: 10.4161/auto.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishikawa T, Tsuno NH, Okaji Y, Sunami E, Shuno Y, Sasaki K, et al. The inhibition of autophagy potentiates anti-angiogenic effects of sulforaphane by inducing apoptosis. Angiogenesis. 2010;13:227–238. doi: 10.1007/s10456-010-9180-2. [DOI] [PubMed] [Google Scholar]
- 35.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Molecular cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Dikic I, Johansen T, Kirkin V. Selective autophagy in cancer development and therapy. Cancer research. 2010;70:3431–3434. doi: 10.1158/0008-5472.CAN-09-4027. [DOI] [PubMed] [Google Scholar]
- 37.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 38.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aguilar-Gallardo C, Rutledge EC, Martinez-Arroyo AM, Hidalgo JJ, Domingo S, Simon C. Overcoming challenges of ovarian cancer stem cells: novel therapeutic approaches. Stem cell reviews. 2012;8:994–1010. doi: 10.1007/s12015-011-9344-5. [DOI] [PubMed] [Google Scholar]
- 40.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bottero F, Tomassetti A, Canevari S, Miotti S, Menard S, Colnaghi MI. Gene transfection and expression of the ovarian carcinoma marker folate binding protein on NIH/3T3 cells increases cell growth in vitro and in vivo. Cancer research. 1993;53:5791–5796. [PubMed] [Google Scholar]
- 42.Figini M, Ferri R, Mezzanzanica D, Bagnoli M, Luison E, Miotti S, et al. Reversion of transformed phenotype in ovarian cancer cells by intracellular expression of anti folate receptor antibodies. Gene Ther. 2003;10:1018–1025. doi: 10.1038/sj.gt.3301962. [DOI] [PubMed] [Google Scholar]
- 43.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The role of autophagy in cancer: therapeutic implications. Molecular cancer therapeutics. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nature reviews Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer research. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 46.Audia S, Samson M, Guy J, Janikashvili N, Fraszczak J, Trad M, et al. Immunologic effects of rituximab on the human spleen in immune thrombocytopenia. Blood. 2011;118:4394–4400. doi: 10.1182/blood-2011-03-344051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer research. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 48.White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Current opinion in cell biology. 2010;22:212–217. doi: 10.1016/j.ceb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamat AA, Coffey D, Merritt WM, Nugent E, Urbauer D, Lin YG, et al. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer. 2009;115:2684–2692. doi: 10.1002/cncr.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubes M, Cordier J, Glowinski J, Girault JA, Chneiweiss H. Endothelin induces a calcium-dependent phosphorylation of PEA-15 in intact astrocytes: identification of Ser104 and Ser116 phosphorylated, respectively, by protein kinase C and calcium/calmodulin kinase II in vitro. Journal of neurochemistry. 1998;71:1307–1314. doi: 10.1046/j.1471-4159.1998.71031307.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.